Summary
Recurrent seizures are a common feature in many neurologic disorders. Seizure examination may help with diagnosis, preclinical study, and development of treatment strategies. Here we detail protocols to prepare and implant electrodes, as well as to record and analyze seizure events in freely moving mice. SCA47 mice exhibit both preclinical seizures (i.e., epileptiform discharges of EEG) starting from ∼14 weeks of age and behavioral seizures (i.e., spontaneous behavioral seizures) starting from ∼22 weeks of age.
For complete details on the use and execution of this protocol, please refer to Gennarino et al. (2018).
Subject areas: Genetics, Model Organisms, Neuroscience, Behavior
Graphical abstract

Highlights
-
•
Construction of electrodes for in vivo recordings
-
•
Electrode implantation for EEG recording
-
•
Behavioral seizure in freely moving mice
-
•
Use of SCA47 mice to monitor both preclinical and spontaneous seizures
Recurrent seizures are a common feature in many neurologic disorders. Seizure examination may help with diagnosis, preclinical study, and development of treatment strategies. Here we detail protocols to prepare and implant electrodes, as well as to record and analyze seizure events in freely moving mice. SCA47 mice exhibit both pre-clinical seizures (i.e., epileptiform discharges of EEG) starting from ∼14 weeks of age and behavioral seizures (i.e., spontaneous behavioral seizures) starting from ∼22 weeks of age.
Before you begin
To conduct electroencephalogram (EEG) recording in freely behaving mice, we need to prepare the following essential elements.
-
1.
A dedicated space with a solder station, vise, and a dissection microscope for electrode construction.
-
2.
Materials for electrode construction (electrode wires, connectors, stainless steel tubing).
-
3.
A procedure suite for mouse survival surgery to implant the electrodes into the mouse brain.
-
4.
A dedicated space and an electrophysiology rig for chronic video-EEG recordings in freely moving mice.
All research and animal care procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Mouse colonies were bred and maintained with standard mouse chow and water ad libitum under a 12 h light / 12 h dark cycle in our on-site AAALAC-accredited facility in the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital. Mice were group-housed before surgery, up to five per cage, and housed individually with nesting material in the cage after surgery to enable undisturbed recovery.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Oxygen | Airgas | UN1072 |
| Saline | Hospira, Inc. | NDC 0409-1966-02 |
| Meloxicam | Covetrus North America | NDC 11695-6936-1 |
| Isoflurane | Covetrus North America | NDC 11695-6777-2 |
| Artificial tears ointment | AKORN | NDC 17478-062-35 |
| Hair remover lotion | Church & Dwight Co., Inc | LL9023 |
| 70% ethanol | VWR | 89370-084 |
| Povidone-iodine | Purdue Products L.P. | NDC 67618-154-16 |
| Lidocaine hydrochloride | Sigma-Aldrich | 1366013 |
| Bupivacaine hydrochloride | Sigma-Aldrich | 1078507 |
| 8% hydrogen peroxide | The OneMinuteMiracle Inc. | #7722-84-1 |
| Dental cement (C&B Metabond) | Parkell | 375-0407 |
| 3M Vetbond tissue adhesive | Amazon | 1469SB |
| Experimental models:Organisms/strains | ||
| Female and male Pum1 mutant mice and wild type littermates in B6/129 mixed background; 12 weeks of age at surgery | Prof. Haifan Lin’s Lab (Yale School of Medicine, New Haven, Connecticut 06520, USA) | (Gennarino et al., 2018) |
| Software and algorithms | ||
| Data acquisition software | Molecular Devices | pCLAMP 10 |
| ANY-maze behavioral tracking software | Stoelting | Version 6.30 |
| Other | ||
| PFA-coated tungsten wire | A-M Systems | Cat. # 795500 |
| PFA-coated silver wire | A-M Systems | Cat. # 786000 |
| Stainless steel tubing, 30 Gauge | A-M Systems | Cat. # 832000 |
| Sandpaper | 3M | 400 Grit |
| Solder flux | MG Chemicals | FLUX H150 |
| Nylon surgical suture | AD Surgical | #S-N518R13 |
| Male connector | Harwin | M22-2580405 |
| Female connector | Harwin | M22-7140442 |
| USB camera | Imaginesource | DMK 22AUC03 |
| Camera lens | FUJINON | YV5x2.7R4B-2 |
