Take Home Message
Urachal carcinoma is a rare cancer. Correct staging is mandatory, and surgical intervention is the treatment in localized cases. Advanced disease requires chemotherapy, even if oncological outcomes remain poor.
Keywords: Urachal carcinoma, Treatment, Cystectomy, Outcomes, Bladder cancer
Abstract
Urachal carcinoma is a rare urological disease. The shortage of data about diagnosis and surgical treatment in literature makes it hard for clinicians to make a decision. Indeed, urachal carcinoma is an aggressive disease that requires prompt staging and treatment to ensure the best outcome for patients. We reviewed the last evidence about the management of urachal carcinoma to provide an easy-to-use guide for clinical practice.
Patient summary
Urachal carcinoma is a rare malignancy. The literature on this challenging disease remains limited. Herein, we provide a practical guide for its management from diagnosis to treatment, which in most cases requires surgical intervention or chemotherapy.
1. Introduction
The urachus is an embryonic remnant that during fetal life connects fetal bladder to allantois and in adult life results in a fibrous cord known as the umbilical ligament, connecting the bladder dome to the umbilicus. Failure of its closure process may allow islets of cells to proliferate, leading to malignancy. Urachal carcinoma (UrC) is a rare malignancy accounting for <1% of all bladder cancers, the prevalence of which in the general population remains unknown [1]. The median age of presentation for UrC is 52–59 yr (range 46–71 yr); in general, this disease affects a younger population than urothelial bladder cancer [2].
UrC is often considered a highly aggressive cancer with a poor prognosis. In the largest series to date, the reported 5-yr overall survival (OS) was about 50% and the 5-yr cancer-specific survival (CSS) was about 35% [3]. Being a rare disease, it lacks large series and/or perspective trials in the literature, and most of the evidence comes from case reports, small case series, and retrospective analyses.
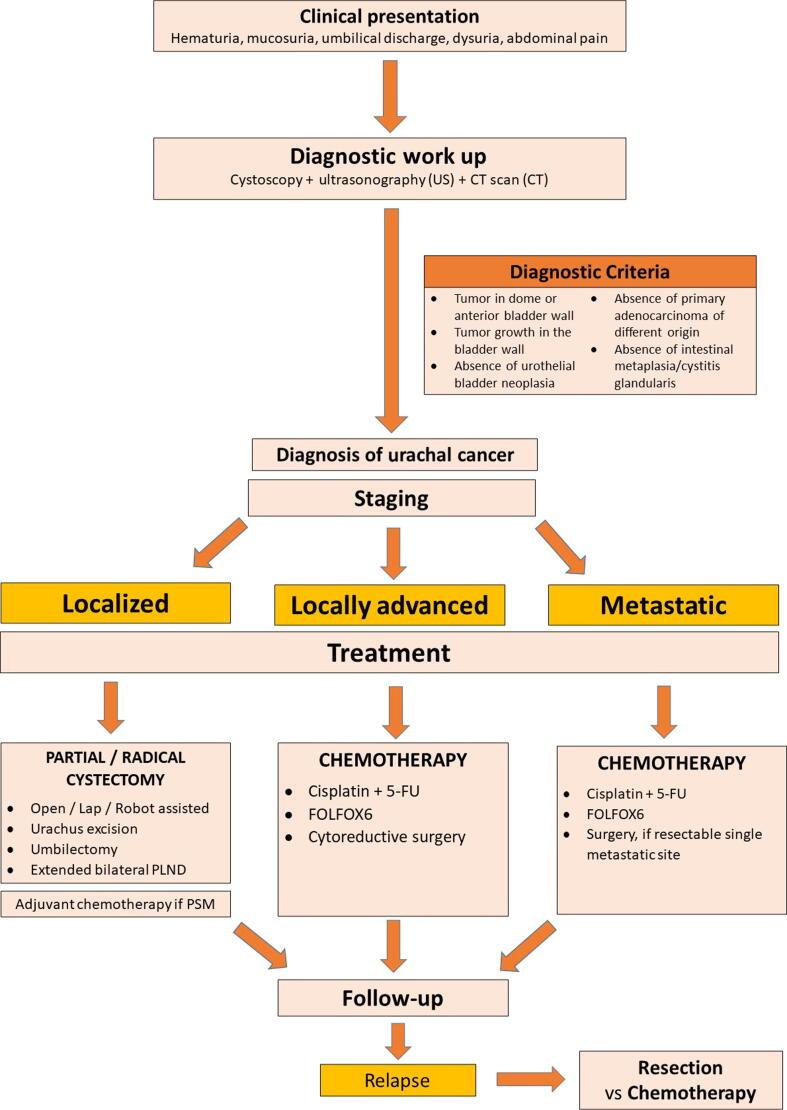
The aim of this review is to summarize the most recent evidence on UrC, focusing on its surgical management, and to provide an easy-to-use guide for clinicians (Fig. 1).
