Abstract
Purpose
Trastuzumab-emtansine (T-DM1), as well as lapatinib plus capecitabine were proven effective in two Phase III studies, following first-line trastuzumab plus a taxane. The introduction of dual HER2 blockade by trastuzumab and pertuzumab as first-line has positioned T-DM1 into second-line, and lapatinib plus capecitabine beyond, without formal evaluation of these strategies.
Methods
ESME Data Platform (NCT03275311) included individual data from all patients aged ≥18 years, in whom first-line treatment for metastatic breast cancer (MBC) was initiated between January 1, 2008 and December 31, 2016 in one of the 18 French Comprehensive Cancer Centers. The efficacy of T-DM1 and lapatinib plus capecitabine combination, following double blockade associating trastuzumab and pertuzumab were evaluated in this national real-life database. Eligibility criteria were: female, MBC, HER2+ tumor, first-line taxane-based chemotherapy and dual HER2-blockage by trastuzumab plus pertuzumab. Cohort A received second-line T-DM1, and Cohort B second-line T-DM1 and third or fourth-line lapatinib plus capecitabine.
Results
Cohort A comprised 233 patients, and Cohort B 47 patients. Median progression-free survival (PFS) was 7.1 months in Cohort A and 4.6 months in Cohort B. Median overall survival were 36.7 months and 12.9 months, respectively. PFS was significantly dependent on the preceding treatment line's duration. In cohort A, HER2 expression status was a significant predictive factor of PFS.
Conclusion
First-line trastuzumab plus pertuzumab do not markedly diminish T-DM1's efficacy in second-line. Similarly, sequential treatment with trastuzumab plus pertuzumab then T-DM1 does not noticeably modify the efficacy of lapatinib plus capecitabine.
Keywords: Dual HER2 blockade, Lapatinib, Metastatic breast cancer, Overall survival, Progression-free survival, T-DM1
Highlights
-
•
French real-life cohort.
-
•
Dual blockade HER2 does not markedly diminish T-DM1's activity in second-line and lapatinib's activity in third or fourth line.
-
•
The second-line (T-DM1) PFS was significantly longer when the first-line treatment with trastuzumab plus pertuzumab was ≥12 months.
1. Introduction
The Epidemiological Strategy and Medical Economics (ESME)-Metastatic Breast Cancer (MBC) cohort has demonstrated a major overall survival (OS) improvement between 2008 and 2017 in HER2 positive MBC thanks to new treatment options [1]. The optimal sequence of anti-HER2 therapies is still an open field, as new agents are approved.
The tyrosine kinase inhibitor lapatinib in combination with capecitabine received approval in 2008 for the management of MBC. This authorization was based on the results of the Phase III EGF 100 151 study, which evaluated this combination's efficacy following failure of first-line treatment combining trastuzumab with a taxane [2]. The capecitabine plus lapatinib combination resulted in a significantly longer progression-free survival (PFS) compared with capecitabine given alone (6.2 months versus 4.3 months, HR = 0.51 [0.35–0.74], p < 0.001).
This position of the lapatinib plus capecitabine combination in the second-line setting for MBC was subsequently taken by trastuzumab emtansine (T-DM1), with lapatinib being pushed beyond the second-line setting.
The antibody drug conjugate (ADC) trastuzumab-emtansine (T-DM1) received approval for MBC management in 2013, on account of the positive results of the Phase III EMILIA study, which evaluated its efficacy following failure of first-line treatment combining trastuzumab with a taxane [3]. Indeed, the EMILIA study demonstrated T-DM1 to be associated with a significantly improved PFS in second-line, compared to the reference treatment consisting of capecitabine plus lapatinib (9.6 months versus 6.4 months, HR = 0.65 [0.55–0.77], p < 0.001).
HER2-positive MBC treatment has been further improved with the advent of pertuzumab given in association with trastuzumab, based on the results of the Phase III CLEOPATRA study conducted in MBC first-line setting [4,5]. Compared to the reference treatment consisting of trastuzumab in association with a taxane, the dual HER2 blockade by trastuzumab plus pertuzumab with a taxane resulted in both a significantly longer PFS (18.5 months versus 12.4 months, HR 0.62 [0.51–0.75], p < 0.001) and a significantly longer overall survival (OS) (56.5 months versus 40.8 months, HR 0.68 [0.58–0.80], p < 0.001).
T-DM1 stayed the best second line choice even formally investigated after first-line dual blockade. Lapatinib has been pushed beyond T-DM1.
Nevertheless, efficacy data concerning these sequences of treatment strategies are scarce, and we employed the ESME cohort to further assess the efficacy of T-DM1 and lapatinib following failure of first-line treatment using trastuzumab plus pertuzumab combined with a taxane in the world.
2. Materials and methods
2.1. Study design
In 2014, the UNICANCER group (composed of 18 French Comprehensive Cancer Centers, managing together over one-third of all breast cancer cases nationwide) launched the ESME academic initiative, in order to investigate real-world data in solid tumors. 22 463 patients were included between January 1, 2008 and December 31, 2016 [6].
