Abstract
Mevalonate kinase deficiency (MKD) is a periodic fever syndrome. Nonsteroidal anti-inflammatory drugs, corticosteroids, and anakinra are the most common treatments. However, colchicine is considered insufficient in disease control. In this case report, we present an 8-month-old infant with an atypical presentation of MKD. She had recurrent fever episodes, diarrhea, and lethargy. Elevated mevalonic acid was not detected in the urine. However, the genetic investigation showed a novel pathogenic heterozygous c.925G>C (p.Gly309Arg) variant and a heterozygous c.1129G>A (p.Val377Ile) mutation in the MVK gene. The patient was treated with colchicine for 8 months. During treatment, no further fever episode had been observed. It should be kept in mind that mevalonic acid excretion may not be present in the urine with mild MKD. Colchicine may be a reasonable option in mild MKD patients for a longer duration of treatment due to favorable adverse event profiles.
Keywords: Mevalonate kinase deficiency, Colchicine, Periodic fever syndromes
Established Facts
Periodic fever syndromes should be investigated in the presence of fever with an undetermined etiology, even if the clinical and laboratory findings are incompatible.
Mevalonate kinase deficiency (MKD) is one of the periodic fever syndromes and is very rare and difficult to diagnose.
Nonsteroidal anti-inflammatory drugs, corticosteroids, and anakinra are the most common treatments.
Novel Insights
No excretion of mevalonic acid in urine in mild MKD patients should be kept in mind for MKD diagnosis.
Colchicine can be used effectively to treat the milder form of the disease without an adverse event.
Introduction
Mevalonate kinase deficiency (MKD) is an autosomal recessive disease caused by mutations in the MVK gene. It is one of the periodic fever syndromes and is very rare and difficult to diagnose. Mevalonate kinase is the enzyme that converts mevalonate into mevalonate phosphate. Mevalonic acid accumulates, and the final product cholesterol cannot be formed in MKD [Prasad et al., 2012; Berody et al., 2015].
Clinical findings play a crucial role in the diagnosis of MKD. In patients with partial mevalonate kinase enzyme deficiency [hyper-immunoglobulin (Ig) D syndrome (HIDS)], fever episodes are accompanied by gastrointestinal complaints, oral ulcers, cervical lymphadenopathy, hepatosplenomegaly, headaches, pharyngitis, arthralgia, and skin rashes. In contrast, in patients with enzyme activity of less than 1%, ocular involvement, neurological symptoms, psychomotor retardation, cerebellar ataxia, prenatal and postnatal growth retardation, and dysmorphic findings (facial dysmorphism) can be determined [Haas and Hoffmann, 2006; Prasad et al., 2012; Berody et al., 2015; Mulders-Manders and Simon, 2015].
Increased mevalonic acid in the urine and acute phase reactants in plasma may be observed. An association with thrombocytopenia has been reported [de Klerk et al., 1988; Prasad et al., 2012]. An increase in Ig D level (>100 IU/mL) is an important finding, while the Ig A level is also high in 80% of the patients [Haraldsson et al., 1992; Klasen et al., 2001].
Nonsteroidal anti-inflammatory drugs, corticosteroids, and anakinra are the most common treatments, although colchicine has been insufficient to control the disease [Haas and Hoffmann, 2006; Zhang, 2016].
In this report, we present the case of a Syrian female infant with an atypical presentation of MKD that was treated successfully with colchicine.
Case Report
An 8-month-old Syrian girl was referred to our intensive care unit due to a coma. The patient had fever, diarrhea, tachypnea, and lethargy before admission to the hospital.
In her medical history, she was born at full term, with a birthweight of 1,500 g, after an uneventful pregnancy of a nonconsanguineous marriage. At 39 days of age, she was admitted to the hospital with fever and treated with antibiotics due to suspected meningitis, although no positive sign was observed in the CSF culture test.
Physical examination revealed a poor general condition and identified a dry mucosa and skin with reduced turgor and tachypnea. The spleen was 5 cm and the liver 6 cm, palpable. Height (62 cm), weight (5,000 g), and head circumference (40 cm) were below the 3rd percentile. We noted no dysmorphic appearance. Other system examinations were unremarkable.
