Abstract
Background:
This study aimed to evaluate the amount of bone regeneration in critical defects of rabbit calvaria filled with magnesium- and strontium-doped bioactive glasses.
Materials and Methods:
In this rabbit critical-size calvarial defects study, 12 male New Zealand white rabbits were randomly divided into two groups. On the calvaria of each rabbit, four lesions (two lesions in the frontal bone and two lesions in the peritoneal bone) were created with a diameter of 8 mm spaced apart. Each lesion was filled in with (1) strontium-doped bioactive glass, (2) magnesium-doped bioactive glass, (3) 45S5 bioactive glass, and (4) empty lesion (control). Six rabbits were sacrificed at the end of 4 weeks, and six rabbits were randomly sacrificed at the end of 8 weeks. Bone sections with a 5-μ thickness of rabbit calvary bone were prepared, and the percentage of new bone, connective tissue, and residual material were calculated in microscopic images. Statistical analysis was performed by two-way ANOVA and Bonferroni additional tests, and the level of significance was set at P < 0.05 in all categories.
Results:
At 4 weeks, magnesium-doped bioactive glass showed the highest new bone formation with a mean of 11.66 ± 2.64, followed by the strontium-doped bioactive glass with the mean of 11.10 ± 1.69 (P = 0.0001). While at week 8, the highest amount of new bone observed in the strontium-doped group with a mean of 28.22 ± 3.19, and then, the magnesium-doped bioactive glass with a mean of 22.55 ± 3.43 (P = 0.0001).
Conclusion:
Doping strontium and magnesium in the structure of bioactive glasses increases new bone regeneration in comparison with 45S5 bioactive glass.
Key Words: Bioactive glass 45S5, bone regeneration, ceramics, magnesium, osteogenesis, strontium
INTRODUCTION
Bone regeneration is a physiological process that comprises a series of organized biological events of stimulation and regulation of bone formation.[1] There is an emergent need for human bone regeneration by tissue engineering methods due to several bone conditions such as bone infections, tumors, and bone loss as a result of trauma. Autogenous bone grafting techniques for regenerating the defect and restoring the contour and function of the lost bone are usually inadequate because of particular problems such as rejection of the graft, donor restrictions, extensive resorption of the graft, increased operating time, and postoperative infection and pain.[2] To eliminate these limitations, over the past decades, many studies have been conducted to find a suitable substitute for bone tissue, and several bone materials have been introduced as bone substitutes.[3] In this regard, the field of medical biomaterials has grown exponentially over the past few years to provide new solutions to reduce the fracture healing period and address other problems encountered in bone regeneration.
Nowadays, Biomaterials have an exceptional place in the regeneration of human bones, and bioactive glasses are one of the state-of-the-art materials intended for this purpose that demonstrates the topmost biological behavior among all biologically active substances. Bioactive glasses encompass a group of calcium phosphate compounds that are capable of creating a strong bond with tissue in a short period.[4,5] Hench et al. first introduced the Bioglass (BG or 45S5 Bioglass) at the University of Florida in 1969.[6] He discovered that this type of glass made such a solid bond with the bone that it was not possible to separate them except by fracturing the bone.[6] Afterward in vivo studies demonstrated that BGs exhibit osteoinductive and osteoconductive properties through the establishment of a carbonated hydroxyl-apatite (CHA) with the bone.[7] Since then, despite much research have taken place in this field, the original Hench and Jones compound is still used extensive.[4]
At present, BGs are manufactured by a method known as sol-gel, which employs a solvent at low temperatures.[8] This approach has several known benefits, including the porous structure and high bioavailability of the produced material, and the possibility of creating a variety of glass-ceramics with different additives.[6,7,8] Perio-Glas® (NovaBone®, Florida, USA 1992) was the first particle-sized (710–90 μ) BG used to reconstruct the jaw and periodontal defects.[9] Since then, BGs in various commercial brands, alone or in combination with different metal ions, have been used to reconstruct jaw-related bone defects.[7]
Bioactive glasses provide a biocompatible response in the interface of bone and tissue and hence have numerous medical applications.[10] The primary purpose of these materials was to improve bone regeneration around dental implants.[4] Several articles have been reported on the positive effects of bioactive glasses on bone growth stimulation and formation.[11,12]
In recent years, the addition of ions such as magnesium, strontium, zinc, and iron to bone replacement grafts has been proposed to enhance the mechanical and biological properties of these materials.[13,14,15,16,17] The rationale for this is the presence of these ions in the natural structure of hard tissues and the indispensable role of these ions in the growth and regeneration of such tissues. It has been shown that magnesium and strontium ions can promote the activity of calcium triphosphate,[18] as well as facilitate the proliferation and adhesion of osteoblasts to scaffolds.[19,20] In particular, magnesium is associated with hard tissue mineralization by direct stimulation of the proliferation of osteoblasts[21] and can reduce the risk of osteoporosis in humans.[22] Magnesium deficiency can affect all stages of the metabolism of skeletal tissue, which leads to reduced bone growth and decreased osteoblastic activity and ultimately causes osteoporosis and bone fragility.[22,23] Strontium is a crucial element in bone turnover, and animal studies have shown that this substance can prevent bone resorption and stimulate bone formation.[24,25] Strontium-doped bioactive glasses have been shown to enhance the osteogenic differentiation of human osteosarcoma cells (SaOS-2), as well as the reduction of osteoclast activity, and thus affect the turnover of the bone.[26] In addition, strontium is a daily supplement to treat osteoporosis. This therapeutic effect is due to the antiresorptive and dual anabolic effects on the bone, which is because of facilitating pre-osteoblastic differentiation and inhibiting osteoclast differentiation.[27] Bioactive glass is thought to be an ideal reservoir for transporting strontium due to its homogenous composition and controlled degradation.[28]
Considering the limited articles on the influence of enhanced bioactive glass on bone regeneration and formation and the demand for a reliable low-cost material to be used in bone grafting procedures, this study was conducted to evaluate the amount of bone regeneration in rabbit calvaria by magnesium- and strontium-doped bioactive glasses and compare it with original Bioglass. The study hypotheses were that if bioactive glass can help the bone regeneration procedure and if the enhanced bioactive glass is capable of promoting bone formation compared to standard bioactive glass.