The ESME research program is managed by R&D Unicancer in accordance with current best practice guidelines and rules [7,8]. The program is supervised by a scientific independent steering committee, which approved the current work. In addition, the study was authorized by the French data protection authority ([Registration ID 1704113 and authorization N_DE-2013.-117], NCT03275311). All data were exclusively obtained retrospectively, with no procedure taken to recover unavailable data by contacting healthcare providers or patients.
For the current study, eligible patients to the first cohort had to meet the following criteria: female gender, MBC, HER2 positive tumor, a first-line treatment with taxane-based chemotherapy and dual HER2-blockade (trastuzumab + pertuzumab), and a second-line with T-DM1 (Cohort A). Among the cohort A, patients treated with a third or a fourth-line by lapatinib plus capecitabine were included in the second cohort (Cohort B).
2.2. Objectives and endpoints
The primary endpoints were the evaluation of PFS and OS in these two cohorts. The secondary endpoint was the evaluation of MBC's hormonal receptor (HR) status, HER2 expression status, presence of hepatic metastasis and duration of previous treatment line as predictive factors for survival outcomes.
2.3. Definitions
HER2 and HR status were derived from existing results involving metastatic tissue sampling where available, or, if not available from last sampling on early disease. Tumors were defined as HR-positive if estrogen receptor or progesterone receptor expression by immunohistochemistry (IHC) was >10% according to French guidelines [9]. An IHC score 3+, IHC score 2+ with a positive fluorescence in situ hybridization (FISH), or chromogenic in situ hybridization classified the cancer as HER2 positive.
PFS was defined as time between the beginning of the second-line treatment (cohort A) or the third/fourth-line treatment (cohort B) and the first event counting for progression or death. Patients without any event were censored at the date of their last news. OS was defined as time between the beginning of the second-line treatment (cohort A) or the third/fourth-line treatment (cohort B) and the death for deceased patients. Patients alive were censored at the date of the last news.
The guidelines used to define HER2-positivity were consistent with the different editions of the American Society of Clinical Oncology/College of American Pathologists criteria in use at the time of sample analysis [[10], [11], [12]].
De novo metastatic disease was defined as regards patients who had metastases at the time of primary tumor diagnosis or within 180 days.
2.4. Statistical analysis
All efficacy analyses were based on patients that met the inclusion criteria and had received at least one T-DM1 or lapatinib plus capecitabine treatment course. Descriptive statistics were utilized to summarize patient demographics, clinical characteristics, and treatment patterns. Categorical variables were expressed as frequency and percentage, and continuous variables as mean and standard deviation or median and range.
OS and PFS were estimated using Kaplan-Meier Method. Statistical analysis was performed using R software, version R 3.6.3 Time-to-event outcomes were analysed using both log-rank tests and Cox univariate and multivariate regression methods with P < 0.05 considered statistically significant.
3. Results
3.1. Patients
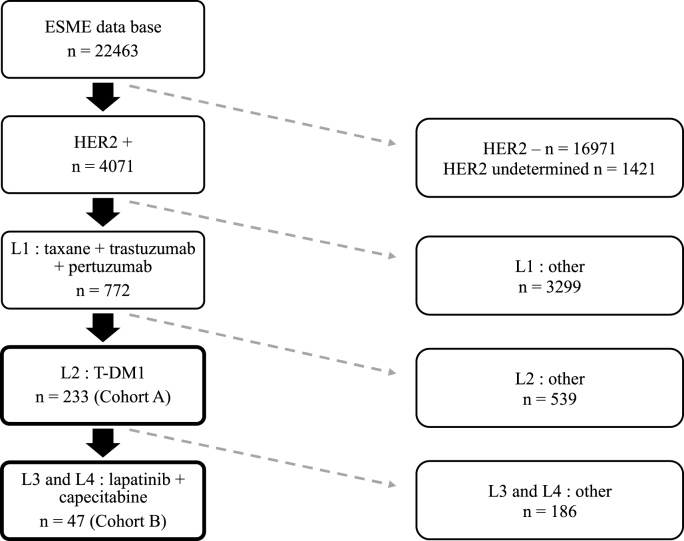
Cohort A treated with T-DM1, comprised 233 patients (Fig. 1), with a median follow-up of 20.8 months (0–61). The median age at metastasis diagnosis was 52 years. Overall, 106 patients (45.5%) exhibited de novo metastatic disease, and 125 (53.6%) recurrent disease (2 missing data). Tumor estrogen receptor status was positive (ER+) in 133 patients (57.6%). The distribution of metastatic sites is provided in Table 1. Liver metastases were observed in 114 patients (48.9%), and brain metastases in 18 patients (7.7%) at metastasis diagnosis. The median duration of first-line treatment (trastuzumab plus pertuzumab with taxane) was 11.3 months (95%CI [9.9; 12.1]). HER2 expression had a score 3+ in 213 patients (91.4%) and a score 2+ (FISH amplified) in 20 patients (8.6%).
Fig. 1.
Flow chart: cohort A and B.
Table 1.
