Abstract
Purpose
Retinal signatures of systemic disease (‘oculomics’) are increasingly being revealed through a combination of high-resolution ophthalmic imaging and sophisticated modelling strategies. Progress is currently limited not mainly by technical issues, but by the lack of large labelled datasets, a sine qua non for deep learning. Such data are derived from prospective epidemiological studies, in which retinal imaging is typically unimodal, cross-sectional, of modest number and relates to cohorts, which are not enriched with subpopulations of interest, such as those with systemic disease. We thus linked longitudinal multimodal retinal imaging from routinely collected National Health Service (NHS) data with systemic disease data from hospital admissions using a privacy-by-design third-party linkage approach.
Participants
Between 1 January 2008 and 1 April 2018, 353 157 participants aged 40 years or older, who attended Moorfields Eye Hospital NHS Foundation Trust, a tertiary ophthalmic institution incorporating a principal central site, four district hubs and five satellite clinics in and around London, UK serving a catchment population of approximately six million people.
Findings to date
Among the 353 157 individuals, 186 651 had a total of 1 337 711 Hospital Episode Statistics admitted patient care episodes. Systemic diagnoses recorded at these episodes include 12 022 patients with myocardial infarction, 11 735 with all-cause stroke and 13 363 with all-cause dementia. A total of 6 261 931 retinal images of seven different modalities and across three manufacturers were acquired from 1 54 830 patients. The majority of retinal images were retinal photographs (n=1 874 175) followed by optical coherence tomography (n=1 567 358).
Future plans
AlzEye combines the world’s largest single institution retinal imaging database with nationally collected systemic data to create an exceptional large-scale, enriched cohort that reflects the diversity of the population served. First analyses will address cardiovascular diseases and dementia, with a view to identifying hidden retinal signatures that may lead to earlier detection and risk management of these life-threatening conditions.
Keywords: ophthalmology, medical ophthalmology, medical retina, health informatics
Strengths and limitations of this study.
AlzEye is a large retrospective cohort dataset linking ophthalmic data from Moorfields Eye Hospital National Health Service (NHS) Foundation Trust in London, UK, with NHS hospital admissions data over a 10-year period in 3 53 157 patients.
The dataset consists of more than six million routinely collected retinal images of seven different modalities across three vendors deterministically linked to prevalent and incident cardiovascular and neurodegenerative disease.
Actively informed by ongoing patient and public engagement, the project leverages a privacy-by-design approach using third-party linkage to facilitate access to high-performance computing while mitigating risks to data privacy.
Introduction
Scientific discovery has increasingly been driven by the availability of large, diverse, high-dimensional datasets providing deeply phenotyping variables in health and disease.1–3 Advances in healthcare informatics, hardware and statistical techniques have uncovered relationships previously unachievable through traditional methods of study design. Thus, rich and voluminous genome sequencing data have provided insight into disease pathogenesis and therapeutic targets,4 5 while radiomic analysis has supported exploration of relationships between quantitative data extracted from medical imaging and disease.6
Crucial to health data research has been the establishment of and accessibility to large prospective epidemiological studies, such as the United Kingdom Biobank (UKBB), the Rotterdam study and the European Prospective Investigation into Cancer and Nutrition study.7–9 While such studies represent exceptionally powerful enablers for discovery science, they are potentially limited for investigations of specific subpopulations of interest (eg, those with rare disease or specific sociodemographic groups) and, where they draw on volunteer participants, also prone to selection bias (eg, over-representation of more healthy subjects). Participants in UKBB are less likely to be obese, smoke or drink alcohol, and accordingly, mortality rates for participants aged 70–74 years in UKBB are 46.2% and 55.5% lower for men and women, respectively, compared with general UK population.10