MATERIALS AND METHODS
Ethical approval
In this rabbit critical-size calvarial defects study, all research related to animals’ use has complied with all the relevant national regulations and institutional policies for the care and use of animals and approved by The Ethical Committee for Animal Research of Islamic Azad University of Tehran, Iran (no. IR.IAU.DENTAL.REC.1396,19). The materials used in this study were supplied by the “Biotechnology Research Center of Baqiyatallah University of Medical Sciences.” The physical and biological properties of the material were tested by that center and confirmed for animal studies.[29,30]
Study population and selection
To determine the sample size, a preliminary study was performed on six New Zealand rabbits according to the statistical population. Results from the initial research were used to determine the number of final samples that was 12 rabbits by Bonferroni formula. Twelve selected rabbits were randomly divided into two groups regarding the period of scarification, which was at 4 weeks and 8 weeks.
Surgical procedure
This original research was conducted on the calvaria of 12 male New Zealand (Oryctolagus Cuniculus-HsdHra: (NZW) SPF) White Rabbits (purchased from Medzist Pishro Co.) weighing approximately 2–2.5 kg between 10 and 12 weeks of age. The rabbits were kept under the same conditions of 12 h of light and 12 h of the dark at 20°C–25°C. Similar water supply and food have been provided to the animals. The rabbits were anesthetized intraperitoneally by 90 mg/kg ketamine combined with 10 mg/kg xylazine. After scrubbing the animal's scalp, the area was cleaned with 7% povidone-iodine and alcohol. After securing the animal's hands and feet to the surgical bed, a 10-cm anterior-posterior incision was created in the midline of the scalp [Figure 1]. The full-thickness flap was slowly elevated, and the rabbit's calvaria were completely exposed. Then, four artificial defects (Two defects in the frontal bone and two defects in the parietal bone) were made by a diameter of 8-mm and a depth of 1-mm in calvaria through Trephine bur [Figure 2]. The size of the defects was determined according to the critical dimension of bone regeneration in the rabbit's calvaria.[31] Anatomical landmarks such as craniocaudal suture and occipital process were used to standardize the defect location.[7,31,32,33] Each defect was filled in as follows [Figure 3]: (1) Strontium-doped bioactive glass, (2) magnesium-doped bioactive glass, (3) standard 45S5 Bioglass (positive control), and (4) unfilled defect (negative control). According to previous studies by the same authors, the particle size of materials was shown to be approximately 20–50 nm.[17,29] All materials have been purchased from Medzist Pishro Co., Tehran, Iran.
Figure 1.
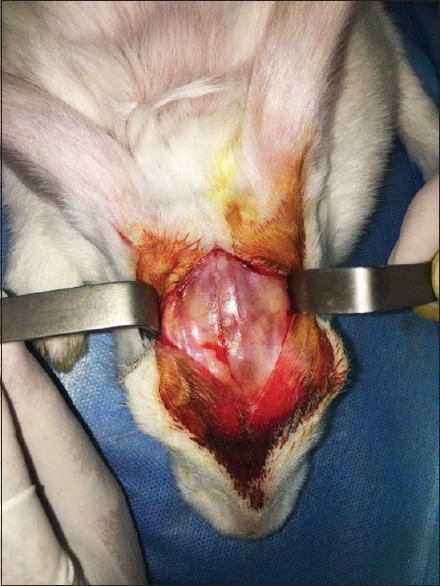
A 10-cm anterior-posterior incision in the midline of the scalp to fully expose the calvaria.
Figure 2.
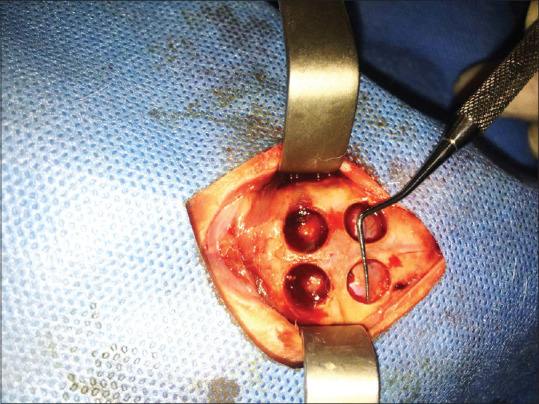
Creating four artificial defects (two defects in the frontal bone and two defects in the parietal bone) by a diameter of 8-mm and depth of 1-mm in calvaria via Trephine bur.
Figure 3.
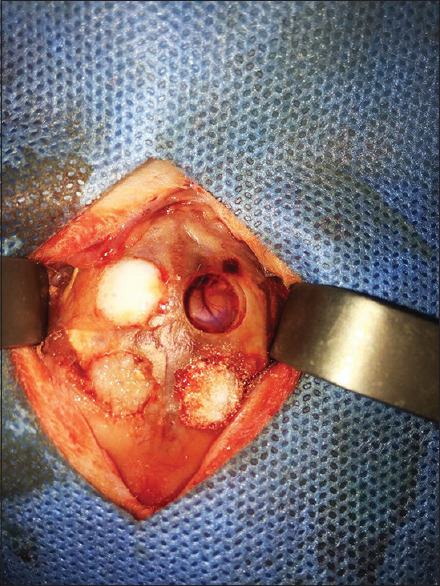
Filling the defects with different types of bioactive glasses.
