Abstract
To improve osseointegration caused by the stress-shielding effect and the inert nature of titanium-based alloys, in this work, we successfully constructed a strontium calcium phosphate (Sr-CaP) coating on three-dimensional (3D)-printed Ti6Al4V scaffolds to address this issue. The energy-dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD) results indicated that the coatings with and without Sr doping mainly consisted of CaHPO4. The bonding strength of Sr doping coating met the required ISO 13 779-4-2018 standard (≥15 MPa). The in vitro results suggested that the Sr-CaP-modified Ti6Al4V scaffolds were found to effectively promote mice bone-marrow stem cell (mBMSC) adhesion, spreading, and osteogenesis. The in vivo experiments also showed that the Sr-CaP-modified Ti6Al4V scaffolds could significantly improve bone regeneration and osseointegration. More importantly, Sr-doped CaP-coated Ti6Al4V scaffolds were found to accelerate bone healing in comparison to CaP-coated Ti6Al4V scaffolds. The Sr-CaP-modified Ti6Al4V scaffolds are considered a promising strategy to develop bioactive surfaces for enhancing the osseointegration between the implant and bone tissue.
1. Introduction
Titanium and its alloys are widely used in dental and orthopedic surgery due to its good biocompatibility, corrosion resistance, and high mechanical strength.1 However, the elastic modulus of titanium-based alloys (≥110 GPa) is significantly higher than that of human bone (cortical bone of 7–30 GPa and cancellous of 1.5–11.2 GPa). This mismatch of elastic modulus between the titanium-based implant and human bone would consequently induce stress shielding, causing the failure of replacement. This is because the bone metabolism including the bone growth and resorption of osteoblasts and osteoclasts generally is regulated via the sensory of proper mechanical stimulation. To address this issue, metal three-dimensional (3D)-printing technology has been used to construct porous scaffolds with low elastic modulus similar to cortical bone, which offers some alternatives to conventional production constraints, such as the uneven distribution of micropores and the pore size.2 Of which, the selective laser melting (SLM) technique used in 3D printing is one of the popular choices for creating Ti6Al4V scaffolds with the ideal structure and pore size.3,4 It has been proven that the 3D-printed Ti6Al4V scaffolds can promote the interaction between cell and implant, thereby enhancing osseointegration.5,6
Although the mismatch of elastic modulus between the titanium-based implant and human bone can be addressed by 3D-printing technology, the inert nature of titanium-based alloys also is one main factor for inducing the failure of cell adhesion. This is because the cell adhesion is greatly associated with the surface morphology and composition of the implant,7 since the implant would be exposed to the physiological environment.8 The ideal way for overcoming the inert nature of titanium-based alloys is improving the bioactivity by surface modification with the intention to promote cell attachment and differentiation.9 Extensive research studies have demonstrated that bioactive coatings can promote bone integration, accelerate patient rehabilitation, and shorten healing time.10 According to Qiao et al, an autologous platelet-rich plasma coating was fabricated on 3D-printed Ti scaffolds to improve the bioactivity of the osseointegration between the Ti implant and bone regeneration, and they found that the autologous platelet-rich plasma-coated Ti scaffolds could significantly favor the attachment and osteogenic differentiation of bone-marrow mesenchymal stem cells (BMSCs) in vitro, while bone regeneration and osseointegration were enhanced by the bioactive interface.11 Chudinova et al. used electrophoretic deposition to produce a calcium phosphate nanoparticles/polyethyleneimine composite coating on 3D-printed porous Ti6Al4V and found that the 3D-printed porous Ti6Al4V with bioactive coating could increase the expression of cell proliferation and alkaline phosphatase (ALP) activity.12 Since the human bone mainly consists of calcium and phosphate, calcium phosphate (CaP)-based coatings have been widely employed in the surface modification of implants,13 which show a good bone induction effect.14 Meanwhile, certain elements required by bone regeneration are doped into the Ca–P coatings to further enhance the biocompatibility of the Ca–P coating.15 For example, as one of the essential trace elements, strontium (Sr) can prevent arteriosclerosis and thrombosis in the human body.16 Many studies have proven that Sr can effectively promote osteoblast proliferation,17 differentiation,18 and mineralization.19 Moreover, Sr can inhibit bone resorption by inducing osteoclast apoptosis20 and encouraging mesenchymal stem cells to differentiate into the bone lineage.21 Given this, Sr has been added to biomaterials,22 bioactive ceramics,23 and glass24 to improve bone formation.
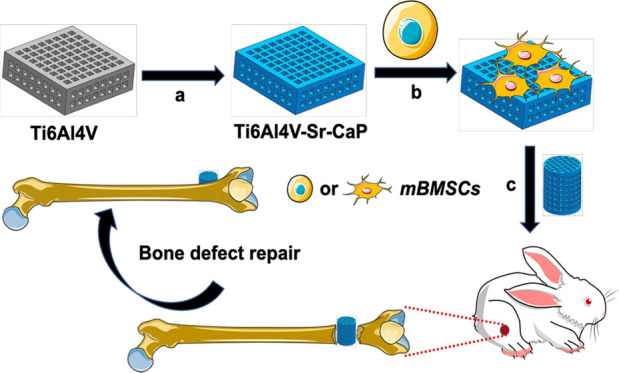
In this study, the Sr-doped CaP coating (Sr-CaP) was produced on 3D-printed Ti6Al4V scaffolds by the hydrothermal method with the aim to improve the biocompatibility of Ti6Al4V scaffolds. The biocompatibility and osteogenic differentiation of the Sr-CaP-coated Ti6Al4V scaffolds were studied in vitro, while the osseointegration was investigated in vivo using the New Zealand white rabbit femur bone deficiency model (Figure 1).
Figure 1.
Diagram of the research route: (a) hydrothermal synthesis was used to coat Ti6Al4V scaffolds with Sr-CaP. (b) In vitro investigation of the osteogenic characteristics of mBMSCs on the Sr-CaP-coated Ti6Al4V scaffold. (c) Examination of the osteogenic characteristics of Sr-CaP-coated Ti6Al4V scaffold in vivo for the purpose of repairing bone defects.
2. Results
2.1. Characterization of Coated Ti6A14V Scaffolds
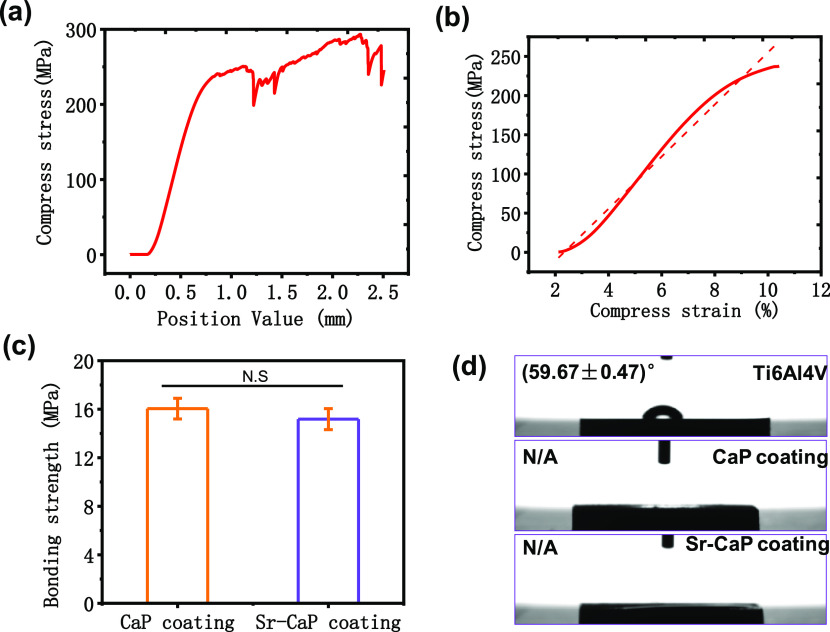
The compressive strength of the Ti6A14V scaffolds was evaluated by the compression test, as shown in Figure 2a. The average value of the maximum compressive force was approximately ∼300 MPa, which was greater than 130–180 MPa of the human bone bear. Additionally, the elastic modulus of porous Ti6A14V scaffolds fitted from the elastic segment was approximately ∼5.19 GPa (Figure 2b), which was significantly lower than that of 4–32 GPa of human bone. The bonding strength between the coating and substrate is shown in Figure 2c, suggesting that the CaP coating (∼16.04 MPa) shows a higher bonding strength compared with a Sr-CaP coating (15.18 MPa). Importantly, both bonding strengths were met the required ISO 13 779-4-2018 standard (≥15 MPa). To determine the hydrophily of the coatings, the coating fabricated on the plates was used to measure the contact angles, as shown in Figure 2d. The contact angle of the Ti6Al4V plate was about 59.67 ± 0.47°, while the contact angle of coatings with and without Sr doping could not be detected. This indicated that the CaP-based coatings could improve the hydrophilicity, which may enhance the ability of cell adhesion on the implant.
Figure 2.
Curves of (a) stress–strain for Ti6Al4V scaffolds and (b) elastic modulus. (c) Bonding strengths of CaP coating and Sr-CaP coating. (d) Contact angle of coatings with and without Sr doping on plates. N.S. means P ≥ 0.05.
