Abstract
ACTG 260 was an open-label, four-arm trial designed to study the safety and anti-human immunodeficiency virus (anti-HIV) activity of delavirdine monotherapy at three ranges of concentrations in plasma compared to those of control therapy with zidovudine or didanosine. Delavirdine doses were adjusted weekly until subjects were within their target trough concentration range (3 to 10, 11 to 30, or 31 to 50 μM). A total of 113 subjects were analyzed. At week 2, the mean HIV type 1 (HIV-1) RNA level declines among the subjects in the three delavirdine arms were similar (0.87, 1.08, and 1.02 log10 for the low, middle, and high target arms, respectively), but by week 8, the subjects in the pooled delavirdine arms showed only a 0.10 log10 reduction. In the subjects in the nucleoside arm, mean HIV-1 RNA level reductions at weeks 2 and 8 were 0.67 and 0.55 log10, respectively. Because viral suppression by delavirdine was not maintained, the trial was stopped early. Rash, which was usually self-limited, developed in 36% of subjects who received delavirdine. Delavirdine monotherapy has potent anti-HIV activity at 2 weeks, but its activity is time limited due to the rapid emergence of drug resistance.
Delavirdine mesylate (U-90152S) is a non-nucleoside human immunodeficiency virus (HIV) type 1 (HIV-1) reverse transcriptase inhibitor that belongs to a class of compounds known as the bisheteroarylpiperazines (5). This agent was discovered by the Pharmacia & Upjohn Co. and has undergone preclinical and phase I safety studies with healthy volunteers. In HIV-infected patients, two multiple-dose, safety, tolerance, and pharmacokinetic studies of delavirdine in combination with zidovudine or zidovudine plus didanosine were initiated in 1993 and demonstrated that delavirdine had antiretroviral activity and was safe (2, 7).
ACTG 260 was a phase I-II, randomized, open-label, concentration-controlled, dose-ranging trial of delavirdine monotherapy. The objectives were to examine the relationship between the concentrations of delavirdine in plasma and the reduction in the HIV burden (as determined by measurement of plasma HIV-1 RNA levels), CD4-cell response, and safety and tolerance.
MATERIALS AND METHODS
Study population.
This study was conducted at eight National Institute of Allergy and Infectious Diseases-sponsored Adult AIDS Clinical Trials Units: Ohio State University, University of Colorado, University of Miami, Northwestern University, Indiana University, Stanford University, University of Rochester, and University of North Carolina. The subjects enrolled in the study had HIV infection as documented by a licensed enzyme-linked immunosorbent assay and CD4-cell counts of 200 to 500/mm3. A total of 120 subjects were to be enrolled, with 30 subjects in each of four treatment arms: low, middle, and high plasma delavirdine concentration arms and a nucleoside analog reverse transcriptase inhibitor (NRTI) arm. Subjects were ≥18 years old and prior to enrollment signed an informed consent approved by the institutional review board of each participating unit. Subjects were antiretroviral agent naive or had used only zidovudine in the past. Other eligibility criteria included the following: Karnofsky performance status, ≥80; hemoglobin concentration, ≥9.2 g/dl for men and ≥8.9 g/dl for women; neutrophil count, ≥1,000/mm3; platelet count, ≥75,000/mm3; alanine aminotransferase and aspartate aminotransferase levels, ≤3.0 times the upper limit of normal; serum creatinine level, ≤1.5 times the upper limit of normal; serum bilirubin level, ≤2.5 mg/dl; and serum amylase level, ≤1.5 times the upper limit of normal. Women of childbearing potential were negative for serum beta-human chorionic gonadotropin in serum within 14 days prior to study entry. Exclusion criteria included prior therapy with any antiretroviral agents other than zidovudine, use of interferon or interleukin therapy within 30 days of study entry, therapy for acute or chronic cytomegalovirus, Mycobacterium avium complex, Toxoplasma gondii, or disseminated fungal infection, active substance abuse that would interfere with compliance, pregnancy or breast-feeding, malignancy other than minimal Kaposi’s sarcoma, and, if the subject was zidovudine experienced, a history of pancreatitis or grade 2 or greater peripheral neuropathy. Because of the potential for drug-drug interactions or hypersensitivity, medications that were disallowed included all investigational medications, rifabutin, rifampin, terfenadine, astemizole, loratadine, trifluoperazine, and piperazine citrate within 30 days of study entry.
Treatment.