Healthcare in England is such that when a patient has a medical event requiring admission, in almost all cases they are admitted under the provisions of the National Health Service (NHS). Routinely collected healthcare administrative data during a patient’s admission are subsequently translated into corresponding International Classification of Diseases (ICD) codes by clinical coders, submitted to the Secondary Uses Service and aggregated by NHS Digital into a unified record-level national repository of Hospital Episode Statistics (HES) data relating to admitted patient care (APC). While the original purpose of HES was the monitoring of service activity and negotiation of financial reimbursement, it is increasingly used for epidemiological research.11 HES data are amenable to research as a sole resource. However, using deterministic linkage where identifiers are matched in a rules based approach in contrast to probabilistic linkage,12 HES can enrich other datasets as in the case of UKBB13 or the European Prospective Investigation into Cancer in Norfolk.14
While these aforementioned studies demonstrate the value of enriching structured datasets through HES linkage, this has not yet been done at scale for routinely collected data of high dimensionality, such as imaging. Thus, we established AlzEye, a large dataset which links routinely collected retinal images and relevant ophthalmic data from an unselected population attending Moorfields Eye Hospital (MEH) NHS Foundation Trust with nationally collected systemic healthcare outcome data provided through the HES APC database. MEH is a tertiary ophthalmic institution incorporating a principal central site, four district hubs and five satellite clinics in London, UK, providing care to a sociodemographically diverse population of six million people (9% of the UK population).15 The aim of AlzEye is to characterise the association between retinal biomarkers and chronic disorders of ageing, particularly dementia and cardiovascular diseases. In addition to describing the characteristics of the AlzEye cohort, we outline the key governance, technical and ethical factors that need to be addressed to support large institution-led individual-level linkage of routinely collected multidimensional data and have enabled us to create an exceptional transdisciplinary resource to explore the retinal signatures of systemic disease.
Cohort description
Study population
The AlzEye project is a retrospective cohort study of patients aged 40 years and over who have attended MEH between 1 January 2008 and 1 April 2018. Patients were included if they had attended the glaucoma, retina, neuro-ophthalmology or emergency ophthalmic services and had valid NHS numbers. Those with invalid NHS numbers, dates of birth or who had previously opted out of their health data being used for purposes of research (described in the NHS as a ‘Type 2 opt-out’) were excluded. Ethnicity group was self-reported by the patient as (1) Asian or Asian British, (2) black or black British, (3) mixed, (4) other ethnic group, (5) white or (6) unknown. Socioeconomic status was categorised using the Index of Multiple Deprivation (IMD) decile, which was estimated by permuting the IMD 2015 rank from the patient’s postcode through Lower Super Output Areas followed by aggregation into deciles.16 Mortality data were derived from the MEH database, which is updated on a 2 weekly basis using reports extracted from the NHS National Spine and is completed on an individual basis by the MEH data quality team to ensure accuracy. Data are completed on any patients who have ever attended MEH. Mortality data up to the end of the study period, 1 April 2018, were included.
Approvals and process
The following key steps in the governance processes were required to provide the necessary ongoing assurance within the research ethics framework of the NHS and the legal framework of the UK. In order to support other researchers wishing to establish similar linked cohorts, we provide an explanation of each stage which outlines the principle which that stage addresses, the UK framework that meets that principle and finally any study-specific considerations that we undertook to not only meet but exceed those requirements (figure 1).
Figure 1.
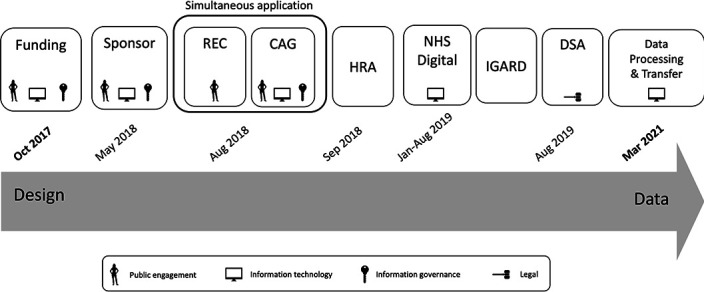
Schematic of the key milestones, prerequisites and approvals with their corresponding achievement dates for the AlzEye dataset. CAG, Confidential Advisory Group; DSA, data sharing agreement; HRA, Health Research Authority; IGARD, Independent Group Advising on the Release of Data; NHS, National Health Service; REC, research ethics committee.
