Previous studies suggest that antibody engagement of red blood cells (RBCs) not only may result in hemolysis but also may induce loss of the target antigen in the setting of autoimmune hemolytic anemia or incompatible RBC transfusion.1,2 Although the mechanism of antibody-induced antigen loss remains to be elucidated, several studies also suggest that antigen modulation may likewise serve as a potential mechanism of antibody-mediated immunosuppression toward RBC alloantigens.2 However, whether anti-RhD alloantibodies (the only alloantibodies that have been used successfully to prevent alloantibody formation in patients) possess the capacity to alter RhD antigen levels after engagement in a human subject remains unknown. As a result, we evaluated the impact of anti-RhD antibody infusion on RhD antigen levels in an RhD-positive patient who was receiving treatment for idiopathic thrombocytopenic purpura (ITP).
A 22-month-old, B-positive male presented to the children’s hospital with a diffuse, purpuric rash and profound thrombocytopenia. Diagnosed with ITP, he was treated with 600 mcg of anti-RhD immunoglobulin (Ig)G (WinRho) and subsequently experienced a hemolytic reaction. In the blood bank, a postinfusion direct anti-globulin test (DAT) was positive (IgG, 3+; C3, 0). Because a preinfusion sample was available, this reaction provided an opportunity to assess RhD antigen expression before and after anti-RhD infusion. To provide a more quantitative evaluation of antibody and antigen levels before and after anti-RhD infusion, DATs were assessed by flow cytometry. As predicted, the pre–anti-RhD infusion DAT was negative, and the post–anti-RhD DAT was positive (Fig. 1A). To evaluate RhD antigen levels, preinfusion and postinfusion samples were incubated in vitro with anti-RhD (with the same lot number used to treat the patient), followed by anti-IgG and anticomplement. Reduced levels of detectable RhD antigen were observed in the post–anti-RhD infusion sample compared with the preinfusion sample (Fig. 1B); however, no complement could be detected on the surface of RBCs either before or after anti-RhD infusion (data not shown).
Fig. 1.
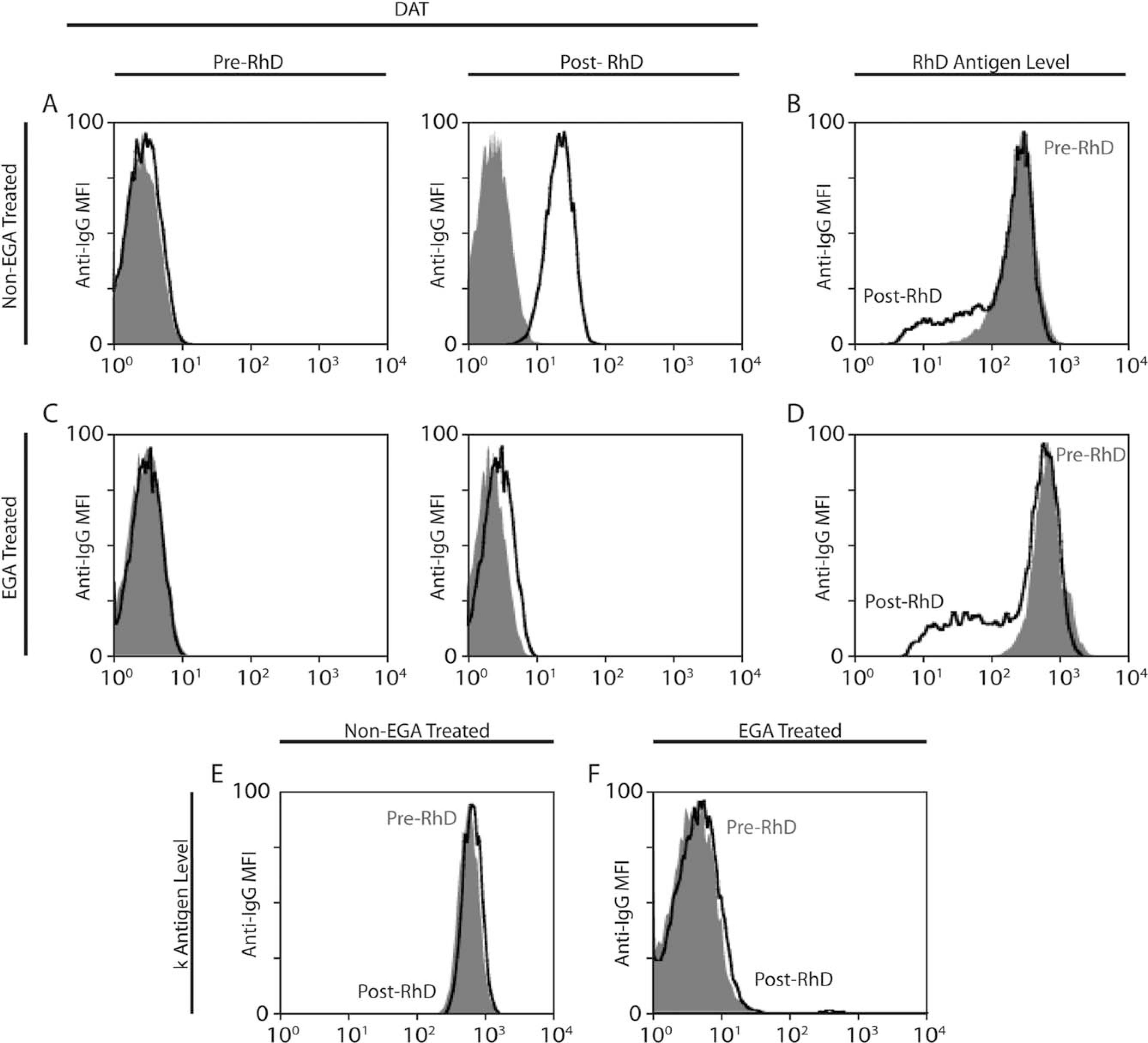
Anti-RhD induces loss of the RhD antigen. (A) DAT results are illustrated before and after anti-RhD exposure (black, patient serum; gray, negative control). (B) RhD antigen levels are illustrated before (gray) and after (black) anti-RhD exposure. (C) DAT results are illustrated before and after anti-RhD exposure (black, patient serum; gray, negative control) following EGA treatment. (D) RhD antigen levels are illustrated before (gray) and after (black) anti-RhD exposure following EGA treatment. (E,F) Cellano (k antigen) levels are illustrated before (gray) and after (black) anti-RhD exposure in the (E) absence or (F) presence of EGA treatment.