| Dissection microscope | A-M Systems | EMZ-5TR |
| Stereo microscope | ZEISS | Stemi 2000-C |
| Soldering station | Weller | Model WES51 |
| Multimeter | Fluke | Fluke 179 |
| 0.5 mL insulin syringes | ADW Diabetes | # SY8290328279 |
| Isoflurane vaporizer | Veterinary Anesthesia Systems, Inc. | Matrx VIP 3000 |
| Fiber optic illuminator | A-M Systems | 725910 |
| Small animal stereotaxic instrument | David Kopf | Model 962 |
| Glass bead sterilizer | Cellpoint Scientific, Inc. | 5-1450 |
| Homeothermic blanket system | Harvard Apparatus | 507220F |
| Animal fur trimmer | WAHL | #9861-900 |
| High speed drill | The Foredom Electric Company | Model 1474 |
| Heat therapy pump | Gaymar Industries, Inc. | Model # TP650 |
| Field potential recording chamber | Pinnacle Technology Inc. | 8228 |
| Faraday cage | Technical Manufacturing Corporation | No.81-344-04 |
| 4-Channel preamplifier | Pinnacle Technology Inc. | 8406-SE4 |
| 8-Channel mouse commutator | Pinnacle Technology Inc. | 8408 |
| Differential AC amplifier | A-M Systems | Model 1700 |
| Digitizer | Molecular Devices | 1440A |
| Batteries | Amazon | Size AA |
| Digital interface for ANY-maze | Stoelting | AMi-2 |
| PC computer | Dell | 64-bit, Windows 10 |
| Surgical tools: fine scissors, scalpel, fine forceps, coarse forceps, 0.7 mm burs | Fine Science Tools | https://www.finescience.com/en-US/ |
| Straight fissure crosscut bur | Dentalaire | https://www.dentalaireproducts.com/products/burs-and-diamond-discs/ |
Materials and equipment
Local anesthetic combo
| Reagent | Final concentration | Amount |
|---|---|---|
| Lidocaine hydrochloride | 0.05% | 0.25 mL |
| Bupivacaine hydrochloride | 0.0125% | 0.25 mL |
| Saline | – | 9.5 mL |
Note: The local anesthetic combo can be made in batch and stored in aliquots of 2 mL at 4°C (6 months). Inject 0.04 mL/10 g body weight subcutaneously along the incision line of the surgical area.
Step-by-step method details
EEG electrode preparation
Timing: 40 min per electrode assembly
The purpose of this step is to construct a multi-channel electrode assembly for EEG recordings in freely moving mice.
Note: The EEG electrode assembly consists of three recording channels (Figure 1). Channel 1 (CH1) and Channel 2 (CH2) record EEG activity from the left frontal cortex and the left parietal cortex, respectively; a shared reference electrode is positioned in the occipital region of the skull. The recording electrodes of these two channels are constructed with Teflon-coated silver wire (127 μm bare diameters). Channel 3 (CH3) uses electrode built with Teflon-coated tungsten wire (50 μm bare diameters) and records local field potentials in the dentate gyrus of the right dorsal hippocampal formation; a reference electrode is positioned in the ipsilateral corpus callosum.
-
1.
Cut 4 pieces of perfluoroalkoxy alkane (PFA)-coated silver wire ∼20 mm long.
-
2.
Use a razor blade to remove 3–4 mm of the Teflon coating at one end of each wire under a dissection microscope.
Note: Take care to avoid cutting through the silver wire. One of the 4 wires is for the ground of the recording preamplifier (see below).
-
3.
Use a surgical tweezer to entwine the exposed portion of the silver wire around the base of the corresponding pins on the female connector (Figure 1A).
-
4.
Solder each connection at the wired place of the connector.
Note: Avoid soldering the connector holes.
-
5.
Cut two pieces of stainless-steel tubing (30 Gauge) that is ∼5 mm long.
-
6.
Use sandpaper to polish both ends to fully open the ends and achieve final tube lengths of ∼4 mm.
-
7.
Flush the tubes with Millipore water and dry them. Solder the tubes together (side by side) to make a bundle.
Note: Use a surgical tweezer to hold the tube while polishing. Remove dust inside the tubes by flicking them occasionally with the tweezer tip; this will avoid blocking the tube ends with dust. Solder briefly in order to bind the two tubes while avoiding blocking them with solder dust.
-
8.
Cut two pieces of PFA-coated tungsten wire that are ∼25 mm long.
-
9.
Under a dissection microscope, insert the wire into each of the two tubes.
