Abstract
The purpose of the current study was to explore the supplementing effects of Moringa oleifera leaf powder (MOLP) on plasma and milk biochemical indices and productive/reproductive performance of goats. A total of 30 healthy pregnant goats were randomly distributed (n = 10) into three experimental groups: control (M0) group (basal diet without MOLP), M2% group (basal diet + 2% MOLP), and M3.5% group (basal diet + 3.5% MOLP). The experiment started 2 months before parturition and continued till the first month of lactation. The plasma flavonoids were significantly increased in the M3.5% group during the entire experiment, whereas the total phenolic contents were enhanced only during the lactation period depending on the supplementation percentage. The amount of vitamin C increased significantly in M2% and M3.5% groups as compared to the M0 group. Supplementation of MOLP improved the plasma total antioxidant capacity by declining malondialdehyde concentration and total oxidant status values. The activities of superoxide dismutase and peroxidase enzymes were modified in M2% and M3.5% supplemented groups throughout the experiment, while the catalase activity was significantly influenced only during the lactation stage. The protein and lycopene contents in plasma were significantly improved in the M3.5% group, whereas the total sugars and carotenoid level was increased in both M2% and M3.5% groups. Dietary supplementation with 3.5% MOLP more effectively enhanced protease and amylase activities as compared to 2% supplementation. MOLP also improved the biochemical indices and antioxidant status of colostrum and milk. The milk yield, weight gain of the kids, and reproductive performance were high in M2% and M3.5% groups in comparison to the M0 group. These findings disclose that supplementing the diet with 3.5% MOLP improves antioxidant status, milk yield, and reproductive performance in goats.
Keywords: Moringa oleifera, plasma biochemistry, antioxidant status, milk composition, productive performance, goat
Introduction
Reproductive performance is a key factor in goat production and is directly related to maternal nutrition. The pariparturient period (late gestation and early lactation) is characterized by depressed feed intake, endocrine, and metabolic changes that interrupt energy balance and anti-oxidant status of the body (1, 2). Nutritional requirements are high during this period due to accelerated digestion rate, tissue mobilization for mammary development, and fetus growth (3). However, maternal malnutrition is common in small ruminants in most parts of the world due to scarcity and high cost of feed stuffs. The farmers, in these regions, mostly depend on conventional grazing and crop residues to meet the requirements of their animals. The natural grazing pastures and crop residues have fluctuating nutritional status, and their feeding alone is not sufficient to satisfy the energy needs of pregnant and lactating animals (4). The energy deficit feed makes the pregnant animals more prone to oxidative stress with enormous production of reactive oxygen species (ROS) that results in a disturbance of the balance between oxidant and antioxidant defense systems of the body (5). All biomolecules including lipids, carbohydrates, and proteins are adversely affected by oxidative stress, which ultimately leads to a decline in reproductive and productive performance (6). Furthermore, the newborn kids may suffer from a variety of diseases that will negatively influence their survival and growth rate (7).
Colostrum is the first secretion produced soon after parturition and is a source of immunity for newborns. Immunoglobulins, minerals, and many other biologically active substances are transferred passively through the colostrum to the kids of sheep, goat, cattle, and horse as they do not get into the embryo's bloodstream (8). The composition and quality of colostrum and milk predominantly depend on the nutrition of the mother (9, 10). The feed should fulfill the nutritional requirements of pregnant animals to get good quality of colostrum and milk from them after parturition. The inadequate supply of nutrients will adversely affect the synthesis and composition of colostrum and milk.
The diet manipulation with phytobiotics (plant-derived feed additives) has been proposed to be an effective approach in managing nutrition-induced oxidative stress during pregnancy and lactation in both small and large ruminants (11–14). Some herbal plants have medicinal values and are nutritionally important to enhance the health status and reproductive performance of goats. Their supplementation with a basal diet can minimize nutrition-related problems in goat production (15).
Moringa oleifera (MO) is an evergreen tree fodder, also known as a “miracle tree,” and is one of the most useful, multi-purpose, fast-growing, and drought-resistant trees. It is well-known for its medicinal importance and nutritional characteristics. Moringa leaves contain a sufficient quantity of vitamins, minerals, and proteins according to the nutritional demands of pregnant and lactating animals (16, 17). Moreover, MO leaves are also a rich source of different bioactive compounds, especially abundant in antioxidants including flavonoids (kaempferol, myricetin, and quercetin), phenolic acids (gallic, ellagic, and chlorogenic acid), Vit C, Vit E, β-carotene, Se, and Zn (18). These substances have been detected separately in various plants, but MO is distinct in having them all in substantial amounts (19).
The MO leaves are readily adapted and easily digested by animals. The favorable impacts of MO have been observed on the anti-oxidant status and reproductive performance in mice and sows (20, 21). However, there is little information about dietary supplementation effects of Moringa oleifera leaf powder (MOLP) during the nutritionally critical stages (late pregnancy and early lactation) in goats. Therefore, the study was designed to evaluate the effects of MOLP as a nutritional supplement on productive and reproductive performance, plasma, and milk biochemical indices in Beetal goats. The results of this study will enlighten the knowledge about the development of different bioactive compounds from MO leaf in the field of goat reproduction.
Materials and Methods
Ethical Statement
The procedures used for study and ethical clearance were approved and granted by Animal Use and Care Research Committee at Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, Pakistan.
Experimental Design and Animal Husbandry
The experiment was conducted at the goat research farm of Nuclear Institute for Agriculture and Biology (NIAB), situated ~7 km from the nucleus of the city Faisalabad, Pakistan (longitude 73.0791° E and latitude 31.4287° N) at an altitude of 184 m from sea level. The average rainfall and temperature were 15.50 mm and 41°C during the months (April to July 2020) of the experiment.
A total of 30 pregnant Beetal goats of 2–3 years age and weighing about 40 kg ± 2.3 were selected exactly 2 months before their predicted delivery. The ultrasonographic examination of goats was performed by the Department of Theriogenology, University of Agriculture, Faisalabad for confirmation of pregnancy. All the goats were randomly divided (n = 10) into the control (M0) group (250 g basal diet without MOLP/animal/day), M2% group (250 g basal diet with 5 g MOLP/animal/day), and M3.5% group (250 g basal diet with 8.75 g MOLP/animal/day). The basal diet was composed of wheat, corn, rice bran, sugarcane molasses, minerals, and soybean meal formulated according to the nutritional requirements of pregnant goats (Table 1) (23). The experiment was initiated 2 months before the estimated due date of kidding and continued till the first month of lactation. All the animals were acclimatized 1 week for the basal diet. Before the start of the experiment, deworming was performed with Albendazole (Zoben, Prix, Lahore, Pakistan) at a dose rate of 2.5 mg/5 kg/BW. The goats were kept in well-ventilated semi-open sheds and offered two times free pasture grazing (Chloris gayana, Leptochloa fusca, Carduus nutans, Chenopodium album, and Cirsium arvense) during morning and evening schedule with free access to clean drinking water.
Table 1.
Formulation of basal diet (% DM).
| Ingredients | Amount (%) |
|---|---|
| Wheat grains | 30 |
| Corn | 20 |
| Wheat bran | 15 |
| Rice bran | 15 |
| Wheat straw | 10 |
| Soyabean meal | 5 |
| Molasses | 3 |
| Dicalcium phosphate | 1.8 |
| Vitamin and mineral premixa | 0.2 |
| Total | 100 |
Vitamin and mineral premix per kg containing: Vit E 300 IU, Vit A 50,000 IU, Vit D3 80,000 IU, Ca 18.5%, Mg 8.2%, P 3.5%, Na 3%, Zn 3,200 mg, Mn 3,333 mg, Cu 800 mg, Se 24 mg, Iodate 68 mg, Co 16 mg (22).
Plant Material
The fresh green leaves of MO (PKM1) were collected from a breeder's farm (Lahore, Pakistan) during the month of November 2019, and their authenticity was assured by an experienced botanist at NIAB. A representative sample of leaves was sent to the institute's herbarium for reference in the future. The leaves were cleaned properly by washing and dried under shade at room temperature for 4 days. The dried leaves were processed to make powder and then stored in airtight containers for use in the experiment.
Compositional Analysis of Diet
The MOLP and basal diet were subjected to proximate chemical compositional analysis by using standardized methods of analytical chemists (24) as also used in our previous study (22). The MOLP was also analyzed for its different biochemical elements spectrophotometrically (UV-VIS U2800, Hitachi, Japan). The compositional analysis of MOLP and basal diet are presented in Tables 2, 3.
Table 2.
Chemical composition of basal diet and MOLP (DM basis).
| Constituents | Basal diet | MOLP |
|---|---|---|
| Mean (%) ±SE | Mean (%) ±SE | |
| Dry matter | 94.8 ± 0.43 | 92.5 ± 0.52 |
| Crude protein | 13.5 ± 0.16 | 18.2 ± 0.06 |
| Crude fat | 4.4 ± 0.23 | 5.5 ± 0.05 |
| Ash | 16.7 ± 0.37 | 11.3 ± 0.03 |
| Nitrogen free extract | 47.3 ± 0.33 | 38.4 ± 0.29 |
| Neutral detergent fiber | 53.2 ± 0.26 | 32.4 ± 0.12 |
| Acid detergent fiber | 18.06 ± 0.13 | 19.1 ± 0.08 |
MOLP, Moringa oleifera dried leaf powder (22).
Table 3.
Nutritional constituent analysis of MOLP.
| Biochemical constituents | Mean ±SE |
|---|---|
| Total phenolic contents (μM/g) | 36,000 ± 3.21 |
| Total Flavonoids (μg/g) | 258.58 ± 2.28 |
| Vitamin C (μg/g) | 546.16 ± 3.06 |
| Lycopene (mg/g) | 9.95 ± 0.17 |
| Total carotenoids (mg/g) | 13.87 ± 0.33 |
| Total sugars (mg/g) | 27.51 ± 1.52 |
| Methionine (% of DM) | 0.42 ± 0.012 |
| Cysteine (% of DM) | 0.52 ± 0.014 |
| Sodium (mg/g) | 2.13 ± 0.075 |
| Calcium (mg/g) | 180 ± 1.154 |
| Potassium (mg/g) | 8.99 ± 0.571 |
| Selenium (mg/g) | 0.31 ± 0.057 |
| Iron (mg/g) | 0.16 ± 0.034 |
MOLP, Moringa oleifera leaf powder (22).