Laboratory evaluation revealed a high white blood cell count (WBC; 21.170 109/L), normal hemoglobin level (10g/dL), thrombocytopenia (85.000 109/L) and elevated ALT (765U/l) AST (3648 U/l) and C-reactive protein (CRP; 148 mg/dL) levels. Metabolic acidosis (pH:7.14 HCO3:9.8 mmol/L PCO2:29.5 mmHg) and hyperlactatemia (8.95 mmol/L, RR:0.45–1.45 mmol/L) were detected. Creatine kinase (1535 U/L) was high, and ammonia (44 mmol/L) values were within the normal range.
Cranial MRI revealed cerebral atrophy, thin corpus callosum, and enlarged lateral ventricles. Cranial MRI spectroscopy was normal. Abdominal ultrasonography showed hepatomegaly, increased liver echogenicity, hepatosteatosis, and splenomegaly. Echocardiographic evaluation was normal.
We initiated peritoneal dialysis due to renal failure and anuria. We started empirical antibiotics and antivirals (vancomycin, meropenem, oseltamivir), although there were no positive signs in blood, urine, or CSF cultures. TORCH [toxoplasmosis, other (syphilis, varicella-zoster, parvovirus B19), rubella, cytomegalovirus, and herpes] serology was normal.
Plasma amino acids, carnitine/acylcarnitine profile, very-long-chain fatty acids panel, biotinidase enzyme activity, lysosomal diseases screening tests (Gaucher, Niemann pick) were within the normal range. We saw no high mevalonic acid level in the urinary organic acid analysis.
During clinical follow-up, persistent, recurrent episodes of fever were observed. Liver function tests were improved. The course of fever was not periodic (Fig. 1). In febrile periods, increases in acute-phase reactants (CRP, WBC, ESR), ALT, and AST levels were noted (Fig. 1). The fever was not accompanied by a rash, arthralgia, lymphadenopathy, or abdominal pain. All blood, urine, and peritoneal fluid cultures were negative. Lymphocyte subsets were unremarkable. As peripheral blood lymphocyte counts, IgG 1280 mg/dL (RR:463–1006), IgM 132 mg/dL (RR:46–169), IgA 191 mg/dL (RR:17–69) levels, and reactive anti-HBs were within normal ranges; immunodeficiencies were ruled out. Repeated urine organic acid analyses were carried out during the febrile periods, and we detected no elevated levels of urinary mevalonic acid.
Fig. 1.
Fever chart and laboratory findings. Gray bars show fever periods.
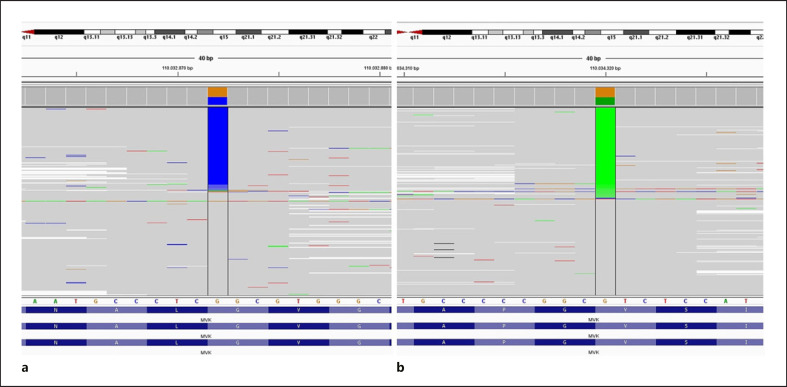
We performed genetic analysis for autoinflammatory syndromes (C1NH, NLRP1, NLRP3, PLCG2, PSMB8, PSTPIP1, TNFRSF1A, MEFV, and MVK genes) due to recurrent episodes of fever and systemic inflammation. Genetic testing was performed using the next-generation sequencing method (Miseq-Illumina). In the MVK gene, a novel pathogenic heterozygous c.925G>C (p.Gly309Arg) variant (according to ACMG criteria) and a heterozygous c.1129G>A (p.Val377Ile) mutation were revealed (Fig. 2).
Fig. 2.
Mutations in the MVK gene. a Novel pathogenic heterozygous c.925G>C (p.Gly309Arg) variant (according to ACMG criteria). b Heterozygous c.1129G>A (p. Val377Ile) mutation.
The patient was diagnosed with HIDS (mevalonate kinase deficiency) by identifying the compound heterozygous mutation in the MVK gene (Fig. 2). We started colchicine treatment at 0.25 mg/day, with recommendations to take a 5 mg/dose (bid) of prednisolone during fever periods. The patient was 25 months old at the last examination. She continued with colchicine treatment for 8 months. No further fever episode related to MKD has been observed.