Subjects were stratified by prior zidovudine exposure and were then randomized to one of the three delavirdine arms or the NRTI arm. Randomization was implemented with a permuted blocks scheme with a box size of 1. In the NRTI arm, zidovudine-naive subjects received zidovudine at 200 mg three times a day and zidovudine-experienced subjects received didanosine at 200 mg twice a day (125 mg twice a day if the subject weighed <60 kg) after discontinuation of zidovudine ≥3 weeks prior to study entry.
In the three delavirdine arms, the targeted trough concentrations in plasma were 3 to 10 (low), 11 to 30 (middle), or 31 to 50 μM (high). These concentrations were selected to meet or exceed the 50% inhibitory concentration (IC50) for the wild-type and mutant virus as determined in previous in vitro studies (6, 14). By using the adult AIDS Clinical Trials Group (ACTG) consensus assay for drug susceptibility (11), the median delavirdine IC50 for 25 clinical isolates from delavirdine-naive subjects was 0.038 μM (range, 0.001 to 0.69 μM) (14). After in vitro exposure of wild-type virus to delavirdine, mutations were found predominately at positions 228, 236, and 273 in the reverse transcriptase gene. The single substitution of proline 236 to leucine conferred high-level drug resistance, with an IC50 of ≈18 μM (6). For recombinant viruses carrying mutations at position 103 or 181 in the reverse transcriptase gene, which are commonly associated with resistance to other nonnucleoside reverse transcriptase inhibitors, the IC50 was ≈8 μM (6).
The volunteers initially received 200, 300, or 400 mg of delavirdine three times a day to achieve the respective low, middle, and high target concentrations in plasma. Trough delavirdine levels (6 to 12 h after administration of the most recent dose) were measured at weekly intervals, and results were available within 48 h. If subjects did not fall within their assigned targeted range after week 2, dose adjustments were made and subjects returned in 7 days to undergo repeat sampling for pharmacologic studies. Dose increases were limited to 50 mg for the low concentration arms and 100 mg for the middle and high concentration arms. When concentrations exceeded target limits, decreases of 50, 100, or 200 mg were applied. The sampling for pharmacologic studies, dose alteration, and repeat sampling usually took place within 7 to 14 days. Delavirdine concentrations were measured by a validated high-pressure liquid chromatography assay in the Laboratory for Antiviral Research, State University of New York at Buffalo, by a method developed by the Pharmacia & Upjohn Co. (17).
After half of the subjects who received delavirdine had been enrolled in the study, it became evident that the initial trough delavirdine concentrations were frequently below the targeted levels, so the protocol was amended so that all subjects who received delavirdine initially received the drug at 400 mg three times a day and then the dosage was adjusted after week 1. With the real-time delavirdine concentration monitoring, daily delavirdine doses higher than 1,200 mg could be administered to achieve the targeted higher concentrations while minimizing the risk of exceeding the levels in plasma known to be safe for more than brief periods. Treatment was to continue for 24 weeks.
Because of the known potential for the rapid emergence of drug resistance with monotherapy with other nonnucleoside reverse transcriptase inhibitors (9, 16), an early-stopping rule was incorporated into the study design. This rule required that at least 30% of the evaluable subjects in a delavirdine arm have a ≥0.7 log10 decrease in their viral burden at week 8 or the arm was to be discontinued. A 30% response rate was chosen as the lower limit of the allowed response to prevent premature elimination of a concentration arm with reasonable antiviral activity.
Clinical and laboratory evaluations.
Subjects were evaluated clinically at entry, at weeks 1, 2, 4, 6, and 8, and at monthly intervals thereafter. At these visits, weight, Karnofsky score, and signs and symptoms were evaluated. In addition, the following laboratory tests were determined at preentry, entry, weeks 2 and 4, and monthly thereafter: complete blood count with differential and platelets, electrolyte tests, liver function tests, amylase test, and urinalysis. Plasma HIV-1 RNA levels and CD3+/CD4+ and CD3+/CD8+ lymphocyte counts (absolute levels or counts and percentages) were determined at preentry, entry, and weeks 2, 4, 8, 12, 16, and 24. In addition, for subjects in the delavirdine arms, an MT-2 cell assay was performed at the baseline to determine the syncytium-inducing phenotypes of their viral isolates.
The plasma HIV-1 RNA level was determined at specified times by quantitative PCR assay (Amplicor) at a centralized laboratory (Lab Corporation of America, Research Triangle Park, N.C.) as the specimens were received. The lower limit of detection was 200 copies/ml (13). For calculation of mean and median values, subjects with undetectable virus were considered to have values of 200 copies/ml. Aliquots of stock virus from qualitative HIV cultures of peripheral blood mononuclear cells were assessed for syncytium-inducing capabilities with MT-2 cells (10). CD4+- and CD8+-cell counts and MT-2 cell assays were performed at the Adult ACTG site laboratories.