Fig. 1.
Management of urachal carcinoma. CT = computed tomography; 5-FU = 5-fluorouracil; PLND = pelvic lymphadenectomy; PSM = positive surgical margin.
2. Clinical findings and diagnosis
2.1. Signs and symptoms
The most common histological type of UrC is adenocarcinoma. It often presents with gross or microscopic hematuria in 73% of cases, sometimes associated to abdominal pain, dysuria, and mucosuria (10–14%) [2]. Seldom, UrC may present other unspecific urinary symptoms (eg, pollakiuria, pyuria, or recurrent urinary tract infections) or umbilical discharge, and an abdominal palpable mass may be found. Systemic nonspecific symptoms may occur (fever, weight loss, and nausea), especially in more advanced stages.
2.2. Diagnostic workup
Diagnosis is based on the medical history and physical examination, and a cystoscopy is mandatory as it represents the most sensitive diagnostic tool (up to 90%) [4]. Indeed, this procedure allows the identification of tumor localization faithfully, which is often described as a broad-based ulcerative mass in the dome or the anterior wall of the bladder. Nevertheless, up to 8% of patients remain asymptomatic; therefore, diagnosis is made incidentally in these cases [2].
Diagnostic workup may include imaging. Ultrasonography can show a supravesical inhomogeneous mass located in the midline, sometimes associated with calcifications that are considered pathognomonic. A computed tomography (CT) scan or magnetic resonance imaging (MRI) is useful for evaluating the mass and performing clinical staging, detecting possible organ invasion, lymph node enlargement, or distant metastasis [3], [5].
Urinary UroVysion FISH has recently been studied as a potential tool for the diagnosis of UrC [5]. It has been found to be positive in a high proportion of pathologically confirmed UrC cases, with sensitivity and specificity of 71.43% and 94.61%, respectively [6]. These promising findings seem to support the use of UroVysion to differentiate UrC from a benign urachal cyst.
Preoperative pathological sampling cannot always be achieved. A transurethral resection (TUR) of the mass can be technically challenging in most cases, and it might carry a risk of bladder rupture. Moreover, it has been reported that, while having high specificity, a TUR is affected by a suboptimal negative predictive value (50%) [6]. Thus, percutaneous biopsy has been evaluated as an option to differentiate an infected urachal cyst from UrC when imaging remains inconclusive [7]. Nevertheless, current diagnostic tools might not be enough, and surgical removal can be indicated on the basis of clinical suspicion and in the absence of a pathology confirmation [6].
Diagnostic criteria have been suggested to standardize the UrC diagnosis. These include the following [8]:
-
1.
Tumor located in the dome or anterior bladder wall.
-
2.
Tumor growth in the bladder wall.
-
3.
Absence of atypical intestinal metaplasia and cystitis/glandularis beyond the dome/anterior wall.
-
4.
Absence of a urothelial bladder neoplasia.
-
5.
Exclusion of a primary adenocarcinoma of a different origin.
Recently, the role of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/CT has also been shown in identifying metastatic sites that are undetectable with other imaging tools, especially during follow-up. However, 18F-FDG PET/CT does not seem to yield additional information compared with CT, which still remains the most reliable tool for initial diagnosis and staging [9].
