Abdominal and thoracic aortic aneurysms are a significant cause of morbidity and mortality. Although the mechanisms leading to abdominal and thoracic aortic aneurysm development differ, there are shared cardinal features—including breakdown of the extracellular matrix, generation of reactive oxygen species, and inflammation1. Aortic aneurysms are typically asymptomatic until they dissect or rupture, the latter of which is associated with a mortality rate of over 80%. According to the current practice guidelines, the mainstay of treatment relies on managing risk factors to prevent rupture, including smoking cessation, blood pressure control, and ultimately, surgical repair for enlarging aneurysms2. Preventive surgeries have associated perioperative morbidity and mortality. Therefore, increased understanding of the mechanisms involved in the pathophysiology of aortic aneurysms has the potential to broaden the scope of targeted medical therapies for prevention and management. The findings of Zhu and colleagues3 in this issue offer insight into an important association between aldosterone and the increased risk of aneurysmal disease.
Zhu et al. conducted a hospital-based electronic database study in which hypertensive patients with aortic aneurysm or dissection, who had plasma aldosterone concentration measurements prior to aortic aneurysm diagnosis, were matched 1:4 by age and sex to those hypertensive patients with plasma aldosterone concentration measurements without aortic aneurysms. In this case-controlled study of adults living in China, the authors demonstrated that higher plasma aldosterone concentration, which was measured on a liberal salt diet(at least 6g NaCl daily), was associated with an increased odds ratio for the subsequent diagnosis of aortic dissection and aneurysms. The study included both abdominal and thoracic aortic aneurysms, although individuals with a known connective tissue disease, vasculitis, and traumatic aortic dissections were excluded. Those in the case group had a higher mean systolic blood pressure and a longer duration of hypertension. However, a secondary analysis which matched individuals 1:1 for duration of hypertension in the two groups continued to show that a higher plasma aldosterone concentration had increased odds ratio for the development of aortic aneurysm/dissection. In a mediation analysis, systolic and diastolic blood pressures were estimated to mediate only 11.5% and 6.4%, respectively, of the association between elevated plasma aldosterone concentration and aortic aneurysm disease, suggesting that the association may in part be independent of aldosterone’s role in blood pressure regulation.
The study excluded individuals with a definite diagnosis of secondary hypertension at the time of aldosterone testing, including renovascular hypertension and aldosterone-producing adenoma greater than 1 cm. A potential limitation of the study is that some, but not all individuals with primary aldosteronism were excluded from the primary analysis. Primary aldosteronism was found in 16.2% of the control group and 8.3% of the case group. In a sensitivity analysis, the authors excluded primary aldosteronism from both the case and control groups, and a higher plasma aldosterone concentration continued to show an increased odds for the presence of aortic aneurysm/dissection. This is important in demonstrating that an elevated plasma aldosterone concentration, which does not necessarily meet the threshold for diagnosis of primary aldosteronism, could pose a significant health burden.
An ongoing question is whether there are clinical implications of an elevated plasma aldosterone concentration that is dependent versus independent of renin. In the present study, stratified analysis by suppressed plasma renin activity(≤0.5ng/ml/h) and unsuppressed plasma renin activity(>0.5ng/ml/h) demonstrated a significant relationship between plasma aldosterone concentration and aortic aneurysm/dissection only among individuals with suppressed plasma renin activity. This finding further emphasizes the potential adverse physiology of a renin-independent elevation in aldosterone.
The data presented by Zhu et al. are associational in nature. Therefore, it would be important to obtain insights from existing animal models to understand potential renin-angiotensin-aldosterone-system(RAAS)-related mechanisms in the pathophysiology of aortic aneurysms. In the hyperlipidemic apolipoprotein E–deficient(ApoE−/−) and LDL receptor knock out(LDLR−/−) murine models, infusion of angiotensin II(Ang II) induced abdominal aortic aneurysm formation, and this process was independent of an increase in blood pressure4. A challenge of the hyperlipidemic/Ang II infusion model is discerning whether Ang II is directly contributing to aneurysmal formation or acting as a potent stimulator of aldosterone and/or inflammation, thereby indirectly contributing to aneurysmal disease.
To explore the role of aldosterone and its receptor in aortic aneurysm formation, Liu et al. studied C57BL/6 mice on a high salt diet. The infusion of mineralocorticoid receptor agonists, deoxycorticosterone acetate(DOCA) or aldosterone, stimulated both abdominal and thoracic aortic aneurysm formation and rupture5. Mineralocorticoid receptor blockade was shown to prevent aortic aneurysm formation indicating that damage was mediated by mineralocorticoid receptor activation. Importantly, this pathology required both DOCA and a high salt diet, as neither condition alone induced aortic aneurysms. Further, aortic aneurysm formation and rupture occurred in the absence of dyslipidemia. A hallmark of human aortic aneurysm formation is chronic perpetual inflammation, impaired healing, and advancement to fibrosis. Importantly, Ang II, DOCA, and aldosterone murine models do show aneurysmal progression and rupture. DOCA/high salt or aldosterone/high salt also increase collagen and elastin degradation, MMP expression, and recruitment of inflammatory cells in the aorta5—biologic changes that can be observed in human pathogenesis of aortic aneurysms1. In this regard, one study conducted using the Ang II/ß-aminopropionitrile model demonstrated treatment with the mineralocorticoid receptor antagonist eplerenone reduced the expression of TNFα, IL-6, MMP-2 and MCP-1 in the aortic wall and protected against aortic aneurysm progression6. One study suggested that aldosterone is synthesized locally in the vascular wall7, which could contribute to aneurysm progression. The mineralocorticoid receptor is also expressed in vascular smooth muscle, endothelial, and myeloid cells. In this regard, it would be informative to investigate whether reducing mineralocorticoid receptor expression in any of these cell types affects the development and progression of aortic aneurysms.