Funding
It was necessary to secure funding to deliver the study and to provide assurance to the sponsor and others that the study would be completed and that the integrity of the study would not be compromised by inadequate resources. In AlzEye, the study was funded through a small grant awarded by Fight for Sight and Alzheimer’s Research UK in October 2017 covering the costs of data storage and linkage fees. The funders had no role in the conception, design or analysis of the study.
Sponsorship
It was necessary to secure a sponsor for the study that would take on ‘overall responsibility for proportionate, effective arrangements being in place to set up, run and report on a research project’. In AlzEye, we sought sponsorship from the relevant NHS Trust (MEH) at which all patients had been seen. Sponsorship confirmation was only sought following internal consultation between data protection, information governance, information security and information technology (IT) teams at both MEH and University College London (UCL). MEH acted as the sponsor, with UCL acting as the trusted third party linking retinal images and HES data and providing computational facilities for data analysis. The study and data governance were approved on 24 May 2018 (internal reference: KEAP1004).
Health Research Authority (HRA) approval
For most research studies in England and Wales, including those limited to working with data for specific projects, HRA approval is required. HRA approvals involve the assessment of governance and legal compliance of a research study with an independent ethics review and opinion from the research ethics committee (REC). Depending on other study characteristics (eg, gene therapy), additional applications may be required to inform HRA approval. In England, limited access to confidential patient information without consent may be granted under the provisions of Section 251 of the NHS Act 2006,17 permitting temporary lifting of the common law duty of confidentiality around confidential patient information ‘in the public interest’ or ‘in the interests of improving patient care’.17 Obtaining Section 251 support requires application to the Confidential Advisory Group (CAG), an independent body providing expert advice to the HRA for research applications and NHS Digital for data dissemination. Applications were accordingly made to the REC (18/LO/1163, approved on 1 August 2018) and the CAG for Section 251 support (18/CAG/0111, approved on 13 September 2018). The NHS HRA gave final approval on 13 September 2018. Approvals thus far granted the legal basis for submitting an application to the Data Access Request Service (DARS) of NHS Digital.18
NHS Digital and the DARS
NHS Digital oversees the DARS, which administers and provides, on application, multiple England-wide datasets from disease-specific audits (eg, National Diabetes Audit Core) to general admissions in secondary care (eg, HES). Applications to DARS require that the organisation have, at a minimum, the following:
Data sharing framework contract for data controllers.
Compliance with minimum-security standards for data processors and data storage locations.
Adequate information security certification (eg, ISO27001).
A legal basis for data access (eg, Section 251).
Applications are then reviewed with an assigned case officer, who will liaise with the applicant on project-specific items. For AlzEye, dialogue between the applicant and NHS Digital data production team revolved around confirmation of data fields and datasets (HES) and the pseudonymisation embedded within the linkage strategy.
Independent Group Advising on the Release of Data (IGARD)
Following internal NHS Digital review and prior to data release, DARS applications are scrutinised by the IGARD in line with Section 263(2) of the Health and Social Care Act 2012, the Code of Practice on confidential information. IGARD is an independent panel with a broad range of expertise, from legal to information governance to epidemiology. Support for AlzEye was given by IGARD in January and August 2019 citing that ‘aspects of the application could be used as an exemplar by NHS Digital to help other researchers with their applications to the DARS’.19
Data sharing agreements (DSA)
Prior to data receipt, DSA must be signed between NHS Digital and the data controller and are overseen by their respective legal departments. AlzEye required an additional DSA between MEH and UCL for the transfer of ophthalmic imaging and clinical data between institutions outlining the purpose and legal basis for sharing.
Data processing and transfer
The dataset was finalised on completion of engineering work parsing manufacturer-specific file formats to non-proprietary data structures amenable to image analysis with appropriate deidentification. A secure cloud-based informatics pipeline was used for transfer of images to UCL from MEH, the establishment of which was delayed by the COVID-19 pandemic. Imaging data were stored (with backup) across dedicated network-attached storage device within the UCL School of Life and Medical Sciences (SLMS) and only accessible to members of the AlzEye research team. All data entities were listed within the UCL SLMS Information Asset Register.