Subsequently, the scalp of the surgical area was sutured with 4/0 vicryl sutures and sterilized with tetracycline spray. Amoxicillin (0.1 mg/kg) was injected into the animals, and after the operation, the animals were kept in a clean cage and transferred to the animal laboratory. The rabbits were kept inside the cage at a suitable temperature and proper nutritional conditions. After surgery, the animals were placed in numbered cages, and their keeping conditions were monitored every 12 h.
Six rabbits were chosen by a randomization computer-software (Sortition, Oxford University Innovation Ltd., UK) and sacrificed at the end of 4 weeks with a high-dose injection of anesthetics. The same procedure was done for the remaining six at the end of 8 weeks.
Histologic examination
The specimens were fixed for 10 days in 10% formalin solution. Decalcification of the samples was carried out in a 5% nitric acid solution for approximately one and a half months. Afterward, they were molded in paraffin by dewatering, clarifying, and impregnating the tissue passage (Leica Germany, 1010TP). Subsequently, cross-sections with the thickness of 5 μm were produced by rotary microtomes and stained with Goldner's Trichrome method (Sheehan and Hrapchak 1980) and H and E, and cellular characteristics were described in this process. Image j software (Java-based image processing, National Institute of Health) calculated the percentage of new bone remodeling, the portion of connective tissue and the amount of residual material in the microscopic images prepared at weeks 4 and 8.
Statistical analysis
The results were analyzed by SPSS version 22 (SPSS Inc., Chicago, Ill., USA) using two-way ANOVA and Bonferroni additional tests. There was no use of two-way ANOVA method for connective tissue percentage so that it was interpreted by Kruskal–Wallis nonparametric methods as well as adjusted P value for comparison of the groups.
RESULTS
In this animal study, the effect of bioactive glasses enhanced either with strontium or magnesium were compared with standard 45S5 Bioglass and a negative control group on three variables in the histologic sections: Bone regeneration, amount of residual material, and connective tissue, and the results are presented in Tables 1-3.
Table 1.
Statistical results of average new bone regeneration based on the utilized materials and time intervals
| Groups | NB formation (% of the area/mm2) | P value (time period) | P value (type of material) |
|---|---|---|---|
| 4 weeks | |||
| St-doped BG | |||
| Mean±SD | 11.10±1.69 | 0.0001* | 0.0001* |
| Minimum | 8.50 | ||
| Maximum | 12.80 | ||
| Range | 4.30 | ||
| Mg-doped BG | |||
| Mean±SD | 11.62±2.64 | ||
| Minimum | 9.00 | ||
| Maximum | 15.70 | ||
| Range | 6.70 | ||
| 45S5 BG | |||
| Mean±SD | 9.92±2.10 | ||
| Minimum | 7.00 | ||
| Maximum | 12.60 | ||
| Range | 5.60 | ||
| Negative control | |||
| Mean±SD | 5.23±1.13 | ||
| Minimum | 3.50 | ||
| Maximum | 6.70 | ||
| Range | 3.20 | ||
| 8 weeks | |||
| St-doped BG | |||
| Mean±SD | 28.32±3.19 | ||
| Minimum | 24.90 | ||
| Maximum | 33.30 | ||
| Range | 8.40 | ||
| Mg-doped BG | |||
| Mean±SD | 22.55±3.43 | ||
| Minimum | 18.50 | ||
| Maximum | 27.40 | ||
| Range | 8.90 | ||
| 45S5 BG | |||
| Mean±SD | 17.82±1.75 | ||
| Minimum | 14.50 | ||
| Maximum | 19.50 | ||
| Range | 5.00 | ||
| Negative control | |||
| Mean±SD | 12.58±1.42 | ||
| Minimum | 10.60 | ||
| Maximum | 14.10 | ||
| Range | 3.50 | ||
| P-value (interaction between time and groups) | 0.0001* |
*Denotes a statistically significant result NB: New bone; BG: Bioactive glass; SD: Standard deviation; St-doped BG: Strontium-doped bioactive glass; Mg-doped BG: Magnesium-doped bioactive glass
Table 3.
Statistical results of the average percentage of connective tissue based on materials and time intervals
| Groups | CT (% of the area/mm2) | P value (time period) | P value (type of material) |
|---|---|---|---|
| 4 weeks | |||
| St-doped BG | |||
| Mean±SD | 33.52±3.37 | 0.0001* | 0.0001* |
| Minimum | 29.00 | ||
| Maximum | 38.80 | ||
| Range | 9.80 | ||
| Mg-doped BG | |||
| Mean±SD | 36.33±5.96 | ||
| Minimum | 29.50 | ||
| Maximum | 46.60 | ||
| Range | 17.10 | ||
| 45S5 BG | |||
| Mean±SD | 36.43±3.90 | ||
| Minimum | 32.40 | ||
| Maximum | 43.00 | ||
| Range | 10.60 | ||
| Negative control | |||
| Mean±SD | 94.77±1.13 | ||
| Minimum | 93.30 | ||
| Maximum | 96.50 | ||
| Range | 3.20 | ||
| 8 weeks | |||
| St-doped BG | |||
| Mean±SD | 58.73±2.56 | ||
| Minimum | 54.20 | ||
| Maximum | 60.60 | ||
| Range | 6.40 | ||
| Mg-doped BG | |||
| Mean±SD | 56.57±4.65 | ||
| Minimum | 51.90 | ||
| Maximum | 61.50 | ||
| Range | 9.60 | ||
| 45S5 BG | |||
| Mean±SD | 51.50±3.89 | ||
| Minimum | 47.90 | ||
| Maximum | 58.80 | ||
| Range | 10.90 | ||
| Negative control | |||
| Mean±SD | 87.42±1.42 | ||
| Minimum | 85.90 | ||
| Maximum | 89.40 | ||
| Range | 3.50 | ||
| P value (interaction between time and groups) | 0.0001* |
*Denotes a statistically significant result. CT: Connective tissue; BG: Bioactive glass; SD: Standard deviation; St-doped BG: Strontium-doped bioactive glass; Mg-doped BG: Magnesium-doped bioactive glass
Based on the results of the two-way ANOVA [Table 1], it was shown that the type of the material, the time interval (4 or 8 weeks), and the interaction between these two variables have a significant influence on the degree of new bone regeneration, the amount of connective tissue and the residual material (P = 0.0001). Accordingly, at week 4, magnesium-doped bioactive glass demonstrated the highest new bone formation with the rate of 11.26% ±2.64%, followed by strontium-doped bioactive glass (11.10% ±1.69%). However, at week 8, for the same variable, the highest value was observed for the strontium-doped bioactive glass (28.32% ±3.19%), followed by the magnesium-doped bioactive glass with the amount of 22.55% ±3.43% of regenerated bone (P = 0.0001).