Patients characteristics.
| Cohort A | Cohort B | |
|---|---|---|
| Number of patients | 233 | 47 |
| Median age (years) | 52 | 48 |
| Median follow-up (months) | 20.8 | 13.8 |
| Hormone receptor status | ||
| ER and/or PR positive | 133 (57.6%) | 23 (50,0%) |
| ER and PR negative | 98 (42.4%) | 23 (50,0%) |
| Unknown | 2 | 1 |
| Metastasis sites at diagnosis MBC | ||
| Bone | 121 (51.9%) | 24 (51.1%) |
| Brain | 18 (7.7%) | 4 (8.5%) |
| Liver | 114 (48.9%) | 20 (42.5%) |
| Lung | 94 (40.3%) | 23 (48,9%) |
| Soft tissuea | 106 (45.5%) | 21 (44.7%) |
| Other | 20 (8.6%) | 5 (10.6%) |
| HER2 status | ||
| HER2 3+ | 213 (91.8%) | 47 (100%) |
| HER2 2+ (FISH amplified) | 19 (8.2%) | 0 (−) |
ER estrogen receptor, PR progesterone receptor.
Soft tissue includes lymph nodes, skin, pleura, and peritoneum.
Cohort B treated with lapatinib plus capecitabine combination, comprised 47 patients (Fig. 1), with a median follow-up of 13.8 months (4.8–31.4). The median age was 48 years. Overall, 17 patients (36.2%) exhibited de novo metastatic disease, and 30 (63.8%) recurrent disease. The lapatinib plus capecitabine association was administered in third-line for 40 patients, and in fourth-line for 7 patients. Tumor was ER+ in 23 patients (50%). The distribution of metastatic sites is provided in Table 1. Liver metastases were observed in 20 patients (42.5%), and brain metastases in 4 patients (8.5%). The median duration of first-line treatment (trastuzumab plus pertuzumab with taxane) was 9.9 months (95%CI [7.5; 11.4]). The median duration of second-line (T-DM1) was 4.1 months (95%CI [3.5; 5.6]).
3.2. Progression-free survival and overall survival
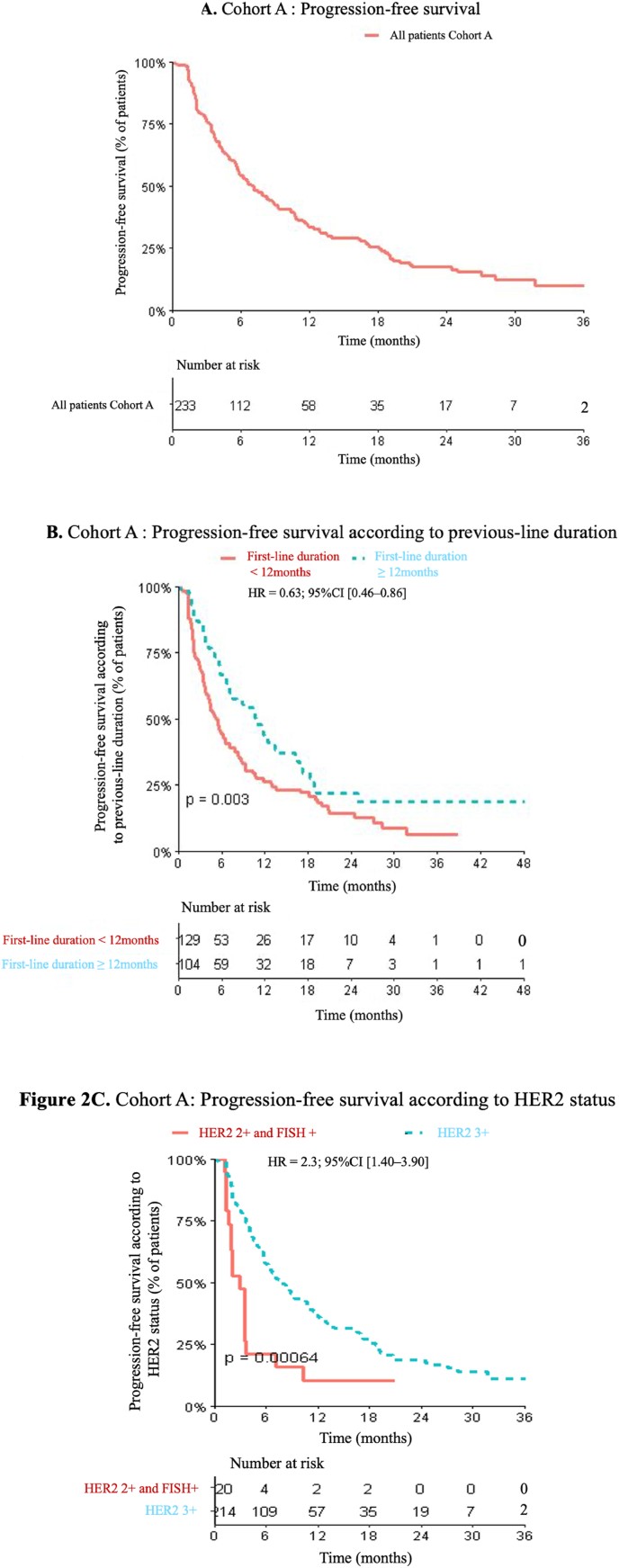
In Cohort A, median PFS was 7.1 months (95%CI [5.72; 8.98]), and median OS was 36.7 months (95%CI [28.3; NA]) (Fig. 2A). No statistically significant differences in PFS were observed depending on the tumor ER status (HR = 1.03; 95%CI [0.76–1.4], p = 0.71). Likewise, PFS was not impacted by the presence of liver metastases, by metastatic de novo status and by trastuzumab treatment in adjuvant or neoadjuvant setting (Table 2). The median OS was 19.1 months (95%CI [10.9–NA]) for patients with brain metastasis at metastasis diagnosis compared to 36.7 months (95%CI [28.3–NA]) for patient without brain metastasis (p = 0.014) (supplementary data 1).