Figure 3a,b shows the scanning electron microscopy (SEM) images of Ti6A14V scaffolds with and without coatings. The observation at the low magnification suggested that the CaP and Sr-CaP coatings were successfully fabricated on the Ti6A14V scaffolds. At high magnification, it can be found that the microstructure was changed when the Sr element was doped into the CaP coating (Figure 3b). Moreover, the crystal size in the CaP coating was greater than that of the Sr-CaP coating. Therefore, the slight decrease in bonding strength may be due to the changing structure of calcium phosphate crystals after the Sr was introduced into CaP coating.25 The corresponding EDS patterns extracted from Figure 3b are displayed in Figure 3c. The main composition of the CaP coating consisted of Ti, Ca, P, and O, while Sr was also presented in the Sr-CaP coating, indicating that Sr was doped into the CaP coating. It should be noted that the ratio of Ca/P and (Ca + Sr/P) was close to 1. Figure 3d presents the XRD patterns of Ti6Al4V, CaP coating, and Sr-CaP coating, which indicated that the main phase of both coatings was CaHPO4. A comparison of XRD patterns ranging from 20 to 40° between the CaP coating and Sr-CaP coating is shown in Figure 3e, which could further confirm the CaHPO4 detail that no obvious change could be found between both coatings, although Sr was confirmed to be introduced into the CaP coating, as indicated by EDS results. The information of the phase could be confirmed by the EDS results that the ratio of Ca/P and (Ca + Sr/P) was close to 1.
Figure 3.
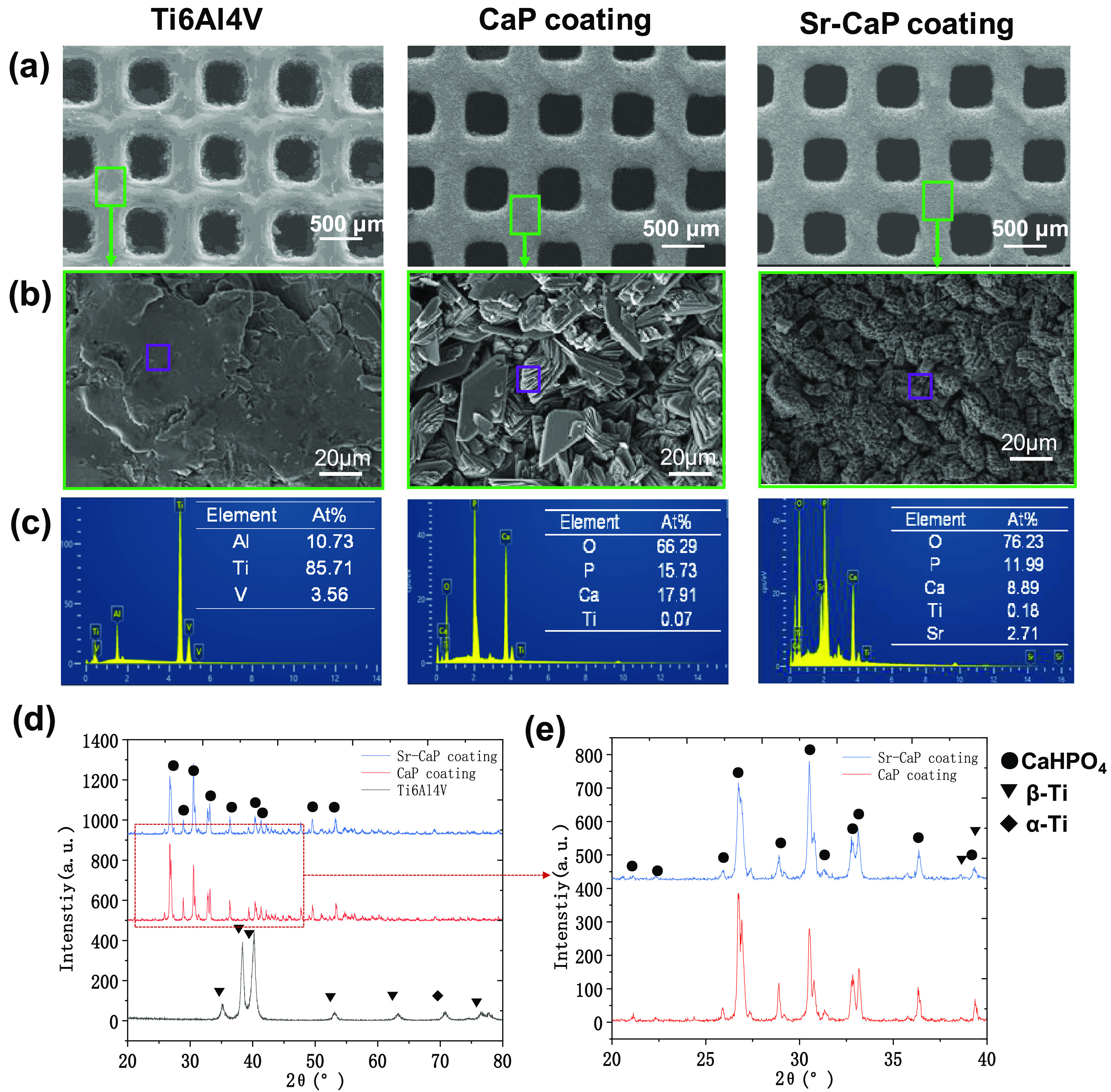
(a) SEM images of samples at low magnification. (b) SEM images at high magnification. (c) The corresponding EDS from (b). (d) XRD patterns of Ti6Al4V, CaP coating, and Sr-CaP coating. (e) Amplification of XRD in the range from 20 to 40°.
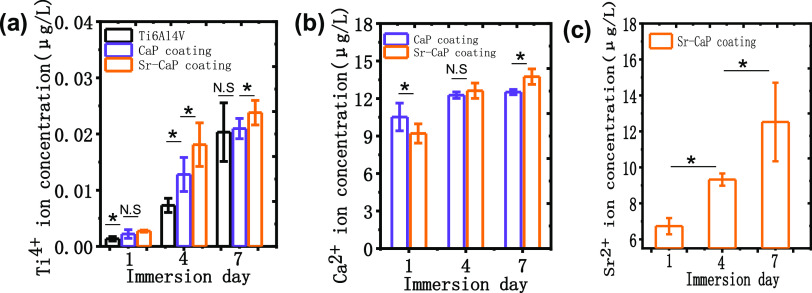
Figure 4 shows the ion-releasing behavior of the samples with and without coatings. Generally, there was an increasing profile of the concentration of each ion with time. The concentration of the Ti ions in the Ti6Al4V groups was lower than that of the coated groups, while the Sr-CaP-coated groups had a higher concentration of Ti ions compared with CaP-coated groups at any time point (Figure 4a). The concentration of Ca ion for the Sr-CaP-coated samples was less than that of the CaP coating on the first day, and there was no significant difference on the fourth day; on the seventh day, the concentration of Ca ion or the Sr-CaP-coated samples was then greater than that of the CaP-coated samples (Figure 4b). As for the Sr ions, an obvious increasing tendency of the concentration could be observed (Figure 4c). It has to be mentioned that why the concentration of the Ti ions in the Ti6Al4V groups was lower than that of the coated groups at any time point should be further investigated in the next study.
Figure 4.
Ion-releasing behavior of the samples with and without coatings: (a) Ti ions, (b) Ca ions, and (c) Sr ions of Sr-CaP after 1, 4, and 7 days of immersion. N.S. means P ≥ 0.05. *P < 0.05.
2.2. Detecting the Biocompatibility of 3D-Printed Porous Ti6A14V Scaffolds with the Sr-CaP Coating
A sufficient quantity of Sr might effectively induce osteogenesis. The CCK-8 test revealed that after 1, 4, and 7 days of culture, the number of mBMSCs in the 15% Sr Ti6Al4V-CaP (Ti6Al4V-15% Sr-CaP) scaffolds decreased considerably, indicating that this group was unable to demonstrate excellent compatibility. However, there was no significant difference in the number of mBMSCs in the 10% Sr Ti6Al4V-CaP (Ti6Al4V-10% Sr-CaP) scaffolds compared to that in the Ti6Al4V scaffolds (Figure S1), showing that it possessed low cytotoxicity and was capable of optimizing the osteogenic impact of Sr; also, we detected the ion-releasing behavior of Ti, Ca, and Sr ions, as shown in Figure S1, which suggested that the Sr concentration for Ti6Al4V-5% Sr-CaP, Ti6Al4V-10% Sr-CaP, and Ti6Al4V-15% Sr-CaP was 4.01, 8.67, and 14.55 μg/L after 1 day of immersion. The Ti6Al4V-5% Sr-CaP group showed the highest Ti concentration, while Ti6Al4V-10% Sr-CaP showed the lowest. The Ca ion concentration increased with the Sr content in the CaP coatings. As a result, we chose a 10% content of Sr for the next study. The fluorescence microscopy live/dead experiment in Figure 5a revealed that all mBMSCs were alive on both unmodified and modified Ti6A14V scaffolds. This was confirmed by the CCK-8 quantification of mBMSC proliferation on various Ti6Al4V scaffolds after 1, 4, and 7 days of culture, as shown in Figure 5c, which indicated that there was no significant cytotoxicity on the Ti6Al4V-CaP scaffolds and the Ti6Al4V-10% Sr-CaP scaffolds as compared to the Ti6A14V scaffolds. Meanwhile, the morphology of mBMSCs revealed no change in the ratio of C/N (total cell spreading area/nuclear area) between the groups (Figure 5b,d). These findings indicated that the Ti6Al4V-10% Sr-CaP scaffolds were biocompatible. In addition, to gain insight into the attached behavior of cells on the scaffolds, the cell morphologies of mBMSCs were stained and observed via a fluorescence microscope after 1 day of culture on scaffolds, as shown in Figure 5e. It can be seen that live mBMSCs were well attached on the scaffolds, where the green represents the cytoplasm and the blue represents the nucleus since the dead cells were removed by phosphate buffered saline (PBS) before the staining processing. At a higher magnification shown in Figure S2, mBMSCs exhibit a pseudopod shape in all scaffolds with and without coatings. This indicated that the Sr-doped coating also presents a good cytocompatibility to mBMSCs for adhesion and migration.