Patient and public involvement and engagement (PPIE)
PPIE support was provided by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at MEH and UCL Institute of Ophthalmology and has been embedded throughout this project from priority setting to plans for dissemination. Feedback has been sought through public engagement events, survey of eye service users and reports within the media. Patients and the public actively contributed to identifying the priority setting of dementia and the acceptability of using routinely acquired eye scans for research purposes and without consent. In addition, two members of the public will sit on the AlzEye working group to contribute to results interpretation and coauthoring and dissemination of research outputs. The members will be supported in selecting the results they find relevant and presenting them to wider patient communities.
Ophthalmic health variables
Patient-level ophthalmic variables were extracted from the MEH data warehouse, which aggregates information from the patient administration system (PAS), electronic health record (EHR) and imaging database, all linked through a unique MEH hospital identification number. Sociodemographic data, including date of birth, sex, ethnicity and postcode as well as patients’ clinic appointments and operation dates, are housed within PAS. Surgical procedures were recorded in the EHR at MEH from 4 September 2012. Operation details, including procedure name, laterality and indication for surgery are contained within the MEH EHR and uploaded to the MEH data warehouse. A patient undergoing the most common operation in the UK, cataract extraction, would therefore have an entry for the typical procedure (phacoemulsification and intraocular lens implant), operated eye (right or left) and indication (cataract).
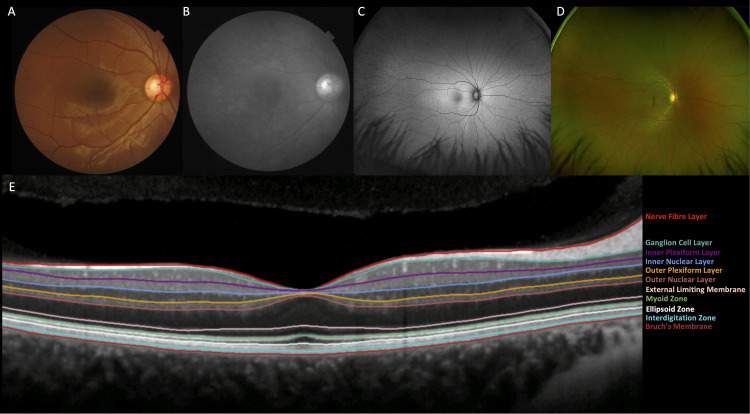
Colour retinal photography (figure 2A) and optical coherence tomography (OCT, figure 2B) images, which represent the majority of retinal images within the database, have been processed through segmentation and feature extraction software. The Vascular Assessment and Measurement Platform for Images of the Retina system provides fully automated segmentation and extraction of retinal vascular indices.20 21 OCT scans are segmented and retinal sublayer thicknesses are computed using the Topcon Advanced Biomedical Imaging Laboratory software.22
Figure 2.
Composite figure showing the major retinal imaging modalities within AlzEye. (A) Colour fundus photograph, (B) red-free photograph, (C) fundus autofluorescence (widefield), (D) pseudocolour photography (widefield) and (E) optical coherence tomography of the central macula illustrating segmentation of the individual sublayers. Consensus nomenclature for the retinal sublayers is indicated.
For the purposes of this report, four common ophthalmic diseases were described—cataract, glaucoma, neovascular age-related macular degeneration (AMD) and proliferative diabetic retinopathy (PDR).
Cataract was defined as any operation code denoting phacoemulsification surgery and the indication of cataract. For the purposes of this report, only first eye cataract surgery was included.
Glaucoma was defined as any patient attending the glaucoma clinic three or more times with ongoing follow-up from 1 January 2010. The first 2 years of the study period were excluded as this may have incorporated patients with previous diagnoses of glaucoma where the maximum follow-up interval can approach 2 years; in contrast, any patient being seen after 2 years since study inception with no previous visit within that 2-year period can be assumed to have/carry a new diagnosis of glaucoma.
Diabetic eye disease represents a special case due to audit procedures mandated by the NHS Diabetic Eye Screening Programme. Coding of eye disease secondary to diabetes mellitus is rigorously validated by a dedicated team within MEH according to the NHS Diabetic Eye Screening Programme criteria,23 at hospital appointment from 12 September 2013 onwards. Dates for onset of PDR dates were recorded as the first appointment for each patient where this diagnosis was first made.