Discussion
In this study, we present an MKD patient with an atypical presentation and reasonable response to colchicine treatment without adverse events. Indeed, several factors made the diagnosis of MKD difficult. Firstly, in more than 50% of patients with MKD, the fever attacks last 3–5 days and occur every 4–6 weeks, and the first fever attack usually appears in the first trimester of life [Berody et al., 2015]. However, in our case, the fever attacks were more frequent and lasted 1–3 days. Additionally, the first fever attack was observed at the age of 8 months. Secondly, clinical features are important for the diagnosis of MKD. In patients with partial mevalonate kinase enzyme deficiency, HIDS, episodes of fever are accompanied by gastrointestinal complaints, oral ulcers, cervical lymphadenopathy, hepatosplenomegaly, headaches, pharyngitis, arthralgia, and skin rashes; in patients with enzyme activity of less than 1%, ocular involvement, neurological symptoms, psychomotor retardation, cerebellar ataxia, prenatal and postnatal growth retardation, and dysmorphic findings (facial dysmorphism) can be determined [Haas and Hoffmann, 2006; Prasad et al., 2012; Berody et al., 2015; Mulders-Manders and Simon, 2015]. In the present case, all of these clinical manifestations other than hepatosplenomegaly were absent, making the diagnosis of MKD difficult.
Another obstacle for diagnosis was the laboratory findings. While the elevated WBC, CRP, ESR, and transaminases levels during the fever attacks were compatible with MKD, they were not specific to the disease. Moreover, the presence of high acute phase reactants during periods without fever was inconsistent with the laboratory features of MKD. Thrombocytopenia with fever has been reported in earlier studies. However, there was no such presentation in our case [de Klerk et al., 1988; Prasad et al., 2012]. The excretion of mevalonic acid in the urine, which is the most indicative finding in the diagnosis of MKD and was controlled 4 times during the febrile periods in our patient, was not detected. This made the diagnosis even more difficult. However, the absence of mevalonic acid excretion in the urine does not rule out an MKD diagnosis, and if there is clinical suspicion, a genetic analysis of the MKV gene is recommended [Jeyaratnam et al., 2016]. One remarkable finding from the laboratory parameters was the serum Ig A level. As mentioned previously, 80% of the patients have a high level of Ig A [Haraldsson et al., 1992; Klasen et al., 2001]. In our case, the elevated serum IgA indicated MKD as a differential diagnosis of recurrent febrile attacks.
In the light of the above, our report emphasizes that in patients with recurrent fever attacks and atypical clinical manifestations that are incompatible with periodic fever syndromes, MKD should be kept in mind.
In MKD, 2 important disease subgroups have been defined, the first of which is HIDS, in which enzyme activity is in the 1.8–28% range and the second is complete enzyme deficiency or mevalonic aciduria, in which enzyme activity is less than 1% [Berody et al., 2015; Jeyaratnam et al., 2016]. No genotype-phenotype relationship has been reported in MKD, although Berody et al. [2015] noted that patients with c1129G>A (p.Val377Ile) mutation have a mild phenotype. A patient with a homozygous c1129G>A (p.Val377Ile) mutation diagnosed at the age of 47 was consistent with the mild phenotype. Furthermore, it was stated that patients detected with the heterozygous p.Val377Ile mutation were identified with an enzyme activity of 1% and above and partial enzyme deficiency [Jeyaratnam et al., 2016]. In the present study, the novel pathogenic heterozygous c.925G>C (p.Gly309Arg) variant (according to ACMG criteria) and heterozygous c.1129G>A (p.Val377Ile) mutation were revealed. The Gly309Arg is a novel variant, but different changes affecting the same amino acid codon (p.Gly309Ser and p.Gly309Val) have been reported in other publications [Bader-Meunier et al., 2011; Qian et al., 2021]. Although we did not assess enzyme activity in the present study, the clinical findings and the genetic analysis results indicated a mild phenotype of HIDS.
There is a lack of consensus in the treatment of MKD. Nonsteroidal anti-inflammatory drugs, corticosteroids, and anakinra are the most commonly used treatments, although Zhang [2016] reported colchicine treatment to be insufficient in 80% of the patients [Haas and Hoffmann, 2006; Zhang, 2016]. Interestingly, in the present case, a complete response was observed upon the initiation of colchicine treatment, suggesting that colchicine may be an effective alternative treatment in mild forms (HIDS). Furthermore, there was no adverse event in our patient during this treatment. In order to prevent fever periods, patients with MKD need to longer duration of treatment. In this context, the treatment options mentioned above can cause adverse events in patients who have received these agents for a long time.