Serum biomarkers have been investigated for UrC. For example, elevated carcinoembryonic antigen serum levels (>3 ng/ml) were found in 59% of UrC cases, and these were associated with worse survival outcomes. Additionally, elevated serum levels of CA19-9 and CA125 were reported in 50.8% and 51.4% of UrC cases, respectively [10]. Despite these intriguing findings, additional studies are needed to recommend their routine use in clinical practice.
2.3. Staging systems
Different staging systems have been proposed to stratify the aggressiveness and prognosis of UrC (Table 1). The TNM system has a limited role, whereas the Mayo staging was reported to be superior to the Sheldon staging based on its simplicity and higher prognostic value in multivariable models [2]. Overall, 59–66% of cases present at diagnosis with extension beyond the bladder, without fulfilling surgical resection criteria [2].
Table 1.
Staging systems of urachal carcinoma
| Stage | Sheldon staging system [2] | Mayo staging system [2] | TNM staging system | |||
|---|---|---|---|---|---|---|
| Localized disease | I | Tumor is limited to the urachal mucosa | Tumor is confined to the urachus and/or bladder | T1 | Tumor invades the subepithelial connective tissue | |
| II | Invasion confined to the urachus itself (not beyond muscle layer) | Extension beyond the muscular layer of the urachus and/or bladder | T2 | Invasion of the muscular layer of the urachus or bladder | ||
| Locally advanced disease | III | IIIA | Local extension to the bladder | Metastasis to regional lymph nodes | T3 | Invasion of the perivisceral soft tissue, prostate, uterus, or vagina |
| IIIB | Local extension to abdominal wall | |||||
| IIIC | Invasion of the peritoneum | |||||
| IIID | Invasion of the local viscera other than the bladder | |||||
| Advanced disease | IV | IVA | Metastasis to lymph nodes | Metastasis to nonregional lymph nodes or other distant sites | T4 | Invasion of the abdominal wall and metastasis to lymph nodes or other distant sites |
| IVB | Distant metastasis | |||||
TNM = tumor, node, metastasis.
3. Treatment
The treatment and oncological outcomes vary depending on the stage of the disease (Table 2) [3], [4], [11], [12].
Table 2.
Overview on recent outcomes of surgical treatment for urachal carcinoma
| Reference | No. of cases | Stage (% of cases) | Surgical treatment (% of cases) | Perioperative chemotherapy, n (% of cases) | Oncological outcomes | Negative prognostic factors |
|---|---|---|---|---|---|---|
| Shao et al (2022) [4] | 59 | TNM stage T1–2: 13.5 T3: 64.4 T4: 22.1 |
Open EPC (72.4) Open PC (17.3) Open RC (17.3) |
18 (30.5) | Median OS: 52.8 mo 3-yr CSS: 69.1% 5-yr CSS: 62.1% |
|
| Yu et al (2021) [3] | 203 | Mayo stage I–II: 77 III: 11.4 IV: 11.4 |
Open or robotic EPC ± LND (82.8) Open RC ± LND (17.3) |
65 (32) | 5-yr OS: 88.3% 5-yr CSS: 83.1% 5-yr RFS: 63.9% |
|
| Duan et al (2020) [11] | 62 | Sheldon stage III: 80.7 IV: 19.3 |
EPC (87) or RC (13) ± LND | 18 (29) | Median DFS: 32.7 mo Mean OS: 114.6 mo |
|
| Mylonas et al (2017) [12] | 420 | TNM stage T1–2: 34.2 T3: 29.5 T4: 30.5 |
Excision/local (20.7) EPC ± LND (52.4) Open RC ± LND (9.8) |
NR | Median OS: 57 mo Median CSS: 105 mo 5-yr OS: 51% 5-yr CSS: 57% |
|
CSS = cancer-specific survival; DFS = disease-free survival; EPC = extended partial cystectomy; LN = lymph node; LND = lymphadenectomy; LVI = lymphovascular invasion; NR = not reported; OS = overall survival; PSM = positive surgical margin; RC = radical cystectomy; RFS = recurrence-free survival; TNM = tumor, node, metastasis.