Figure 5.
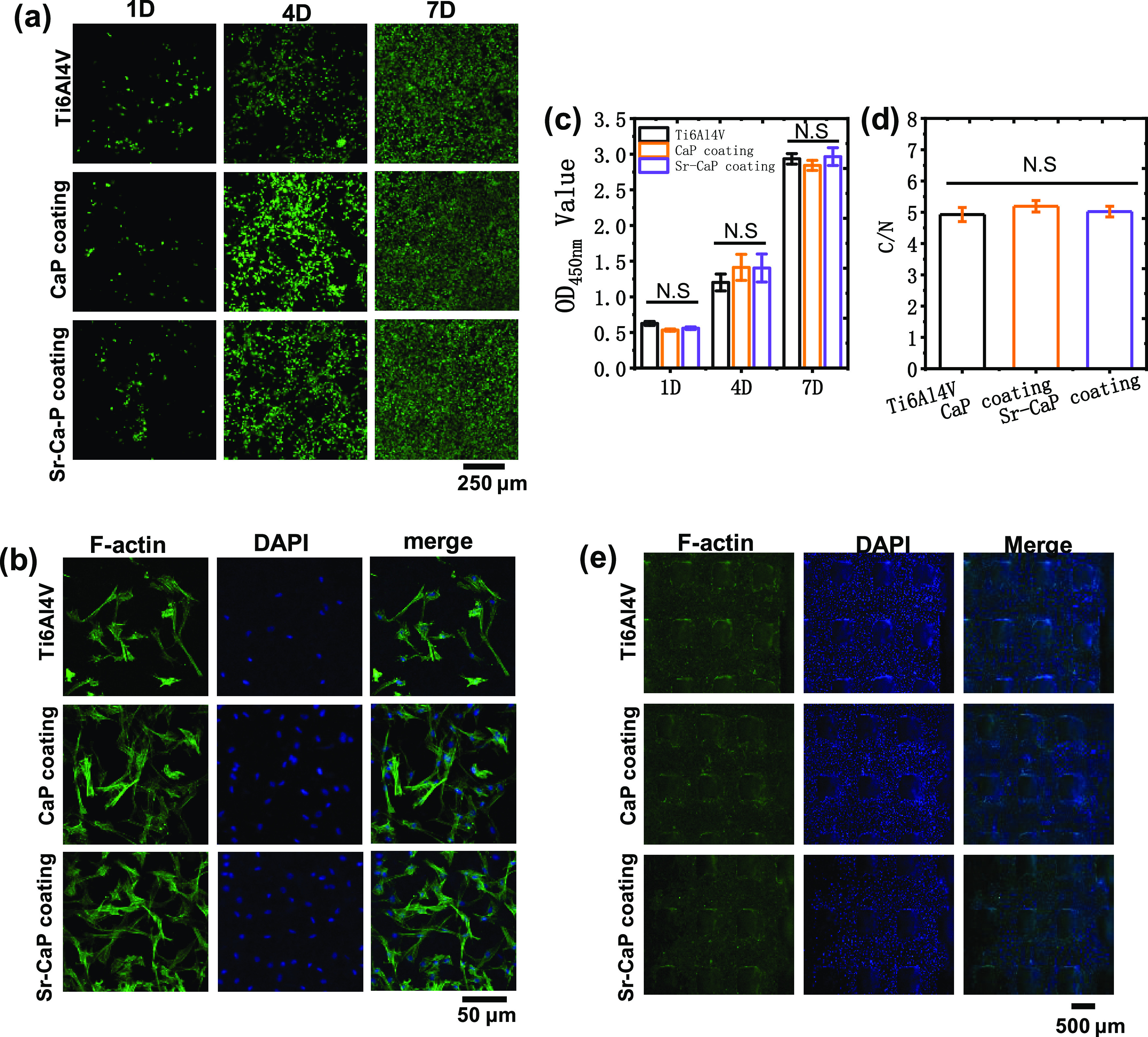
(a) Viability of mBMSCs cultured on various Ti6Al4V scaffolds after 1, 4, and 7 days. (b) Cell spreading of mBMSCs on various Ti6Al4V scaffolds after 1 day of culture. (c) CCK-8 quantification of mBMSC proliferation on various Ti6Al4V scaffolds after 1, 4, and 7 days of culture (n = 3). (d) Quantitative C/N ratio (the total cell spreading area divided by the nuclear area) on various Ti6Al4V scaffolds after 1 day of culture (n = 3). (e) Images of mBMSCs stained via fluorescence after 1 day of culture on scaffolds. N.S. means P ≥ 0.05. *P < 0.05.
2.3. Osteogenesis Differentiation of mBMSCs on 3D-Printed Porous Ti6Al4V-10%Sr-CaP Scaffolds In Vitro
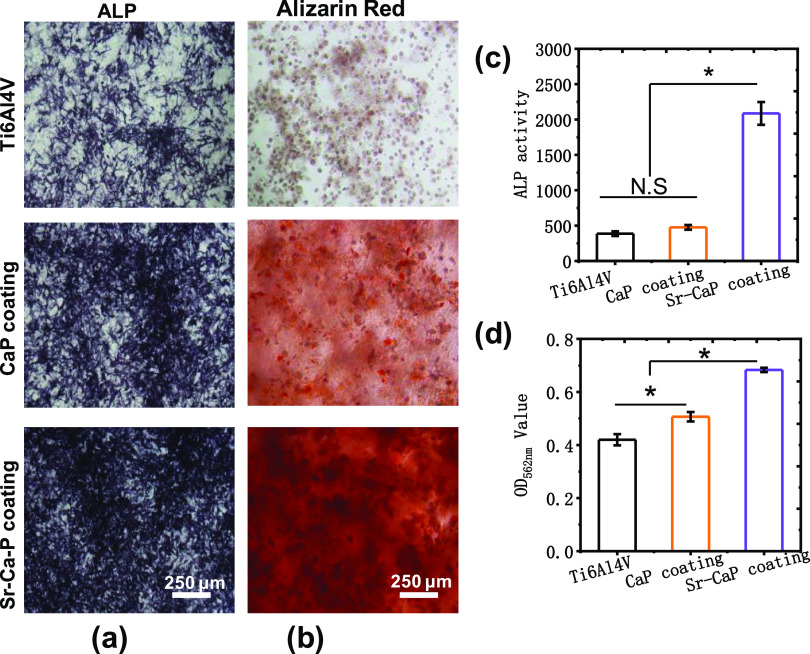
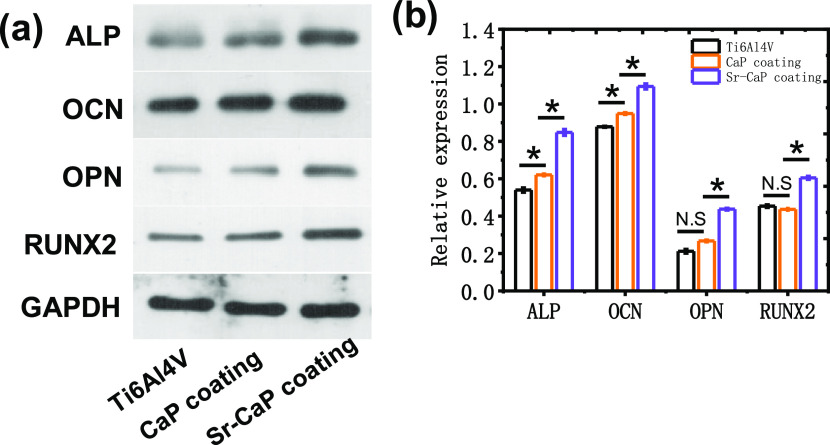
Figure 6 shows the ALP staining, alizarin red staining, and the corresponding quantitative analysis of ALP activities, and calcium ion deposition for cmBMSCs on various Ti6Al4V scaffolds after 14 days of culture. The staining of ALP activity tests discovered a statistical difference in ALP activity after 14 days compared to the other groups (Figure 6a,c). Also, the osteogenesis of Ti6Al4V-10% Sr-CaP scaffolds using alizarin red staining indicated that Ti6Al4V-10% Sr-CaP scaffolds had higher calcium ion deposition than other groups, as indicated by the analysis in Figure 6b and d. To further detect the expression of osteogenesis differentiation, the gene expression of osteogenesis differentiation of mBMSCs on Ti6Al4V-10%Sr-CaP scaffolds, including ALP, COL-1, OCN, OPN, and RUNX2, were tested after 7, 14, and 21 days of culture (Figure 7a–e). The gene expression of ALP was showed an obvious increasing expression after 7 and 14 days of culturing in the Ti6Al4V-10% Sr-CaP scaffolds, and the Sr-containing group showed the highest expression level (Figure 7a). However, the ALP expression of all groups decreased after 21 days of culture. As for the OCN and OPN genes, no significant difference could be found, but substantial upregulation of the OCN and OPN genes was detected between Ti6Al4V-10% Sr-CaP scaffolds and other groups after 14 and 21 days (Figure 7b,c). In the case of the COL-1 gene, the Sr-containing group exhibited the highest expression of COL-1, which was then downregulated after 14 and 21 days of culture. Regarding RUNX2, a higher expression level of the Sr-containing group was found on 7 and 14 days (Figure 7d,e). These results revealed that doping Sr into coating could favor the osteogenesis differentiation, which was also further confirmed by a Western blot test. The Western blot test showed that these associated proteins (ALP, COL-1, OCN, OPN, and RUNX2) in mBMSCs grown on Ti6Al4V-10% Sr-CaP scaffolds followed the same pattern as the quantitative real-time polymerase chain reaction (qRT-PCR) results (Figure 8a,b). Therefore, the above findings indicated that Sr could promote the osteogenic ability of scaffolds in vitro.