Given the potential confounding effect of antibody already bound in vivo on the detection of the RhD antigen post–anti-RhD infusion, we removed bound anti-RhD antibody using ethylenediaminetetraacetate glycine acid (EGA) (ImmucorGamma), which dissociates bound IgG from the RBC surface. After EGA treatment, DATs before and after anti-RhD infusion revealed minimal reactivity (Fig. 1C). Using this approach, antigen loss after anti-RhD infusion was still apparent when directly comparing RBCs after and before anti-RhD infusion (Fig. 1D). Finally, to determine whether reduction in the level of detectable antigen was specific to the RhD antigen, we evaluated cellano (k) antigen levels before and after anti-RhD exposure. Unlike the observed alterations of RhD, no difference in k antigen was detected before or after anti-RhD infusion (Fig. 1E). Because EGA is known to disrupt the k antigen, no k antigen was detected post-EGA treatment from pre–anti-RhD or post–anti-RhD infusion samples (Fig. 1F).
Because most autoimmune hemolytic anemia or hemolytic transfusion reactions are not anticipated, it is difficult to examine potential changes in target antigen before and after antibody engagement. However, this case provided a unique opportunity to examine the impact of antibody engagement on a RBC antigen using defined anti-RhD reagents used not only to treat the patient but also to define changes in RhD antigen levels. Although it is certainly possible that the apparent impact of anti-RhD on RhD antigen levels may reflect undetectable masking by anti-RhD Fab fragments, the ability of EGA treatment to largely eliminate the binding of intact IgG strongly suggests that potential Fab fragments would be similarly removed. Furthermore, because anti-RhD antibodies do not typically fix complement, examination of RBCs before and after anti-RhD exposure provides an opportunity to determine the impact of antibody engagement in the absence of complement fixation. Our case demonstrates that, in the clinical setting, anti-RhD antibodies can induce specific alterations in the levels of detectable RhD antigen, consistent with previous studies suggesting that similar alterations can occur in the setting of passive antibody administration.3 Although antibody engagement can result in complete antigen loss,3 similar to experimental models, anti-RhD antibodies do not induce complete loss of detectable antigen, nor does antibody engagement reduce antigen levels in every RBC.4 Alterations in antigen levels, coupled with potential changes in the saturation of the mononuclear phagocyte system after anti-RhD antibody administration, may impact both RBC clearance and additional antigen changes after anti-RhD antibody engagement. Taken together, these results suggest that anti-RhD can induce the loss of detectable antigen independent of complement, and thus may influence the rate and magnitude of RBC clearance in addition to the availability of the RhD antigen in the setting of RBC alloimmunization. Understanding mechanisms whereby antibody can induce alterations in the levels of detectable antigen may provide a useful strategy to intentionally protect cells in settings of incompatible RBC transfusion and autoimmune hemolytic anemia while also providing insight into potential mechanisms of anti-RhD immunoprophylaxis.
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Contributor Information
Harold C. Sullivan, Center for Transfusion Medicine and Cellular Therapies, Department of Laboratory Medicine and Pathology, Emory University School of Medicine, Atlanta, GA.
Connie M. Arthur, Center for Transfusion Medicine and Cellular Therapies, Department of Laboratory Medicine and Pathology, Emory University School of Medicine, Atlanta, GA.
Louisa Thompson, Center for Transfusion Medicine and Cellular Therapies, Department of Laboratory Medicine and Pathology, Emory University School of Medicine, Atlanta, GA.
Seema R. Patel, Center for Transfusion Medicine and Cellular Therapies, Department of Laboratory Medicine and Pathology, Emory University School of Medicine, Atlanta, GA.
Sean R. Stowell, Center for Transfusion Medicine and Cellular Therapies, Department of Laboratory Medicine and Pathology, Emory University School of Medicine, Atlanta, GA.
Jeanne E. Hendrickson, Yale Cooperative Center of Excellence in Hematology, Yale University, New Haven, CT.
Alan H. Lazarus, Department of Laboratory Medicine and Pathobiology, St Michael’s Hospital/Research Institute, University of Toronto, Toronto, Ontario, Canada.
REFERENCES
- 1.Zimring JC, Cadwell CM, Spitalnik SL. Antigen loss from antibody-coated red blood cells. Transfus Med Rev 2009;23:189–204. [DOI] [PubMed] [Google Scholar]
- 2.Girard-Pierce KR, Stowell SR, Smith NH, et al. A novel role for C3 in antibody-induced red blood cell clearance and antigen modulation. Blood 2013;122:1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan HC, Gerner-Smidt C, Nooka AK, et al. Daratumumab (anti-CD38) induces loss of CD38 on red blood cells. Blood 2017;129:3033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stowell SR, Liepkalns JS, Hendrickson JE, et al. Antigen modulation confers protection to red blood cells from antibody through Fcγ receptor ligation. J Immunol 2013;191:5013–25.<image> [DOI] [PubMed] [Google Scholar]