Based on the results of the two-way ANOVA [Table 2], at the 4th-week interval, the highest amount of residual material observed in the defects filled with the strontium-doped bioactive glass (55.38% ±2.51%). At the 8th week, for the same variable, the standard 45S5 Bioglass showed the highest value (34.35% ±4.07%) (P = 0.0001).
Table 2.
Statistical results of the average percentage of different residual materials based on time intervals
| Groups | RM (% of the area/mm2) | P value (time period) | P value (type of material) |
|---|---|---|---|
| 4 weeks | |||
| St-doped BG | |||
| Mean±SD | 55.38±2.51 | 0.0001* | 0.0001* |
| Minimum | 52.70 | ||
| Maximum | 58.50 | ||
| Range | 5.80 | ||
| Mg-doped BG | |||
| Mean±SD | 52.05±4.89 | ||
| Minimum | 44.40 | ||
| Maximum | 56.00 | ||
| Range | 11.60 | ||
| 45S5 BG | |||
| Mean±SD | 53.65±4.40 | ||
| Minimum | 45.50 | ||
| Maximum | 58.60 | ||
| Range | 13.10 | ||
| Negative control | |||
| Mean±SD | 0.00 | ||
| Minimum | 0.00 | ||
| Maximum | 0.00 | ||
| Range | 0.00 | ||
| 8 weeks | |||
| St-doped BG | |||
| Mean±SD | 12.95±2.73 | ||
| Minimum | 10.50 | ||
| Maximum | 17.70 | ||
| Range | 7.20 | ||
| Mg-doped BG | |||
| Mean±SD | 20.88±2.15 | ||
| Minimum | 18.50 | ||
| Maximum | 24.00 | ||
| Range | 5.50 | ||
| 45S5 BG | |||
| Mean±SD | 34.35±4.07 | ||
| Minimum | 28.80 | ||
| Maximum | 39.60 | ||
| Range | 10.80 | ||
| Negative control | |||
| Mean±SD | 0.00 | ||
| Minimum | 0.00 | ||
| Maximum | 0.00 | ||
| Range | 0.00 | ||
| P value (interaction between time and groups) | 0.0001* |
*Denotes a statistically significant result. RM: Residual material; BG: Bioactive glass; SD: Standard deviation; St-doped BG: Strontium-doped bioactive glass; Mg-doped BG: Magnesium-doped bioactive glassa
Based on the results of the two-way ANOVA [Table 3], it was found that in both time intervals (4 and 8 weeks), the negative control group (unfilled defect) showed the highest amount of connective tissue that was 94.17% ±13.1% and 42.47 ± 1.42, respectively (P = 0.0001).
In pair-wise comparison of new bone regeneration consistent with the Bonferroni test [Table 4] in the 4th week, the highest mean difference belonged to magnesium-doped bioactive glass compared to the negative control group (6.38 ± 1.13) (P = 0.0001), followed by strontium-doped bioactive glass compared to the negative control group (5.86 ± 1.13) (P = 0.0001). However, the amount of bone regeneration in the 4th week between magnesium-doped and strontium-doped bioactive glass was not significantly different (P = 1), and neither was compared to standard 45S5 Bioglass (P = 0.903). In the 8th week, the highest mean difference belonged to the strontium-doped bioactive glass in comparison to the negative control group (15.73 ± 1.49) (P = 0.0001). In addition, bone regeneration was significantly higher in strontium-doped bioactive glass group compared to the magnesium-doped group in this time interval (P = 0.006).
Table 4.
Pairwise comparison of the percentage new bone regeneration based on the type of materials and time intervals according to the Bonferroni test
| Time period | (I) group | (J) group | Mean difference (I−J) | SE | Significant | 95% CI (lower bound–upper bound) |
|---|---|---|---|---|---|---|
| Week 4 | St-doped BG | 45S5 | 1.18333 | 1.13717 | 1.000 | -2.1453-4.5120 |
| Negative | 5.86667* | 1.13717 | 0.0001* | 2.5380-9.1953 | ||
| Mg-doped BG | Strontium | 0.51667 | 1.13717 | 1.000 | -2.8120-3.8453 | |
| 45S5 | 1.70000 | 1.13717 | 0.903 | -1.6286-5.0286 | ||
| Negative | 6.38333* | 1.13717 | 0.0001* | 3.0547-9.7120 | ||
| 45S5 BG | Negative | 4.68333* | 1.13717 | 0.003* | 1.3547-8.0120 | |
| Week 8 | St-doped BG | Magnesium | 5.76667* | 1.49889 | 0.006* | 1.3792-10.1541 |
| 45S5 | 10.50000* | 1.49889 | 0.0001* | 6.1126-14.8874 | ||
| Negative | 15.73333* | 1.49889 | 0.0001* | 11.3459-20.1208 | ||
| Mg-doped BG | 45S5 | 4.73333* | 1.49889 | 0.030* | 0.3459-9.1208 | |
| Negative | 9.96667* | 1.49889 | 0.0001* | 5.5792-14.3541 | ||
| 45S5 BG | Negative | 5.23333* | 1.49889 | 0.014* | 0.8459-9.6208 |
*Denotes a statistically significant result. CI: Confidence interval; BG: Strontium-doped bioactive glass; Mg-doped BG: Magnesium-doped bioactive glass; BG: Bioactive glass; SE: Standard error
In pairwise comparison (According to Bonferroni test) of the amount of residual material [Table 5], in the 4th week, the highest mean difference belonged to the strontium-doped bioactive glass compared to magnesium-doped bioactive glass (3/33 ± 2.34), which was not statistically significant (P = 0.528). In the 8th week, the highest mean difference was observed in the strontium-doped bioactive glass compared to standard 45S5 Bioglass (21.40 ± 1.78) (P = 0.0001), followed by the magnesium-doped bioactive glass compared to 45S5 Bioglass (13.46 ± 1.78), both of which were significant (P = 0.0001).
