Abstract
Bi-allelic mutations in the TUBGCP4 gene have been recently associated with autosomal recessive microcephaly with chorioretinopathy. However, little is known about the genotype-phenotype characteristics of this disorder. Here, we describe a 5-year-old male patient with autism and a normal occipitofrontal circumference. No retinal abnormalities were observed. Brain MRI revealed the presence of enlarged sheaths of both tortuous optic nerves; both eyes had shorter axial lengths. Whole-exome sequencing in trio revealed synonymous TUBGCP4 variants in homozygous state: c.1746G>T; p.Leu582=. This synonymous variant has been previously described and probably leads to skipping of exon 16 of TUBGCP4. These results broaden the clinical spectrum of this new syndrome and suggest that TUBGCP4 bi-allelic mutations may underlie complex neurodevelopmental disorders.
Keywords: TUBGCP4, Recessive syndrome, Autism, Intellectual disability
Established Facts
Bi-allelic TUBGCP4 mutations have been related to a new autosomal recessive syndrome.
This syndrome is characterized by autosomal recessive microcephaly with chorioretinopathy and global developmental delay/intellectual disability.
To our knowledge, only 5 caseswith TUBGCP4 mutations have been reported worldwide.
All of these were caused by compound heterozygous TUBGCP4 variants.
No formal developmental/intellectual assessments have been performed.
Novel Insights
We report a 5-year-old boy with a homozygous mutation in TUBGCP4.
He presented with autism and ectasia of the optic nerve sheaths, but not microcephaly with chorioretinopathy or global developmental delay/intellectual disability.
This new case widens the clinical spectrum of this new syndrome.
TUBGCP4 mutations may underlie neurodevelopmental disorders without some clinical manifestations previously reported.
Introduction
Microtubules are polarized polymers of tubulin that are involved in a wide range of cellular processes including cell structure, intracellular transport, organelle positioning, cell division, cell-cell signaling, and cell motility [Farache et al., 2018; Rossello et al., 2018; Tovey and Conduit, 2018]. In humans, there are 5 known tubulin isoforms: α-tubulin, β-tubulin, γ-tubulin, δ-tubulin, and ε-tubulin. In eukaryotes, the α-tubulin and β-tubulin families participate in the microtubules assembling, and 2 main complexes containing γ-tubulin − the γ-tubulin small complex or γ-TuSC, and the γ-tubulin ring complex or γ-TuRC − are necessary for microtubule nucleation [Farache et al., 2018; Rossello et al., 2018; Tovey and Conduit, 2018]. The γ-TuRC is a complex resulting from the assembly of γ-tubulin with γ-tubulin complex proteins GCP2, GCP3, GCP4, GCP5, and GCP6 (encoded by TUBGCP2, TUBGCP3, TUBGCP4, TUBGCP5, and TUBGCP6). Infrequent bi-allelic mutations in some of these genes cause microcephaly without clear structural brain anomalies [Puffenberger et al., 2012; Scheidecker et al., 2015; Mitani et al., 2019; Da Palma et al., 2020].
In 2015, Scheidecker et al. [2015] reported 4 children with microcephaly and chorioretinopathy caused by compound heterozygous TUBGCP4 (tubulin-gamma complex-associated protein 4) mutations. After this initial report, only one more case with bi-allelic TUBGCP4 mutations and the same phenotype has been published [Da Palma et al., 2020]. Interestingly, all reported cases had different truncating mutations but shared the same synonymous variant (c.1746G>T; p.Leu582=); this previously published pathogenic variant generates a new cryptic splice site that produces exon 16 skipping [Scheidecker et al., 2015].
Here, we present a 5-year-old Spanish boy with autism, with homozygous TUBGCP4 variants, and without microcephaly or chorioretinal dysplasia.