-
10.
Then, bend each tungsten wire by ∼90º in the middle and remove 4–5 mm of Teflon coating from the tips not inserted through the tubes.
-
11.
Vertically attach the tube bundle with tungsten wires on the corresponding pin of the female connector by soldering them briefly (Figures 1A–1C).
-
12.
Use a surgical tweezer to entwine the Teflon-free portion of the tungsten wire around the base of the corresponding pins on the female connector and solder each connections of the wired place (Figure 1A).
Note: Solder flux can help make the soldering more efficient.
-
13.
Under a scaled dissection microscope, use surgical scissors to cut the two coated tungsten wires that came out of the stainless-steel tubes so that the wires extend past the tube tip by 1.8 mm and 0.9 mm, respectively.
-
14.
Use a multimeter to check/verify each wire connection and avoid crosstalk between different channels.
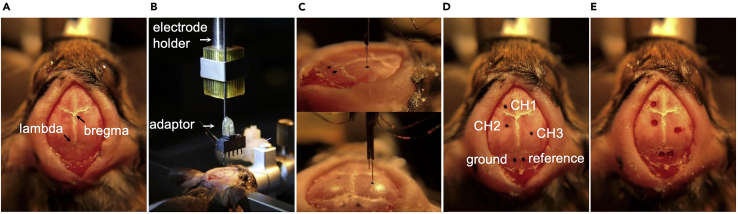
Figure 1.
Recording channel layout and electrode wiring
(A) Diagram of the channel layout on a female connector (bottom view) and the wiring of each recording electrode.
(B) Diagram of a female connector (top view) with wired electrodes. The matched double male connector enables the connection during recordings.
(C) Diagram of the electrode assembly mounted on the mouse head.
Surgical implantation of the EEG electrodes
Timing: 1.5 h
This step outlines how to implant electrode probes into mouse brains. All the procedures should be performed under aseptic conditions that include appropriate personal protective equipment, autoclaved instruments, and the use of a glass-bead sterilizer.
-
15.
Autoclave all necessary surgical instruments on a stainless-steel tray.
-
16.
Inject the mouse with Meloxicam (5.0 mg/kg, S.C.) 30 min before administering anesthesia.
-
17.
Use an isoflurane vaporizer to anesthetize the 12-week-old mouse with 2.5% isoflurane in oxygen (1.0 L/min) in the induction chamber until it is fully anesthetized (does not respond to tail- or foot-pinch).
-
18.
Remove the mouse from the induction chamber and lay it flat on its stomach on a diaper. Carefully shave the top of its head.
-
19.
Evenly apply hair remover lotion on the shaved surgical area for ∼10 s to completely remove the fur from the skin.
-
20.
Put the mouse back into the induction chamber until it again loses pinch reflexes.
-
21.
Quickly mount the anesthetized mouse onto the stereotaxic frame and switch the isoflurane anesthesia to the nose connection of the frame. The mouse should rest comfortably on a heating blanket (see step 23).
-
22.
Reduce the isoflurane concentration to 1.0%–2.0% to maintain the anesthesia level throughout the procedure.
-
23.
Use a feedback-controlled heating blanket to maintain the mouse’s body temperature at 35°C–36°C.
-
24.
Insert the temperature sensor probe with a lubricated tip part 7–8 mm into the rectum.
-
25.
Cover the mouse's eyes with ophthalmic ointment to protect them from dryness and bright light during the surgery.
-
26.
Disinfect the surgical region with 75% ethanol followed by betadine, three times.
-
27.
Inject a 50/50 mix of 2% lidocaine and 0.5% bupivacaine diluted 1:20 in sterile saline (0.04 mL/10 g body weight) subdermally along the incision line.
-
28.
Wait for 2–3 min before making the incision.
-
29.
Use a scalpel to make an incision along the midline between the eyes and the occiput (∼15 mm long) and pull the skin to the sides.
-
30.
Use a scalpel blade to clear all the connecting tissues and then a cotton swab soaked in 8% hydrogen peroxide to expose the skull surface.
-
31.
Let it dry so that the suture lines (bregma, lambda, etc.) on the skull are clearly visible (Figure 2A).
-
32.
Attach the electrode assembly to the electrode holder of the stereotaxic arm with a custom-made adaptor (Figure 2B).
-
33.