Table 5.
Pairwise comparison of the percentage of residual material based on the time intervals according to the Bonferroni test
| Time period | (I) group | (J) group | Mean difference (I−J) | SE | Significant | 95% CI (lower bound–upper bound) |
|---|---|---|---|---|---|---|
| Week 4 | St-doped BG | Magnesium | 3.33333 | 2.34804 | 0.528 | -2.9917-9.6583 |
| 45S5 | 1.73333 | 2.34804 | 1.000 | -4.5917-8.0583 | ||
| 45S5 BG | Magnesium | 1.60000 | 2.34804 | 1.000 | -4.7250-7.9250 | |
| Week 8 | Mg-doped BG | Strontium | 7.93333* | 1.78499 | 0.001* | 3.1250-12.7416 |
| 45S5 BG | Strontium | 21.40000* | 1.78499 | 0.0001* | 16.5917-26.2083 | |
| Magnesium | 13.46667* | 1.78499 | 0.0001* | 8.6584-18.2750 |
*Denotes a statistically significant result. CI: Confidence interval; BG: Strontium-doped bioactive glass; Mg-doped BG: Magnesium-doped bioactive glass; BG: Bioactive glass; SE: Standard error
In pairwise comparison (Kruskal–Wallis test with adjusted P value) of the amount connective tissue based on the type of material and the time interval [Table 6], the amount of connective tissue was only statistically significant in the 4th week and between all materials compared to the negative control group, (P = 0.0001), but the difference was not significant in the 8th week.
Table 6.
Pairwise comparison of the amount of connective tissue (percentage of the assessed area/mm2) based on the time intervals according to the Kruskal–Wallis test
| Time periods/materials | Test statistics | SE | Standard test statistic | Significant | Adjusted significant |
|---|---|---|---|---|---|
| 4 weeks/St-doped BG | -38.250 | 8.082 | -4.733 | 0.0001* | 0.0001 |
| 4 weeks/negative control | |||||
| 4 weeks/Mg-doped BG | -35.167 | 8.082 | -0.4351 | 0.0001* | 0.0001 |
| 4 weeks/negative control | |||||
| 4 weeks/45S5 BG | -34.583 | 8.082 | -4.279 | 0.0001* | 0.001 |
| 4 weeks/negative control |
*Denotes a statistically significant result. St-doped BG: Strontium-doped bioactive glass; Mg-doped BG: Magnesium-doped bioactive glass; BG: Bioactive glass; SE: Standard error
Histological examination (qualitative evaluation)
Samples were fixed in 10% formalin, and following the tissue preparations; they were molded in paraffin. Afterward, samples were cut with a 5-micrometer rotary microtome and placed on a slide. The slides were stained with H and E and Tri-chromium Mason coloring [Figure 4].
Figure 4.
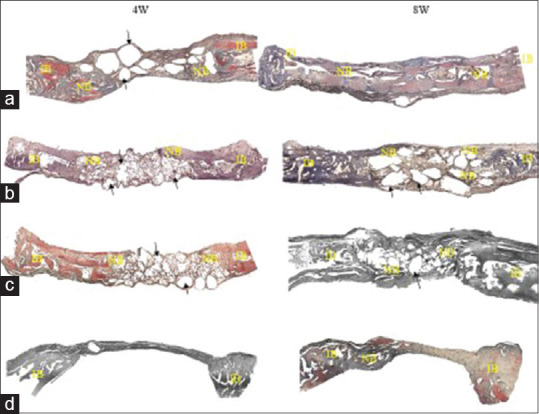
Comparison of newly formed bone (NB) in experimental groups. (a) Strontium-doped bioactive glass, (b) magnesium-doped bioactive glass, (c) standard 45S5 bioglass and (d) unfilled defect (negative control).
Four weeks - Standard 45S5 bioglass
Four weeks after inserting the 45S5 Bioglass into the created defect in the rabbit's calvaria, there was some new osseous tissue (NB) visible near the borders adjacent to the old bone. The remainder of the Bioglass granules was enclosed by the connective tissue, which is seen at the distant border of the defect [Figure 4c - black arrow].
Four weeks - Magnesium-doped bioactive glass
There were new bone fragments near the two ends adjacent to the old bone, which shows a higher bone density compared to 45S5 Bioglass. Residual Mg-BG granules enclosed by the connective tissue are seen at the distant end of the defect [Figure 4b - black arrow].
Four weeks - Strontium-doped bioactive glass
There was greater new bone tissue in Sr-BG samples compared to the 45S5 Bioglass and BG-Mg samples. The remainder of the Sr granules enclosed by the connective tissue is seen at the distant border of the defect [Figure 4a - black arrow].
Four weeks - Unfilled defect (negative control)
The control sample is covered by a thin fibrous tissue at the end of 4 weeks following surgery [Figure 4d].
Eight weeks - Standard 45S5 bioglass
Eight weeks after the introduction of 45S5 Bioglass into the defect, there was greater osseous tissue near the two ends adjacent to the old bone compared to the 4-week interval (NB). The remaining Bioglass granules enclosed by connective tissue can be seen at the distant border of the defect [Figure 4c - black arrow].