Clinical Report
Clinical Features of the Patients and Previous Diagnostic Studies
A 5-year-old male, the second child from nonconsanguineous healthy parents of Spanish origin, was referred to our clinic. Partum occurred via uncomplicated vaginal delivery after 40 weeks of pregnancy. Apgar scores were 9-10 at 1 and 5 min, respectively. Birth weight was 2,890 g (15th centile), length 51 cm (80th centile), and OFD 33.5 cm (20th centile). OFD was 46,5 (60th centile) and 49 (65th centile) at the age of 1 and 2 years, respectively. Family history was not relevant; his parents and his brother were healthy.
He walked unsupported at 12 months; the first bisyllabic words occurred at 28 months. At the age of 5 years, no advances were observed in verbal and nonverbal communication abilities; echolalia, neologisms, a stereotyped and idiosyncratic language associated with hyperkinetic behavior was associated with minimal eye contact and tendency to play alone.
At the age of 3 years, a neuropsychological assessment to measure intellectual functioning and development, as well as language abilities, was conducted. Specifically, the following tests and scales were used: the Leiter Internation Performance Scale, the Merrill Palmer-Revised scale of development (MP-R), the Peabody Picture Vocabulary Test (PPVT), the Reynell Developmental Language Scales (RDLS), the Autism Diagnostic Interview-Revised (ADI-R), the Autism Diagnostic Observation Scale (ADOS), and the Vineland Adaptive Behavior Scales (VABS). Notably, Leiter scale revealed the presence of an IQ of 100 (50th centile) and Cognitive Index of MP-R was 80 (normal; below average); verbal skills were severely compromised, not being possible to assess them with PPVT or RDLS. VABS total score was 73 (communication, daily living skills, social skills and motor skills scores were 66, 83, 65, and 93, respectively), according to his clinical communication and social problems. ADI-R and ADOS algorithm scores were 18 and 21, meeting the criteria for autism spectrum disorder on both instruments.
The clinical examination disclosed a weight of 19.8 kg (70th centile), a height of 111 cm (60th centile), and an OFD of 52 cm (80th centile), without dysmorphic features. Observation of the patient revealed verbal and nonverbal communication deficits, echolalia, neologisms, a stereotyped and idiosyncratic language associated with hyperkinetic behavior.
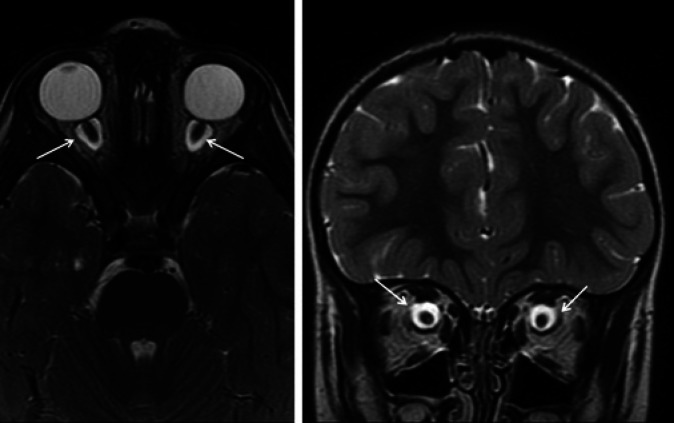
Routine laboratory screening including thyroid function and neurometabolic tests were within the normal range. Sleep video-EEG test and auditory evoked potentials displayed normal results. The ophthalmologic studies performed by 2 different ophthalmologists displayed no retinal anomalies. Brain 1.5T MRI did not reveal any significant structural brain malformations; however, the presence of a clear dilation of the subarachnoid space around both optic nerves was observed, as well as a significant tortuosity of the optic nerves (Fig. 1, 2). These alterations were associated with a smaller size of the orbit and eye size; to objectify this finding, the axial lengths of the patient's eyes were measured, as well as the axial length of both eyes in other 6 boys of the same age. The measurement was taken from the retina-choroid complex to the anterior surface of the cornea. The axial length of both eyes of the patient was 20.2 mm versus 23.5 mm measured in the control group.