Fig. 2.
A. Cohort A: Progression-free survival. B. Cohort A: Progression-free survival according to previous-line duration, C. Cohort A: Progression-free survival according to HER2 status.
Table 2.
Univariate and multivariate analysis of risk factors for progression: cohort A.
| Factors | n | HR | Univariate |
pvalue | Multivariate |
P multi |
|---|---|---|---|---|---|---|
| IC95% (HR) | HR IC95% (HR) | |||||
| Age at metastatic diagnosis | 233 | 1 | [0.99; 1.02] | 0.723 | 1.00 [0.99; 1.01] | 0.942 |
| Hormone receptor status | ||||||
| 1 - Négative | 98 | 1 | 0.709 | 1 | 0.800 | |
| 2 - Positive | 133 | 1.03 | [0.76; 1.40] | 1.08 [0.76; 1.48] | ||
| 3 - unknown | 2 | 1.92 | [0.47; 7.86] | 1.48 [0.36; 6.18] | ||
| Liver metastasis | ||||||
| 0 - no | 119 | 1 | 0.371 | 1 | 0.093 | |
| 1 - yes | 114 | 1.15 | [0.85; 1.55] | 1.32 [0.95; 1.83] | ||
| First line duration | ||||||
| <12 mois | 129 | 1 | 0.003 | 1 | 0.017 | |
| ≥12 mois | 104 | 0.63 | [0.46; 0.86] | 0.66 [0.49; 0.91] | ||
| HER2 status | ||||||
| HER2 3+ | 212 | 1 | 0.004 | 1 | 0.001 | |
| HER2 2+ (FISH amplified) | 20 | 2.29 | [1.38; 3.88] | 2.28 [1.36; 3.84] | ||
| De novo/recurrent | ||||||
| De novo | 106 | 1 | 0.285 | 1 | 0.1027 | |
| Recurrent | 125 | 1.18 | [0.87; 1.60] | 1.44 [0.94; 2.22] | ||
| Trastuzumab in neo/adjuvant setting | ||||||
| No | 152 | 1 | 0.745 | 1 | 0.582 | |
| Yes | 81 | 1.05 | [0.77; 1.45] | 0.88 [0.57; 1.37] |
The duration of first-line treatment (trastuzumab plus pertuzumab combined with taxane) exerted a significant impact on the PFS length under T-DM1 (Fig. 3). For the patients that displayed a first-line duration <12 months, the median PFS under T-DM1 was 5.4 months (95%CI [4.08; 7.07]). For those patients exhibiting a first-line duration ≥12 months, the median PFS was 10.8 months (95%CI [7.47; 13.91]). This between-group difference was significant (HR = 0.63; 95%CI [0.46–0.86], p = 0.003) (Fig. 2B).
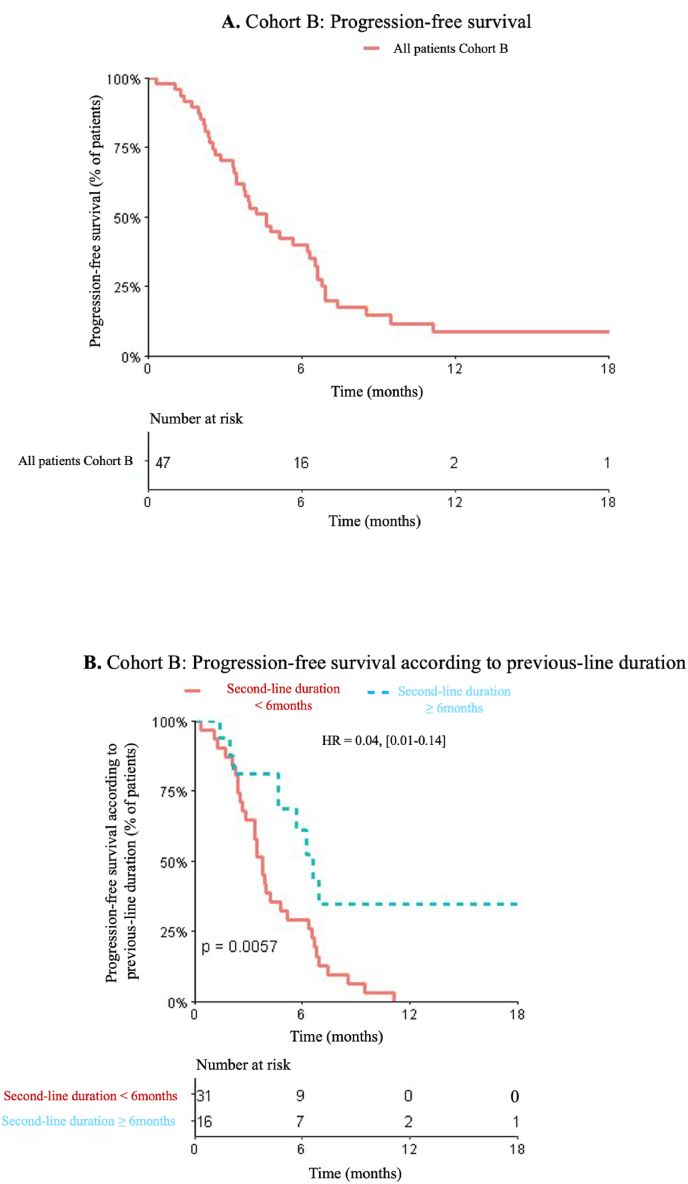
Fig. 3.