Eight weeks - Magnesium-doped bioactive glass
The new osseous tissue is seen near the two ends of the defect adjacent to the old bone, which is greater compared to 45S5 Bioglass sample. Residual Mg-BG granules enclosed by the connective tissue are seen at the distant end of the defect [Figure 4b - black arrow].
Eight weeks - Strontium-doped bioactive glass
There was greater new bone tissue in Sr-BG samples compared to the 45S5 Bioglass and BG-Mg samples at the 8-week interval. The remainder of the Sr granules enclosed by the connective tissue is seen at the distant border of the defect [Figure 4a - black arrow].
Eight weeks - Unfilled defect (negative control)
The control sample is covered by a fibrous tissue at the end of 8 weeks following surgery [Figure 4d]. In addition, tiny amounts of new bone are seen at the borders of the defect.
DISCUSSION
Today, biomaterials are in the center of focus in the regeneration of human bones. One of the newest materials proposed for bone regeneration is bioactive glasses which show the best biological behavior and highest bioavailability among all biologically active substances.[5,6] Bioactive glasses bind strongly to the bone through the formation of hydroxyapatite layer, so that separation of the glass and the bone is not possible except by breaking the bone.[10] Studies have shown that 45S5 Bioglass and other silica-based bioactive glasses are not only able to bind to the bone, but also can stimulate angiogenesis and neovascularization.[9,34] Several recent articles have also reported the positive effects of bioactive glasses on bone growth and osteoblast cell stimulation[16,35,36] but there are few articles on the impact of doped bioactive glasses on bone regeneration. In this study, the effect of magnesium-doped and strontium-doped bioactive glass was investigated on the rate of bone regeneration in rabbit's calvaria. The rationale for the selection of magnesium and strontium ions was the specific biological and mechanical properties of these materials. However, in the present study, only their biological effects were investigated.
Magnesium plays a vital role in many biological processes in the human body[37] and is one of the most critical ions associated with bone mineralization. Magnesium stimulates osteoblasts and amplifies their proliferation and differentiation.[38] Magnesium also regulates the active transport of calcium and plays a role in phagocytosis.[22] Magnesium deficiency leads to a reduction in osteoclastic and osteoblastic activity, causing bone fragility and impaired growth.[22] Similarly, strontium is recommended as a daily supplement for the treatment of osteoporosis, which is due to its anti-resorptive and dual anabolic effects on the bone that facilitates the differentiation of preosteoblasts, inhibition of osteoclast differentiation, and ultimately reduction in the activity of osteoclasts.[27] In addition, strontium at low doses stimulates osteoblastic proliferation and bone formation.[24] Bioactive glasses are thought to be an ideal reservoir for transporting strontium due to its amorphous structure and controlled degradation over time.[24,26,39,40]
The results of this study showed that doping bioactive glasses with either strontium or magnesium increases their regenerative properties and leads to the formation of more significant amounts of new bone compared to both 45S5 Bioglass and unfilled defect (negative control). However, the highest degree of bone formation was observed in the strontium-doped BG group. Therefore, these new materials can be potentially used to reconstruct periodontal and peri-implant bone defects. The increase in the regenerative activity of bioactive glasses doped with strontium or magnesium can be explained by several factors, which are discussed below.
In this study, it was found that all types of bioactive glasses (doped and non-doped) significantly increase bone regeneration compared to the unfilled defect (negative control group) in the 4th week, but the bone regeneration rate in this interval between doped-bioactive glass groups was not statistically significant. In other words, the addition of magnesium and strontium ions into the structure of the bioactive glass in the 4th week did not have a substantial effect on bone regeneration compared to 45S5 bioactive glass. This occurrence can be attributed to the fact that the strontium and magnesium ions have a significant impact on the increase in osteoblastic activity and thus in the 4th week have not yet fully demonstrated their effect.[16] It should be noted, however, that in the 4th week, in groups doped with strontium and magnesium, the rate of bone regeneration was higher (although insignificant) than 45S5 Bioglass, which could be due in part to the effect of magnesium and strontium ions on inhibition of osteoclastic activity.[26,27,41] The study of Wei et al. In 2014 showed that the number of osteoclasts decreased in the 4th week in the strontium-doped bioactive glass group, but this decrease was not significant between the different groups.[36] The results from Wei et al.'s study can justify increasing the rate of bone regeneration in strontium-doped groups in the current study.
In the 8th week, the rate of bone regeneration in the strontium-doped bioactive glass group was significantly higher than both the magnesium-doped bioactive glass and 45S5 Bioglass group. This finding is in line with several studies that have stated that bone formation in the presence of strontium ion increases.[39,42] A study by Zhang et al. in 2015 showed that in cement composed of strontium-and chitosan-doped bioactive glass particles, in comparison to the same cement but without strontium, bone formation and also the proliferation and differentiation of marrow stem cells (MSCs) derived from the brain and bone was higher.[39] Another remarkable finding in the 8th week was a more significant increase in bone regeneration in the strontium-doped group compared to the magnesium-doped group. One of the possible causes of this finding may be the release of more strontium ions in comparison to magnesium in the 8th week, as studies have shown that bioactive glass is a structurally suitable carrier for the accurate and controlled release of strontium ions.[28] In a similar survey, Poh et al., in 2016, created a new scaffold by adding 50% by weight of standard Bioglass and strontium-doped bioactive glass particles to PCL.[43] When the scaffolds were immersed in the medium, silicon and strontium ions were released in culture media for up to 10 weeks. This study showed the excellent bioavailability of the scaffold in vitro conditions. Furthermore, this scaffold in osteogenic media was able to enhance the adhesion, growth, and proliferation of cells. The explanation for this finding requires further research on the kinetics of ion release from bioactive glass and the exact effect of magnesium and strontium ions on bone formation. It should also be noted that in the 8th week, standard Bioglass significantly increased bone regeneration compared to the negative control group (unfilled defect). This phenomenon can be due to the natural effect of Bioglass on increasing angiogenesis and stimulating the secretion of growth factors and osteogenesis.[34]
In the current study, in the 4th week, the amount of residual material was not significantly different between experimental groups. In other words, osteogenesis was still in the early stages, and the percentage of absorbed material was low. The slow absorption of bioactive glass doped with either strontium or magnesium increases the amount of its tissue integration over time. In the 8th week, the residual material content in the standard Bioglass group was significantly higher than that of magnesium and strontium-doped groups. This finding can be due to the effect of strontium and magnesium ions on increasing the regeneration and, consequently, the conversion of material to osseous tissue.[19]
The amount of connective tissue was higher in all experimental groups compared to the negative control group only at the 4th week interval, which could be because the bioactive glass had induced angiogenesis and connective tissue formation.[34,44] In the 8th week, there was no significant difference between any of the groups. The explanation for this finding is that the transitional stage, namely, the conversion of the provisional connective tissue to the woven bone, was complete, and therefore, there was no difference in the degree of residual connective tissue between the groups.