Figure 6.
(a) ALP staining, (b) alizarin red staining, (c) quantitative analysis of ALP activities, and (d) calcium ion deposition for cmBMSCs on various Ti6Al4V scaffolds after 14 days of culture (n = 3). N.S. means P ≥ 0.05. *P < 0.05.
Figure 7.
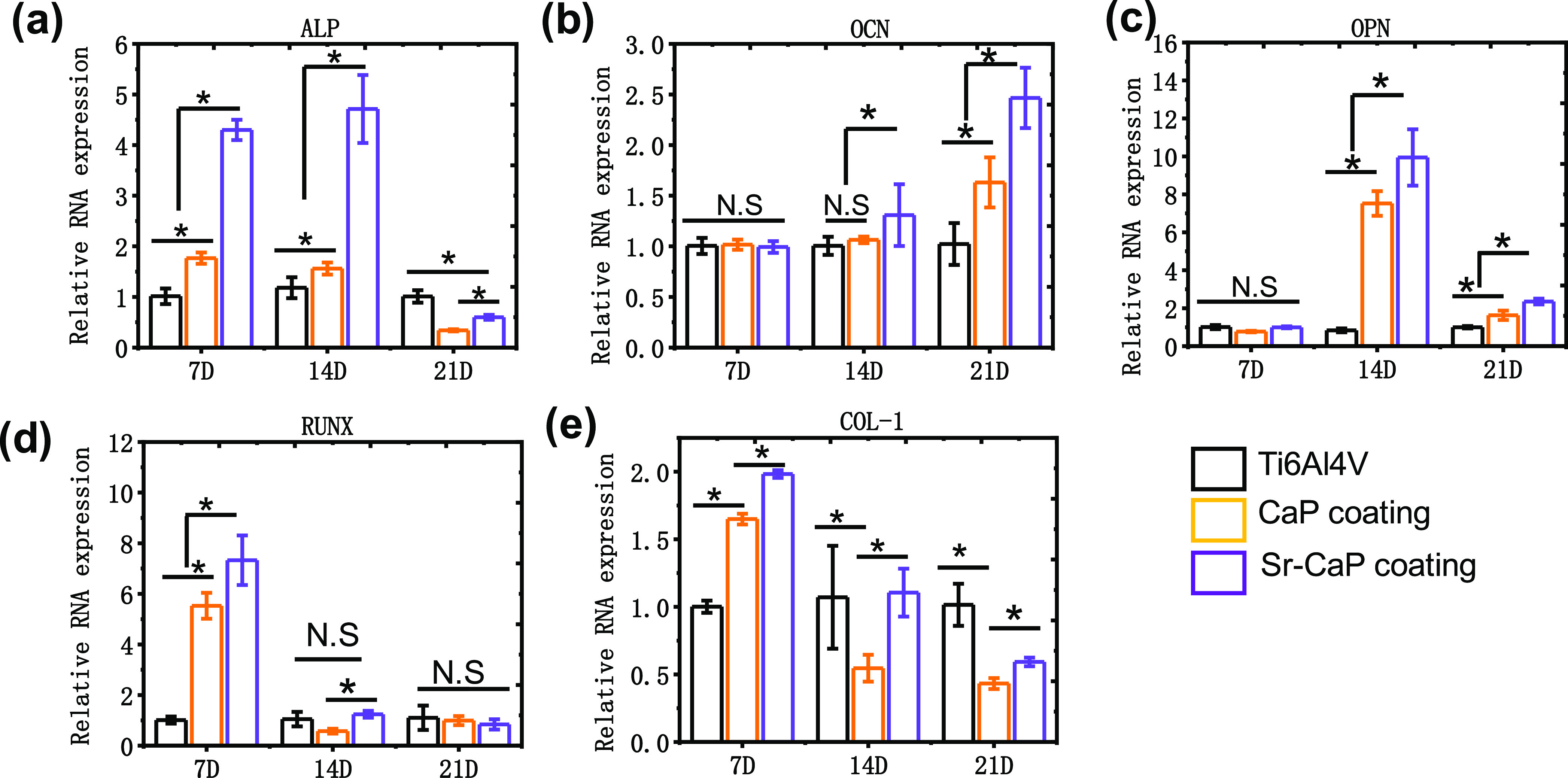
Real-time polymerase chain reaction (PCR) test for mBMSCs grown on various Ti6Al4V scaffolds after 7, 14, and 21 days: (a) ALP, (b) OCN, (c) OPN, (d) RUNX2, and (e) COL-1 (n = 3). N.S. means P ≥ 0.05. *P < 0.05.
Figure 8.
(a) Western blot assay and (b) its corresponding quantitative analysis of mBMSCs cultured on different Ti6Al4V scaffolds after 14 days (n = 3). N.S. means P ≥ 0.05. *P < 0.05.
2.4. 3D-Printed Ti6A14V Scaffolds with the Sr-CaP Coating Promote Bone Regeneration In Vivo
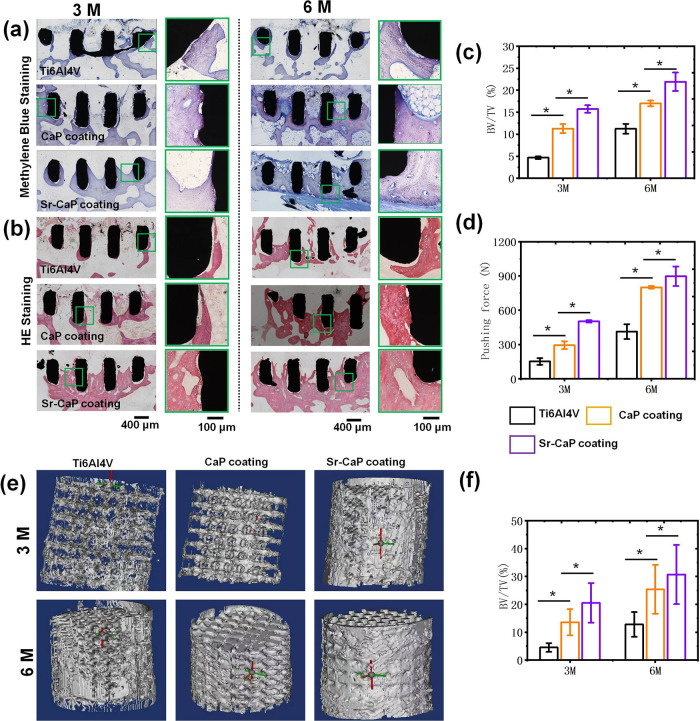
Furthermore, we studied the osteogenesis characteristics of different Ti6A14V scaffolds in vivo using the New Zealand rabbit femur defect model of bone and the hard tissue sections for methylene blue, HE staining. The corresponding quantitative analysis is displayed in Figure 9. The findings of the hard tissue sections for methylene blue and HE staining (Figure 9a,b) after 3 and 6 months of implanting revealed that more new bone tissue was formed in Ti6Al4V-10%Sr-CaP scaffolds compared with the other groups. The quantitative analysis of methylene blue in Figure 9c indicates that the BV/TV of Ti6Al4V-10%Sr-CaP scaffolds was greater than that of the other groups after 3 and 6 months of implantation. Also, the results from the pushing force test for different Ti6A14V scaffolds suggested that the pushing force of all groups increased when the implanting period was extended, and the Ti6Al4V-10% Sr-CaP scaffolds presented the greatest pushing force with respect to the other groups (Figure 9d). Micro-CT analysis was the most straightforward approach for evaluating peri- and interimplant bone tissue development. The outcomes from the micro-CT analysis and the BV/TV result further indicated that an excessive amount of new bone was generated in the Ti6Al4V-10% Sr-CaP scaffolds (Figure 9e,f). Overall, the in vivo assay demonstrated that the Ti6Al4V-10% Sr-CaP scaffolds had the highest osteogenic activity for bone defect healing.
Figure 9.
(a, b) After three and six months of implanting, hard tissue slices were stained with methylene blue and HE staining. (c) BV/TV ratio was quantified using ImageJ (n = 6). (d) Pushing force of various Ti6Al4V scaffolds with and without coatings after three and six months of implantation. (e) 3D micro-CT of different Ti6Al4V scaffolds after implanting for 3 and 6 months. (f) Results of BV/TV (n = 4). N.S. means P ≥ 0.05. *P < 0.05.
3. Discussion
Titanium (Ti) has been frequently utilized in implants because of its outstanding mechanical property and biocompatibility.1 To validate the feasibility of our approach, we employed orthopedic Ti6Al4V implants as substrate materials. There were several ways for preparing porous Ti6Al4V scaffolds, such as metal injection moulding26 and polymer space scaffolding,27 and so on, but traditional technology was unable to create porous scaffolds with consistent micropores and pore sizes.3 Surprisingly, additive manufacturing (3D printing) technologies such as SLM could precisely control the micropore and pore width of scaffolds.5 The SLM technique is commonly utilized in the fabrication of porous metal scaffolds,28 e.g., Ti alloys29 and magnesium (Mg) alloys.30 The SLM technology was utilized to print Ti6Al4V scaffolds with ideal pore size. The outcomes from the compressive yield strength (300 MPa) and elastic modulus (5.19 GPa) showed that the 3D-printed Ti6Al4V scaffolds combined good mechanical properties and the biofuction of promoting bone growth by reducing stress shielding. Moreover, 3D-printed porous structures could well promote bone growth by increasing the contact area between the implant and bone by favoring the flow of oxygen and nutrients. The presence of pores could support the bone tissue to grow into the scaffold. Consequently, good osseointegration could be generated via a strong enough three-dimensional mechanical lock between the 3D-printed porous structure and tissues.