3.1. Surgery
Surgery represents the mainstay of treatment for localized disease. Excision of the urachus and the umbilicus, and partial/radical cystectomy, combined with extended bilateral pelvic lymphadenectomy (PLND), are usually offered [2]. The most commonly employed surgical approach is the open one, with laparoscopy as a safe and feasible alternative [4].
The prognostic role of lymph node metastasis in OS has recently been shown [3], [11], [13]. However, the therapeutic role of PLND remains controversial, as previous evidence suggests that it does not improve OS, while carrying a higher complication rate and lymph node positivity of only 17% [1], [2]. To the best of our knowledge, PLND is not a predictor of OS; however, the prognostic impact of lymph node metastasis and the accuracy of PLND to assess the pathological stage should encourage clinicians to perform this procedure [11], [13]. Recent findings confirm the impact of omphalectomy in improving OS and CSS in these patients [11], [13]. An umbilical-sparing management approach might be considered in patients with localized disease [14]. However, the lack of strong evidence should discourage clinicians from performing this procedure routinely.
The main goal in surgical treatment of UrC is to achieve negative surgical margins, especially after partial cystectomy. Indeed, the prognostic impact of positive surgical margins (PSMs) on OS and CSS has consistently been demonstrated [3], [15].
More recently, it has been shown that robot-assisted partial cystectomy might represent a feasible and safe procedure to treat UrC [16], [17], as it offers the same rate of PSMs as the open approach, with lower perioperative morbidity. Furthermore, new technological tools, such as intraoperative indocyanin green fluorescence, have been tested to facilitate the targeting of surgical landmarks and identification of surgical margins [18].
3.2. Chemotherapy
Advanced stages have a poor prognosis, and perioperative chemotherapy has a limited role. Neoadjuvant chemotherapy treatment could be employed in cases of unresectable disease only when achieving a response may lead to surgical consolidation with a negative margin resection [19].
Recently, its effectiveness in decreasing overall mortality and cancer-specific mortality has been shown in a metastatic cohort, especially in younger patients (≤70 yr) [20]. Commonly employed regimens are 5-fluorouracil and cisplatin or FOLFOX6 (folinic acid, 5-fluorouracil, and oxaliplatin), with gemcitabine increasingly being included in therapeutic protocols [12]. To better standardize chemotherapy regimens, a recent study has demonstrated higher effectiveness of platinum-based chemotherapy than that without platinum in disease control, while there is no significant difference in progression-free survival and OS between groups using fluorouracil and paclitaxel as first-line regimen [21].
Chemotherapy associated with cytoreductive surgery has also been evaluated, demonstrating satisfactory long-term oncological outcomes [22]. Furthermore, UrC local recurrence occurs in about 15–18% of patients after primary treatment [23], [24], and chemotherapy should be considered a suitable option to control the relapse when not surgically resectable [24]. However, the benefit of these treatment modalities has yet to be evaluated in adequately powered studies.
3.3. Radiotherapy
Radiotherapy is rarely employed in UrC management: a Surveillance, Epidemiology, and End Results (SEER)-based population study shows that it has been used in only 10% of patients [12]. The limited available evidence on the use of radiotherapy in this setting suggests it to be ineffective as local treatment [23].
4. Metastatic disease
UrC is usually diagnosed at advanced stages, with >20% of patients having distant metastasis at presentation [2]. Moreover, up to 59% of patients may manifest metastasis at some point in the evolution of disease [24], with the lung, bone, peritoneum, and liver being the most commonly involved organs [2], [23]. Obviously, the presence of metastasis is associated with a worse prognosis, and chemotherapy is considered the best option of treatment to prolong OS [2]. Surgical resection of metastasis in slow growing disease may be considered, but only in single organ resectable metastasis, after disease control with systemic chemotherapy [19].