Limitations and suggestions
One of the limitations of this study was that the impossibility of accurately measuring the release of strontium and magnesium ions from bioactive glass and the kinetic profile of materials. It is suggested that by kinetic laboratory studies, the release of strontium and magnesium ions be compared.
CONCLUSION
Considering the limitations, the findings of this study show that strontium and magnesium-doped bioactive glasses lead to increased bone regeneration compared to standard Bioglass, and the rate of new bone formation in the strontium-doped bioactive glass was significantly higher than all tested materials in the 8 weeks.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
REFERENCES
- 1.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: Current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Hong J, Zheng Q, Guo X, Lan S, Cui F, et al. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. J Orthop Res. 2011;29:1745–52. doi: 10.1002/jor.21439. [DOI] [PubMed] [Google Scholar]
- 3.Fricain JC, Schlaubitz S, Le Visage C, Arnault I, Derkaoui SM, Siadous R, et al. A nano-hydroxyapatite-pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials. 2013;34:2947–59. doi: 10.1016/j.biomaterials.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 4.Hench LL, Jones JR. Bioactive glasses: Frontiers and challenges. Front Bioeng Biotechnol. 2015;3:194. doi: 10.3389/fbioe.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Miao X. Sol-gel derived bioglass as a coating material for porous alumina scaffolds. Ceram Int. 2004;30:1781–5. [Google Scholar]
- 6.Hench LL. The story of bioglass. J Mater Sci Mater Med. 2006;17:967–78. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 7.Abbasi Z, Bahrololoom M, Shariat M, Bagheri R. Bioactive glasses in dentistry: A review. J Dent Biomater. 2015;2:1–9. [Google Scholar]
- 8.Li J, Cai S, Xu G, Li X, Zhang W, Zhang Z. In vitro biocompatibility study of calcium phosphate glass ceramic scaffolds with different trace element doping. Mater Sci Eng C. 2012;32:356–63. [Google Scholar]
- 9.Wilson J, Pigott GH, Schoen FJ, Hench LL. Toxicology and biocompatibility of bioglasses. J Biomed Mater Res. 1981;15:805–17. doi: 10.1002/jbm.820150605. [DOI] [PubMed] [Google Scholar]
- 10.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 11.Burke JF, Didisheim P, Goupil D, Heller J, Kane JB, Katz JL, et al. Biomaterials Science. Ch. 7. San Diego: Academic Press; 1996. Application of materials in medicine and dentistry; pp. 283–388. [Google Scholar]
- 12.Schepers EJ, Ducheyne P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: A 1-24 month experiment with several materials and particle sizes and size ranges. J Oral Rehabil. 1997;24:171–81. [PubMed] [Google Scholar]
- 13.Cacciotti I, Bianco A. High thermally stable Mg-substituted tricalcium phosphate via precipitation. Ceram Int. 2011;37:127–37. [Google Scholar]
- 14.Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Perez-Amodio S, Barrère-de Groot FY, Everts V, van Blitterswijk CA, Habibovic P. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials. 2010;31:2976–89. doi: 10.1016/j.biomaterials.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Bellucci D, Sola A, Salvatori R, Anesi A, Chiarini L, Cannillo V. Role of magnesium oxide and strontium oxide as modifiers in silicate-based bioactive glasses: Effects on thermal behaviour, mechanical properties and in-vitro bioactivity. Mater Sci Eng C Mater Biol Appl. 2017;72:566–75. doi: 10.1016/j.msec.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 17.Esfahanizadeh N, Nourani MR, Bahdaor A, Akhondi N, Montazeri M. The anti-biofilm activity of nanometric zinc doped bioactive glass against putative periodontal pathogens: An in vitro study. Biomed Glasses. 2018;4:95–107. [Google Scholar]
- 18.de Groot K. Boca Raton, Florida, United States: Taylor and Francis Group, an Informa Company, CRC Press; 1983. Bioceramics of Calcium Phosphate. [Google Scholar]
- 19.Banerjee SS, Tarafder S, Davies NM, Bandyopadhyay A, Bose S. Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of beta-TCP ceramics. Acta Biomater. 2010;6:4167–74. doi: 10.1016/j.actbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Xue W, Dahlquist K, Banerjee A, Bandyopadhyay A, Bose S. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J Mater Sci Mater Med. 2008;19:2669–77. doi: 10.1007/s10856-008-3395-4. [DOI] [PubMed] [Google Scholar]
- 21.Martini LA. Magnesium supplementation and bone turnover. Nutr Rev. 1999;57:227–9. doi: 10.1111/j.1753-4887.1999.tb06948.x. [DOI] [PubMed] [Google Scholar]
- 22.Rude RK. Magnesium deficiency: A cause of heterogeneous disease in humans. J Bone Miner Res. 1998;13:749–58. doi: 10.1359/jbmr.1998.13.4.749. [DOI] [PubMed] [Google Scholar]