Determine the coordinates for each recording channel (CH1, left frontal cortex: A1.8L1.8; CH2, left parietal cortex: P0.8L1.5; CH3, dentate gyrus: P2.0R1.0H1.9) (Paxinos and Franklin, 2001) and mark each point on the skull with a fine black marker (Figures 2C and 2D).
-
34.
Use a surgical high-speed drill (0.7 mm bur tip diameter) to make openings (∼0.8 mm diameter) at each mark on the skull (Figure 2E).
-
35.
Then, rough the rest of the exposed skull surface with drill mounted with fissure crosscut bur for better cementing contact. All the cortical electrodes should be placed in the subdural space.
-
36.
Slowly lower the CH3 electrode to their planned depth (0.2 mm per step).
Note: Make sure that the bregma and lambda are at the same vertical level before determining the coordinates (Figure 2A).
-
37.
Apply dental cement (C&B Metabond) sequentially at the skull openings with electrode wires, the rest of the skull surface, and the space between the skull and the bottom side of the female connector. Make sure that all of the wires and wire connections on the connector pins are embedded into the cement.
-
38.
After the cement hardens (∼20 min), suture the incised skin at the rostral and caudal ends. Attach the skin edges at both sides on the cement surface using tissue glue.
-
39.
Withdraw the ear bars and loosen the nose piece. Carefully detach the implanted head posts from the connected adaptor.
-
40.
Transfer the implanted mouse into a pre-warmed cage that is heated by a heat-therapy pump. After the mouse awakes from the anesthesia, house it individually in the animal facility for 14 days to recover from the surgery.
-
41.
Administer Meloxicam (5.0 mg/kg, S.C.) daily for at least 3 days post-surgery. Carefully observe the post-surgical recovery of the mouse.
Note: Help the mice to begin eating again after surgery by setting drinking-water-softened food in a petri dish on the cage floor for the first few days. Offer fresh softened food each day for at least 2 days, or until the mouse has completely recovered. We gauge recovery by the mouse returning to normal eating and drinking, showing normal activity levels in the home cage, responding as it did before surgery to the experimenter opening the cage, and, very importantly, showing no signs of infection or pain. Obviously, this means the experimenter needs to spend time closely observing the mice and gently handling them prior to surgery and carefully checking on them daily after the surgery. In fact, we recommend weighing the mice after the surgery daily for at least a week, to provide opportunities to gently interact with them and to make sure they don't lose too much body weight from the surgery (2–3 weeks after the surgery their weight is usually within 5% of its value on the surgery day).
Figure 2.
Surgical implantation of the electrodes
(A) The bregma and lambda are identified after the skull is cleaned and dried.
(B) Electrode assembly mounted on the electrode holder of the stereotaxic arm through a custom-made adaptor.
(C and D) The sites of the three recording channels, the reference, and the ground are determined on the skull.
(E) Small openings are made with a high-speed drill.
Video-EEG recording
Timing: 28 h over 7 days
This step describes in detail how to record EEG activities synchronized with behavior in freely moving mice (Figure 3).
-
42.
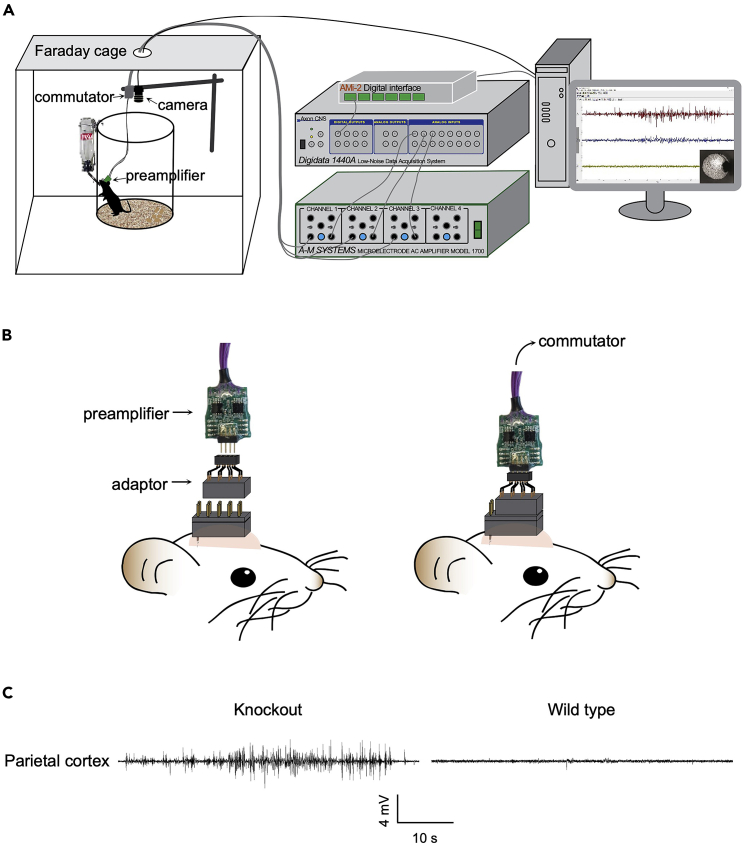
The EEG recording system requires the following components: Faraday cage, recording chamber, head-mounted preamplifier, commutator, differential AC amplifier, Axon digitizer, and Axon data acquisition software pClamp 10. The behavioral monitoring system consists of an overhead USB camera, ANY-tracking software, and an interface (AMi-2 digital interface). The output signal from the EEG recording system uses AMi-2 to trigger the video tracking system and synchronize the EEG and video recordings. Video and EEG data are stored in the computer for off-line analysis.