In the precision-medicine era, systemic treatment may be shifted toward molecular-targeted therapy. Recently, UrC has been characterized in its genetic signature through a genome-wide analysis. The main common genetic and molecular mutations have been identified, such as KRAS, BRAF, and NRAF, which could be useful in potential treatment tailoring [25], [26]. Furthermore, from a molecular standpoint, UrC may show mutation in epidermal growth factor receptor (EGFR) as colon cancer, and the response to EGFR inhibitors such as gefitinib or cetuximab has already been shown in two single reports [2], [27]. Immune and targeted therapies for UrC seem to be promising, but their role remains to be determined [19], [28].
5. Conclusions
UrC is a rare clinical entity, but its management can represent a clinical challenge, given the limited evidence in the literature. The importance of accurate clinical staging is the first step to establish a tailored treatment plan. Surgery remains the mainstay of treatment for nonmetastatic disease, via open, laparoscopic, or more recently, robotic-assisted techniques. Treatment for metastatic and advanced disease relies on systemic chemotherapy, but no standardized protocols have been established.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Loizzo.
Acquisition of data: Loizzo, Ferro.
Analysis and interpretation of data: Loizzo, Guruli.
Drafting of the manuscript: Loizzo, Pandolfo.
Critical revision of the manuscript for important intellectual content: Autorino, Imbimbo.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: Paul, Crocerossa, Lucarelli.
Supervision: Autorino, Ditonno.
Other: None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: M. Carmen Mir
References
- 1.Bruins H.M., Visser O., Ploeg M., Hulsbergen-van de Kaa C.A., Kiemeney L.A.L.M., Witjes J.A. The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol. 2012;188:1102–1107. doi: 10.1016/j.juro.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Szarvas T., Módos O., Niedworok C., et al. Clinical, prognostic, and therapeutic aspects of urachal carcinoma—a comprehensive review with meta-analysis of 1,010 cases. Urol Oncol. 2016;34:388–398. doi: 10.1016/j.urolonc.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Yu Y.D., Ko Y.H., Kim J.W., et al. The prognosis and oncological predictor of urachal carcinoma of the bladder: a large scale multicenter cohort study analyzed 203 patients with long term follow-up. Front Oncol. 2021;11:1893. doi: 10.3389/fonc.2021.683190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao G., Xu C., Liu J., et al. Clinical, pathological, and prognostic analysis of urachal carcinoma. Urol Int. 2022;106:199–208. doi: 10.1159/000518028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Z., Ke C., Liu Z., et al. Evaluation of UroVysion for urachal carcinoma detection. Front Med (Lausanne) 2020;7:437. doi: 10.3389/fmed.2020.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meeks J.J., Herr H.W., Bernstein M., Al-Ahmadie H.A., Dalbagni G. Preoperative accuracy of diagnostic evaluation of the urachal mass. J Urol. 2013;189:1260–1262. doi: 10.1016/j.juro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 7.Wilson A.L., Gandhi J., Seyam O., et al. Urachal anomalies: a review of pathological conditions, diagnosis, and management. Transl Res Anat. 2019;16:100041. [Google Scholar]
- 8.Paner G.P., Lopez-Beltran A., Sirohi D., Amin M.B. Updates in the pathologic diagnosis and classification of epithelial neoplasms of urachal origin. Adv Anat Pathol. 2016;23:71–83. doi: 10.1097/PAP.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 9.Stokkel L.E., Stokkel M.P.M., Donswijk M.L., et al. The diagnostic value of FDG-PET/CT for urachal cancer. Clin Genitourin Cancer. 2021;19:373–380. doi: 10.1016/j.clgc.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Reis H., Krafft U., Niedworok C., et al. Biomarkers in urachal cancer and adenocarcinomas in the bladder: a comprehensive review supplemented by own data. Dis Markers. 2018;2018:e7308168. doi: 10.1155/2018/7308168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan F., Zhai W., Zhang B., Guo S. Urachal carcinoma: impact of recurrence pattern and lymphadenectomy on long-term outcomes. Cancer Med. 2020;9:4166–4174. doi: 10.1002/cam4.