A. Cohort B: Progression-free survival. B. Cohort B: Progression-free survival according to previous-line duration.
The HER2 status was a statistically significant prognostic biomarker, with 6-months PFS of 57.4% and 21.1% for patients with HER2 3+ and HER2 2+ status (FISH+), respectively (HR = 2.3, 95%CI [1.4–3.9], p = 0.0013). The median PFS was 2.8 months (95%CI [1.1–7.1]) for HER2 2+ and 7.9 months (95%CI [6.4–10.7]) for HER2 3+ (HR = 2.3 95%CI [1.4–3.9] p = 0.0013) (Fig. 2C) (Table 2).
In Cohort B, the median PFS was 4.6 months (95%CI [3.45; 6.55]), and the median OS was 12.9 months (95%CI [6.94; NA]) (Fig. 3A). For the 40 patients who were treated with lapatinib plus capecitabine in third-line, the median PFS was 4.7 months (95%CI [3.75–6.61]). The PFS length was not significantly impacted by either ER status (HR = 1.21, 95%CI [0.65; 2.26], p = 0.83) or presence/absence of liver metastases (HR = 0.91, 95%CI [0.49; 1.7], p = 0.77).
The PFS length under lapatinib plus capecitabine was significantly longer in those patients in whom the T-DM1 duration was superior to 6 months (HR = 0.04, [0.01–0.14], p = 0.0057). The median PFS was 3.8 months (95%CI [2.83; 6.32]) if the T-DM1 duration <6 months versus 6.2 months (95%CI [4.64; NA]) if the T-DM1 duration was ≥ 6 months (Fig. 3B) (Table 3).
Table 3.
Univariate and multivariate analysis of risk factors for progression: cohort B.
| Factors | n | HR | Univariate |
pvalue | Multivariate |
pvalue |
|---|---|---|---|---|---|---|
| IC95% (HR) | HR IC95% | |||||
| Hormone receptor status | ||||||
| 1 - Négative | 23 | 1 | 0.828 | 0.850 | ||
| 2 - Positive | 23 | 1.21 | [0.65; 2.26] | 0.83 [0.43; 1.61] | ||
| 3 - unknown | 1 | 0.99 | [0.13; 7.49] | 1.1 [0.14; 8.88] | ||
| Liver metastasis | ||||||
| 0 - no | 27 | 1 | 0.774 | 0.950 | ||
| 1 - yes | 20 | 0.91 | [0.49; 1.70] | 1.02 [0.5; 2.08] | ||
| First line duration | ||||||
| <12 mois | 33 | 1 | 0.244 | 0.212 | ||
| ≥12 mois | 14 | 0.67 | [0.33; 1.34] | 0.64 [0.31; 1.31] | ||
| TDM1 duration | ||||||
| <6 mois | 28 | 1 | 0.006 | 0.035 | ||
| ≥6 mois | 19 | 0.04 | [0.01; 0.14] | 0.47 [0.23; 0.98] |
4. Discussion
In this real-life cohort of HER2 positive MBC patients, we found survival data that were consistent with the previously reported results from the two pivotal studies. In Cohort A, the median PFS was 7.1 months in patients treated with T-DM1, following progression upon dual HER2-blockade by trastuzumab and pertuzumab combined with taxane-based chemotherapy (Fig. 2A). The EMILIA study reported a median PFS of 9.6 months after prior taxane-based chemotherapy plus trastuzumab [3]. The median OS was 36.7 months in our study, compared to the 29.9 months obtained in the EMILIA study for T-DM1-treated patients [13].
Our analysis did not reveal any difference in PFS outcome according to tumor HR status. In the EMILIA study, the superiority of T–DM1 in comparison with the reference treatment was similarly observed in both ER-positive (HR = 0.72 [0.58–0.91]) and ER-negative tumors (HR = 0.56 [0.44–0.72]) [3]. Likewise, the presence or absence of hepatic metastases had no impact on PFS in our series.
In Cohort A, the second-line (T-DM1) PFS was significantly longer when the first-line treatment with trastuzumab plus pertuzumab was ≥12 months (10.8 months versus 5.4 months, HR = 0.63; 95%CI [0.46–0.86], p = 0.003) (Fig. 2).