In conclusion, based on the present study's findings, periodic fever syndromes should be investigated in the presence of fever with an undetermined etiology, even if the clinical and laboratory findings are incompatible. No excretion of mevalonic acid in the urine in mild MKD patients should be kept in mind. Colchicine can be used effectively to treat the mild form of MKD patients without serious adverse events. We believe that more case reports will help reveal the genotype-phenotype relationship and clarify the clinical manifestations of the disease.
Statement of Ethics
Informed consent for genetic analysis and publication of clinical reports and photographs were obtained from the patient or his parent/guardian in accordance with the national ethics regulation. There is no name or number indicating the patient's identity.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no funding for this study.
Author Contributions
All authors contributed equally to this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (M.K.Y) upon reasonable request.
Acknowledgement
We thank the patient and her family for their valuable participation in this study.
References
- Bader-Meunier B, Florkin B, Sibilia J, Acquaviva C, Hachulla E, Grateau G, et al. Mevalonate kinase deficiency: a survey of 50 patients. Pediatrics. 2011;128:e152–9. doi: 10.1542/peds.2010-3639. [DOI] [PubMed] [Google Scholar]
- Berody S, Galeotti C, Koné-Paut I, Piram M. A restrospective survey of patients's journey before the diagnosis of mevalonate kinase deficiency. Joint Bone Spine. 2015;82:240–4. doi: 10.1016/j.jbspin.2014.12.011. [DOI] [PubMed] [Google Scholar]
- de Klerk JB, Duran M, Dorland L, Brouwers HA, Bruinvis L, Ketting D. A patient with mevalonic aciduria presenting with hepatosplenomegaly, congenital anaemia, thrombocytopenia and leukocytosis. J Inherit Metab Dis. 1988;11((Suppl 2)):233–6. doi: 10.1007/BF01804244. [DOI] [PubMed] [Google Scholar]
- Haas D, Hoffmann GF. Mevalonate kinase deficiencies: from mevalonic aciduria to hyperimmunoglobulinemia D syndrome. Orphanet J Rare Dis. 2006;1:13. doi: 10.1186/1750-1172-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsson A, Weemaes CM, De Boer AW, Bakkeren JA, Stoelinga GB. Immunological studies in the hyper-immunoglobulin D syndrome. J Clin Immunol. 1992;12:424–8. doi: 10.1007/BF00918854. [DOI] [PubMed] [Google Scholar]
- Jeyaratnam J, Ter Haar NM, de Sain-van der Velden MG, Waterham HR, van Gijn ME, Frenkel J. Diagnostic Value of Urinary Mevalonic Acid Excretion in Patients with a Clinical Suspicion of Mevalonate Kinase Deficiency (MKD) JIMD Rep. 2016;27:33–8. doi: 10.1007/8904_2015_489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasen IS, Göertz JH, van de Wiel GA, Weemaes CM, van der Meer JW, Drenth JP. Hyper-immunoglobulin A in the hyperimmunoglobulinemia D syndrome. Clin Diagn Lab Immunol. 2001;8:58–61. doi: 10.1128/CDLI.8.1.58-61.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders-Manders CM, Simon A. Hyper-IgD syndrome/mevalonate kinase deficiency: what is new? Semin Immunopathol. 2015;37:371–6. doi: 10.1007/s00281-015-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad C, Salvadori MI, Rupar CA. Severe phenotypic spectrum of mevalonate kinase deficiency with minimal mevalonic aciduria. Mol Genet Metab. 2012;107:756–9. doi: 10.1016/j.ymgme.2012.10.019. [DOI] [PubMed] [Google Scholar]
- Qian W, Wu J, Tang H, Zhen Q, Ge H, Gao J, et al. Mutation Analysis of the MVD Gene in a Chinese Family with Disseminated Superficial Actinic Porokeratosis and a Chinese Literature Review. Indian J Dermatol. 2021 Mar-Apr;66((2)):126–31. doi: 10.4103/ijd.IJD_226_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. Natural history of mevalonate kinase deficiency: a literature review. Pediatr Rheumatol Online J. 2016;14:30. doi: 10.1186/s12969-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (M.K.Y) upon reasonable request.