Collection of Blood and Milk Samples
Blood samples (5 ml) were collected in sterile EDTA tubes (Vacutainer, Xinle) from the jugular vein with 20-day intervals after the start of the experimental diet from day 90 of gestation. Plasma was separated from blood samples by centrifugation at 3,000 rpm/4°C and stored at −20°C till further analysis. Colostrum samples were obtained within 2 h after parturition. Milk samples were collected with 1-week interval for 4 consecutive weeks and stored at −20°C for further biochemical analyses. Blood and milk samples were collected at 7–8 a.m. during the whole experiment. The defatting of colostrum and milk samples was done by centrifugation at 2,500 × g for 15 min for enzymatic and non-enzymatic antioxidant estimation.
Analysis of Blood Plasma
Non-enzymatic Antioxidants
Total Flavonoids
Total flavonoids (TF) in plasma samples were estimated by AlCl3 colorimetric assay and rutin was used as standard (25). The samples were mixed with 100 μl of 10% AlCl3, 100 μl of 1 M potassium acetate, and 275 μl of deionized water. The contents were incubated for 40 min at room temperature and then absorbance was measured at 415 nm by using a double beam spectrophotometer (UV-VIS U2800, Hitachi, Japan). The TFs were computed with the help of a standard curve and expressed as μg RE (retinol equivalents) per milliliter of sample.
Total Phenolic Content
Total phenolic contents (TPC) were assessed using a modified Folin-Ciocalteu procedure (26). The 100-μl blood plasma sample was vortexed with 100 μl of Folin-Ciocalteu reagent for 15 s and then incubated for 1 h at room temperature after adding 700 mM Na2CO3 (800 μl). The absorbance of reaction was read at 765 nm and TPCs were calculated from a linear regression equation.
Vitamin C
Vitamin C was determined by using a standardized protocol of Moeslinger et al. (27). Briefly, vitamin C causes reduction of a colored compound 2,6 Dichlorophenolindophenol (DCPIP) into DCPIPH2 (colorless compound). This reaction was monitored by fall-off absorbance at 520 nm. The concentration of vitamin C in plasma samples was measured by using a standard calibration curve.
Malondialdehyde
Malondialdehyde (MDA) concentration in blood plasma samples was assessed colorimetrically by using MDA as standard (28). Plasma sample (25 μl) was homogenized in 0.1% trichloroacetic acid and centrifuged exactly for 5 min at 14,000 × g. Then, trichloroacetic acid (20%) containing thiobarbituric acid (0.05%) was added in 1 ml aliquot of the supernatants and heated for 30 min by placing in boiling water bath. The reaction mixture was cooled after removing from the water bath and centrifuged for 10 min at 14,000 × g. The absorbance of clear supernatants was read at 535 nm and the value of non-specific absorbance (600 nm) was subtracted from it. The MDA contents were measured by a coefficient of extinction 155 per mM per cm.
Total Antioxidant Capacity
Total antioxidant capacity (TAC) of plasma samples was measured by an assay based on the reduction of a blue radical cation (ABTS∙+) to its original ABTS colorless form by antioxidants (29). The assay solution to measure TAC is composed of reagent R1 (CH3COONa buffer and glacial acetic acid), R2 (H2O2, Na3PO4 buffer, glacial acetic acid, and ABTS), and sample. The contents of the reaction solution were incubated for about 6 min at room temperature and then absorbance was read at 660 nm. The TAC value was computed from a standard ascorbic acid calibration curve and represented as ascorbic acid (μM) equivalent per milliliter of sample.
Total Oxidant Status
The method for the determination of total oxidant status (TOS) values of plasma samples is based on the oxidation of Fe2+ into Fe3+ by oxidants present in the sample (29). A specific color appeared when Fe3+ ions react with xylenol and the magnitude of color is directly related to the quantity of oxidant molecules that were measured spectrophotometrically. The reaction solution for the determination of TOS value is composed of R1, xylenol solution (0.38 g in 500 μl of 25 mM H2SO4), R2 [ferrous ammonium sulfate (II) 0.0196 g, o-dianisidine 0.0317 g, glycerol 500 μl, and NaCl 0.4 g], and sample. The absorbance value was read after 5 min of adding R2. Hydrogen peroxide (H2O2) was used to calculate the final value of TOS that was expressed in μM H2O2 equivalent per milliliter.
Enzymatic Antioxidants
Superoxide Dismutase Activity
The plasma samples were assayed for superoxide dismutase (SOD) activity by an inhibition assay that works on the base of SOD ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) into formazan (30). The reaction solution for the study of inhibition assay is composed of 50 mM potassium phosphate buffer (pH 7.8), 13 mM L-methionine, 57 μM NBT, Triton X-100 (0.025%), riboflavin (0.004%), and 50 μl of blood plasma sample in a total volume of 3 ml. The photoreaction was performed in a box lined with aluminum (Al) foil and having a 15-W lamp as a light source. The absorbance of the reduction reaction of NBT to formazan was taken at 560 nm and a unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of NBT.
Peroxidase Activity
Plasma peroxidase (POD) activity was estimated by using the method of Agostini et al. (31) with some necessary modifications and using guaiacol as substrate. The assay solution to measure POD activity contained guaiacol (200 mM), H2O2 (400 mM), 545 μl of distilled water, 200 mM potassium phosphate buffer (pH 7.0), and 15 μl of blood plasma sample. The reaction was initiated immediately after the addition of the plasma sample, and the absorbance of the reaction solution was measured after every 20 s for 1 min at 470 nm. One unit of POD activity was narrated as the quantity of enzyme that catalyzed the oxidation of guaiacol.
Catalase Activity
Catalase (CAT) activity of blood plasma samples was measured by mixing the samples with 50 mM potassium phosphate buffer (pH 7.0) and dithiothreitol (1 mM) as described by Beers and Sizer (32). The reaction mixture to study CAT activity contained 59 mM H2O2, 50 mM phosphate buffer (pH 7), and a 100-μl plasma sample. The decreasing pattern of absorbance was measured after every 20 s for 1 min at 240 nm and a unit of CAT activity was described as a change in absorbance in 0.01 min.
Biochemical Parameters
Total Soluble Protein
The quantitative protein estimation of plasma samples was performed by dye-binding method as described by Bradford (33). The plasma sample (5 μl) was mixed and homogenized with 0.1 N NaCl. The reaction solution was incubated for 5 min at room temperature after adding 1 ml Bradford dye to form a protein–dye complex. Thereafter, absorbance was measured at 420 nm.
Total Sugar
The total sugar level of plasma samples was enacted by Folin's (34) protocol with few desired modifications. Briefly, the samples were mixed with sulfuric acid (H2SO4) and neutralized by using sodium carbonate (Na2CO3). The contents were then filtered and absorbance was read spectrophotometrically at 415 nm for estimation of sugar contents.
Lycopene and Carotenoids
Lycopene and carotenoids in blood plasma samples were assayed according to the standardized procedure of Nagata and Yamashita (35). For estimation of lycopene and carotenoids, 1 ml of blood plasma sample was thoroughly homogenized with 10 ml of hexane and acetone solution (6:4). The assay solution was incubated for 5 min at 37°C and then filtered. The absorbance was measured at 453, 505, and 663 nm, and finally the quantities of lycopene and carotenoids were calculated with the help of the following formulae:
Lycopene = −0.0458A663 + 0.372A505
Carotenoids = 0.216A663 – 0.304A505 + 0.452A453
Hydrolytic Enzymes
Protease
The protease activity was measured by the casein digestion method (36). Protease enzyme releases an amino acid “tyrosine” after digestion of casein. The reaction of tyrosine Folin's reagent results in the formation of a blue color product that is quantified at 660 nm. A standard calibration curve of tyrosine was used to compute the protease activity in plasma samples. One unit of enzyme activity was defined as the amount of enzyme that causes the release of soluble acid fragments equivalent to 0.001 A 280 nm in 1 min at pH 7.8.
Esterase
The esterase (alpha and beta) enzyme activity was estimated by utilizing naphthyl acetate (α and β) as substrate as described by Van Asperen (37). The reaction mixture consisting of plasma (enzyme extract), phosphate buffer (0.04 M, pH 7), 30 mM naphthyl acetate (α and β), and 1% acetone was incubated in the dark at 37°C for 15 min. Then, 1 ml of staining solution composed of fast blue BB (1%) and sodium dodecyl sulfate (5%) was added in both blank control (phosphate buffer and substrate solution) and reaction mixture and again incubated for 15 min in the dark at room temperature. The absorbance of the assay was recorded at 590 nm, and the enzyme activity was calculated in μM min−1 ml−1 of a sample using a standard curve.
Amylase
The activity of the amylase enzyme in plasma samples was assessed by using 0.2 M tris-malate (pH 7.2) buffer as an extraction cum assay medium (38). For estimation of enzyme activity, 1 ml of substrate solution (0.15% starch) was homogenized with 1 ml of plasma and incubated at 37°C for 10 min. The OD of the reaction mixture was measured at 620 nm after adding quenching reagent and enzyme activity was represented in milligrams of starch degraded per minute per milliliter of blood plasma sample.
Analysis of Milk
Milk samples were analyzed for chemical composition by a milk analyzer (Julie Z7, Scope Electric, Regensburg, Germany). The defatted milk samples were used for estimation of non-enzymatic (TPC, TAC, and vitamin C) and enzymatic (SOD, POD, and CAT) antioxidants by a spectrophotometer (UV-VIS U2800, Hitachi, Japan) as described above for plasma samples.
Reproductive and Productive Performance
The reproductive and productive performance was evaluated in terms of birth weight, weight gain per week, survival rate of newly born kids, shedding time of placenta, the onset of first postpartum estrus, and milk yield in goats.
Statistical Analysis
All the statistical analyses were carried out by using SPSS version 20. The experimental procedures were performed in triplicate, and data obtained were analyzed by one-way analysis of variance (ANOVA) with repeated measures under the shade of LSD to access the differences among different treatment means on specific days. The results were expressed in the tables as mean ± SE, and the values with p < 0.05 were considered statistically significant.