In addition, the surface property is a key factor for determining the adhesion and migration behavior of cells on the implants. Although the porous scaffolds constructed by 3D-printing technology could address the mismatch of elastic modulus between the titanium-based implant and human bone, the failure of cell adhesion induced by the inert nature of titanium-based alloys also is one main issue. This is because cell adhesion is greatly associated with the surface morphology and composition of the implant. After implantation, biomaterial surfaces directly interacted with cells and tissues, substantially influencing a variety of cellular activities such as adhesion,31 spreading,32 migration,33 proliferation,34 and differentiation,35 as well as the result of tissue repair and regeneration.36 Therefore, the implant surface biological properties also should be considered during the development and design of implants as well as the suitable porous structure37,38 and mechanical characteristics.39 Given this, extensive studies concerning surface modification on titanium-based alloys have been conducted to improve the cytocompatibility, such as producing CaP-based coating on the Ti6Al4V scaffolds,40 but limited studies have been conducted to modify the surface of Ti6Al4V scaffolds using Sr ion doping. Sr, as one of the most important trace elements, has the ability to both stimulate new bone growth and prevent bone resorption.41 Recently, most studies have found that Sr can be used in place of calcium silicate to increase cell survival and expression of osteoblast-related genes to a certain level.42 The inclusion of Sr may effectively improve the biological osteogenic properties of hard tissue repair implanting materials.29,43 As a result, it is reasonable to assume that bioactive coatings doped with Sr are beneficial for the development of novel bioactive biomaterials, particularly for bone tissue production and regeneration. Peng et al. displayed that Sr ions activated all kinds of signaling pathways to improve their biological functions, such as the Ras/MAPK signaling pathway.44 Another study indicated that it could bind with calcium-sensing receptors and then activate the MAPK/Erk 1/2 signaling pathway to stimulate osteogenesis.45 Furthermore, it was reported that Sr exhibited angiogenic capability by increasing vascular endothelial growth factor (VEGF) expression in the dermal fibroblasts,46 which released vasodilator nitric oxide (NO) by activating the PI3K and MAPK downstream pathways and then upregulating prostacyclin (PGI2) and endothelial nitric oxide synthase (eNOS).47 In this study, it is found that the Sr-doped CaP layer dominated by CaHPO4 was successfully produced on the 3D-printed Ti6Al4V scaffolds via the hydrothermal method, which possessed ideal bonding strength between the coating and substrate (Figure 1c). The in vitro results revealed that the Sr-CaP coating showed excellent biocompatibility to mBMSCs. This was confirmed by the upregulated expression of osteogenic genes and proteins (Figures 7 and 8), which showed that the osteogenic property of mBMSCs on the Ti6Al4V-10%Sr-CaP scaffolds was considerably greater than that of the Ti6Al4V and Ti6Al4V-CaP scaffolds. Furthermore, the in vivo investigation also demonstrated that the Ti6Al4V-10%Sr-CaP scaffolds could promote more growth of new bone tissue into scaffolds (Figure 9a–c). This behavior then led to better osseointegration than that of the other groups, as indicated by the pushing force (Figure 9d), which was found to be the highest pushing in the Ti6Al4V-10%Sr-CaP scaffolds. These findings suggested that the Sr-CaP-coated Ti6Al4V scaffolds could increase their osteogenic bioactivity.
4. Conclusions
In this work, the Sr-CaP coating was effectively produced on the Ti6Al4V scaffolds with controlled porosity and low elastic modulus of 5.19 GPa via the hydrothermal method. The main phase of the coatings with and without Sr doping was CaHPO4, which could well bond to the substrate. The bonding strength of both coatings met the required ISO 13 779-4-2018 standard. The in vitro demonstrated that the Ti6Al4V-10% Sr-CaP scaffolds showed good biocompatibility and osteogenic activity, while the in vivo suggested that the Ti6Al4V-10%Sr-CaP scaffolds could promote the growth of new bone tissue into scaffolds. These findings indicate that 3D-printed Ti6Al4V scaffolds with the Sr-CaP coatings are a promising biomaterial for bone growth and regeneration.
5. Materials and Methods
5.1. Materials
Ti6Al4V powders were obtained from AP&C, Sweden. Cell counting kit-8 (CCK-8) was obtained from Dojindo, Japan. The mice bone mesenchymal stem cells (mBMSCs) were obtained from ATCC (Invitrogen). Calcein-AM/PI was purchased from Molecular Probes. All of the cell experiment reagents were bought from Gibco, Sigma, and Beyotime Biotechnology Ltd. (China). Guangzhou Chemical Reagent Factory (Guangzhou, China) supplied all of the additional chemicals required to produce the Ti6Al4V scaffolds (Guangzhou, China).
5.2. Preparation and Characterization of Scaffolds
Designing and Manufacturing Porous Ti6A14V Scaffold
At the start of the study, computer-assisted design (CAD) software was used to create a model of a porous Ti6A14V scaffold with an aperture diameter of 700 μm, a rod diameter of 400 μm, and a porosity of 62.5%. Two types of porous Ti6A14V scaffolds were created for in vitro and in vivo testing (Figure 10 taken by ShenghuiSu). The scaffolds used in the in vitro experiment were cuboid (10 mm × 10 mm × 3 mm), whereas the scaffolds used in the in vivo experiment were cylindrical (Φ 7 mm × 8 mm). The data was then transferred into the computer terminal of a selective laser melting (SLM) metal printer (Renishaw, U.K.), and the prepared Ti6Al4V powder and substrate were fed into the machine. After preheating the substrate, layer-by-layer printing began. When the current layer sintering was finished, the laser sintering process was likewise finished, and the substrates were shifted down to 30 μm. For 30 μm, the equipment was repainted. The sintering process was then proceeded according to the design scheme for the following layers, and the porous Ti6Al4V scaffold was produced layer by layer. The interior atmosphere of the equipment was constantly shielded by high-purity argon throughout this procedure. Finally, the scaffold materials were washed for 15 min each using acetone, deionized water, and 100% ethanol in an ultrasonic washing machine.
Figure 10.
3D-printed Ti6Al4V scaffolds.
Preparation of the Sr-Doped CaP Coating on Porous Ti6A14V Scaffolds
CaCl2, SrCl2, NaH2PO4, and Na2EDTA·2H2O (without SrCl2 in the CaP group) were sequentially added into 25 mL of deionized water with continuous stirring for 30 min until the solution became clear at room temperature. The solution pH was adjusted to 3.5 by adding HCl and NH3·H2O. Then, 15 mL of the prepared solution and Ti6Al4V porous scaffolds were placed into a 25 mL poly(tetrafluoroethylene) lining stainless steel reactor. The reactor was then sealed and placed in an oven that was preheated to various temperatures for 16 h. The reactor was removed from the oven and cooled to room temperature in the air after the reaction. After removing from the reactor, the samples were gently rinsed with deionized water and finally dried in an oven at 70 °C. Finally, after the bioactive coating on the porous Ti6A14V scaffold materials, the scaffold material was cleaned in an ultrasonicator with acetone and anhydrous ethanol, respectively, for 15 min. The cleaned samples were then sterilized using 60Co irradiation.
Characterization of Porous Ti6A14V Scaffolds
To investigate the mechanical characteristics of porous scaffolds with varying pore structures, we utilized an electromechanical universal testing machine (MTS) (WDW-10, OZ, Guangzhou, China) at room temperature at a speed of 1 mm/min. The maximum compressive strength of Ti6Al4V scaffolds was determined by multiplying the highest load by the cross-sectional area. The linear area of the stress–strain curve was used to determine the elastic modulus. The porous Ti6A14V scaffolds and porous Ti6A14V scaffolds modified with bioactive coating were analyzed by high-resolution field emission scanning electron microscopy (S-4800, HITACHI, Japan) and energy-dispersive X-ray spectroscopy (Rigaku D/max 2500PC, Japan). Prior to SEM imaging, the porous Ti6A14V scaffolds were coated with gold using an E-1010 sputtering equipment. The contact angles of the coating were detected using a contact angle meter (DSA-25E, Germany).
Bonding Test
According to ISO 13 779-4-2018 and the reference,48 a universal testing machine was used to evaluate the bonding strength between the coating and the substrate. The tested specimen was bonded on the head of chucking using an E-7 glue. Then, the specimen with chucking was fixed on the universal testing machine and applied to tensile tests with a speed of 2 mm/min.
Immersion Test
The immersion test was conducted in a Dulbecco’s modified Eagle’s medium (DMEM) solution at 37 °C in an oven to detect the concentration of Ti, Sr, and Ca ions released from samples with and without coatings. The ratio of weight to solution volume (mL) is 0.1 g/ml according to ISO 10993-12:2012. After 1, 4, and 7 days of immersion, the concentration of Ti, Sr, and Ca ions in solution was measured using an inductively coupled plasma atomic emission spectrometer (ICP-AES, Ultima 2).
5.3. In Vitro Cell Assay
Cell Culture
The mBMSCs were cultured with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum in a 5% CO2 incubator. Passages of 3–5 were used prior to any cell assay.