Toxicity monitoring.
The National Institute of Allergy and Infectious Diseases Division of AIDS Table for Grading Adult Adverse Experiences was used at all study sites. Toxicity was graded on a scale of from 1 to 4, with severe and life-threatening events graded as 3 and 4, respectively. The study team reviewed all grade 3 and 4 toxicities, and in the absence of other known causes, toxicities were assumed to be drug related. For cutaneous toxicity, grade 2 was a diffuse rash, grade 3 was diffuse rash with fever, blistering, or mucosal lesions, and grade 4 was diffuse rash with bullae or two or more sites of mucosal ulceration. For any subject with grade 3 toxicity judged to be potentially delavirdine related, study medication was held until the toxicity returned to grade 2 or less and then the study medication was resumed at a 100-mg-per-dose reduction. For subjects with grade 4 rashes, the subjects were permanently discontinued from study medications. An arm was to be discontinued if 5 of 30 subjects suffered a drug-related toxicity which led to treatment discontinuation.
Statistical analysis.
The study was designed to assess the rate of dose-limiting toxicity and changes in plasma HIV-1 RNA levels from the baseline to weeks 8 and 24. An interim analysis suggested that the strongest antiviral response in the delavirdine arms was at week 2, so additional analyses were included with data obtained for samples collected at this time point. A prolonged RNA response was defined as a ≥0.5 log10 decline in RNA level from the baseline level to week 8 or a ≥0.5 log10 decline from the baseline level at two consecutive visits post-week 8. Exact binomial confidence intervals were computed for the toxicity rates, and linear trend in the rate of toxicity associated with dosing arms was evaluated by the Cochran-Armitage exact test. Single-arm assessments of changes from the baseline were by t tests and Wilcoxon signed-rank tests, as appropriate. Differences among treatment arms were evaluated by analysis of covariance or Kruskal-Wallis tests, as appropriate. Predictors of antiviral response were assessed by linear regression models, and predictors for the incidence of rash and prolonged response were evaluated by logistic regression analysis. All models included the design variables treatment arm, prior zidovudine experience, and protocol version. Logarithmic transformations were used, when necessary, in order to more closely satisfy model assumptions. All reported P values were two-tailed.
RESULTS
Study population.
One hundred fifteen subjects were enrolled in ACTG 260 from October 1994 to July 1995 and were randomized to one of the four treatment arms. Data for two subjects, one each from the low- and middle-range delavirdine arms, were excluded from analysis; one received no drug and the other withdrew after the receipt of one dose, with no evaluations subsequently performed. Of the 113 subjects, 55 were zidovudine naive and 58 were zidovudine experienced with a median duration of use of 21 months. Most subjects (85%) were male, with a median age of 36 years, and most (88%) had no history of intravenous drug abuse. The study population was 69% white, 20% African American, and 11% Hispanic. The subjects were healthy, with a median Karnofsky score of over 90. The subjects in the delavirdine arms who enrolled in the amended version of the protocol, in which the initial delavirdine dosage in all arms was increased to 400 mg three times a day, were more likely to be zidovudine naive (26 of 42). Table 1 shows the baseline laboratory values for the 113 subjects analyzed.
TABLE 1.
Baseline laboratory characteristics of study populationa
| Group | Median no. (%) of CD4 cells/mm3 | Median no. (%) of CD8 cells/mm3 | Median no. of HIV-1 RNA copies/ml | % Subjects MT-2 cell assay positiveb |
|---|---|---|---|---|
| Delavirdine concn arm | ||||
| Low (3–10 μM) (n = 27) | 321 (23) | 890 (56) | 4.48 | 27 |
| Middle (11–30 μM) (n = 28) | 333 (21) | 1,034 (58) | 4.70 | 12 |
| High (31–50 μM) (n = 29) | 315 (20) | 877 (59) | 4.74 | 17 |
| Pooled (n = 84) | 322 (21) | 912 (58) | 4.62 | 19 |
| NRTI arm (n = 29) | 335 (18) | 939 (59) | 4.70 | NTd |
| All subjects (n = 113) | 322 (248–401)c [20 (16–25)]c | 926 (732–1,185) [58 (52–66)] | 4.63 (4.23–5.03) |
For CD4 counts and HIV RNA levels, the means of preentry and entry values were used to determine baseline values for individual patients. Screening CD4 counts were excluded from the calculation of baseline values.