Fig. 1.
Axial and coronal fast spin echo T2-weighted planes. Dilation of the subarachnoid space (arrows) surrounding both optic nerves with enlarged sheaths. Absence of hyperintensities in the optic nerve.
Fig. 2.
Sagittal T1-weighted planes. Tortuosity of optic nerves: increased curvature of the nerve (arrows) with deviation within the axial plane.
Previous genetic studies (karyotype, CGH-arrays) did not show anomalies. Whole-exome trio analysis was performed; it revealed synonymous TUBGCP4 variants in homozygous state: c.1746G>T; p.Leu582= . The variant was confirmed by Sanger sequencing. DANN score was 0.80 and the Combined Annotation-Dependent Depletion (CADD) score of the variant was 13.92 [Rentzsch et al., 2019]. This variant was not found in international databases in homozygosis.
Discussion
We describe a new case with autism and microphthalmia, associated with homozygous TUBGCP4 mutations Five cases with microcephaly and chorioretinopathy caused by compound heterozygous TUBGCP4 variants have been previously reported (Table 1); multiple punched-out lesions, sometimes associated with chorioretinal folds, were observed in fundus examination in all cases in their first years of life [Scheidecker et al., 2015; Da Palma et al., 2020]. All of them suffered developmental delay and/or learning disabilities; no formal cognitive evaluations have been reported in these cases. Patients with microcephaly and chorioretinopathy may also show microphthalmia, intellectual disability, brain malformations, short stature and/or dysmorphic features [Jones et al., 2014; Mears et al., 2015; Scheidecker et al., 2015; Balikova et al., 2016; Da Palma et al., 2020; Shurygina et al., 2020]. Interestingly, all reported individuals had been previously referred to specialized centers for genetic eye diseases because of chorioretinopathy associated with congenital microcephaly; this cohort selection might itself be an important problem to assess the whole clinical spectrum of this new autosomal recessive disorder.
Table 1.
Genetic and clinical features of the case reported here and the 4 previously reported cases with TUBGP4 mutations
| Scheidecker et al., 2015 |
Da Palma et al., 2020 | Described case | ||||
|---|---|---|---|---|---|---|
| case 1 | case 2 | case 3 | case 4 | case 5 | ||
| Genetics | ||||||
|
| ||||||
| TUBGCP4 mutations | c.579dup; c.1746G>T | c.579dup; c.1746G>T | c.1732-?_*544+?del; c.1746G>T | c.298del;c.1746G>T | c.1380G>A;c.1746G>T | c.1746G>T;c.1746G>T |
|
| ||||||
| Protein changes | p.Gly194TrpfsTer8; p.Leu582= | p.Gly194TrpfsTer8; p.Leu582= | p.? p.Leu582= | p.Tyr100IlefsTer27; p.Leu582= | p.Trp460Ter; p.Leu582= p.Leu582=; p.Leu582= | |
|
| ||||||
| Neurological anomalies | ||||||
|
| ||||||
| Microcephaly | + | + | + | + | + | − |
|
| ||||||
| Neuropsychological phenotype | Lower range of intellectual ability Attends regular educatior | Walks at 18 months Mild learning difficulties | Development delay Learning difficulties | Speech delay Learning difficulties | Learning difficulties | Speech delay Nonverbal IQ: 100 Autism |
|
| ||||||
| Brain MRI | Thin corpus callosum | No abnormalities | No abnormalities | No abnormalities | No abnormalities | No abnormalities |
|
| ||||||
| Ocular anomalies | ||||||
|
| ||||||
| Microphthalmia | + | + | − | + | + | + |
|
| ||||||
| Chorioretinopathy | + | + | + | + | + | − (not yet) |
|
| ||||||
| Optic nerve (ON) | No abnormalities | No abnormalities | No abnormalities | ON hypoplasia | No abnormalities | Tortuous ON Dural ectasia of both ON sheaths |