Three retrospective studies have evaluated T-DM1 efficacy following dual HER2-blockade by trastuzumab and pertuzumab. The retrospective American study involved 78 patients, with 32% receiving T-DM1 as first- and second-line line therapy, and 48% receiving it as fourth-line or later therapy. The median duration of therapy was 4 months (95%CI [0.46–0.86]) [14]. An Italian study, involving 77 patients, found a median PFS of 6.3 months (95%CI [4.80–7.70]) [15]. In patients with a prior pertuzumab-based therapy duration ≥1 year, the median PFS with T-DM1 was 8 months versus a median PFS of 6 months with prior pertuzumab-based therapy <1 year. This between-group difference was not significant. In a Japanese study involving 42 patients, the median PFS was 2.8 months (95%CI [1.70–4.80]) in the group who received pertuzumab plus trastuzumab (n = 18) versus 7.8 months (95%CI [(5.50–11.90]) in the group who received only trastuzumab in first-line (n = 24) [16].
In the exploratory biomarker analyses of EMILIA and TH3RESA, which recruited patients with previously treated HER2-positive MBC, single-agent T-DM1 was associated with numerically longer PFS in the subgroup of patients with higher versus lower levels of HER2 mRNA [17,18]. Previous studies have demonstrated that more HER2 heterogeneity was reported in IHC 2+ versus IHC 3+ tumors [19,20]. In the present study, HER2 expression is a strong prognostic factor with significant shorter PFS for patients with HER2 2+ tumors (2.8 months) versus patients with HER2 3+ tumors (7.9 months) (Fig. 2C).
Cohort B comprised 47 patients treated with capecitabine plus lapatinib, resulting in a median PFS of 4.6 months (Fig. 3A). For the 40 patients who received the capecitabine plus lapatinib combination in third-line following T-DM1, the median PFS was 4.7 months. In the EMILIA study, the median PFS in patients treated by capecitabine plus lapatinib in second-line was 6.4 months [3].
Accordingly, the disease control duration pertaining to the preceding treatment line (T-DM1 in second-line) exerted a significant impact on the efficacy of the third-line treatment (Fig. 3B).
Several studies have already investigated the efficacy of the capecitabine plus lapatinib combination in patients that had previously been treated using trastuzumab. In the randomized Phase III EGF 100 151 study that compared capecitabine and capecitabine plus lapatinib, the median PFS was 6.2 months in patients treated with capecitabine and lapatinib [2]. Two randomized Phase II studies have revealed PFS data of 6.8 and 7 months, respectively [21,22]. The randomized Phase III NALA study was designed to compare the standard arm comprising capecitabine plus lapatinib against an experimental arm consisting of capecitabine plus neratinib [23]. In this study, 35% of patients had previously been treated with trastuzumab plus pertuzumab, and then T-DM1. Median PFS in the standard arm was 5.5 months.
Rapidly evolving data are still changing standards of care [24]. In the DESTINY-Breast03 phase III trial, the novel ADC trastuzumab-deruxtecan (T-DXd), compared to T-DM1, led to a significant increase of PFS in second line (HR = 0.28, p = 7.8 × 10−22) [25]. This benefit was observed across all pre-specified subgroups, including patients with brain metastasis (HR = 0.37). In the T-DM1 arm, the median PFS was 6.8 months, with 60% of patients previously treated with the trastuzumab and pertuzumab combination. The novel tyrosine kinase inhibitor tucatinib was evaluated in combination with capecitabine and trastuzumab in the HER2CLIMB phase III trial [26]. There was a significant improvement of PFS (HR = 0.57, p < 0.00001) and OS (HR = 0.73, p = 0.004) favoring tucatinib over placebo. The median PFS of the standard treatment (capecitabine and trastuzumab) was 4.9 months, with nearly 100% of the patients previously treated with both T-DM1 and pertuzumab. For patients with brain metastasis, median OS was longer in tucatinib group (18.1 vs 12 months) [27].
The strengths of our study are the high quality-levels of the data collection and validation including a robust quality control methodology [8], and the academic, independent nature of this work in a country where access to new drugs is guaranteed for all patients and fully covered by the national insurance system [28]. The limitation of this study is the selection of patients from French Comprehensive Cancer Centers, which may not fully reflect all French patients, nor all patients from other countries. Another study limitation is the retrospective and observational design employed.
Nevertheless, the administration of pertuzumab plus trastuzumab in first-line does not appear to alter notably the activity of T-DM1 in second-line. Likewise, administration of the dual HER2 blockade based on trastuzumab + pertuzumab in first-line, followed by T-DM1 in second-line, does not markedly modify the activity of the capecitabine plus lapatinib combination. The disease control duration pertaining to the previous treatment line seems to exert a predictive value concerning the subsequent treatment's efficacy. Our data confirm the biomarker analyses of EMILIA and TH3RESA with a reduced T-DM1 activity in HER2 2+ tumors compared of HER2 3+ tumors. These observations derived from our dataset are useful for clinical practice as the best sequence of anti-HER2 agents in the continuum of care of HER2+ metastatic breast cancer patients are still debated.