Thirteen (32%) of 41 zidovudine-experienced and 2 (5%) of 40 zidovudine-naive subjects were MT-2 cell assay positive (P = 0.003); samples from 3 subjects were not tested by the MT-2 cell assay.
Values in parentheses are the 25 to 75% interquartile ranges.
NT, not tested.
Drug concentrations.
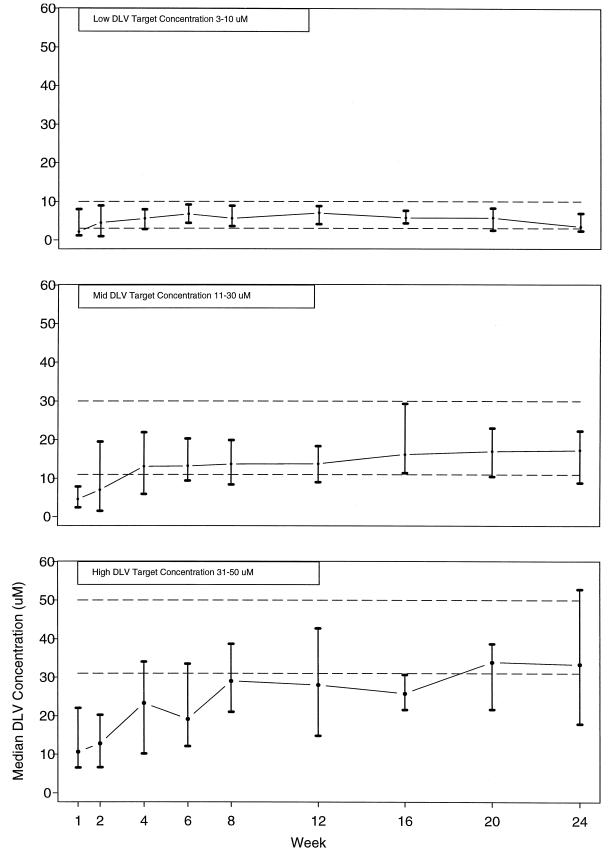
Figure 1 shows the median and interquartile ranges of the trough plasma delavirdine concentrations for each of the delavirdine arms. At 2 weeks, median concentrations for the low, middle, and high concentration arms were 5, 7, and 12 μM, respectively. Most subjects in the low concentration arm (3 to 10 μM) achieved concentrations above the minimum target level at week 2, and most subjects in the middle concentration arm (11 to 30 μM) achieved concentrations above the minimum target level at week 4, but only 47% of subjects in the high delavirdine arm (31 to 50 μM) achieved concentrations above the minimum target level by week 8. Among subjects who exceeded their minimum target concentrations, the mean current delavirdine dosages were 344, 385, and 567 mg three times a day at weeks 2, 4, and 8 for the low, middle, and high concentration arms, respectively.
FIG. 1.
Median achieved delavirdine (DLV) trough concentration by delavirdine treatment arm. The error bars indicate the interquartile ranges. The dashed lines are target concentration ranges for each treatment arm.
Viral responses.
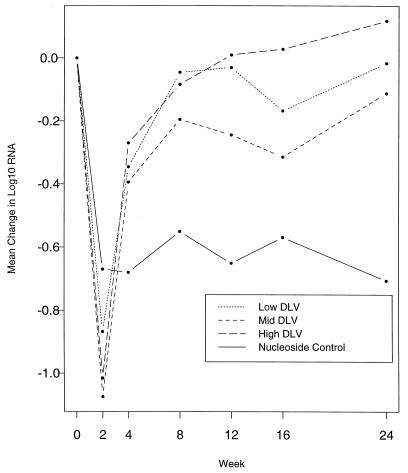
The plasma HIV-1 RNA responses in the subjects in the three delavirdine arms and the NRTI arm are shown in Fig. 2. There were no clear differences among the three delavirdine arms at week 2 (P = 0.47). At that time, subjects receiving delavirdine monotherapy had a mean fall in the plasma HIV-1 RNA level of 0.99 log10, which was significantly greater than the mean fall of 0.67 log10 in subjects in the nucleoside arm (P = 0.004). The antiviral effect of delavirdine monotherapy was short-lived, with a rapid rebound of HIV-1 RNA levels by week 4 and a return to near baseline levels at week 8. In the pooled delavirdine arms, the mean plasma HIV-1 RNA level at week 8 was 0.10 log10 lower than that of the baseline (P = 0.008); at week 24 it returned to the baseline level (P = 0.81).
FIG. 2.
Changes in median log10 plasma HIV-1 RNA level by treatment arm. DLV, delavirdine.