Results
Non-enzymatic Antioxidant Parameters
The response of plasma non-enzymatic antioxidant parameters to MOLP supplementation during pregnancy and early lactation period is shown in Table 4. Plasma TFs were increased significantly from day 110 of pregnancy to day 20 of lactation in the M3.5% group (p < 0.05). The impact of supplementation was non-significant on M2% group plasma TFs as compared to the control (M0) group (p > 0.05). The values of plasma TPC of the M3.5% and M2% groups were non-significant in the pregnancy stage, while they became significant during the early lactation period of the experiment (p < 0.05). The increase in plasma Vit C contents was significant (p < 0.05) from the 130th day of pregnancy till day 20 of lactation in both M3.5% and M2% supplemented groups. The MOLP supplementation significantly improved the plasma TAC by declining MDA concentration and TOS values near parturition and early lactation stage of the experiment in contrast to the control (M0) group (p < 0.05).
Table 4.
Plasma non-enzymatic antioxidant indices of Beetal goats.
| Non-enzymatic antioxidants | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| Gestation day 110 | |||||
| Total flavonoids (μg/ml) | 239.93 ± 0.91b | 241.06 ± 0.74b | 253.91 ± 0.72a | 0.43 | 0.030 |
| Total phenolic contents (μM/ml) | 5,821.75 ± 0.85b | 5,832.61 ± 1.60a | 5840 ± 2.45a | 1.19 | 0.028 |
| Vitamin C (μg/ml) | 803.75 ± 1.55 | 805.50 ± 1.04 | 810.25 ± 1.44 | 0.84 | 0.221 |
| Malondialdehyde (μM/ml) | 5.90 ± 0.33 | 5.76 ± 0.37 | 5.59 ± 0.26 | 0.17 | 0.627 |
| Total anti-oxidant capacity (μM/ml) | 1.41 ± 0.02c | 1.55 ± 0.01b | 1.67 ± 0.01a | 0.01 | 0.002 |
| Total oxidant status (μM/ml) | 1,566.25 ± 1.11 | 1,559.75 ± 0.63 | 1,555.50 ± 2.10 | 1.04 | 0.065 |
| Gestation day 130 | |||||
| Total flavonoids (μg/ml) | 251.31 ± 0.59b | 256.14 ± 0.76b | 272.79 ± 1.07a | 0.29 | 0.013 |
| Total phenolic contents (μM/ml) | 5,819 ± 0.82b | 5,844.75 ± 1.31a | 5,848.25 ± 0.95a | 0.65 | 0.007 |
| Vitamin C (μg/ml) | 795 ± 1.08c | 811.25 ± 1.32b | 819.50 ± 0.96a | 0.42 | 0.008 |
| Malondialdehyde (μM/ml) | 7.26 ± 0.09b | 5.28 ± 0.23a | 5.06 ± 0.27a | 0.18 | 0.024 |
| Total anti-oxidant capacity (μM/ml) | 1.49 ± 0.01c | 1.73 ± 0.02b | 1.86 ± 0.02a | 0.01 | 0.002 |
| Total oxidant status (μM/ml) | 1,585.25 ± 0.63c | 1,533 ± 0.71b | 1,502.5 ± 0.96a | 0.39 | <0.001 |
| Gestation day 150 | |||||
| Total flavonoids (μg/ml) | 258.93 ± 1.13b | 264.66 ± 0.68b | 278.84 ± 0.58a | 0.67 | 0.001 |
| Total phenolic contents (μM/ml) | 5,796.50 ± 0.86b | 5,853 ± 0.57a | 5,859.25 ± 2.05a | 1.14 | <0.001 |
| Vitamin C (μg/ml) | 780 ± 0.41c | 816.75 ± 0.85b | 827.75 ± 0.48a | 0.39 | <0.001 |
| Malondialdehyde (μM/ml) | 8.31 ± 0.12b | 4.55 ± 0.22a | 4.19 ± 0.18a | 0.06 | 0.004 |
| Total anti-oxidant capacity (μM/ml) | 1.37 ± 0.02c | 1.81 ± 0.01b | 1.96 ± 0.04a | 0.02 | 0.007 |
| Total oxidant status (μM/ml) | 1,605 ± 1.08c | 1,515.75 ± 0.85b | 1,481 ± 0.82a | 0.86 | <0.001 |
| Lactation day 20 | |||||
| Total flavonoids (μg/ml) | 265.28 ± 1.23b | 273.19 ± 2.46b | 291.44 ± 0.63a | 1.06 | 0.013 |
| Total phenolic contents (μM/ml) | 5,811.50 ± 0.64c | 5,866 ± 0.71b | 5,884 ± 1.08a | 0.68 | <0.001 |
| Vitamin C (μg/ml) | 783 ± 0.39c | 825.51 ± 0.48b | 838.69 ± 1.25a | 0.44 | <0.001 |
| Malondialdehyde (μM/ml) | 7.94 ± 0.25c | 4.01 ± 0.04b | 2.74 ± 0.29a | 0.11 | 0.013 |
| Total anti-oxidant capacity (μM/ml) | 1.53 ± 0.01c | 1.94 ± 0.01b | 2.16 ± 0.04a | 0.02 | 0.001 |
| Total oxidant status (μM/ml) | 1,598.50 ± 0.65c | 1,492.50 ± 1.04b | 1,459.75 ± 0.48a | 0.42 | <0.001 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05.
Enzymatic Antioxidants
The MOLP supplementation impacts on plasma enzymatic antioxidants are presented in Table 5. The SOD activity of M3.5% and M2% groups increased significantly throughout the experiment in response to supplementation (p < 0.05) and reached its peak on day 20 of lactation in the M3.5% group. The effect of supplementation levels (3.5 and 2%) was non-significant on POD activity (p > 0.05). However, there was a significant difference between the POD activities of supplemented (M3.5% and M2%) and control (M0) groups during the entire experiment (p < 0.05). A non-significant increase in the plasma CAT activity was noticed till day 150 of gestation (p > 0.05), but soon after kidding, the CAT activity was enhanced significantly during the lactation period according to the supplementation levels in M3.5% and M2% groups as compared to the control (M0) group (p < 0.05).
Table 5.
Plasma enzymatic antioxidant indices of Beetal goats.
| Enzymatic antioxidants (Units/ml) | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| Gestation day 110 | |||||
| SOD | 21.29 ± 0.52c | 25.82 ± 0.28b | 32.08 ± 0.39a | 0.23 | 0.011 |
| POD | 195.82 ± 0.61b | 207.16 ± 0.79a | 212.24 ± 1.30a | 0.45 | 0.002 |
| CAT | 40 ± 0.82 | 41 ± 1.29 | 44.75 ± 0.48 | 0.52 | 0.062 |
| Gestation day 130 | |||||
| SOD | 19.88 ± 0.63c | 28.20 ± 0.57b | 37.77 ± 0.73a | 0.29 | 0.015 |
| POD | 209.65 ± 0.88b | 231.79 ± 1.16a | 238.17 ± 1.06a | 0.34 | 0.001 |
| CAT | 42 ± 0.41 | 44.75 ± 0.85 | 45.25 ± 0.25 | 0.24 | 0.100 |
| Gestation day 150 | |||||
| SOD | 13.76 ± 0.38c | 28.88 ± 0.64b | 39.54 ± 0.89a | 0.47 | 0.001 |
| POD | 215.21 ± 0.74b | 244.11 ± 0.89a | 247.07 ± 0.98a | 0.51 | 0.005 |
| CAT | 45.50 ± 0.96 | 47 ± 0.71 | 49.50 ± 0.94 | 0.49 | 0.154 |
| Lactation day 20 | |||||
| SOD | 17.97 ± 0.43c | 36.17 ± 1.05b | 42.76 ± 0.36a | 0.30 | 0.001 |
| POD | 226.91 ± 0.69b | 261.79 ± 1.55a | 268.11 ± 0.72a | 0.67 | 0.001 |
| CAT | 50.48 ± 0.87c | 55.50 ± 0.29b | 63.39 ± 0.86a | 0.38 | 0.023 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05. SOD, superoxide dismutase; POD, peroxidase; CAT, catalase.
Biochemical Indices
The change in plasma biochemical indicators in response to MOLP supplementation is given in Table 6. The supplementation initially showed no pronounced effect on plasma TSP contents till the 130th day of pregnancy. Thereafter, the plasma TSP contents increased significantly on day 150 of pregnancy and day 20 of lactation in M3.5% group in comparison to M2% and M0 groups (p < 0.05). The plasma sugar level of both M3.5% and M2% supplemented groups increased significantly from the beginning of the experiment till day 20 of lactation (p < 0.05). A drastic increase in plasma sugar level was observed soon after kidding in supplemented groups in contrast to the control group. The concentration of carotenoids in plasma was significantly enhanced throughout the experiment in the M3.5% group as compared to the control (M0) group (p < 0.05), whereas the lycopene contents were improved from day 130 of pregnancy to day 20 of lactation in the M3.5% as well as M2% group (p < 0.05).
Table 6.
Plasma biochemical parameters of Beetal goats.
| Biochemicals | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| Gestation day 110 | |||||
| Total soluble proteins (mg/ml) | 60.98 ± 0.73 | 63.87 ± 0.34 | 65.09 ± 1.12 | 0.59 | 0.118 |
| Total sugars (mg/ml) | 4.58 ± 0.05c | 5.40 ± 0.04b | 6.18 ± 0.03a | 0.03 | 0.003 |
| Carotenoids (μg/ml) | 165.77 ± 0.79b | 169.09 ± 1.07b | 176.56 ± 0.62a | 0.25 | 0.013 |
| Lycopene (μg/ml) | 98.75 ± 2.66 | 103 ± 1.15 | 108 ± 3.02 | 0.53 | 0.207 |
| Gestation day 130 | |||||
| Total soluble proteins (mg/ml) | 59.56 ± 3.17 | 66.23 ± 0.57 | 69.16 ± 1.78 | 1.63 | 0.066 |
| Total sugars (mg/ml) | 4.43 ± 0.05c | 5.80 ± 0.04b | 6.63 ± 0.06a | 0.02 | 0.004 |
| Carotenoids (μg/ml) | 170.79 ± 0.96b | 177.45 ± 0.83b | 185.41 ± 0.61a | 0.49 | 0.001 |
| Lycopene (μg/ml) | 95 ± 1.08c | 111 ± 0.57b | 119.75 ± 0.85a | 0.51 | 0.010 |
| Gestation day 150 | |||||
| Total soluble proteins (mg/ml) | 55.96 ± 3.04b | 61.19 ± 1.49b | 71.02 ± 0.69a | 1.56 | 0.042 |
| Total sugars (mg/ml) | 3.88 ± 0.13c | 5.90 ± 0.04b | 6.93 ± 0.05a | 0.03 | 0.005 |
| Carotenoids (μg/ml) | 168 ± 2.19b | 175.93 ± 0.68b | 187.13 ± 1.30a | 0.85 | 0.032 |
| Lycopene (μg/ml) | 89.75 ± 0.47c | 116.78 ± 1.03b | 125.69 ± 0.75a | 0.47 | 0.001 |
| Lactation day 20 | |||||
| Total soluble proteins (mg/ml) | 63.50 ± 1.56b | 67.18 ± 0.35b | 75.40 ± 0.73a | 0.78 | 0.016 |
| Total sugars (mg/ml) | 4.03 ± 0.04c | 6.30 ± 0.05b | 7.35 ± 0.07a | 0.04 | 0.002 |
| Carotenoids (μg/ml) | 172.42 ± 1.02b | 178.74 ± 1.32b | 193.55 ± 0.89a | 0.73 | <0.001 |
| Lycopene (μg/ml) | 93 ± 0.91c | 118.25 ± 0.85b | 129.5 ± 0.95a | 0.69 | <0.001 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05.