-
43.Set up the video-EEG motoring system (Figure 3A).
-
a.Inside a Faraday cage, prepare the mouse recording chamber (8-inch-high Plexiglass cylinder with a 10-inch diameter) with a water bottle, bedding, and food on the chamber floor
-
b.Set up the overhead camera with proper zoom and focus. Connect the camera to a USB port of the computer.
-
c.Connect the preamplifier to its power supply (± 4.5 V) and to the differential AC amplifier inputs. Use high pass filter at 0.1 Hz and low pass filter at 1 kHz. Set the overall gain (preamplifier and amplifier together) at 100×.
-
d.Connect the outputs of the differential AC amplifier to the analog inputs of the digitizer. Then, connect the USB output of the digitizer to a USB port of the computer.
-
e.Connect a digital output of the digitizer to the input of AMi-2 interface and AMi-2 to a USB port of the computer to synchronize EEG and behavior recordings.
-
f.Build up the protocol for EEG recording in pClamp software and the protocol for video motoring in ANY-maze tracking system.
-
a.
-
44.Data collection:
-
a.For each recording session, transfer the mouse to the recording room and wait at least 30 min before recording.
- b.
-
c.Open pCLAMP and ANY-maze software and load corresponding protocols.
-
d.Start the video-EEG recording.
-
a.
Note: In this protocol, one of these two pins is used to mechanically hold the CH3 electrode wires only (Figures 1A and B). Leave the two pins at the caudal head post free when connecting the preamplifier to the head post (Figure 3B). Make sure that the Faraday cage and the recording system are properly grounded to reduce environmental noise.
Figure 3.
Video-EEG recording system and connection between head post and pre-amplifier
(A) Schematic of equipment wiring for video-EEG monitoring in freely moving mice.
(B) Illustration of the connection between head post and pre-amplifier via a custom-made adaptor.
(C) Representative EEG traces adapted from (Gennarino et al., 2018) under permission. The knockout mice showed generalized epileptiform spikes typically lasted over 10 s. Wild type littermates do not show neither hyperexcitability discharges nor electrographic seizure.
Figure 4.
Photograph of a freely moving mouse with preamplifier in an EEG recording session
The recording chamber locates inside a Faraday cage, with mouse chow and water ad libitum. Therefore, the setup allows 24/7 chronic video-EEG monitoring.
Seizure analysis of the video-EEG data
Timing: 15 h
This step details how to use the recorded EEG and behavioral data to identify seizure events.
-
45.
Open the EEG data files using Clampfit (a built-in pCLAMP software program). Open the corresponding mouse-behavior video files using ANY-maze software.
-
46.
Seizure events at the EEG or behavioral level are identified visually by experimenters blind to mouse genotype and/or treatment. Electrographic seizure activities were visually identified and matched with the behavioral seizure, if applicable. The continuous field potential recording traces are composed of regular EEG with smaller amplitude fluctuations and superimposed higher amplitude spike discharges, if any (Figure 3C). To qualify as an electrographic seizure event, the event must show at least 10 s of epileptiform discharges that are positive or negative deflections exceeding twice the baseline activity (Roberson et al., 2011).
Expected outcomes
Successful EEG recording allows chronic video-EEG monitoring (both cortical EEG and subcortical local field potentials) for days, weeks, or even longer. Outcomes of the EEG data may include electrographic seizure events, power spectrum density, or cross-frequency coupling, according to the design of the project.