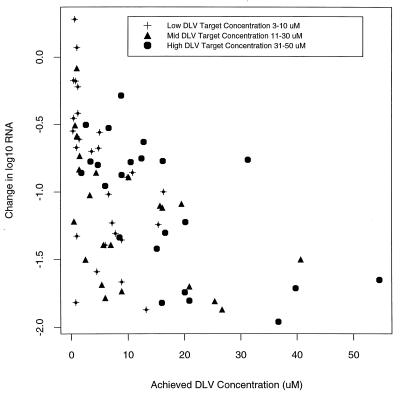
Although there was no correlation between the plasma HIV-1 RNA level reduction at week 2 with the targeted delavirdine concentration arm, the higher delavirdine concentrations that were achieved were associated with greater declines in RNA levels independent of the treatment arm (P < 0.0001) (Fig. 3). This held after adjusting individually for other variables including baseline plasma HIV-1 RNA level, CD4-cell count, and MT-2 cell assay result. A doubling of the achieved delavirdine concentration within the observed range of values was associated with a 0.17 log10 decline in the plasma HIV-1 RNA level. When considered separately, protocol version, zidovudine-naive status, and baseline MT-2 cell assay results were also significant determinants of a reduction in the plasma HIV-1 RNA level at week 2 (P < 0.05), but none of these variables remained significant predictors after adjusting for plasma delavirdine concentration.
FIG. 3.
Concentration of delavirdine (DLV) achieved at week 2 versus change in plasma HIV-1 RNA level at week 2 from that at the baseline.
The correlation of viral suppression at week 2 with plasma delavirdine concentrations did not mean that those subjects with higher concentrations were more likely to maintain viral suppression. Among the 63 subjects with delavirdine levels at week 2, 8 subjects had high delavirdine levels (>20 μM) but only one had prolonged plasma HIV-1 RNA suppression. Whereas among the other 55 subjects with delavirdine levels of less than 20 μM at week 2, there were 11 with prolonged plasma HIV-1 RNA suppression. A prolonged response was, however, associated with lower baseline plasma HIV-1 RNA levels (P = 0.05).
CD4 responses.
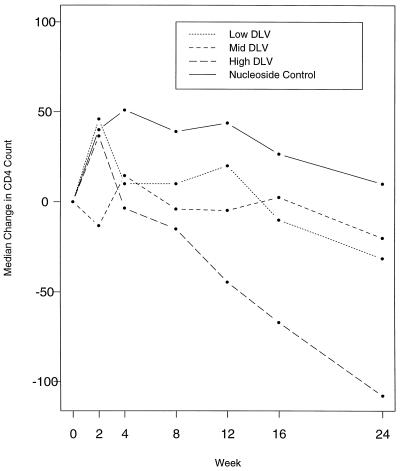
The CD4 responses among the subjects in the three delavirdine arms and the nucleoside arm are shown in Fig. 4. There was no difference among the subjects in the three delavirdine arms at week 2 or week 8 (P > 0.16). For the pooled delavirdine arms, there was a transient median increase of 25 cell/mm3 from the baseline to week 2 (P = 0.03), but this declined to below the baseline by week 8 and ended with a median decrease of 40 cells by week 24 (P = 0.001). The subjects in the nucleoside arm demonstrated a median increase of 40 CD4 cells from the baseline to week 2 (P = 0.007), and this increase was similar to that for the pooled delavirdine arms. The median CD4+-cell count in the nucleoside arm slowly fell after 4 weeks and was 10 cells above the baseline count at 24 weeks.
FIG. 4.
Changes in CD4-cell count by treatment arm. DLV, delavirdine.
Adverse reactions.
Sixty-nine subjects completed the 24-week study. Failure to complete the study was due to early study termination for 23 subjects and early withdrawal from the study for 21 (3 were withdrawn because of protocol-defined toxicities, 3 were withdrawn because of physician requests, 9 subjects requested withdrawal, 3 were withdrawn because of clinical endpoints from major intercurrent illnesses, 1 was withdrawn because of the use of prohibited concomitant medication, and 2 were lost to follow-up).