Funding source
The ESME MBC database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai and Daiichi Sankyo). Data collection, analysis and publication are managed entirely by UNICANCER independently of the industrial consortium
Declaration of competing interest
T.P. reports grants from Pfizer, Novartis, AstraZeneca, Lilly, and Pierre Fabre, but which were unrelated to the submitted work. C.P. reports board from Lilly and non-financial support from Novartis, Pfizer, Mundi Pharma, but which were unrelated to the submitted work. T.B. reports grants, personal fees and non-financial support from Novartis, Pfizer and AstraZeneca; personal fees from Seattle Genetics and personal fees and non-financial support from Roche. W.J. reports personal fees and non-financial support from Eisai, Novartis, Roche, Pfizer and Lilly; grants, personal fees and non-financial support from AstraZeneca; personal fees from MSD; non-financial support from Chugai. M.-A.M.-R. reports other from Novartis, Lilly, Pfizer, Roche, Pierre Fabre and MSD, outside the submitted work. F.D. reports boards from Novartis, Lilly, Pfizer and AstraZeneca. C.C. reports grants from Pfizer, Roche, MSD, AstraZeneca, Eisai and Daiichi. A.G. reports non-financial support from Roche, Novartis, AstraZeneca and Pfizer. PC reports grants from Pfizer and Novartis, personal fees from Pfizer and Eli-Lilly, and non-financial support from Pfizer, outside the submitted work. The other authors declare that they have no conflict of interest to disclose.
Acknowledgements
We thank the 18 French Comprehensive Cancer Centers for providing the data and each ESME local coordinator for managing the project at the local level. Moreover, we thank the ESME Scientific Group and Strategic Committee for their ongoing support
18 Participating French Comprehensive Cancer Centers (FCCC): I. Curie, Paris/Saint-Cloud, G. Roussy, Villejuif, I. Cancérologie de l’Ouest, Angers/Nantes, C. F. Baclesse, Caen, ICM Montpellier, C. L. Bérard, Lyon, C. G-F Leclerc, Dijon, C. H. Becquerel, Rouen; I. C. Regaud, Toulouse; C. A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C. E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C. J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C. P. Strauss, Strasbourg; I. J. Godinot, Reims; C. O. Lambret, Lille.
Program director: Anne-Laure Martin.
Data Management team: Coralie Courtinard, Emilie Nguyen, Olivier Payen, Irwin Piot, Dominique Schwob and Olivier Villacroux.
Operational team: Nathalie Bouyer, Michaël Chevrot, Daniel Couch, Patricia D'Agostino, Pascale Danglot, Levent Dinc, Tahar Guesmia, Elodie Kupfer, Harmonie Oulie, Frédéric Roy, Gaëtane Simon and Toihiri Said.
Software designer Team: Matou Diop, Blaise Fulpin, Fréja Messo, José Paredes and Alexandre Vanni.
ESME local coordinators: Thomas Bachelot, Delphine Berchery, Etienne Brain, Mathias Breton, Loïc Campion, Emmanuel Chamorey, Sandrine Dabakuyo, Valérie Dejean, Stéphanie Delaine, Anne-Valérie Guizard, Anne Jaffré, Lilian Laborde, Carine Laurent, Marie-Paule Lebitasy, Agnès Loeb, Damien Parent, Geneviève Perrocheau, Marie-Ange Mouret-Reynier, Michel Velten.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.03.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Grinda T., Antoine A., Jacot W., Blaye C., Cottu P.-H., Diéras V., Dalenc F., Gonçalves A., Debled M., Patsouris A., et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort. ESMO Open. 2021;6:100114. doi: 10.1016/j.esmoop.2021.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron D., Casey M., Oliva C., Newstat B., Imwalle B., Geyer C.E. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncol. 2010;15:924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., Pegram M., Oh D.-Y., Diéras V., Guardino E., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swain S.M., Baselga J., Kim S.-B., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.-M., Schneeweiss A., Heeson S., et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain S.M., Miles D., Kim S.-B., Im Y.-H., Im S.-A., Semiglazov V., Ciruelos E., Schneeweiss A., Loi S., Monturus E., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 6.Pérol D., Robain M., Arveux P., Mathoulin-Pélissier S., Chamorey E., Asselain B., Berchery D., Gourgou S., Breton M., Delaine-Clisant S., et al. The ongoing French metastatic breast cancer (MBC) cohort: the example-based methodology of the epidemiological Strategy and medical Economics (ESME) BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg M., Chevalier A., Leclerc A., Lesieur S. Banque de données en santé publique; 2007. Recommandations de déontologie et bonnes pratiques en épidémiologie. - Résultats de votre recherche. [Google Scholar]
- 8.Guidelines for good pharmacoepidemiology practices (GPP) - International Society for Pharmacoepidemiology. https://www.pharmacoepi.org/resources/policies/guidelines-08027 Available online.