Hydrolytic Enzymes
The activities of plasma hydrolytic enzymes in supplemented (M3.5% and M2%) and control (M0) groups are illustrated in Table 7. The protease enzyme activity was modified more effectively from day 130 to 150 of gestation in the M3.5% group in comparison to the M2% group. However, the supplementation of MOLP significantly influenced plasma protease activity in the M2% group during the lactation period (p < 0.05). However, the supplementation did not show any significant impact on esterase activity during the experiment (p > 0.05). The amylase activity was slightly improved in the M3.5% group from day 150 of gestation in comparison to the control (M0) group (p < 0.05).
Table 7.
Plasma hydrolytic enzymes activities of Beetal goats.
| Enzymes | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| Gestation day 110 | |||||
| Protease (U/ml) | 233.25 ± 0.95 | 235.50 ± 0.64 | 239.25 ± 1.18 | 0.79 | 0.065 |
| Esterase (μM/min/ml) | 643.75 ± 0.85 | 641 ± 1.08 | 645.75 ± 0.63 | 0.37 | 0.084 |
| Amylase (mg/min/ml) | 1.31 ± 0.02 | 1.34 ± 0.01 | 1.39 ± 0.02 | 0.01 | 0.369 |
| Gestation day 130 | |||||
| Protease (U/ml) | 235 ± 1.08b | 238.50 ± 0.29b | 243 ± 0.41a | 0.38 | 0.009 |
| Esterase (μM/min/ml) | 638.25 ± 0.95 | 639.50 ± 0.96 | 641.75 ± 0.48 | 0.41 | 0.252 |
| Amylase (mg/min/ml) | 1.35 ± 0.02 | 1.39 ± 0.03 | 1.42 ± 0.01 | 0.02 | 0.110 |
| Gestation day 150 | |||||
| Protease (U/ml) | 238.50 ± 0.51b | 240.75 ± 0.75b | 247 ± 0.82a | 0.44 | 0.036 |
| Esterase (μM/min/ml) | 634.50 ± 0.87 | 636.50 ± 0.64 | 637 ± 0.91 | 0.25 | 0.486 |
| Amylase (mg/min/ml) | 1.36 ± 0.03b | 1.43 ± 0.01b | 1.51 ± 0.01a | 0.01 | 0.036 |
| Lactation day 20 | |||||
| Protease (U/ml) | 242.75 ± 0.85c | 249.75 ± 0.63b | 254.50 ± 0.29a | 0.47 | 0.008 |
| Esterase (μM/min/ml) | 639.25 ± 0.63 | 642 ± 0.82 | 644 ± 0.56 | 0.33 | 0.059 |
| Amylase (mg/min/ml) | 1.41 ± 0.01b | 1.44 ± 0.01b | 1.57 ± 0.02a | 0.04 | 0.016 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05.
Milk Biochemical Composition
The alterations in milk biochemical composition as a result of MOLP supplementation are expressed in Table 8. The colostrum and milk protein contents were increased significantly in M3.5% group. However, the supplementation in the M2% group exhibited a significant impact on days 21 and 28 milk protein contents (p < 0.05). The milk fat percentage was not affected by supplementation in either the M3.5% or M2% group up to day 14 of lactation (p > 0.05), but on days 21 and 28, the milk fat was significantly increased in the M3.5% group as compared to the control (M0) group (p < 0.05). There was no influence of supplementation on the lactose contents of colostrum and mature milk samples of M3.5% and M2% groups (p > 0.05). Total carotenoids were significantly high in the milk samples of the M3.5% group as compared to M2% and M0 groups from day 0 to day 28 of lactation (p < 0.05).
Table 8.
Effect of Moringa oleifera leaf powder supplementation on milk composition.
| Biochemical | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| Day 0 (Colostrum) | |||||
| Protein (%) | 8.93 ± 0.37b | 10.18 ± 0.28b | 13.53 ± 0.53a | 0.24 | 0.043 |
| Fat (%) | 8.95 ± 0.40 | 9.18 ± 0.48 | 9.53 ± 0.34 | 0.37 | 0.092 |
| Lactose (%) | 2.93 ± 0.29 | 3.09 ± 0.31 | 3.27 ± 0.13 | 0.21 | 0.663 |
| Carotenoids (μg/ml) | 15.22 ± 0.83b | 16.71 ± 0.40b | 18.53 ± 0.39a | 0.49 | 0.031 |
| Day 7 | |||||
| Protein (%) | 6.84 ± 0.09b | 7.11 ± 0.31b | 8.51 ± 0.25a | 0.16 | 0.015 |
| Fat (%) | 7.38 ± 0.86 | 7.94 ± 0.62 | 8.72 ± 0.30 | 0.61 | 0.233 |
| Lactose (%) | 3.43 ± 0.07 | 3.62 ± 0.21 | 3.71 ± 0.16 | 0.05 | 0.514 |
| Carotenoids (μg/ml) | 6.91 ± 0.19b | 7.36 ± 0.07b | 9.82 ± 0.36a | 0.15 | 0.041 |
| Day 14 | |||||
| Protein (%) | 5.08 ± 0.36b | 5.40 ± 0.25b | 7.94 ± 0.46a | 0.28 | 0.045 |
| Fat (%) | 6.56 ± 0.35 | 6.87 ± 0.39 | 7.19 ± 0.52 | 0.28 | 0.645 |
| Lactose (%) | 3.97 ± 0.14 | 4.01 ± 0.23 | 4.14 ± 0.20 | 0.09 | 0.273 |
| Carotenoids (μg/ml) | 4.68 ± 0.41b | 4.94 ± 0.16b | 6.37 ± 0.08a | 0.2 | 0.022 |
| Day 21 | |||||
| Protein (%) | 4.03 ± 0.13c | 5.15 ± 0.08b | 6.56 ± 0.20a | 0.08 | 0.012 |
| Fat (%) | 4.94 ± 0.34b | 5.32 ± 0.11b | 6.29 ± 0.16a | 0.17 | 0.024 |
| Lactose (%) | 4.37 ± 0.51 | 4.51 ± 0.58 | 4.89 ± 0.78 | 0.1 | 0.748 |
| Carotenoids (μg/ml) | 2.73 ± 0.21b | 3.02 ± 0.13b | 4.36 ± 0.11a | 0.09 | 0.007 |
| Day 28 | |||||
| Protein (%) | 3.43 ± 0.19c | 4.27 ± 0.11b | 5.82 ± 0.40a | 0.22 | 0.027 |
| Fat (%) | 4.87 ± 0.13b | 3.76 ± 0.15b | 3.41 ± 0.02a | 0.09 | 0.003 |
| Lactose (%) | 4.95 ± 0.80 | 5.08 ± 0.19 | 5.35 ± 0.49 | 0.15 | 0.917 |
| Carotenoids (μg/ml) | 1.38 ± 0.36b | 1.61 ± 0.25b | 3.04 ± 0.08a | 0.17 | 0.025 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05.
Milk Enzymatic and Non-enzymatic Antioxidants
The enzymatic and non-enzymatic antioxidant parameters of the colostrum and milk samples are presented in Table 9. The TPCs in the colostrum and milk samples of the M3.5% group were significantly increased in response to supplementation. There was no effect of supplementation on the TPCs of colostrum from the M2% group, while these TPCs were improved significantly in the milk samples of the M2% group from day 14 to 28 of lactation (p < 0.05). The TAC of the milk from M3.5% and M2% groups was significantly increased from the beginning to day 28 of lactation in comparison to the control (M0) group (p < 0.05). The significant impact of MOLP on colostrum and milk Vit C level was noticed only in the M3.5% group (p < 0.05). Both the supplementation levels (3.5 and 2%) showed a significant impact on the SOD activity of colostrum and milk samples as compared to the non-supplemented (M0) group (p < 0.05). Initially, the POD activity was increased significantly irrespective of the level of supplementation. However, from day 21 to 28 of lactation, a significant difference was observed in the improvement of POD activity depending on levels of supplementation. The increase in the activity of CAT enzyme was non-significant up to day 14 of lactation in both M3.5% and M2% groups (p > 0.05). However, on days 21 and 28, the supplementation resulted in a significant enhancement in the CAT activity of milk samples from the M3.5% group in comparison to the control (M0) group (p < 0.05).
Table 9.