Thirty (36%) of the subjects who received delavirdine and none of the subjects who received an NTRI developed rashes. All grade 3 and 4 rashes were thought to be drug related, and lesser-grade rashes were included if they were thought to be drug related. The median time to the onset of the rash was 10 days (25th and 75th percentiles, 8 and 11 days, respectively). Twenty-three subjects had rashes of grade 2 or less and were able to continue delavirdine therapy uninterrupted or were successfully rechallenged. Seven (8%) of the subjects who received delavirdine had a grade 3 or 4 rash. The one subject with a grade 4 rash had oral mucosal lesions which resolved completely after the delavirdine was discontinued. Grade 3 fever occurred in one subject with a rash in the delavirdine group. The median concentration of delavirdine at week 1 tended to be higher among subjects with rash (any grade) compared to those without rash (8.6 versus 4.6 μM). In addition, the concentration of delavirdine achieved at week 1 was a significant predictor of rash (P = 0.04), with a 10 μM increase in concentration associated with a twofold increase in the odds of developing a rash.
Overall rates of grade 3 or 4 toxicities were 7% in the low-concentration delavirdine arm, 14% in the middle-concentration delavirdine arm, 24% in the high-concentration delavirdine arm, and 3% in the NRTI arm. Due to the small sample sizes, there was no evidence of a trend in the rate of grade 3 or 4 toxicities or grade 3 or 4 rash with increasing target delavirdine levels (P = 0.10 and 0.23, respectively). However, in the initial version of the protocol, which varied the initial delavirdine dose by treatment arm, there was a trend in the rate of grade 1 or higher rash associated with the increasing targeted delavirdine level (P = 0.02). This effect was not observed under the modified version of the protocol, which set the initial dose as the same for all three delavirdine arms (P = 0.71). In addition to rashes, six subjects had grade 3 or 4 increases in liver function tests, but the increases for three subjects were later determined to be due to viral hepatitis and the increase for one subject was related to lymphoma. The two subjects with liver function abnormalities that were possibly or probably related to delavirdine completed 24 weeks of the trial after temporarily stopping drug. Other grade 3 toxicities that resolved with continuation of the delavirdine occurred in three subjects, with one each having thrombocytopenia, nausea, or fatigue. In addition, one subject in the delavirdine group with grade 4 headache withdrew from treatment, and one subject in the nucleoside control arm experienced grade 3 nausea.
DISCUSSION
This clinical trial was the first to evaluate the anti-HIV activity and safety of monotherapy with the nonnucleoside reverse transcriptase inhibitor delavirdine. The two major objectives of the trial were to determine if higher plasma delavirdine levels were safe and if they could enhance and maintain viral suppression by exceeding the in vitro IC90 concentration for drug-resistant mutant virus.
Although the initial antiviral activity of delavirdine was concentration dependent and was greater than that observed with zidovudine or didanosine, it was transient even at higher concentrations in plasma. Antiviral activity could not be correlated with concentration target groups because the time to reach the targeted concentration range exceeded the duration of antiviral activity. Viral rebound occurred within the first month of delavirdine dosing and correlated with the development of reverse transcriptase mutations, predominately K103N and Y181C, and decreased in vitro susceptibility (4). The CD4 responses to delavirdine and nucleosides mirrored the HIV-1 RNA responses. Initial CD4 increases with delavirdine therapy were comparable to those observed with nucleoside therapy, but those observed with delavirdine were short-lived. The rapid onset and loss of antiviral activity and immunologic response from delavirdine monotherapy are similar to those observed with nevirapine monotherapy (3, 9).
This study was one of the first to use HIV-1 RNA assays that were performed and whose results were analyzed while the trial was ongoing. By design, the protocol included an interim analysis which permitted discontinuation of an ineffective treatment. On the basis of the results of the HIV-1 RNA levels for the first 75 delavirdine recipients, which demonstrated the failure to achieve prolonged viral suppression, the trial was terminated. The current availability of real-time viral RNA assays should facilitate the development of future trials that limit the time that subjects continue on ineffective regimens. Such designs could enhance subject recruitment into trials of new experimental anti-HIV therapies even when the standard therapies are rapidly improving.
The concentration-controlled study design allowed the attainment of the desired concentrations in the plasma of the majority of subjects. However, the time needed to reach the targeted levels increased with the magnitude of the desired concentration. In part, this was due to the small dose increments allowed for delavirdine. The logistics of this concentration-controlled study design were also complex. While dose modifications to attain the desired concentration were made within a week of sampling for drug concentration determination, the very rapid outgrowth of drug-resistant virus complicated the interpretation of the concentration-response curve. This was evidenced in our trial. Although there was a drug concentration-viral response relationship at week 2, overall, we could not demonstrate a relationship between the assigned delavirdine arm and the virologic response. On the other hand, monitoring of levels of therapeutic antiviral drugs such as delavirdine might prove to be useful in a trial design which uses potent combinations of antiviral agents to more completely suppress viral replication. Drug-resistant virus would be less likely to emerge during the dose-adjustment period, and a more reliable concentration-response curve could be generated.