Selection of Sr Concentration Assay
For 1, 4, and 7 days, the extracting solutions of different Sr content in CaP-coated Ti6Al4V scaffolds were placed into a 24-well plate with 5 × 103 CFU mBMSCs. After that, 10 μL of the CCK-8 working solution was added to each well for 3 h. Finally, 100 μL of the solution was added to the 96-well plate for absorbance measurement at a wavelength of 450 nm.
Cell Live/Dead Assay and CCK-8 Assay
Prior to cell seeding, the extracting solutions of different Ti6Al4V scaffolds were initially placed into a 24-well plate. Furthermore, 5 × 103 CFU was added to each well for the cell live/dead assay. After 1, 4, and 7 days, each well was dyed with a calcein-AM/PI working solution for 15 min in an incubator. Following that, each well was washed twice with PBS and examined using the FITC and PI channels (SP-8, Leica, Germany). Additionally, we used CCK-8 tests to demonstrate the biocompatibility, after 1, 4, and 7 days. In each well, 10 μL of the CCK-8 working solution was added. After 3 h of culture, 100 μL of the solution was added into the 96-well plate for absorbance measurement at 450 nm.
Cell Morphology Assay
After co-culturing with the extracting solutions of different Ti6Al4V scaffolds, the morphology of mBMSCs was characterized after 1 day. The cells in each well were washed with PBS twice and fixed with 4 vol % neutral paraformaldehyde solutions for 60 min at 4 °C. The following solutions of 0.1% Triton, F-actin, and 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) were added and incubated for 10, 60, and 8 min, respectively. Finally, the cells in each well were washed with PBS twice. The fixed cells were then visualized using fluorescence microscopy (SP-8, Leica, Germany). Meanwhile, 5 × 104 cells/ml were cultured on samples in 24-well plates and for 1 day and subsequently rinsed with PBS. Thereafter, the cell microfilament was stained by 1.0% (v/v) FITC-phalloidin dye for 30 min, and then nuclei were stained by 1 mg/mL DAPI for 10 min at 37 °C. The cell morphology was analyzed by a fluorescence microscope.
Alkaline Phosphatase (ALP) Assay
The extracting solutions of different Ti6Al4V scaffolds were cocultured with 5 × 103 CFU mBMSCs in 24-well plates for 14 days. To quantify the amount of ALP, the cells were lysed with a Beyotime RIPA lysis buffer. The lysed cell solutions were then centrifuged at 8000 rcf for 3 min, and the supernatants were further collected. The ALP activity was calculated by dividing the BCA proteins with cell numbers. After 14 days of culture, 200 μL of ALP staining solutions was added to each well and incubated in the dark for 30 min at 37 °C. Finally, each well was observed with fluorescence microscopy in a bright field (SP-8, Leica, Germany).
Alizarin Red (ARS) Assay
Steps similar to those used in the ALP assay were used in the ARS assay. After 14 days of culture, 4 vol % neutral paraformaldehyde solutions were added to each well and incubated at 4 °C for 60 min. Following that, 200 μL of alizarin reds was placed in the wells to stain for 5 min before being rinsed with deionized water to remove the stain. Fluorescence microscopy in a bright field was used to inspect the wells (SP-8, Leica, Germany). For the quantitative alizarin red assay, 500 μL of 10% hexadecyl pyridinium chloride was added to the stained wells for 30 min. Further, 100 μL of the solution was added to the 96-well plate for 562 nm absorbance measurement.
qRT-PCR Assay
In 24-well plates, the extracting solution of Ti6Al4V samples was incubated with 5 × 103 CFU of mBMSCs. qRT-PCR was used to detect osteogenic markers (RUNX2, ALP, OCN, COL-1, and OPN) after 7, 14, and 21 days of culture. In brief, we quantified the isolated mRNA using the NanoDrop2000 device (Thermo). After reverse transcription of RNA into complementary DNA (cDNA) using the PrimeScriptTM DRR047A (TaKaRa, Japan) reagent Kit, cDNA was amplified using the two-step cycling settings of a QuantStudio 6 Flex system (Life Technologies). The primer sequences used for qRT-PCR gene expression analysis are listed in Table 1.
Table 1. Primer Sequences Used for qRT-PCR Gene Expression Analysis.
| genes | 5′-3′ | primer sequences |
|---|---|---|
| GAPDH | sense | 5′-AAATGGTGAAGGTCGGTGTGAAC-3′ |
| antisense | 5′-CAACAATCTCCACTTTGCCACTG-3′ | |
| RUNX2 | sense | 5′-TGCAAGCAGTATTTACAACAGAGG-3′ |
| antisense | 5′-GGCTCACGTCGCTCATCTT-3′ | |
| COL-I | sense | 5′-GACATGTTCAGCTTTGTGGACCTC-3′ |
| antisense | 5′-GGGACCCTTAGGCCATTGTGTA-3′ | |
| ALP | sense | 5′-GCAGTATGAATTGAATCGGAACAAC-3′ |
| antisense | 5′-ATGGCCTGGTCCATCTCCAC-3′ | |
| OPN | sense | 5′-TACGACCATGAGATTGGCAGTGA-3′ |
| antisense | 5′-TATAGGATCTGGGTGCAGGCTGTAA-3′ | |
| OCN | sense | 5′-AGCAGCTTGGCCCAGACCTA-3′ |
| antisense | 5′-TAGCGCCGGAGTCTGTTCACTAC-3′ |
Western Blot Assay
After extracting the Ti6Al4V samples, they were cultivated for 7, 14, and 21 days with mBMSCs. The BCA Protein Assay Kit was used to quantify the RUNX2, ALP, OCN, and OPN proteins in mBMSCs. Following that, the proteins were transferred to poly(vinylidene fluoride) membranes and incubated for 2 h at 37 °C with primary antibodies against proteins ((RUNX2: Affinity Biosciences), (ALP: Proteintech, Chicago), (OCN: Affinity Biosciences), and (OPN: Affinity Biosciences)). Following three rounds of PBST washing, the membranes were labeled with a second antibody (IgG (H&L) HRP, Proteintech, Chicago) specific for RUNX2, ALP, OCN, and OPN.
5.4. In Vivo Assay
Animal Survey
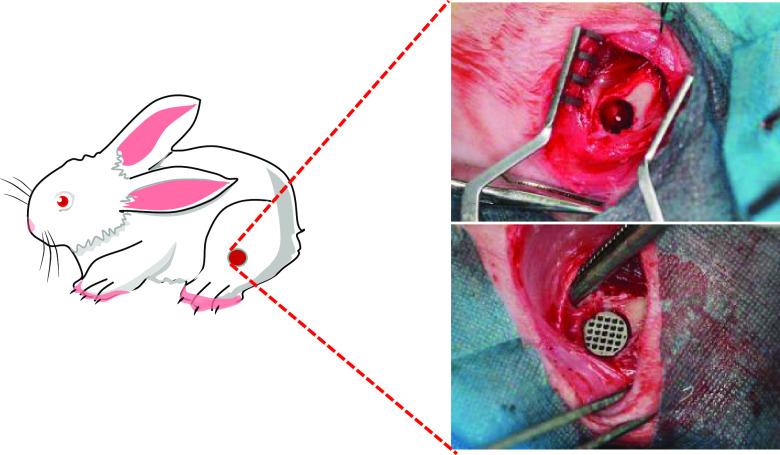
All animal treatments were authorized by South Medical University’s Institutional Animal Care and Use Committee (Guangzhou, China, No.NFYY-2019-237). For the in vivo experiment, 12-week-old New Zealand white rabbits weighing 1.5–2 kg were used. Before implantation, all rabbits were anesthetized with isoflurane at a rate of 3 mL/min. We investigated the osseointegration of the various Ti6Al4V scaffolds in vivo using a bone defect model. Typically, a single hole was made in the distal femur (Φ 7 mm × 8 mm), and the various Ti6Al4V scaffolds were implanted (Figure 11 taken by ShenghuiSu).
Figure 11.
Schematic representation of the surgical procedures.
Push Out Force Assay
The Ti6Al4V samples were cemented in a specific mold using denture powder after 3 and 6 months. The metal implant’s long axis was perpendicular to the MTS’s push rod (WDW-10, OZ, Guangzhou, China). To determine the maximal pushing force, the implants were pushed out of the bone tissue at a rate of 1 mm/min.
Micro-CT Scanning
To identify osteogenesis, the peri- and interimplant bone development was studied using micro-CT, and the specimens were rebuilt and evaluated using mimics.
Pathological Analysis
Methylene blue and hematoxylin-eosin (HE) staining were used to color the femurs with implants. The development of new bone in several types of porous Ti6Al4V scaffolds was detected in bright-field using a fluorescent microscope (SP-8, Leica, Germany). The whole unified region was imaged. Additionally, we applied ImageJ 2.0 analysis to manage the gathered pictures and determine the area % of new bone in the whole Ti6Al4V scaffolds. We calculated the area percentage of new bone in scaffolds using bone volume/total volume (BV/TV).
5.5. Statistics and Reproducibility
All results presented here were collected through at least three separate experiments. SPSS 20.0 was used to determine the statistical significance. P < 0.05 was considered as a statistically significant difference.
Acknowledgments
This study was supported by the National Engineering Research Center for Tissue Restoration and Reconstruction, South China University of Technology, Guangzhou, China.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05908.
Information about the optimal content of Sr in the coatings and the attached behavior of cells on the scaffolds (PDF)
This work was financially supported by the National Natural Science Foundation of China (Grant No. 81272022) and the Innovation Foundation of Zengcheng, Guangzhou, China (Grant No. 185. 2019).