Effect of Moringa oleifera leaf powder supplementation on milk antioxidant parameters.
| Non-enzymatic antioxidants | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| Day 0 (Colostrum) | |||||
| Total phenolic contents (μM/ml) | 2,826.75 ± 3.49b | 2,838.50 ± 0.64b | 2,875.50 ± 1.55a | 1.06 | 0.002 |
| Total anti-oxidant capacity (μM/ml) | 0.92 ± 0.02c | 1.42 ± 0.08b | 1.78 ± 0.13a | 0.07 | 0.005 |
| Vitamin C (μg/ml) | 457.75 ± 1.65b | 464.75 ± 1.79b | 483 ± 0.82a | 0.97 | 0.005 |
| SOD (Units/ml) | 69.86 ± 0.46c | 88.65 ± 0.69b | 96.53 ± 0.32a | 0.18 | 0.001 |
| POD (Units/ml) | 291.25 ± 0.63b | 309.22 ± 0.85a | 314.02 ± 1.04a | 0.37 | 0.003 |
| CAT (Units/ml) | 71.75 ± 0.85 | 76.75 ± 1.11 | 84 ± 1.78 | 1.01 | 0.060 |
| Day 7 | |||||
| Total phenolic contents (μM/ml) | 2,608.85 ± 2.38b | 2,622 ± 0.71b | 2,669.25 ± 1.88a | 0.21 | 0.005 |
| Total anti-oxidant capacity (μM/ml) | 0.81 ± 0.05c | 1.39 ± 0.12b | 1.73 ± 0.11a | 0.09 | 0.016 |
| Vitamin C (μg/ml) | 421.75 ± 1.97b | 427.25 ± 1.32b | 436.75 ± 0.75a | 0.78 | 0.035 |
| SOD (Units/ml) | 75.75 ± 0.63c | 81.52 ± 0.26b | 94.4 ± 0.39a | 0.16 | 0.001 |
| POD (Units/ml) | 263.45 ± 0.87b | 277.56 ± 1.32a | 285 ± 1.65a | 0.28 | 0.015 |
| CAT (Units/ml) | 66.75 ± 1.37 | 69.75 ± 1.18 | 74 ± 0.41 | 0.35 | 0.103 |
| Day 14 | |||||
| Total phenolic contents (μM/ml) | 2,471.50 ± 1.32c | 2,493.25 ± 1.03b | 2,547 ± 2.55a | 1.03 | <0.001 |
| Total anti-oxidant capacity (μM/ml) | 0.74 ± 0.10c | 1.29 ± 0.13b | 1.64 ± 0.12a | 0.12 | 0.017 |
| Vitamin C (μg/ml) | 412.70 ± 1.31b | 416.40 ± 1.28b | 429.60 ± 0.93a | 0.71 | 0.016 |
| SOD (Units/ml) | 61.52 ± 0.31c | 74.40 ± 0.41b | 83.45 ± 0.55a | 0.33 | <0.001 |
| POD (Units/ml) | 251.67 ± 0.79b | 270.92 ± 1.37a | 276.05 ± 0.96a | 0.19 | 0.008 |
| CAT (Units/ml) | 50.61 ± 1.51 | 56.75 ± 1.43 | 62 ± 1.92 | 0.69 | 0.072 |
| Day 21 | |||||
| Total phenolic contents (μM/ml) | 2,289 ± 1.47c | 2,326 ± 0.91b | 2,391.50 ± 0.65a | 0.32 | 0.001 |
| Total anti-oxidant capacity (μM/ml) | 0.65 ± 0.04c | 1.24 ± 0.11b | 1.53 ± 0.09a | 0.08 | 0.013 |
| Vitamin C (μg/ml) | 385.75 ± 2.46b | 391.50 ± 1.50b | 408.25 ± 0.75a | 0.57 | 0.021 |
| SOD (Units/ml) | 58.65 ± 0.69c | 63.45 ± 0.91b | 75.04 ± 0.48a | 0.51 | 0.003 |
| POD (Units/ml) | 227.02 ± 1.08c | 243.01 ± 0.56b | 259.66 ± 0.73a | 0.25 | 0.008 |
| CAT (Units/ml) | 37.95 ± 1.31b | 41.98 ± 0.32b | 53.30 ± 0.61a | 0.21 | 0.007 |
| Day 28 | |||||
| Total phenolic contents (μM/ml) | 2,133 ± 0.82c | 2,173.25 ± 1.70b | 2,205.75 ± 1.25a | 0.59 | <0.001 |
| Total anti-oxidant capacity (μM/ml) | 0.61 ± 0.13c | 1.11 ± 0.04b | 1.37 ± 0.07a | 0.07 | 0.007 |
| Vitamin C (μg/ml) | 374.45 ± 1.04b | 378.70 ± 0.85b | 395.25 ± 0.69a | 0.52 | 0.001 |
| SOD (Units/ml) | 51.70 ± 0.77c | 59.65 ± 1.05b | 66 ± 0.41a | 0.57 | 0.003 |
| POD (Units/ml) | 202.66 ± 0.68 | 216.02 ± 1.12 | 231.01 ± 1.31 | 0.55 | 0.006 |
| CAT (Units/ml) | 29.75 ± 0.43b | 31.36 ± 0.59b | 46 ± 0.42a | 0.26 | 0.002 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05. SOD, superoxide dismutase; POD, peroxidase; CAT, catalase.
Productive and Reproductive Performance
The supplementation of MOLP increased the milk production in M3.5% and M2% groups as compared to the control (M0) group from day 7 to 28 of lactation (p < 0.05) as is presented in Table 10. Similarly, the weight gain of kids in supplemented groups was significantly high (Table 11). The results of reproductive parameters (Figures 1, 2) showed that the shedding time of the placenta and the time of onset of first postpartum estrus was less in the goats of M3.5% and M2% groups as compared to the goats of the (M0) control group. The survival rate and initial birth weight of the kids of M3.5%- and M2%-supplemented goats were higher than M0 group goats (Figures 3, 4).
Table 10.
Effect of Moringa oleifera leaf powder supplementation on milk production (liters) in Beetal goats.
| Days | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| 07 | 1.01 ± 0.03c | 1.27 ± 0.05b | 1.41 ± 0.04a | 0.04 | 0.007 |
| 14 | 1.32 ± 0.14c | 1.48 ± 0.11b | 1.65 ± 0.09a | 0.11 | 0.033 |
| 21 | 1.54 ± 0.07c | 1.73 ± 0.06b | 2.01 ± 0.07a | 0.06 | 0.031 |
| 28 | 1.87 ± 0.16c | 2.11 ± 0.13b | 2.72 ± 0.15a | 0.14 | 0.04 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05.
Table 11.
Effect of feeding Moringa oleifera leaf powder supplemented goat milk on body weight (kg) of their kids.
| Days | Levels of Moringa oleifera leaf powder supplementation | SEM | p -value | ||
|---|---|---|---|---|---|
| M0 | M2% | M3.5% | |||
| 0 | 2.14 ± 0.49c | 2.81 ± 0.52b | 3.53 ± 0.36a | 0.45 | 0.037 |
| 7 | 2.91 ± 0.17c | 3.77 ± 0.32b | 4.71 ± 0.28a | 0.25 | 0.016 |
| 14 | 3.64 ± 0.22c | 4.51 ± 0.29b | 5.88 ± 0.12a | 0.2 | 0.001 |
| 21 | 4.49 ± 0.42c | 5.72 ± 0.18b | 6.96 ± 0.14a | 0.24 | 0.021 |
| 28 | 5.03 ± 0.16c | 6.61 ± 0.24b | 8.36 ± 0.35a | 0.16 | 0.044 |
Means with superscript letters (a, b, c) within the same row differ significantly at p ≤ 0.05.
Figure 1.
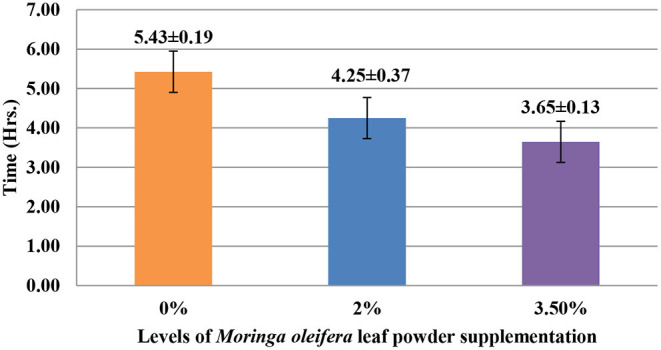
Effect of Moringa oleifera leaf powder supplementation on shedding time (hours) of placenta in Beetal goats.
Figure 2.
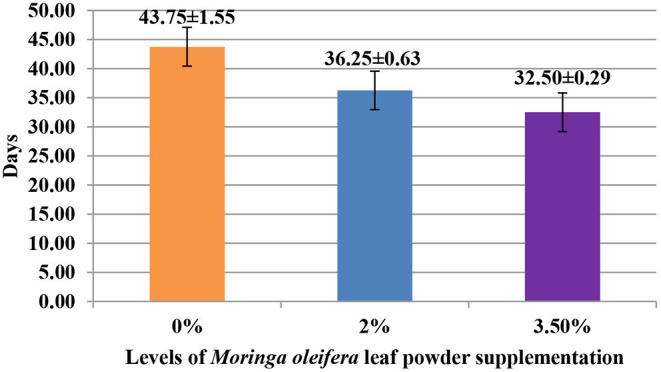
Effect of Moringa oleifera leaf powder supplementation on onset time (days) of first postpartum estrus after kidding in Beetal goats.
Figure 3.
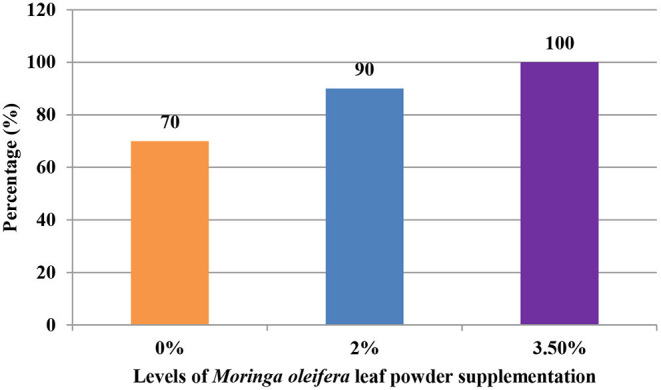
Survival rate (%) of kids in response to milk feeding of goats supplemented with Moringa oleifera leaf powder.
Figure 4.
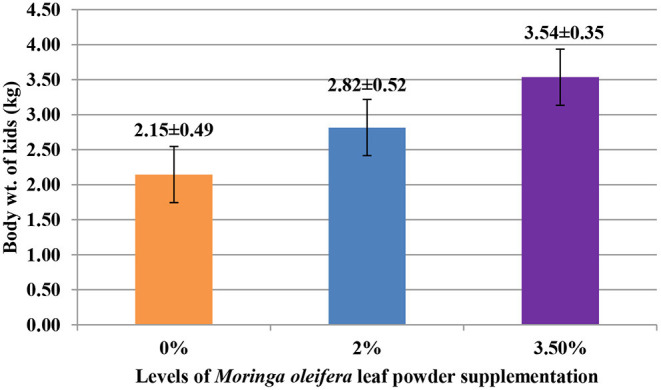
Effect of Moringa oleifera leaf powder supplementation on birth weight (kg) of kids in Beetal goats.