Delavirdine was tolerated by most subjects, although rash was problematic. Rash was seen in about one-third of recipients and was responsible for an 8% discontinuation rate. Its overall frequency, but not the likelihood of serious reactions, was related to observed drug concentrations. A high (25 to 40%) incidence of drug rash and related 7% drug discontinuation rate have also been seen with nevirapine (1, 15). The incidence of rash from nevirapine was reduced by initiating therapy at a reduced dose. Fever, which has been reported in nevirapine recipients with severe rash, was rare with delavirdine (1).
The transient viral suppression and immunologic improvement from delavirdine monotherapy should not be interpreted as indicative of a lack of benefit with this agent. In this study, subjects with low baseline HIV-1 RNA levels were more likely to demonstrate prolonged viral suppression with delavirdine monotherapy, and a recent phase I-II trial showed prolonged suppression in some patients who received delavirdine with zidovudine and didanosine (2). In other studies of delavirdine in combination with zidovudine or zidovudine and lamivudine, subjects with lower baseline HIV-1 RNA levels were more likely to have an HIV-1 RNA level of <400 copies/ml at 24 weeks (8). Delavirdine-containing antiviral combination regimens which result in more complete viral suppression would likely maintain antiviral activity longer and would reduce the development of drug-resistant isolates, as has been demonstrated with nevirapine (12). The concentration-response curve for delavirdine also suggests that even higher concentrations of delavirdine might be more effective in such regimens in which complete viral suppression is possible.
ACKNOWLEDGMENTS
This study was supported by U.S. Public Health Service contracts from the National Institute of Allergy and Infectious Diseases to the Adult AIDS Clinical Trials Group (contract U01-AI-38858 [to the central Adult ACTG] and contract AI-25924 [to the Ohio State University] and the National Center for Research Services, which funds the General Clinical Research Centers used at select sites. Viral load assays and drug assays were supported by the Pharmacia & Upjohn Co., and antiviral drugs were supplied by their manufacturers.
Appendix
The Adult ACTG 260 team members who also contributed to the design and execution of the protocol were Caroline Whitacre (Ohio State University Columbus), Janie Reese (University of Miami, Miami, Fla.), Ana Martinez and Irene Fishman, (Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, Md.), Joseph Timpone (DC General Hospital, Washington, D.C.), and Bruce Peel (Pharmacia & Upjohn, Kalamazoo, Mich.).
The following individuals and institutions also participated in the performance of this trial: Mark Shelton, John Adams, Linda Bartos, Ross G. Hewitt, and Michele Lewis, State University of New York at Buffalo; Thomas C. Merigan, Jr., Pat Cain, and Virginia Tallman, Stanford University; W. Jeffrey Fessel and Gretchen Van Raalte, Stanford at Kaiser; Richard C. Reichman and Carol Greisberger, University of Rochester Medical Center; Donald C. Blair and Linda Brasington, State University of New York at Syracuse; Judith Neidig and Amy Fetzer, Ohio State University; Lawrence Joseph Wheat, Beth Zwickl, and Kris Todd, Indiana University; John P. Phair, Baiba L. Berzins, and Robert Murphy, Northwestern University; Joseph J. Pulvirenti and Ed Goodwin, Cook County Hospital; Joseph J. Eron and Barbara Longmire, University of North Carolina; Peter Anthony Leone, Wake County Department of Health; and Dan Kuritzkes, and M. Graham Ray, and Dara Torre, University of Colorado Health Sciences Center.