Taken together, emerging evidence from human and animal studies lead to the concept that mineralocorticoid receptor activation is integral to aortic aneurysm disease and progression. As shown in Figure 1, aldosterone activation of mineralocorticoid receptor in the aorta could promote aneurysmal disease through direct effects on the vessel, potentially by affecting structural integrity and promoting localized inflammation. Excess aldosterone is well-known to have a critical impact on blood pressure, which also could contribute to aortic aneurysm rupture.
Figure 1. Possible Mediators of Aneurysmal Disease.
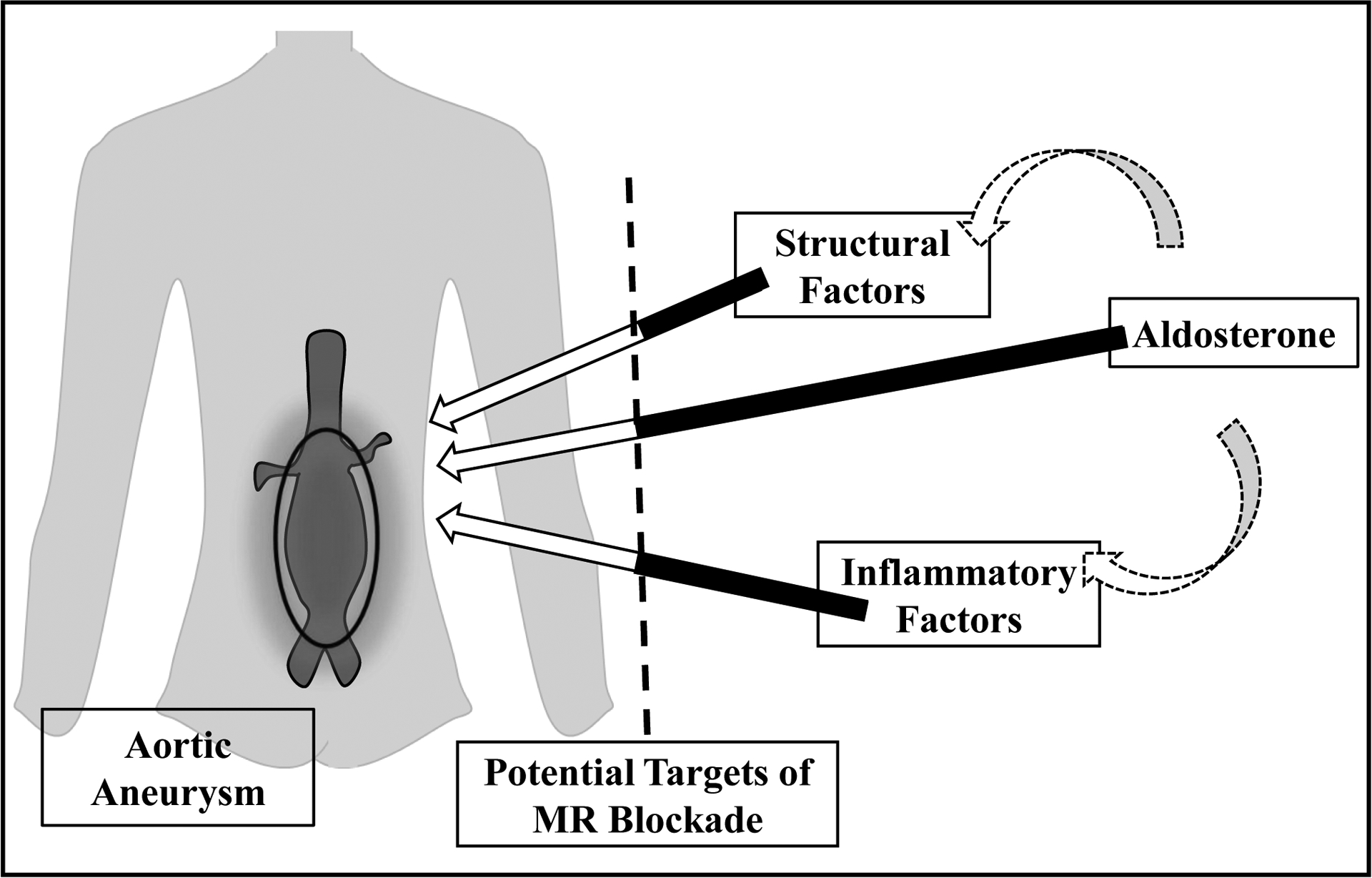
Aldosterone may directly contribute to aneurysmal formation and growth. In addition, aldosterone may indirectly affect structural and inflammatory factors, leading to an imbalance in matrix metalloproteinases, an excess production of reactive oxygen species, and an enhanced local inflammatory response. Mineralocorticoid receptor (MR) is expressed in vascular smooth muscle cells, endothelial cells and inflammatory cells. Excess aldosterone is linked to hypertensive disease which could promote aneurysmal rupture. If further studies support aldosterone’s role in the pathogenesis of aneurysmal disease, there is potential to intervene with mineralocorticoid receptor blockade.