Discussion
The animals are more prone to oxidative stress during the transition period because an increase in energy requirements to support developing fetuses and subsequent lactation coincide with depressed feed intake. The nutritional quality of feedstuffs is very important to regulate the pregnancy and lactation performance of goats. The MO leaves are well-known for their nutritional and therapeutic properties that have been attributed to their various phytochemical constituents (39, 40).
The pregnancy and lactation stress results in the excessive production of hydroxyl (OH) and nitric oxide (NO) radicals. The animal body is well-equipped with different protective mechanisms to neutralize the harmful effects of OH and NO radicals. The antioxidant protective system is naturally suppressed near parturition due to certain physiological changes in the body (41). The MOLP supplementation to pregnant goats resulted in an increase in their plasma TFs and TPCs that control the immense production of OH and NO radicals via Haber-Weiss and Fenton reactions to minimize the detrimental effects of oxidative stress during pregnancy and lactation (42). The regulation of antioxidant defense system of the body under different stress conditions through MO leaf extract supplementation was also reported in rats (43).
Vitamin C is generally regarded as the first line of defense to provide protection from the damaging effects of oxidative stress in pregnancy especially near parturition (44). A constant decline in plasma vit C level near parturition aggravates the situation that makes the animals more vulnerable to oxidative damage (45). A significant improvement in plasma vit C level during this study in supplemented goats showed that MOLP as a rich source of vit C has the ability to protect the pregnant goats from the deleterious effects of oxidative stress by suppressing the action of free radicals. The increase in plasma vit C concentration in response to MOLP supplementation is in accordance with the findings of a study on Aardi goats (14).
Lipids act as substrate for reactive nitrogen and oxygen species (RNS and ROS) to start the process of lipid peroxidation (LPO) (46, 47). The balance between the production and elimination of ROS and RNS from the body is sustained by antioxidant defense system in healthy animals. Any untoward disturbance in this balance may enhance the plasma TOS and MDA concentration. The high values of TOS and MDA represent the state of oxidative stress (48). It is evident from the results of this study that the supplementation of MOLP increased the plasma TAC in treated goats by suppressing the process of LPO. This consequently reduces the values of plasma TOS and MDA. The enzymatic (SOD, POD, and CAT) antioxidants also have a key role in limiting LPO, as both enzymatic and non-enzymatic components of antioxidant defense system work in collaboration to maintain the conditions suitable for mother and developing fetus by minimizing the parturition and early lactation stress (49).
The physiological changes in maternal body during the transition period especially near parturition result in excessive production of ROS (50). These ROS are converted into H2O2 by SOD enzyme, while POD and CAT enzymes further degrade the H2O2 into water and molecular oxygen (51, 52). The successful completion of parturition stage and start of healthy lactation depend on the activities of SOD, POD, and CAT enzymes (53). High antioxidant (SOD, POD, and CAT) enzyme activities indicate that the supplementation of MOLP in late pregnancy improved the plasma enzymatic antioxidants that was also reported in some other studies conducted with different supplementation levels of MO in rabbits, poultry, and dairy cows (54–56).
Blood biochemical parameters are the established indicators to provide information about the health status of pregnant animals for their successful transfer from gestation to lactation stage (57). Late pregnancy is characterized by severe metabolic changes and a rapid decrease in plasma protein level was also noticed in different other animal species during this period (58–60). The plasma protein level drops promptly in the last trimester due to high amino acid requirements for developing fetus and preparation of the mammary system for subsequent lactation stage (61). However, after parturition, the plasma protein level starts to increase due to immense production of immunoglobulins (62). It is evident from the findings of this study that the plasma protein level was high in supplemented groups as compared to the control group of the experiment during the peripartum period. The supplementation of MOLP increased the plasma protein contents to satisfy the high protein requirements of pregnancy and lactation. These results support the findings of other studies in Jersey cattle and sows (21, 63). The presence of high amount of protein in MOLP enhances the synthesis of selenocysteine-based selenoproteins. These proteins have been reported to play a role in the modification of antioxidant defense system and improvement of reproductive functions (64).
The decrease in plasma sugar level near parturition is typical for goats and ewes. A reduction in feed intake occurs during late pregnancy due to the squeezing of the rumen by rapid fetal growth (65). The mobilization of the body fats starts if the energy requirements of the animals are not fulfilled with the advancement in pregnancy through provision of appropriate feed supplement (66). The negative energy balance in this stage may lead to the development of ketosis and some other metabolic diseases. The feeding of MOLP increased the plasma sugar level of supplemented groups to provide sufficient amount of energy for parturition and early lactation. The revival of gluconeogenesis process after parturition in response to certain endocrine changes rapidly increase the plasma sugar level (67, 68). Furthermore, the supplementation also improved the plasma lycopene and carotenoids status to regulate the synthesis of inflammatory cytokines that reduces the chances of complications at the time of parturition (69, 70).
A proteolytic enzyme system in the body helps in the removal of worthless and damaged biomolecules to maintain hemostasis during pregnancy and lactation (71). The significant increase in the activity of protease enzyme in MOLP-supplemented groups depicted its defensive effects at the cellular level. The proteolytic enzyme system is of great importance because it also has the ability to act as a secondary antioxidant defense system when the primary antioxidant system is unable to protect the body from oxidative stress (72). The supplementation of MOLP also resulted in an improvement in the amylase activity. This enzyme enhances the conversion of carbohydrates into glucose to produce energy according to the requirements of body during pregnancy and lactation (66, 73).
The major outcome of the study was that supplementing the goats' ration with MOLP markedly influenced their colostrum and milk composition. The preparations for colostrum synthesis start in the last month of pregnancy (74). Colostrum provides energy and maternal immunity along with different other growth factors to maintain health status and development of newly born kids. The findings of this study showed that supplementation of MOLP increased the protein contents of colostrum and milk in supplemented groups. The concentration of protein is generally high in colostrum than normal milk due to the presence of Igs in huge amount (75). The consumption of Igs protects the newly born kids from different diseases and thereby enhances their survival rate. The supplementation also improved the fat percentage of mature milk, which is in accordance with the findings of Kholif et al. (76). MOLP stimulates the production of acetate that acts as a major precursor for the biosynthesis of fat (77). Similarly, a significant increase in carotenoids of colostrum and milk was also noticed with 3.5% supplementation. The concentration of carotenoids in milk mostly depends on the type of feed and MOLP being a rich source of carotenoids has positive effects on the carotenoid contents of colostrum and milk in both supplemented groups. The carotenoids have been reported to play an important role in the improvement of milk quality by preventing the process of auto-oxidation (78).
The presence of antioxidants in appropriate amount prolongs the shelf life of milk and reduces its chances of microbial spoilage. The antioxidants in milk also protect the suckling kids from various health hazards by strengthening their immune system (79). The supplementation of diet with MOLP increased the colostrum and milk TAC as was also reported previously in cows by Kekana et al. (63). This could be due to the synergistic effects of flavonoids, phenolics, Se, and vit C present in MOLP. Generally, milk is not considered a good source of vit C. However, the results of this study showed the presence of an appreciable amount of vit C in the milk of goats supplemented with MOLP. Thus, feeding the goats with MOLP-supplemented diet positively influenced the vit C contents in their milk. The presence of functional antioxidants in MOLP also improved the enzymatic (SOD, POD, and CAT) antioxidant status of the milk to fulfill the demands of both milk producers and consumers for healthier dairy products. The favorable impacts of MO supplementation on milk composition were also reported in some other studies performed on dairy animals (80, 81).
The beneficial effects of MOLP supplementation on reproductive performance parameters observed in this study were due to its high nutritional profile. The birth weight of kids in supplemented groups was high as compared to the control group. The supplementation of MOLP in advance stage of pregnancy increases the provision of protein to developing fetus for its further growth and also improves the protein contents of colostrum and milk (82). The high level of protein contents in the milk of supplemented goats promoted the weight gain in their kids. The presence of therapeutic compounds in MOLP increased the survival rate of the kids of supplemented goats by protecting them from various diseases (83). Similar findings were reported by Qwele et al. (84), who disclosed that feeding of MO-supplemented diet is beneficial for the animals to protect them from oxidative stress-induced diseases. High milk production in early lactation stage often results in negative energy balance (NEB), if the nutrient supply is inadequate to lactating animals. The NEB is the major cause of delay in shedding of placental membranes and resumption of postpartum estrus after parturition (85). MOLP supplementation improved the energy status of the body to ensure the revival of ovarian activity and reduces postpartum anestrus interval. The shortening of postpartum anestrus duration by dietary modifications has also been reported previously in cows (86). The high proportion of protein and Se contents in MO strengthened the uterine muscle contractions for timely shedding of placenta and thus protects the reproductive tract from different infections.
Conclusion
The results of the current study revealed that supplementing 3.5% MOLP improved maternal health and milk quality in terms of antioxidant status and biochemical composition. Furthermore, this supplementation level also increased the milk yield, kids' growth rate, and reproductive performance of goats. These findings propose that MOLP has the potential to improve the productive/reproductive performance of goats. However, further studies are required with different feeding levels of MOLP to explore the molecular aspects of improving productive and reproductive performance in large herds of animals.
Author's Note
This experiment/research paper is a part of Ali Afzal PhD study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The procedures used for study and ethical clearance was approved and granted by Animal Use and Care Research Committee at Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, Pakistan.
Author Contributions
AA: conduct experiment, data aggregation, statistical analysis, and wrote manuscript. AH: lab analysis. TH: design experiment, monitoring experiment, and revised manuscript. MS and MM: methodology. GY: manuscript editing and revision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors pay their special thanks to NIAB-C, PIEAS management for their help to complete the research work.