- 9.Recommendations for the immunohistochemistry of the hormonal receptors on paraffin sections in breast cancer. Update 1999. Group for Evaluation of Prognostic Factors using Immunohistochemistry in Breast Cancer (GEFPICS-FNCLCC) Ann Pathol. 1999;19:336–343. [PubMed] [Google Scholar]
- 10.Wolff A.C., Hammond M.E.H., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., Dowsett M., Fitzgibbons P.L., Hanna W.M., Langer A., et al. American society of clinical oncology/college of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 11.Wolff A.C., Hammond M.E.H., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., Allred D.C., Bartlett J.M.S., Bilous M., Fitzgibbons P., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 12.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., Bilous M., Ellis I.O., Fitzgibbons P., Hanna W., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 13.Diéras V., Miles D., Verma S., Pegram M., Welslau M., Baselga J., Krop I.E., Blackwell K., Hoersch S., Xu J., et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzimitrowicz, H.; Berger, M.; Vargo, C.; Hood, A.; Abdelghany, O.; Raghavendra, A.S.; Tripathy, D.; Valero, V.; Hatzis, C.; Pusztai, L.; et al. T-DM1 activity in metastatic human epidermal growth factor receptor 2–positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol 8. [DOI] [PMC free article] [PubMed]
- 15.Conte B., Fabi A., Poggio F., Blondeaux E., Dellepiane C., D'Alonzo A., Buono G., Arpino G., Magri V., Naso G., et al. T-DM1 efficacy in patients with HER2-positive metastatic breast cancer progressing after a taxane plus pertuzumab and trastuzumab: an Italian multicenter observational study. Clin Breast Cancer. 2019 doi: 10.1016/j.clbc.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Noda-Narita S., Shimomura A., Kawachi A., Sumiyoshi-Okuma H., Sudo K., Shimoi T., Noguchi E., Yonemori K., Shimizu C., Fujiwara Y., et al. Comparison of the efficacy of trastuzumab emtansine between patients with metastatic human epidermal growth factor receptor 2-positive breast cancers previously treated with combination trastuzumab and pertuzumab and with trastuzumab only in Japanese population. Breast Cancer. 2019;26:492–498. doi: 10.1007/s12282-019-00949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baselga J., Lewis Phillips G.D., Verma S., Ro J., Huober J., Guardino A.E., Samant M.K., Olsen S., de Haas S.L., Pegram M.D. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res. 2016;22:3755–3763. doi: 10.1158/1078-0432.CCR-15-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S.-B., Wildiers H., Krop I.E., Smitt M., Yu R., Lysbet de Haas S., Gonzalez-Martin A. Relationship between tumor biomarkers and efficacy in TH3RESA, a phase III study of trastuzumab emtansine (T-DM1) vs. Treatment of physician's choice in previously treated HER2-positive advanced breast cancer. Int J Cancer. 2016;139:2336–2342. doi: 10.1002/ijc.30276. [DOI] [PubMed] [Google Scholar]
- 19.Seol H., Lee H.J., Choi Y., Lee H.E., Kim Y.J., Kim J.H., Kang E., Kim S.-W., Park S.Y. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012;25:938–948. doi: 10.1038/modpathol.2012.36. [DOI] [PubMed] [Google Scholar]
- 20.Brunelli M., Manfrin E., Martignoni G., Miller K., Remo A., Reghellin D., Bersani S., Gobbo S., Eccher A., Chilosi M., et al. Genotypic intratumoral heterogeneity in breast carcinoma with HER2/Neu amplification: evaluation according to ASCO/CAP criteria. Am J Clin Pathol. 2009;131:678–682. doi: 10.1309/AJCP09VUTZWZXBMJ. [DOI] [PubMed] [Google Scholar]
- 21.Martin M., Bonneterre J., Geyer C.E., Ito Y., Ro J., Lang I., Kim S.-B., Germa C., Vermette J., Wang K., et al. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer. 2013;49:3763–3772. doi: 10.1016/j.ejca.2013.07.142. [DOI] [PubMed] [Google Scholar]
- 22.Takano T., Tsurutani J., Takahashi M., Yamanaka T., Sakai K., Ito Y., Fukuoka J., Kimura H., Kawabata H., Tamura K., et al. A randomized phase II trial of trastuzumab plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxanes: WJOG6110B/ELTOP. Breast. 2018;40:67–75. doi: 10.1016/j.breast.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol 38, 23. [DOI] [PMC free article] [PubMed]
- 24.Tarantino P., Prat A., Cortes J., Cardoso F., Curigliano G. Third-line treatment of HER2-positive advanced breast cancer: from No standard to a pandora's box. Biochim Biophys Acta Rev Cancer. 2021;1875:188487. doi: 10.1016/j.bbcan.2020.188487. [DOI] [PubMed] [Google Scholar]
- 25.DESTINY Breast03 second-line trastuzumab deruxtecan for metastatic HER2-positive breast cancer. https://ascopost.com/news/september-2021/destiny-breast03-second-line-trastuzumab-deruxtecan-for-metastatic-her2-positive-breast-cancer/ The ASCO Post Available online.
- 26.Curigliano G., Mueller V., Borges V., Hamilton E., Hurvitz S., Loi S., Murthy R., Okines A., Paplomata E., Cameron D., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33:321–329. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Lin N.U., Borges V., Anders C., Murthy R.K., Paplomata E., Hamilton E., Hurvitz S., Loi S., Okines A., Abramson V., et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38:2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le parcours Du Médicament en France n.d. Https://Www.Has-Sante.Fr/Upload/Docs/Application/Pdf/201903/Le_parcours_du_medicaments_en_france.Pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.