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mylonas K.S., O’Malley P., Ziogas I.A., El-Kabab L., Nasioudis D. Malignant urachal neoplasms: a population-based study and systematic review of literature. Urol Oncol. 2017;35:33.e11–33.e19. doi: 10.1016/j.urolonc.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Jia Z., Chang X., Li X., Wang B., Zhang X. Urachal carcinoma: are lymphadenectomy and umbilectomy necessary? Med Sci Monit. 2020;26:e927913. doi: 10.12659/MSM.927913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavelescu C., Pavelescu A., Surcel C., et al. Surgical management of urachal tumors: can the umbilicus be sparred in localized disease? Rare Tumors. 2019;11 doi: 10.1177/2036361319847283. 2036361319847283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D., Li Y., Yu Z., et al. Investigating urachal carcinoma for more than 15 years. Oncol Lett. 2014;8:2279–2283. doi: 10.3892/ol.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James K., Vasdev N., Mohan-S G., Lane T., Adshead J.M. Robotic partial cystectomy for primary urachal adenocarcinoma of the urinary bladder. Curr Urol. 2015;8:183–188. doi: 10.1159/000365714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owyong M., Koru-Sengul T., Miao F., et al. Impact of surgical technique on surgical margin status following partial cystectomy. Urol Oncol. 2019;37:870–876. doi: 10.1016/j.urolonc.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito K., Takahashi T., Kanno T., Okada T., Higashi Y., Yamada H. Indocyanine green fluorescence-guided partial cystectomy and pelvic lymphadenectomy for urachal carcinoma. J Endourol Case Rep. 2020;6:275–277. doi: 10.1089/cren.2020.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siefker-Radtke A. Urachal adenocarcinoma: a clinician’s guide for treatment. Semin Oncol. 2012;39:619–624. doi: 10.1053/j.seminoncol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Flammia R.S., Chierigo F., Würnschimmel C., et al. Survival benefit of chemotherapy in a contemporary cohort of metastatic urachal carcinoma. Urol Oncol. 2021 doi: 10.1016/j.urolonc.2021.09.008. In press. [DOI] [PubMed] [Google Scholar]
- 21.Chen M., Xue C., Huang R.Q., et al. Treatment outcome of different chemotherapy in patients with relapsed or metastatic malignant urachal tumor. Front Oncol. 2021;11:739134. doi: 10.3389/fonc.2021.739134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mertens L.S., Behrendt M.A., Mehta A.M., et al. Long-term survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with peritoneal metastases of urachal cancer. Eur J Surg Oncol. 2019;45:1740–1744. doi: 10.1016/j.ejso.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Herr H.W., Bochner B.H., Sharp D., Dalbagni G., Reuter V.E. Urachal carcinoma: contemporary surgical outcomes. J Urol. 2007;178:74–78. doi: 10.1016/j.juro.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Ashley R.A., Inman B.A., Sebo T.J., et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer. 2006;107:712–720. doi: 10.1002/cncr.22060. [DOI] [PubMed] [Google Scholar]
- 25.Lee S., Lee J., Sim S.H., et al. Comprehensive somatic genome alterations of urachal carcinoma. J Med Genet. 2017;54:572–578. doi: 10.1136/jmedgenet-2016-104390. [DOI] [PubMed] [Google Scholar]
- 26.Módos O., Reis H., Niedworok C., et al. Mutations of KRAS, NRAS, BRAF, EGFR, and PIK3CA genes in urachal carcinoma: occurrence and prognostic significance. Oncotarget. 2016;7:39293–39301. doi: 10.18632/oncotarget.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collazo-Lorduy A., Castillo-Martin M., Wang L., et al. Urachal carcinoma shares genomic alterations with colorectal carcinoma and may respond to epidermal growth factor inhibition. Eur Urol. 2016;70:771–775. doi: 10.1016/j.eururo.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N., Khosla D., Kumar R., et al. Urachal carcinoma: clinicopathological features, treatment and outcome. J Cancer Res Ther. 2014;10:571–574. doi: 10.4103/0973-1482.137955. [DOI] [PubMed] [Google Scholar]