References
- 1.Abuelo A, Hernández J, Benedito JL, Castillo C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants. (2019) 8:20–39. 10.3390/antiox8010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sordillo LM. Nutritional strategies to optimize dairy cattle immunity. J Dairy Sci. (2016) 99:4967–82. 10.3168/jds.2015-10354 [DOI] [PubMed] [Google Scholar]
- 3.Berchieri-Ronchi CB, Presti PT, Ferreira AL, Correa CR, Salvadori DM, Damasceno DC, et al. Effects of oxidative stress during human and animal reproductions: a review. Int J Nutr. (2015) 8:6–11. 10.1055/s-0040-170506326606158 [DOI] [Google Scholar]
- 4.Idamokoro EM, Muchenje V, Afolayan AJ, Hugo A. Comparative fatty-acid profile and atherogenicity index of milk from free grazing Nguni, Boer and non-descript goats in South Africa. Pastoralism. (2019) 9:1–8. 10.1186/s13570-019-0138-8 [DOI] [Google Scholar]
- 5.Obrador E, Liu-Smith F, Dellinger RW, Salvador R, Meyskens FL, Estrela JM. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol Chem. (2019) 400:589–612. 10.1515/hsz-2018-0327 [DOI] [PubMed] [Google Scholar]
- 6.Vitale SG, Capriglione S, Peterlunger I, La Rosa VL, Vitagliano A, Noventa M, et al. The role of oxidative stress and membrane transport systems during endometriosis: a fresh look at a busy corner. Oxid Med Cell Longev. (2018) 2018:1–14. 10.1155/2018/7924021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colitti M, Stefanon B, Gabai G, Gelain ME, Bonsembiante F. Oxidative stress and nutraceuticals in the modulation of the immune function: current knowledge in animals of veterinary interest. Antioxidants. (2019) 8:28. 10.3390/antiox8010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernández-Castellano LE, Morales-delaNuez A, Sánchez-Macías D, Moreno-Indias I, Torres A, Capote J, et al. The effect of colostrum source (goat vs sheep) and timing of the first colostrum feeding (2 h vs 14 h after birth) on body weight and immune status of artificially reared newborn lambs. J Dairy Sci. (2015) 98:204–10. 10.3168/jds.2014-8350 [DOI] [PubMed] [Google Scholar]
- 9.Zachwieja A, Szulc T, Potkański A, Mikuła R, Kruszyński W, Dobicki A. Effect of different fat supplements used during dry period of cows on colostrum physicochemical properties. Biotechnol Anim Husb. (2007) 23:67–75. 10.2298/BAH0702067Z [DOI] [Google Scholar]
- 10.Hyrslova I, Krausova G, Bartova J, Kolesar L, Curda L. Goat and bovine colostrum as a basis for new probiotic functional foods and dietary supplements. J Microb Biochem Technol. (2016) 8:56–9. 10.4172/1948-5948.1000262 [DOI] [Google Scholar]
- 11.Kekana TW, Nherera-Chokuda VF, Baloyi JJ, Muya CM. Immunoglobulin G response and performance in Holstein calves supplemented with garlic powder and probiotics. S Afr J Anim Sci. (2020) 50:263–70. 10.4314/sajas.v50i2.9 [DOI] [Google Scholar]
- 12.Kholif AE, Morsy TA, Gouda GA, Anele UY, Galyean ML. Effect of feeding diets with processed Moringa oleifera meal as protein source in lactating Anglo-Nubian goats. Anim Feed Sci Technol. (2016) 217:45–55. 10.1016/j.anifeedsci.2016.04.012 [DOI] [Google Scholar]
- 13.Abou-Elkhair R, Mahboub H, Sadek K, Ketkat S. Effect of prepartum dietary energy source on goat maternal metabolic profile, neonatal performance, and economic profitability. J Adv Vet Anim Res. (2020) 7:566–74. 10.5455/javar.2020.g454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Juhaimi FY, Alsawmahi ON, Abdoun KA, Ghafoor K, Babiker EE. Antioxidant potential of Moringa leaves for improvement of milk and serum quality of Aardi goats. S Afr J Bot. (2020) 129:134–37. 10.1016/j.sajb.2019.03.022 [DOI] [Google Scholar]
- 15.Patra AK, Amasheh S, Aschenbach JR. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds–a comprehensive review. Crit Rev Food Sci Nutr. (2019) 59:3237–66. 10.1080/10408398.2018.1486284 [DOI] [PubMed] [Google Scholar]
- 16.Abbas RK, Elsharbasy FS, Fadlelmula AA. Nutritional values of Moringa oleifera, total protein. amino acid, vitamins, minerals, carbohydrates, total fat and crude fiber, under the semi-arid conditions of Sudan. J Microb Biochem Technol. (2018) 10:56–8. 10.4172/1948-5948.1000396 [DOI] [Google Scholar]
- 17.Kou X, Li B, Olayanju JB, Drake JM, Chen N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients. (2018) 10:343. 10.3390/nu10030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleem A, Saleem M, Akhtar MF. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: an ethnomedicinal plant of Moringaceae family. S Afr J Bot. (2020) 128:246–56. 10.1016/j.sajb.2019.11.023 [DOI] [Google Scholar]
- 19.Vats S, Gupta T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol Mol Biol Plants. (2017) 23:239–48. 10.1007/s12298-016-0407-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng B, Luo J, Wang P, Yang L, Chen T, Sun J, et al. The beneficial effects of Moringa oleifera leaf on reproductive performance in mice. Food Sci Nutr. (2019) 7:738–46. 10.1002/fsn3.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun JJ, Wang P, Chen GP, Luo JY, Xi QY, Cai GY, et al. Effect of Moringa oleifera supplementation on productive performance, colostrum composition and serum biochemical indexes of sow. J Anim Physiol Anim Nutr. (2020) 104:291–9. 10.1111/jpn.13224 [DOI] [PubMed] [Google Scholar]
- 22.Afzal A, Hussain T, Hameed A. Moringa oleifera supplementation improves antioxidant status and biochemical indices by attenuating early pregnancy stress in Beetal goats. Front Nutr. (2021) 8:700957. 10.3389/fnut.2021.700957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NRC . Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids. Washington DC: National Academy Press; (2007). [Google Scholar]
- 24.AOAC . Association of Official Analytical Chemist. Official Methods of Analysis. 21st ed. Vol. 222. Washington, DC: Association of Official Analytical Chemists; (2005). [Google Scholar]
- 25.Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. (2007) 101:140–7. 10.1016/j.foodchem.2006.01.014 [DOI] [Google Scholar]
- 26.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat protoc. (2007) 2:875–77. 10.1038/nprot.2007.102 [DOI] [PubMed] [Google Scholar]
- 27.Moeslinger T, Brunner M, Volf I, Spieckermann PG. Spectrophotometric determination of ascorbic acid and dehydroascorbic acid. Clin Chem. (1995) 41:1177–81. 10.1093/clinchem/41.8.1177 [DOI] [PubMed] [Google Scholar]
- 28.Dhindsa RS, Plumb-Dhindsa PA, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. (1981) 32:93–101. 10.1093/jxb/32.1.93 [DOI] [Google Scholar]
- 29.Dikilitas M, Guldur ME, Deryaoglu A, Ozcan ER. Antioxidant and oxidant levels of pepper (Capsicum annuum cv. Charlee) infected with pepper mild mottle virus. Not Bot Horti Agrobo. (2011) 39:58–63. 10.15835/nbha3925881 [DOI] [Google Scholar]
- 30.Beyer WF Jr, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. (1987) 161:559–66. 10.1016/0003-2697(87)90489-1 [DOI] [PubMed] [Google Scholar]
- 31.Agostini E, Medina MI, Milrad de Forchetti SR, Tigier H. Properties of two anionic peroxidase isoenzymes from turnip (Brassica napus L.) roots. J Agric Food Chem. (1997) 45:596–8. 10.1021/jf960259u [DOI] [Google Scholar]
- 32.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. (1952) 195:133–40. 10.1016/S0021-9258(19)50881-X [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. (1976) 72:248–54. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 34.Folin O. Two revised copper methods for blood sugar determination. J Biol Chem. (1929) 82:83–93. 10.1016/S0021-9258(18)63690-7 [DOI] [Google Scholar]
- 35.Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi. (1992) 39:925–8. 10.3136/nskkk1962.39.925 [DOI] [Google Scholar]
- 36.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. (1977) 83:346–56. 10.1016/0003-2697(77)90043-4 [DOI] [PubMed] [Google Scholar]
- 37.Van Asperen K. A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol. (1962) 8:401–16. 10.1016/0022-1910(62)90074-4 [DOI] [Google Scholar]
- 38.Filner P, Varner JE. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci USA. (1967) 58:1520. 10.1073/pnas.58.4.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crops Prod. (2013) 44:566–71. 10.1016/j.indcrop.2012.09.021 [DOI] [Google Scholar]
- 40.Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. (2009) 47:2196–201. 10.1016/j.fct.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 41.Sharma N, Singh NK, Singh OP, Pandey V, Verma PK. Oxidative stress and antioxidant status during transition period in dairy cows. Asian-Austr J Anim Sci. (2011) 24:479–84. 10.5713/ajas.2011.10220 [DOI] [Google Scholar]
- 42.Zhang LY, Cosma G, Gardner H, Vallyathan V, Castranova V. Effect of chlorogenic acid on hydroxyl radical. Mol Cell Biochem. (2003) 247:205–10. 10.1023/a:1024103428348 [DOI] [PubMed] [Google Scholar]
- 43.Aekthammarat D, Pannangpetch P, Tangsucharit P. Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine. (2019) 54:9–16. 10.1016/j.phymed.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 44.Hassan HM, El-Moniary MM, Hamouda Y, El-Daly EF, Youssef AW, Abd El-Azeem NA. Effect of different levels of Moringa oleifera leaves meal on productive performance, carcass characteristics and some blood parameters of broiler chicks reared under heat stress conditions. Asian J Anim Vet Adv. (2016) 11:60–6. 10.3923/ajava.2016.60.66 [DOI] [Google Scholar]
- 45.Rao GM, Sumita P, Roshni M, Ashtagimatt MN. Plasma antioxidant vitamins and lipid peroxidation products in pregnancy induced hypertension. Indian J Clin Biochem. (2005) 20:198–200. 10.1007/BF02893070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mello LD, Kubota LT. Biosensors as a tool for the antioxidant status evaluation. Talanta. (2007) 72:335–48. 10.1016/j.talanta.2006.11.041 [DOI] [PubMed] [Google Scholar]