REFERENCES
- 1.D’Aquila R T, Hughes M D, Johnson V A, Fischl M A, Sommadossi J P, Liou S, Timpone J, Myers M, Basgoz N, Niu M, Hirsh M S. Nevirapine, zidovudine, and didanosine compared to zidovudine, and didanosine in patients with HIV-1 infection. Ann Intern Med. 1996;1214:1019–1030. doi: 10.7326/0003-4819-124-12-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Davey R T, Chaitt D G, Reed G F, Freimuth W W, Herpin B R, Metcalf J A, Eastman P S, Falloon J, Kovacs J A, Polis M A, Walker R E, Masur H, Boyle J, Coleman S, Cox S R, Wathen L, Daenzer C L, Lane H C. Randomized, controlled phase I/II trial of combination therapy with delavirdine (U-90152S) and conventional nucleosides in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 1996;40:1657–1664. doi: 10.1128/aac.40.7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeJong M D, Vella S, Carr A, et al. High-dose nevirapine in previously untreated human immunodeficiency virus type-1 infected persons does not result in sustained suppression of viral replication. J Infect Dis. 1997;175:966–970. doi: 10.1086/514002. [DOI] [PubMed] [Google Scholar]
- 4.Demeter L, Shafer R, Para M, Morse G, Freimuth W, Merigan T, Reichman R. Program and abstracts of the Third Conference on Retroviruses and Opportunistic Infections. 1996. Delavirdine susceptibility of HIV-1 isolates obtained from patients receiving delavirdine monotherapy-ACTG 260, abstr. 322; p. 113. [Google Scholar]
- 5.Dueweke T L, Kezdy F J, Waszak G A, Deibel M R, Tarpley W G. The binding of a novel bisheteroarylpiperazine mediates inhibition of human immunodeficiency virus type 1 reverse transcriptase. J Biol Chem. 1992;267:27–30. [PubMed] [Google Scholar]
- 6.Dueweke T J, Pushkarskaya T, Poppe S M, Swaney S N, Zhao J Q, Chen I S Y, Stevenson M, Tarpley W G. A mutation in reverse transcriptase of bis(heteroaryl)piperazine-resistant human immunodeficiency virus type 1 that confers increased sensitivity to other non-nucleoside inhibitors. Proc Natl Acad Sci USA. 1993;90:4713–4717. doi: 10.1073/pnas.90.10.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freimuth W W, Davey R, Batts D, Lane C, Cox S, Wathen L, Peel B, Daenzer C, Morse G, Chaitt D, et al. Program and abstracts of the X International Conference on AIDS/V STD World Congress. 1994. Phase II clinical trials of delavirdine mesylate combination therapy, abstr. 512B; p. 59. [Google Scholar]
- 8.Green S, Para M F, Daly P W, Freimuth W W, Getchel L D, Greenwald C A, Wathen L K. Program and abstracts of the Twelfth World AIDS Conference. 1998. Interim analysis of plasma viral burden reductions and CD4 increases in HIV-1 infected patients with delavirdine + Retrovir (ZDV) + Epivir (3TC), abstr. 12219. [Google Scholar]
- 9.Havlir D, Cheeseman S H, McLaughline H M, Murphy R, Erice A, Spector S A, Greenough T C, Sullivan J L, Hall D, Myers M, Lamson M, Richman D D. High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J Infect Dis. 1995;171:537–545. doi: 10.1093/infdis/171.3.537. [DOI] [PubMed] [Google Scholar]
- 10.Japour A, Fiscus S, Arduino J-M, Mayers D L, Reichelderfer P S, Kuritzkes D R. Standardized microtiter assay for determination of syncytium-inducing phenotypes of clinical human immunodeficiency virus type 1 isolates. J Clin Microbiol. 1994;32:2291–2294. doi: 10.1128/jcm.32.9.2291-2294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japour A J, Myers D L, Johnson V A, Kuritzkes D R, Beckett L A, Arduino J, Lane J, Black R J, Reichelderfer P S, D’Aquila R T, Crumpacker C S the RV-43 Study Group; the AIDS Clinical Trials Group Virology Committee Resistance Working Group. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother. 1993;37:1095–1101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montaner J S G, Reiss P, Cooper D, Vella S, Harris M, Conway B, Wainberg M, Smith D, Robinson R, Hall D, Myers M W, Lange J for the INCAS Study Group. A randomized double-blind trial comparing combinations of nevirapine, didanosine and zidovudine for HIV-infected patients: the INCAS trial. JAMA. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 13.Mulder J, McKinney N, Christopherson C, Snisky J, Greenfield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pharmacia & Upjohn Company. Delavirdine package insert. Bridgewater, N.J: Pharmacia & Upjohn Company; 1997. [Google Scholar]
- 15.Roxane Laboratories. Nevirapine package insert. Columbus, Ohio: Roxane Laboratories; 1998. [Google Scholar]
- 16.Saag M S, Emini E A, Laskin O L, et al. A short-term clinical evaluation of L-697,661, a non-nucleoside inhibitor of HIV-1 reverse transcriptase. N Engl J Med. 1993;329:1065–1072. doi: 10.1056/NEJM199310073291502. [DOI] [PubMed] [Google Scholar]
- 17.Staton B A, Johnson M G, Friis J M, Adams W J. Simple, rapid, and sensitive high-performance liquid chromatographic determination of delavirdine and its N-desisopropyl metabolite in human serum. J Chromatogr Biomed Appl. 1995;668:99–106. doi: 10.1016/0378-4347(95)00045-k. [DOI] [PubMed] [Google Scholar]