More than 200 missense TUBGCP4 variants at a frequency of <0.1% have been registered in international databases in the control population [Karczewski et al., 2020]. The pLI [Lek et al., 2016] and the haploinsufficiency scores [Huang et al., 2010] of TUBGCP4 are 0.0 and 13.88, respectively, and the Z score for missense mutation is 2.58 [Karczewski et al., 2020]. These data support the deleterious effect only of biallelic variants as demonstrated in all the cases reported so far. These 5 individuals had a nonsense or frameshift mutation, but all shared the same synonymous variant (c.1746G>T; p.Leu582=), located in C-terminal domain [Guillet et al., 2011]. According to reported studies, all truncated variants are expected to lead to a new reading frame ending in a stop codon, and mRNA transcripts with these premature stop codons would be eliminated by nonsense-mediated mRNA decay. In relation to the synonymous variant, this is present in healthy controls with a frequency of 0.0003 [Karczewski et al., 2020] and was present in our patient in homozygous state. Scheidecker et al. [2015] demonstrated that this variant generates a new cryptic splice site than induces exon 16 skipping, contributing to a residual production of non-spliced cDNA containing exon 16, and therefore to decreased levels of gamma-tubulin complex protein-4 (GCP4), abnormal microtubule organization, and altered cell morphology. These anomalies suggest that the synonymous variant may be the only one to be expressed [Scheidecker et al., 2015].
This group used the zebrafish model to demonstrate the role of GCP4 in embryogenic development; the translation block of its mRNA was associated with a smaller head and eyes in these embryos. However, 25% of the embryos did not show a reduced size of the head and eyes. A similar finding is observed in patients with tubulinopathies, where 25% of the affected cases do not show microcephaly [Bahi-Buisson and Cavallin, 2021]. This phenotypic variability might explain the absence of microcephaly in our case. However, we could demonstrate the presence of associated ophthalmologic abnormalities, not only related to the size of the ocular globes, but also in the structure of both optic nerves.
The presence of autism, ectasia of the optic nerve sheaths, without associated microcephaly or chorioretinopathy, widens the phenotypic variability of this new autosomal recessive syndrome. The presence of biallelic TUBGCP4 mutations may underlie severe neurodevelopmental disorders, autism or intellectual disability, without the presence of other typical manifestations so far, such as microcephaly or chorioretinal dysplasia. Nevertheless, chorioretinopathy may appear at anolder age and should be monitored regularly in these cases. The selection of the cases reported to date could have determined the true clinical dimension of this syndrome.
Statement of Ethics
The study was carried out in accordance with the Declaration of Helsinki of the World Medical Association and approved by the Local Ethics Committee (Madrid, Spain; Ref 30,062,019). Informed consent was obtained from the parents, after full explanation of the procedures.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Spanish Ministerio de Economía y Competitividad, MINECO [grant number PSI2017-84922-R], and the Comunidad de Madrid [grant number SI1/PJI/2019-00061].
Author Contribution
A.J.D., P.T., D.M.F.-M., A.L.F.-P., and B.C.-P.: investigation, writing - review and editing. S.L.-M.: investigation, writing - original draft, writing - review and editing, visualization. J.A.: investigation, writing - original draft, writing - review and editing, visualization, funding acquisition. M.J.P.: software, formal analysis. M.M. and S.A.: software, formal analysis. A.F.-J.: conceptualization, methodology, validation, formal analysis, investigation, resources, writing - original draft, writing - review and editing, visualization, supervision, project administration.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.
Acknowledgments
We thank the parents for their participation and helpful cooperation in this study. We also thank Dr. Jean Muller at the Hôpitaux Universitares de Strasbourg and Dr. Anthony Moore, at the University of California for their medical advice.