Limitations
This video-EEG study is performed in freely moving mice. It is more complicated and technically challenging than experiments using anesthetized mice. To properly set up and record EEG signals requires expertise in electrophysiology. Commercially available video-EEG motoring systems do exist (e.g., the Sirenia system from Pinnacle Technologies Inc.) and tend to be a little more user-friendly for labs that do not regularly perform electrophysiology experiments. We recommend that practitioners undertake at least basic training to properly identify epileptic seizure events in EEG data.
Troubleshooting
Problem 1
Difficulty soldering the stainless-steel tubes and the tungsten wires when constructing the electrode assemblies (step EEG electrode preparation, 4, 7, 11, 12).
Potential solutions
We recommend setting the soldering temperature to 450°C. Using solder flux helps. Entwining the tungsten wire on the connector pin for a few runs before soldering may increase the contact surface and reinforce the connection between the two parts.
Problem 2
Environmental noise during EEG recordings (step video-EEG recording, 43, 44).
Potential solutions
First, ground the Faraday cage and other equipment properly. Second, make sure only the animal and the battery-powered preamplifier are inside the Faraday cage when recording EEG in freely moving mice. Third, twist the active and reference cables may help reduce the noise. Fourth, use the notch filter of the amplifier if applicable. Please note that notch filter will remove the 60 Hz EEG component as well.
Problem 3
An electrode post falls off the mouse (step video-EEG recording, 44).
Potential solutions
This is a rare occurrence, but when it happens, we have to terminate experiment and euthanize the mouse. To prevent from or reduce this problem, we suggest making rough on the exposed skull surface for better cementing contact. Gently touch the skull surface with the rolling edge of a straight fissure crosscut bur mounted to the surgical drill to make it rough evenly. Clean the skull with a cotton swab lightly soaked with 8% hydrogen peroxide and let it dry before electrode insertion.
Problem 4
Occasionally, transgenic mice could be super active or even manic. There could be a mouse jumping up, grabbing on the recording wires, and even biting off the wires (step video-EEG recording, 44).
Potential solutions
Try to avoid too long wires between mouse and the commutator. In the worst-case scenario, we recommend removing and euthanize the animal.
Problem 5
EEG analysis in depth (e.g., power spectrum density, cross-frequency coupling) that is out of capability for a non-computational laboratory (step seizure analysis of the video-EEG data, 46).
Potential solutions
We recommend collaborating with investigator who has computational neuroscience background/expertise.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Jianrong Tang (jtang1@bcm.edu).
Materials availability
This study did not generate unique reagents.
Acknowledgments
We thank members of the Gennarino and Tang lab for helpful discussions and the staff of the In Vivo Neurophysiology Core of the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital for their support. We also thank V. Brandt for essential input on the manuscript. The Pum1 mouse model was a generous gift of Prof. Haifan Lin of Yale University. This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS; R01NS109858 to V.A.G.); the Paul A. Marks Scholar Program, Columbia University Vagelos College of Physicians and Surgeons (V.A.G.); NINDS (R01NS100738 to J.T.); and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD083092, P50HD103555 to Baylor College of Medicine Intellectual and Developmental Disabilities Research Center, Neuroconnectivity Core and Circuit Modulation Core).
Author contributions
V.A.G. and J.T. devised the original protocol. Q.W. and N.d.P. wrote the manuscript and contributed to the technical sections. V.A.G. and J.T. edited the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jianrong Tang, Email: jtang1@bcm.edu.
Vincenzo A. Gennarino, Email: vag2138@cumc.columbia.edu.
Data and code availability
No software was generated for this project. All software used in this study is publicly available and links are provided as appropriate in different sections of the methods and key resources table.
References
- Gennarino V.A., Palmer E.E., McDonell L.M., Wang L., Adamski C.J., Koire A., See L., Chen C.A., Schaaf C.P., Rosenfeld J.A., et al. A mild PUM1 mutation is associated with adult-onset ataxia, whereas haploinsufficiency causes developmental delay and seizures. Cell. 2018;172:924–936.e11. doi: 10.1016/j.cell.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. Academic Press; 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Roberson E.D., Halabisky B., Yoo J.W., Yao J., Chin J., Yan F., Wu T., Hamto P., Devidze N., Yu G.Q., et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J. Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No software was generated for this project. All software used in this study is publicly available and links are provided as appropriate in different sections of the methods and key resources table.