- 47.Kale M, Rathore N, John S, Bhatnagar D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol Lett. (1999) 105:197–205. 10.1016/S0378-4274(98)00399-3 [DOI] [PubMed] [Google Scholar]
- 48.Konvičná J, Vargová M, Paulíková I, Kováč G, Kostecká Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Veterinaria Brno. (2015) 84:133–40. 10.2754/avb201584020133 [DOI] [Google Scholar]
- 49.Gong J, Xiao M. Effect of organic selenium supplementation on selenium status, oxidative stress, and antioxidant status in selenium-adequate dairy cows during the periparturient period. Biol Trace Elem Res. (2018) 186:430–40. 10.1007/s12011-018-1323-0 [DOI] [PubMed] [Google Scholar]
- 50.Radin L, Šimpraga M, Vince S, Kostelić A, Milinković-Tur S. Metabolic and oxidative status of Saanen goats of different parity during the peripartum period. J Dairy Res. (2015) 82:426–33. 10.1017/S0022029915000552 [DOI] [PubMed] [Google Scholar]
- 51.Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. (2010) 42:1634–50. 10.1016/j.biocel.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 52.El-Demerdash FM. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J Trace Elem Med Biol. (2004) 18:113–21. 10.1016/j.jtemb.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 53.Karapehlivan M, Kaya I, Sag A, Akin S, Ozcan A. Effects of early and late lactation period on plasma oxidant/antioxidant balance of goats. Kafkas Univ Vet Fak Derg. (2013) 19:529–33. [Google Scholar]
- 54.Kekana TW, Marume U, Muya MC, Nherera-Chokuda FV. Periparturient antioxidant enzymes, haematological profile and milk production of dairy cows supplemented with Moringa oleifera leaf meal. Anim Feed Sci Technol. (2020) 268:114606. 10.1016/j.anifeedsci.2020.114606 [DOI] [Google Scholar]
- 55.Sun B, Zhang Y, Ding M, Xi Q, Liu G, Li Y, et al. Effects of Moringa oleifera leaves as a substitute for alfalfa meal on nutrient digestibility, growth performance, carcass trait, meat quality, antioxidant capacity and biochemical parameters of rabbits. J Anim Physiol Anim Nutr. (2018) 102:194–203. 10.1111/jpn.12678 [DOI] [PubMed] [Google Scholar]
- 56.Cui YM, Wang J, Lu W, Zhang HJ, Wu SG Qi GH. Effect of dietary supplementation with Moringa oleifera leaf on performance, meat quality, and oxidative stability of meat in broilers. Poultry Sci. (2018) 97:2836–44. 10.3382/ps/pey122 [DOI] [PubMed] [Google Scholar]
- 57.Guo J, Peters RR, Kohn RA. Effect of a transition diet on production performance and metabolism in periparturient dairy cows. J Dairy Sci. (2007) 90:5247–58. 10.3168/jds.2007-0326 [DOI] [PubMed] [Google Scholar]
- 58.Janku L, Pavlata L, Mišurová L, Filípek J, Pechová A, Dvorák R. Levels of protein fractions in blood serum of periparturient goats. Acta Veterinaria Brno. (2011) 80:185–90. 10.2754/avb20118002018522079706 [DOI] [Google Scholar]
- 59.Ibrahim MA, Abdelrahman H, Elmetwaly H. Hormonal profile, antioxidant status and some biochemical parameters during pregnancy and periparturient period in dromedary she camel. Egypt J Vet Sci. (2017) 48:81–94. 10.21608/ejvs.2017.2040.1022 [DOI] [Google Scholar]
- 60.Tóthová C, Nagy O, Nagyová V, Kováč G. Serum protein electrophoretic pattern in dairy cows during the periparturient period. J Appl Anim Res. (2018) 46:33–8. 10.1080/09712119.2016.1256293 [DOI] [Google Scholar]
- 61.Ateya A, El-Sayed A, Mohamed R. Gene expression and serum profile of antioxidant markers discriminate periparturient period time in dromedary camels. Mammal Res. (2021) 66:603–13. 10.1007/s13364-021-00578-3 [DOI] [Google Scholar]
- 62.Tharwat M, Ali A, Al-Sobayil F. Hematological and biochemical profiles in goats during the transition period. Comp Clin Path. (2015) 24:1–7. 10.1007/s00580-013-1842-1 [DOI] [Google Scholar]
- 63.Kekana TW, Marume U, Muya CM, Nherera-Chokuda FV. Lactation performance and blood metabolites in lactating dairy cows micro-supplemented with Moringa oleifera leaf meal. S Afr J Anim Sci. (2019) 49:709–16. 10.4314/sajas.v49i4.12 [DOI] [Google Scholar]
- 64.Mehdi Y, Hornick JL, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. (2013) 18:3292–311. 10.3390/molecules18033292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castagnino DD, Härter CJ, Rivera AR, Lima LD, Silva HG, Biagioli B, et al. Changes in maternal body composition and metabolism of dairy goats during pregnancy. Brazilian J Anim Sci. (2015) 44:92–102. 10.1590/S1806-92902015000300003 [DOI] [Google Scholar]
- 66.Caré S, Trevisi E, Minuti A, Ferrari A, Loor JJ, Calamari L. Plasma fructosamine during the transition period and its relationship with energy metabolism and inflammation biomarkers in dairy cows. Livest Sci. (2018) 216:138–47. 10.1016/j.livsci.2018.08.003 [DOI] [Google Scholar]
- 67.Hashizume T, Takahashi Y, Numata M, Sasaki K, Ueno K, Ohtsuki K, et al. Plasma profiles of growth hormone, prolactin and insulin-like growth factor-I during gestation, lactation and the neonatal period in goats. J Reprod Dev. (1999) 45:273–81. 10.1262/jrd.45.273 [DOI] [Google Scholar]
- 68.Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. (1997) 2:265–78. 10.1023/A:1026336505343 [DOI] [PubMed] [Google Scholar]
- 69.Ishola IO, Yemitan KO, Afolayan OO, Anunobi CC, Durojaiye TE. Potential of Moringa oleifera in the treatment of benign prostate hyperplasia: role of antioxidant defence systems. Med Princ Prac. (2018) 27:15–22. 10.1159/000486349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kilany OE, Abdelrazek HM, Aldayel TS, Abdo S, Mahmoud MM. Anti-obesity potential of Moringa olifera seed extract and lycopene on high fat diet induced obesity in male Sprauge Dawely rats. Saudi J Biol Sci. (2020) 27:2733–46. 10.1016/j.sjbs.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petushkova AI, Zamyatnin AA. Redox-mediated post-translational modifications of proteolytic enzymes and their role in protease functioning. Biomolecules. (2020) 10:650. 10.3390/biom10040650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bota DA, Davies KJ. Protein degradation in mitochondria: implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion. (2001) 1:33–49. 10.1016/S1567-7249(01)00005-8 [DOI] [PubMed] [Google Scholar]
- 73.Khan JR, Ludri RS. Changes in blood glucose, plasma non-esterified fatty acids and insulin in pregnant and non-pregnant goats. Trop Anim Health Prod. (2002) 34:81–90. 10.1023/a:1013798114081 [DOI] [PubMed] [Google Scholar]
- 74.Banchero GE, Clariget RP, Bencini R, Lindsay DR, Milton JT, Martin GB. Endocrine and metabolic factors involved in the effect of nutrition on the production of colostrum in female sheep. Reprod Nutr Dev. (2006) 46:447–60. 10.1051/rnd:2006024 [DOI] [PubMed] [Google Scholar]
- 75.Moreno-Indias I, Sánchez-Macías D, Castro N, Morales-delaNuez A, Hernández-Castellano LE, Capote J, Argüello A. Chemical composition and immune status of dairy goat colostrum fractions during the first 10 h after partum. Small Rumin Res. (2012) 103:220–4. 10.1016/j.smallrumres.2011.09.015 [DOI] [Google Scholar]
- 76.Kholif AE, Gouda GA, Olafadehan OA, Abdo MM. Effects of replacement of Moringa oleifera for berseem clover in the diets of Nubian goats on feed utilisation, and milk yield, composition and fatty acid profile. Animal. (2018) 12:964–72. 10.1017/S1751731117002336 [DOI] [PubMed] [Google Scholar]
- 77.Babiker EE, Juhaimi FA, Ghafoor K, Mohamed HE, Abdoun KA. Effect of partial replacement of alfalfa hay with Moringa species leaves on milk yield and composition of Najdi ewes. Trop Anim Health Prod. (2016) 48:1427–33. 10.1007/s11250-016-1111-9 [DOI] [PubMed] [Google Scholar]
- 78.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med. (2003) 24:345–51. 10.1016/S0098-2997(03)00030-X [DOI] [PubMed] [Google Scholar]
- 79.Aguiar SC, Cottica SM, Boeing JS, Samensari RB, Santos GT, Visentainer JV, et al. Effect of feeding phenolic compounds from propolis extracts to dairy cows on milk production, milk fatty acid composition, and the antioxidant capacity of milk. Anim Feed Sci Tech. (2014) 193:148–54. 10.1016/j.anifeedsci.2014.04.006 [DOI] [Google Scholar]
- 80.Zeng B, Sun JJ, Chen T, Sun BL, He Q, Chen XY, et al. Effects of Moringa oleifera silage on milk yield, nutrient digestibility and serum biochemical indexes of lactating dairy cows. J Anim Physiol Anim Nutr. (2018) 102:75–81. 10.1111/jpn.12660 [DOI] [PubMed] [Google Scholar]
- 81.Dong L, Zhang T, Diao Q. Effect of dietary supplementation of Moringa oleifera on the production performance and fecal methanogenic community of lactating dairy cows. Animals. (2019) 9:262. 10.3390/ani9050262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kholif AE, Gouda GA, Morsy TA, Salem AZ, Lopez S, Kholif AM. Moringa oleifera leaf meal as a protein source in lactating goat's diets: feed intake, digestibility, ruminal fermentation, milk yield and composition, and its fatty acids profile. Small Rumin Res. (2015) 129:129–37. 10.1016/j.smallrumres.2015.05.007 [DOI] [Google Scholar]
- 83.Asaolu V, Binuomote R, Akinlade J, Aderinola O, Oyelami O. Intake and growth performance of West African Dwarf goats fed Moringa oleifera, Gliricidia sepium and Leucaena leucocephala dried leaves as supplements to cassava peels. J Biol Agric Healthc. (2012) 2:76–88. [Google Scholar]
- 84.Qwele K, Hugo A, Oyedemi SO, Moyo B, Masika PJ, Muchenje V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. (2013) 93:455–62. 10.1016/j.meatsci.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 85.Butler WR. Nutritional interactions with reproductive performance in dairy cattle. Anim Reprod Sci. (2000) 60:449–57. 10.1016/S0378-4320(00)00076-2 [DOI] [PubMed] [Google Scholar]
- 86.Vu DD, Dung CA, Hai PH. Use of urea–molasses–multinutrient block and urea-treated rice straw for improving dairy cattle productivity in Vietnam. Prev Vet Med. (1999) 38:187–93. 10.1016/S0167-5877(98)00124-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