The authors declare no competing financial interest.
Notes
⊥ S.S. and W.C. contributed equally to this work.
Supplementary Material
References
- a Bosshardt D. D.; Chappuis V.; Buser D. Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions. Periodontol 2000 2017, 73, 22–40. 10.1111/prd.12179. [DOI] [PubMed] [Google Scholar]; b Geetha M.; Singh A. K.; Asokamani R.; Gogia A. K. Ti based biomaterials, the ultimate choice for orthopaedic implants – A review. Prog. Mater. Sci. 2009, 54, 397–425. 10.1016/j.pmatsci.2008.06.004. [DOI] [Google Scholar]
- a Lykov P. A.; Baitimerov R. M.; Panfilov A. V.; Guz A. O. The manufacturing of TiAl6V4 implants using selective laser melting technology. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 248, 012004 10.1088/1757-899x/248/1/012004. [DOI] [Google Scholar]; b Yang J.; Yu X.; Zhang Z.; Xu R.; Wu F.; Wang T.; Liu Y.; Ouyang J.; Deng F. Surface modification of titanium manufactured through selective laser melting inhibited osteoclast differentiation through mitogen-activated protein kinase signaling pathway. J. Biomater. Appl. 2020, 35, 169–181. 10.1177/0885328220920457. [DOI] [PubMed] [Google Scholar]
- Duan M.; Wu X.; Yuan L.; Zhang Z.; Zhang Y.; Zhou Y. Fabrication and In vitro Bioactivity of Robust Hydroxyapatite Coating on Porous Titanium Implant. Chem. Res. Chin. Univ. 2019, 35, 686–692. 10.1007/s40242-019-9101-x. [DOI] [Google Scholar]
- Sidambe A. T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Mater 2014, 7, 8168–8188. 10.3390/ma7128168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov I. V.; Deev R. V.; Bozo I. I.; Fedotov A. Y.; Gurin A. N.; Mamonov V. E.; Kravchuk A. D.; Popov V. K.; Egorov A. A.; Komlev V. S. Octacalcium phosphate coating for 3D printed cranioplastic porous titanium implants. Surf. Coat. Technol. 2020, 383, 125192 10.1016/j.surfcoat.2019.125192. [DOI] [Google Scholar]
- Park J.-H.; Odkhuu M.; Cho S.; Li J.; Park B.-Y.; Kim J.-W. 3D-printed titanium implant with pre-mounted dental implants for mandible reconstruction: a case report. Maxillofac. Plast. Recons. 2020, 42, 28 10.1186/s40902-020-00272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jandt K. D.; Finke M.; Cacciafesta P. Aspects of the physical chemistry of polymers, biomaterials and mineralised tissues investigated with atomic force microscopy (AFM). Colloid Surface B 2000, 19, 301–314. 10.1016/S0927-7765(00)00139-9. [DOI] [PubMed] [Google Scholar]; b Kargozar S.; Ramakrishna S.; Mozafari M. Chemistry of biomaterials: future prospects. Curr. Opin Biomed. Eng. 2019, 10, 181–190. 10.1016/j.cobme.2019.07.003. [DOI] [Google Scholar]; c Ke Y.; Liu C.; Zhang X.; Xiao M.; Wu G. Surface Modification of Polyhydroxyalkanoates toward Enhancing Cell Compatibility and Antibacterial Activity. Macromol. Mater. Eng. 2017, 302, 1700258 10.1002/mame.201700258. [DOI] [Google Scholar]; d Chang S.-H.; Hsiao Y.-C. Surface and Protein Adsorption Properties of 316L Stainless Steel Modified with Polycaprolactone Film. Polymers 2017, 9, 545 10.3390/polym9100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Tian Y.; Ding S.; Peng H.; Lu S.; Wang G.; Xia L.; Wang P. Osteoblast growth behavior on porous-structure titanium surface. Appl. Surf. Sci. 2012, 261, 25–30. 10.1016/j.apsusc.2012.07.035. [DOI] [Google Scholar]; b Elias C. N.; Rocha F. A.; Nascimento A. L.; Coelho P. G. Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J. Mech. Behav. Biomed. Mater 2012, 16, 169–180. 10.1016/j.jmbbm.2012.10.010. [DOI] [PubMed] [Google Scholar]; c Gittens R. A.; McLachlan T.; Olivares-Navarrete R.; Cai Y.; Berner S.; Tannenbaum R.; Schwartz Z.; Sandhage K. H.; Boyan B. D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy Y.; Wu C.; Zreiqat H. Orthopedic coating materials: considerations and applications. Expert Rev. Med. Devices 2009, 6, 423–430. 10.1586/erd.09.17. [DOI] [PubMed] [Google Scholar]
- a Wu C.; Chen Z.; Yi D.; Chang J.; Xiao Y. Multidirectional Effects of Sr-, Mg-, and Si-Containing Bioceramic Coatings with High Bonding Strength on Inflammation, Osteoclastogenesis, and Osteogenesis. ACS Appl. Mater. Interfaces 2014, 6, 4264–4276. 10.1021/am4060035. [DOI] [PubMed] [Google Scholar]; b Wu C.; Chen Z.; Wu Q.; Yi D.; Friis T.; Zheng X.; Chang J.; Jiang X.; Xiao Y. Clinoenstatite coatings have high bonding strength, bioactive ion release, and osteoimmunomodulatory effects that enhance in vivo osseointegration. Biomaterials 2015, 71, 35–47. 10.1016/j.biomaterials.2015.08.027. [DOI] [PubMed] [Google Scholar]; c Huang Y.; Wu C.; Zhang X.; Chang J.; Dai K. Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration. Acta Biomater. 2018, 66, 81–92. 10.1016/j.actbio.2017.08.044. [DOI] [PubMed] [Google Scholar]
- Qiao S.; Sheng Q.; Li Z.; Wu D.; Zhu Y.; Lai H.; Gu Y. 3D-printed Ti6Al4V scaffolds coated with freeze-dried platelet-rich plasma as bioactive interface for enhancing osseointegration in osteoporosis. Mater. Des. 2020, 194, 108825 10.1016/j.matdes.2020.108825. [DOI] [Google Scholar]
- Chudinova E. A.; Surmeneva M. A.; Timin A. S.; Karpov T. E.; Wittmar A.; Ulbricht M.; Ivanova A.; Loza K.; Prymak O.; Koptyug A.; et al. Adhesion, proliferation, and osteogenic differentiation of human mesenchymal stem cells on additively manufactured Ti6Al4V alloy scaffolds modified with calcium phosphate nanoparticles. Colloid Surface B 2019, 176, 130–139. 10.1016/j.colsurfb.2018.12.047. [DOI] [PubMed] [Google Scholar]
- a Su Y.; Cockerill I.; Zheng Y.; Tang L.; Qin Y.-X.; Zhu D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bio Mater. 2019, 4, 196–206. 10.1016/j.bioactmat.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hwang J.-W.; Lee E.-U.; Lee J.-S.; Jung U.-W.; Lee I.-S.; Choi S.-H. Dissolution behavior and early bone apposition of calcium phosphate-coated machined implants. J. Periodontal Implant Sci 2013, 43, 291–300. 10.5051/jpis.2013.43.6.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez M. A.; Farooq A.; Zang A.; Saleem A.; Deen K. M. Phosphate chemical conversion coatings for magnesium alloys: a review. J. Coat. Technol. Res. 2020, 17, 827–849. 10.1007/s11998-020-00335-2. [DOI] [Google Scholar]
- Ma J.; Wang C.; Huang B.; Zhao X.; Chen C.; Yu H. In vitro degradation and apatite formation of magnesium and zinc incorporated calcium silicate prepared by sol-gel method. Mater. Technol. 2021, 36, 420–429. 10.1080/10667857.2020.1762352. [DOI] [Google Scholar]
- Zhao D.-W.; Liu C.; Zuo K.-Q.; Su P.; Li L.-B.; Xiao G.-Y.; Cheng L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chem. Eng. J. 2021, 408, 127362 10.1016/j.cej.2020.127362. [DOI] [Google Scholar]
- Atkins G. J.; Welldon K. J.; Halbout P.; Findlay D. M. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporosis Int. 2009, 20, 653–664. 10.1007/s00198-008-0728-6. [DOI] [PubMed] [Google Scholar]
- Liang W.; Li L.; Cui X.; Tang Z.; Wei X.; Pan H.; Li B. Enhanced Proliferation and Differentiation Effects of a CGRP- and Sr-Enriched Calcium Phosphate Cement on Bone Mesenchymal Stem Cells. J. Appl. Biomater. Funct. Mater. 2016, 14, 431–440. 10.5301/jabfm.5000295. [DOI] [PubMed] [Google Scholar]
- Wang R.; Song F.; Li S.; Ding L.; Xu Z.; Zhang B.; Wang J.; Li M.; Liu B.; Qiu Y. Polydopamine-assisted Sr immobilization to improve the osteogenesis of a three-dimensional reduced graphene oxide/polypyrrole composite scaffold. Mater. Lett. 2019, 246, 182–185. 10.1016/j.matlet.2019.03.065. [DOI] [Google Scholar]
- Hurtel-Lemaire A. S.; Mentaverri R.; Caudrillier A.; Cournarie F.; Wattel A.; Kamel S.; Terwilliger E. F.; Brown E. M.; Brazier M. The Calcium-sensing Receptor Is Involved in Strontium Ranelate-induced Osteoclast Apoptosis: New Insights into the Associated Signaling Pathways. J. Biol. Chem. 2009, 284, 575–584. 10.1074/jbc.M801668200. [DOI] [PubMed] [Google Scholar]