References
- Bahi-Buisson N, Cavallin M. Tubulinopathies Overview. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet] University of Washington, Seattle, 1993, 2021; Available from: https://www.ncbi.nlm.nih.gov/books/NBK350554/ [PubMed] [Google Scholar]
- Balikova I, Robson AG, Holder GE, Ostergaard P, Mansour S, Moore AT. Ocular manifestations of microcephaly with or without chorioretinopathy, lymphedema or intellectual disability (MCLID) syndrome associated with mutations in KIF11. Acta Ophthalmol. 2016;94((1)):92–8. doi: 10.1111/aos.12759. [DOI] [PubMed] [Google Scholar]
- Da Palma MM, Motta FL, Takitani GEDS, Salles MV, Lima LH, Ferraz Sallum JM. TUBGCP4 - associated microcephaly and chorioretinopathy. Ophthalmic Genet. 2020;41((2)):189–93. doi: 10.1080/13816810.2020.1747084. [DOI] [PubMed] [Google Scholar]
- Farache D, Emorine L, Haren L, Merdes A. Assembly and regulation of γ-tubulin complexes. Open Biol. 2018;8((3)):170266. doi: 10.1098/rsob.170266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet V, Knibiehler M, Gregory-Pauron L, Remy MH, Chemin C, Raynaud-Messina B, et al. Crystal structure of γ-tubulin complex protein GCP4 provides insight into microtubule nucleation. Nat Struct Mol Biol. 2011;18((8)):915–9. doi: 10.1038/nsmb.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Lee I, Marcotte EM, Hurles ME. Characterising and predicting haploinsufficiency in the human genome. PLoS Genet. 2010;6:e1001154. doi: 10.1371/journal.pgen.1001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GE, Ostergaard P, Moore AT, Connell FC, Williams D, Quarrell O, et al. Microcephaly with or without chorioretinopathy, lymphoedema, or mental retardation (MCLMR): review of phenotype associated with KIF11 mutations. Eur J Hum Genet. 2014;22:881–7. doi: 10.1038/ejhg.2013.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears K, Bakall B, Harney LA, Penticoff JA, Stone EM, et al. Autosomal Dominant Microcephaly Associated With Congenital Lymphedema and Chorioretinopathy Due to a Novel Mutation in KIF11. JAMA Ophthalmol. 2015;133((6)):720–1. doi: 10.1001/jamaophthalmol.2015.199. [DOI] [PubMed] [Google Scholar]
- Mitani T, Punetha J, Akalin I, Pehlivan D, Dawidziuk M, Coban Akdemir Z, et al. Bi-allelic Pathogenic Variants in TUBGCP2 Cause Microcephaly and Lissencephaly Spectrum Disorders. Am J Hum Genet. 2019;105((5)):1005–15. doi: 10.1016/j.ajhg.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One. 2012;7:e28936. doi: 10.1371/journal.pone.0028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47((D1)):D886–D894. doi: 10.1093/nar/gky1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossello CA, Lindstrom L, Eklund G, Corvaisier M, Kristensson MA. gamma-Tubulin-gamma-Tubulin Interactions as the Basis for the Formation of a Meshwork. Int J Mol Sci. 2018;19((10)):3245. doi: 10.3390/ijms19103245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidecker S, Etard C, Haren L, Stoetzel C, Hull S, Arno G, et al. Mutations in TUBGCP4 alter microtubule organization via the γ-tubulin ring complex in autosomal-recessive microcephaly with chorioretinopathy. Am J Hum Genet. 2015;96((4)):666–74. doi: 10.1016/j.ajhg.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurygina MF, Simonett JM, Parker MA, Mitchell A, Grigorian F, Lifton J, et al. Genotype Phenotype Correlation and Variability in Microcephaly Associated With Chorioretinopathy or Familial Exudative Vitreoretinopathy. Invest Ophthalmol Vis Sci. 2020;61:2. doi: 10.1167/iovs.61.13.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey CA, Conduit PT. Microtubule nucleation by γ-tubulin complexes and beyond. Essays Biochem. 2018;62((6)):765–80. doi: 10.1042/EBC20180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.