- 23.Kim SR, Lee JH, Kim YT, Riu DH, Jung SJ, Lee YJ, et al. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials. 2003;24:1389–98. doi: 10.1016/s0142-9612(02)00523-9. [DOI] [PubMed] [Google Scholar]
- 24.Buehler J, Chappuis P, Saffar JL, Tsouderos Y, Vignery A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys (Macaca fascicularis) Bone. 2001;29:176–9. doi: 10.1016/s8756-3282(01)00484-7. [DOI] [PubMed] [Google Scholar]
- 25.Pors Nielsen S. The biological role of strontium. Bone. 2004;35:583–8. doi: 10.1016/j.bone.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Gentleman E, Fredholm YC, Jell G, Lotfibakhshaiesh N, O’Donnell MD, Hill RG, et al. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials. 2010;31:3949–56. doi: 10.1016/j.biomaterials.2010.01.121. [DOI] [PubMed] [Google Scholar]
- 27.Rizzoli R. A new treatment for post-menopausal osteoporosis: Strontium ranelate. J Endocrinol Invest. 2005;28:50–7. [PubMed] [Google Scholar]
- 28.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 29.Hafezi F, Hosseinnejad F, Fooladi AA, Mafi SM, Amiri A, Nourani MR. Transplantation of nano-bioglass/gelatin scaffold in a non-autogenous setting for bone regeneration in a rabbit ulna. J Mater Sci Mater Med. 2012;23:2783–92. doi: 10.1007/s10856-012-4722-3. [DOI] [PubMed] [Google Scholar]
- 30.Koudehi MF, Fooladi AA, Mansoori K, Jamalpoor Z, Amiri A, Nourani MR. Preparation and evaluation of novel nano-bioglass/gelatin conduit for peripheral nerve regeneration. J Mater Sci Mater Med. 2014;25:363–73. doi: 10.1007/s10856-013-5076-1. [DOI] [PubMed] [Google Scholar]
- 31.Naito Y, Terukina T, Galli S, Kozai Y, Vandeweghe S, Tagami T, et al. The effect of simvastatin-loaded polymeric microspheres in a critical size bone defect in the rabbit calvaria. Int J Pharm. 2014;461:157–62. doi: 10.1016/j.ijpharm.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 32.Rokn AR, Khodadoostan MA, Reza Rasouli Ghahroudi AA, Motahhary P, Kharrazi Fard MJ, Bruyn HD, et al. Bone formation with two types of grafting materials: A histologic and histomorphometric study. Open Dent J. 2011;5:96–104. doi: 10.2174/1874210601105010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidlin PR, Nicholls F, Kruse A, Zwahlen RA, Weber FE. Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin Oral Implants Res. 2013;24:149–57. doi: 10.1111/j.1600-0501.2011.02315.x. [DOI] [PubMed] [Google Scholar]
- 34.Day RM. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005;11:768–77. doi: 10.1089/ten.2005.11.768. [DOI] [PubMed] [Google Scholar]
- 35.Sabareeswaran A, Basu B, Shenoy SJ, Jaffer Z, Saha N, Stamboulis A. Early osseointegration of a strontium containing glass ceramic in a rabbit model. Biomaterials. 2013;34:9278–86. doi: 10.1016/j.biomaterials.2013.08.070. [DOI] [PubMed] [Google Scholar]
- 36.Wei L, Ke J, Prasadam I, Miron RJ, Lin S, Xiao Y, et al. A comparative study of Sr-incorporated mesoporous bioactive glass scaffolds for regeneration of osteopenic bone defects. Osteoporos Int. 2014;25:2089–96. doi: 10.1007/s00198-014-2735-0. [DOI] [PubMed] [Google Scholar]
- 37.Soulié J, Nedelec JM, Jallot E. Influence of Mg doping on the early steps of physico-chemical reactivity of sol-gel derived bioactive glasses in biological medium. Phys Chem Chem Phys. 2009;11:10473–83. doi: 10.1039/b913771h. [DOI] [PubMed] [Google Scholar]
- 38.Rude RK, Olerich M. Magnesium deficiency: Possible role in osteoporosis associated with gluten-sensitive enteropathy. Osteoporos Int. 1996;6:453–61. doi: 10.1007/BF01629578. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Cui X, Zhao S, Wang H, Rahaman MN, Liu Z, et al. Evaluation of injectable strontium-containing borate bioactive glass cement with enhanced osteogenic capacity in a critical-sized rabbit femoral condyle defect model. ACS Appl Mater Interfaces. 2015;7:2393–403. doi: 10.1021/am507008z. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Meng G, Wang S, Wu F, Huang W, Gu Z. Zn and Sr incorporated 64S bioglasses: Material characterization, in-vitro bioactivity and mesenchymal stem cell responses. Mater Sci Eng C Mater Biol Appl. 2015;52:242–50. doi: 10.1016/j.msec.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 41.Ła̧czka M, Cholewa K, Ła̧czka-Osyczka A. Gel-derived powders of CaO P2O5 SiO2 system as a starting material to production of bioactive ceramics. J Alloys Compd. 1997;248:42–51. [Google Scholar]
- 42.Gorustovich AA, Steimetz T, Cabrini RL, Porto López JM. Osteoconductivity of strontium-doped bioactive glass particles: A histomorphometric study in rats. J Biomed Mater Res A. 2010;92:232–7. doi: 10.1002/jbm.a.32355. [DOI] [PubMed] [Google Scholar]
- 43.Poh PS, Hutmacher DW, Holzapfel BM, Solanki AK, Stevens MM, Woodruff MA. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016;30:319–33. doi: 10.1016/j.actbio.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–20. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]