- a Hu P.; Du J.; Zhang S.; Wang T.; Li J.; Chen G.; Zhou G. Oral Administration of Strontium Gluconate Effectively Reduces Articular Cartilage Degeneration Through Enhanced Anabolic Activity of Chondrocytes and Chondrogenetic Differentiation of Mesenchymal Stromal Cells. Biol. Trace Elem. Res. 2020, 193, 422–433. 10.1007/s12011-019-01711-9. [DOI] [PubMed] [Google Scholar]; b Zhang Y.; McGillicuddy F. C.; Hinkle C. C.; O’Neill S.; Glick J. M.; Rothblat G. H.; Reilly M. P. Adipocyte Modulation of High-Density Lipoprotein Cholesterol. Circulation 2010, 121, 1347–1355. 10.1161/CIRCULATIONAHA.109.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejón-Alonso L.; Mochales C.; Nascimento L.; Müller W.-D. Electrochemical deposition of Sr and Sr/Mg-co-substituted hydroxyapatite on Ti-40Nb alloy. Mater. Lett. 2019, 248, 65–68. 10.1016/j.matlet.2019.03.141. [DOI] [Google Scholar]
- Wu C.; Ramaswamy Y.; Kwik D.; Zreiqat H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials 2007, 28, 3171–3181. 10.1016/j.biomaterials.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Özarslan A. C.; Yücel S. Fabrication and characterization of strontium incorporated 3-D bioactive glass scaffolds for bone tissue from biosilica. Mater. Sci. Eng., C 2016, 68, 350–357. 10.1016/j.msec.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Bigi A.; Boanini E.; Capuccini C.; Gazzano M. Strontium-substituted hydroxyapatite nanocrystals. Inorg. Chim. Acta 2007, 360, 1009–1016. 10.1016/j.ica.2006.07.074. [DOI] [Google Scholar]
- Wiranegara H.; Syahputra D.; Arief T. M. Injection process parameter analysis of metal injection molding for green part orthopedic implants. IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1034, 012177 10.1088/1757-899x/1034/1/012177. [DOI] [Google Scholar]
- Dean D.; Mott E.; Luo X.; Busso M.; Wang M. O.; Vorwald C.; Siblani A.; Fisher J. P. Multiple initiators and dyes for continuous Digital Light Processing (cDLP) additive manufacture of resorbable bone tissue engineering scaffolds. Virtual Phys. Prototyping 2014, 9, 3–9. 10.1080/17452759.2013.873337. [DOI] [Google Scholar]
- a Li L.; Chen Y.; Lu Y.; Qin S.; Huang G.; Huang T.; Lin J. Effect of heat treatment on the corrosion resistance of selective laser melted Ti6Al4V3Cu alloy. J. Mater. Res. Technol. 2021, 12, 904–915. 10.1016/j.jmrt.2021.03.041. [DOI] [Google Scholar]; b Lu Y.; Zhao W.; Yang C.; Liu Y.; Xiang H.; Yang K.; Lin J. Improving mechanical properties of selective laser melted Co29Cr9W3Cu alloy by eliminating mesh-like random high-angle grain boundary. Mater. Sci. Eng., A 2020, 793, 139895. 10.1016/j.msea.2020.139895. [DOI] [Google Scholar]
- Zhang S.; Sun X.; Kang C.; Yang M.; Zhao Y.; Wang C. Study on repairing canine mandibular defect with porous Mg–Sr alloy combined with Mg–Sr alloy membrane. Regener. Biomater. 2020, 7, 331–336. 10.1093/rb/rbz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Mao J.; Zhou Y.; Ji F.; Chen X. Mechanical properties and osteoblast proliferation of complex porous dental implants filled with magnesium alloy based on 3D printing. J. Biomater. Appl. 2021, 35, 1275–1283. 10.1177/0885328220957902. [DOI] [PubMed] [Google Scholar]
- Steller D.; Herbst N.; Pries R.; Juhl D.; Klinger M.; Hakim S. G. Impacts of platelet-rich fibrin and platelet-rich plasma on primary osteoblast adhesion onto titanium implants in a bisphosphonate in vitro model. J. Oral Pathol. Med. 2019, 48, 943–950. 10.1111/jop.12944. [DOI] [PubMed] [Google Scholar]
- Carmine M. D.; Toto P.; Feliciani C.; Scarano A.; Tulli A.; Strocchi R.; Piattelli A. Spreading of Epithelial Cells on Machined and Sandblasted Titanium Surfaces: An In Vitro Study. J. Periodontol. 2003, 74, 289–295. 10.1902/jop.2003.74.3.289. [DOI] [PubMed] [Google Scholar]
- Hamilton D. W.; Chehroudi B.; Brunette D. M. Comparative response of epithelial cells and osteoblasts to microfabricated tapered pit topographies in vitro and in vivo. Biomaterials 2007, 28, 2281–2293. 10.1016/j.biomaterials.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Balderrama I. de. F.; Cardoso M. V.; Stuani V. T.; Oliveira R. C.; Matos A. A.; Greghi S. L. A.; Sant’Ana A. C. P. Residual decontamination chemical agents negatively affect adhesion and proliferation of osteoblast-like cells on implant surface. Int. J. Implant Dent. 2020, 6, 84 10.1186/s40729-020-00278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. B.; Zaharias R.; Seabold D.; Keller J.; Stanford C. Differentiation of preosteoblasts is affected by implant surface microtopographies. J. Biomed. Mater. Res., Part A 2004, 69A, 462–468. 10.1002/jbm.a.30016. [DOI] [PubMed] [Google Scholar]
- Erggelet C.; Endres M.; Neumann K.; Morawietz L.; Ringe J.; Haberstroh K.; Sittinger M.; Kaps C. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J. Orthop. Res. 2009, 27, 1353–1360. 10.1002/jor.20879. [DOI] [PubMed] [Google Scholar]
- Pfeifer R.; Hustedt M.; Wesling V.; Hurschler C.; Olender G.; Mach M.; Gösling T.; Müller C. W. Noninvasive induction implant heating: An approach for contactless altering of mechanical properties of shape memory implants. Med. Eng. Phy. 2013, 35, 54–62. 10.1016/j.medengphy.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Lee L. Y.; Ranganath S. H.; Fu Y.; Zheng J. L.; Lee H. S.; Wang C.-H.; Smith K. A. Paclitaxel release from micro-porous PLGA disks. Chem. Eng. Sci. 2009, 64, 4341–4349. 10.1016/j.ces.2009.07.016. [DOI] [Google Scholar]
- Bachour Y.; Oei L. J.; Van der Veen A. J.; Vos B. E.; Louis A.; Heukelom S.; Ritt M. J. P. F.; Niessen F. B.; Koken P. W.; Winters H. A. H. The Influence of Radiotherapy on the Mechanical Properties of Silicone Breast Implants. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1172 10.1097/GOX.0000000000001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J. L.; Bessho K.; Cavin R.; Carnes D. L. Bone response to radio frequency sputtered calcium phosphate implants and titanium implants in vivo. J. Biomed. Mater. Res. 2002, 59, 184–190. 10.1002/jbm.1232. [DOI] [PubMed] [Google Scholar]
- a Zhang W.; Tian Y.; He H.; Chen R.; Ma Y.; Guo H.; Yuan Y.; Liu C. Strontium attenuates rhBMP-2-induced osteogenic differentiation via formation of Sr-rhBMP-2 complex and suppression of Smad-dependent signaling pathway. Acta Biomater. 2016, 33, 290–300. 10.1016/j.actbio.2016.01.042. [DOI] [PubMed] [Google Scholar]; b Li Z.; Wang S.; Xu G.; Hu X.; Han L.; Zhao Y. Synergy effect of Sr and rhBMP-2: A potential solution to osteolysis caused by rhBMP-2. Med. Hypotheses 2020, 144, 109895 10.1016/j.mehy.2020.109895. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Li B.; Lu S.; Zhang L.; Han Y. Regulation of osteoblast proliferation and differentiation by interrod spacing of Sr-HA nanorods on microporous titania coatings. ACS Appl. Mater. Interfaces 2013, 5, 5358–5365. 10.1021/am401339n. [DOI] [PubMed] [Google Scholar]
- Udduttula A.; Li J.; Zhao P.-Y.; Wang G.-C.; Zhang J. V.; Ren P.-G. Sol-gel derived nanosized Sr5(PO4)2SiO4 powder with enhanced in vitro osteogenesis and angiogenesis for bone regeneration applications. Ceram Int. 2019, 45, 3148–3158. 10.1016/j.ceramint.2018.10.215. [DOI] [Google Scholar]
- Peng S.; Zhou G.; Luk K. D.; Cheung K. M.; Li Z.; Lam W. M.; Zhou Z.; Lu W. W. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Cell. Physiol. Biochem. 2009, 23, 165–174. 10.1159/000204105. [DOI] [PubMed] [Google Scholar]
- Saidak Z.; Marie P. J. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216–226. 10.1016/j.pharmthera.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Guo X.; Wei S.; Lu M.; Shao Z.; Lu J.; Xia L.; Lin K.; Zou D. Dose-dependent Effects of Strontium Ranelate on Ovariectomy Rat Bone Marrow Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells. Int. J. Biol. Sci. 2016, 12, 1511–1522. 10.7150/ijbs.16499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi M.; Mousa S. A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.; Xie Y.; Ji H.; Huang L.; Zheng X. Excellent stability of plasma-sprayed bioactive Ca3ZrSi2O9 ceramic coating on Ti–6Al–4V. Appl. Surf. Sci. 2010, 256, 4677–4681. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.