The concept that mineralocorticoid receptor activation is involved in aortic aneurysm formation is consistent with our knowledge of its involvement in other cardiovascular diseases. Large scale clinical trials of patients on optimal therapies, including angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapies, have shown that treatment with mineralocorticoid receptor blockade reduces mortality in heart failure with reduced ejection fraction and post-myocardial ischemia, and in patients with diabetes, mineralocorticoid receptor blockade reduces progression of renal disease and cardiovascular mortality8. Furthermore, mineralocorticoid receptor blockade is used to treat hypertension and primary aldosteronism.
To date, there are no specific medical treatments to prevent aortic aneurysm progression. Data from human studies investigating angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in aortic aneurysms have been equivocal and do not show a clear benefit9. Findings from Zhu et al. identify aldosterone as a novel target that warrants further investigation of mineralocorticoid receptor blockade, and potentially aldosterone synthase inhibition, for treatment of aortic aneurysms.
We do not have validated clinical tools to identify who may be at risk for aortic aneurysm formation or progression. Only D-dimer and plasmin-antiplasmin have been consistently associated with abdominal aortic aneurysm growth10. The work by Zhu et al. offers the possibility that aldosterone, potentially in the context of a low renin, has utility as a biomarker of subclinical aneurysmal disease or progression. Ideally, combining radiographic measures with informative circulating biomarkers would enhance clinical care of patients for risk stratification, diagnosis, and management of aortic aneurysms.
In conclusion, data from Zhu et al. highlight that aldosterone, whose action is regulated by mineralocorticoid receptor activation, may be important to aortic aneurysm formation and progression. These preliminary findings should prompt more mechanistic studies to fully understand the role of mineralocorticoid receptor activation in aortic aneurysm pathogenesis and consideration of large scale randomized clinical trials designed to test the efficacy of mineralocorticoid receptor blockade on aortic aneurysm progression.
Funding:
YEY was supported by NIH T32 HL007609. Funding was also provided by NIH K24 HL103845 to GKA and NIH K23 HL136262 to SS. Funding sources had no role in the writing of the manuscript.
Footnotes
Disclosure Statement:
YEY has nothing to declare. SS was the recipient of a Gilead Sciences Research Scholars award.
References
- 1.Quintana RA,Taylor WR. Cellular Mechanisms of Aortic Aneurysm Formation. Circulation Research. 2019;124(4):607–618.doi: 10.1161/CIRCRESAHA.118.313187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaikof EL,Dalman RL,Eskandari MK,et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of Vascular Surgery. 2018;67(1):2–77.e2.doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 3.Zhu Q,Heizhati M,Wang M,et al. Higher plasma aldosterone concentrations are associated with elevated risk of aortic dissection and aneurysm: a case-control study. Hypertension. 2022. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Cassis LA,Gupte M,Thayer S, et al. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. May2009;296(5):H1660–5.doi: 10.1152/ajpheart.00028.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S,Xie Z,Daugherty A,et al. Mineralocorticoid receptor agonists induce mouse aortic aneurysm formation and rupture in the presence of high salt. Arterioscler Thromb Vasc Biol. Jul2013;33(7):1568–79.doi: 10.1161/atvbaha.112.300820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurobe H,Hirata Y,Matsuoka Y,et al. Protective effects of selective mineralocorticoid receptor antagonist against aortic aneurysm progression in a novel murine model. doi: 10.1016/j.jss.2013.05.002 [DOI] [PubMed]
- 7.Hatakeyama H,Miyamori I,Fujita T,Takeda T,Takeda R,Yamamoto H. Vascular aldosterone. Biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 1994. Sep 30;269(39):24316–20. [PubMed] [Google Scholar]
- 8.Agarwal R,Kolkhof P,Bakris G,et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. European Heart Journal. 2021;42(2):152–161.doi: 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindeman JH,Matsumura JS. Pharmacologic Management of Aneurysms. Circ Res. Feb15 2019;124(4):631–646.doi: 10.1161/circresaha.118.312439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanhainen A,Mani K,Golledge J. Surrogate Markers of Abdominal Aortic Aneurysm Progression. Arterioscler Thromb Vasc Biol. Feb2016;36(2):236–44.doi: 10.1161/atvbaha.115.306538 [DOI] [PubMed] [Google Scholar]