AMD can be categorised into two major types—dry and neovascular (‘wet’). Given dry AMD is slowly progressive and has no active hospital intervention currently available, it is MEH standard practice for patients to be discharged with lifestyle and monitoring advice (self-monitoring and standard optometric review). In contrast, neovascular AMD requires treatment through intravitreal anti-vascular endothelial growth factor (VEGF) injections, and therefore remains under active follow-up. The diagnostic codes for neovascular AMD were based on extensive previous work in which all patients with neovascular AMD at MEH were manually validated up to 2018.24 25
Systemic health variables
Systemic health data were derived from HES APC data, with a focus on cardiovascular disease and all-cause dementia. Diagnostic codes in HES APC are reported in line with the 10th revision of ICD.26 In line with previous reports, myocardial infarction was defined as code I21 or I22.27–29 Similarly, stroke was defined using stroke definitions from UKBB.30 Dementia was defined as ICD codes E512 (Wernicke’s encephalopathy), F00 (Dementia in Alzheimer disease), F01 (Vascular dementia), F02 (Dementia in other diseases classified elsewhere), F03 (Unspecified dementia), F10.6 (Mental and behavioural disorders due to psychoactive substance use, Amnesic syndrome), F10.7 (Mental and behavioural disorders due to psychoactive substance use, Residual and late-onset psychotic disorder), G30 (Alzheimer disease) or G31.0 (Other degenerative diseases of nervous system, not elsewhere classified), derived from previous work evaluating the agreement between HES APC data and primary care data, through general practitioner surveys and the Clinical Practice Research Datalink.31
Data linkage and transfer
The linkage strategy was designed through collaboration between experts in information governance, IT, computer scientists and clinicians based at MEH, UCL and NHS Digital (figure 3). A third-party linkage approach was used for two main reasons. First, it enhanced privacy preservation as the data originator, MEH, never received HES admissions data and the third party, UCL, did not receive personally identifiable information. Second, it enabled the linked dataset to be accessible within a site with sufficient high-performance computing capability to undertake the proposed analyses, a function significantly beyond almost all NHS facilities. Patient link identifiers consisting of a unique NHS identification number, sex and date of birth originating from MEH were transferred to NHS Digital in conjunction with a unique study ID generated using a cryptographic hash function (random pseudonymisation). Ophthalmic covariates, mortality data and patient sociodemographics with study ID were transferred to UCL. Ophthalmic imaging data pertaining to the patients within the study were extracted and deidentified during conversion from their proprietary format to Digital Imaging and Communications in Medicine (DICOM) format before transfer to UCL. Following linkage with HES, NHS Digital transferred HES data to the UCL Data Safe Haven, a ‘walled garden’ trusted research environment certified and externally audited to ISO27001 information security standards.32 33
Figure 3.
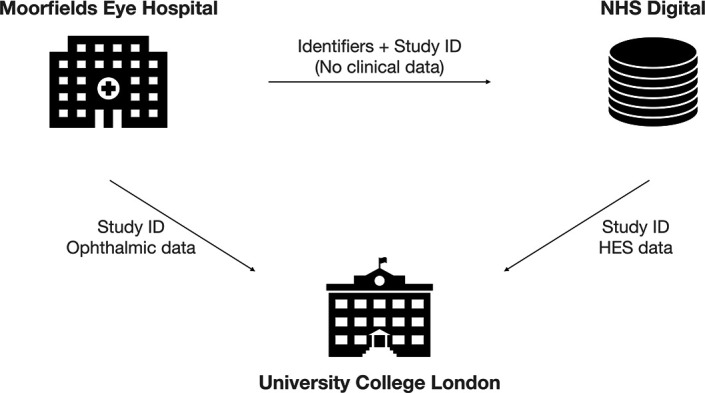
Linkage approach of AlzEye. Moorfields Eye Hospital (MEH) NHS Foundation Trust securely transfers a spreadsheet of identifiers with a study ID to NHS Digital and separately transfers the study ID with ophthalmic data, including diagnoses and retinal images, to University College London (UCL). NHS Digital links the identifiers with the Hospital Episode Statistics (HES) database and returns the admissions data with the study ID (and no identifiable data) to UCL. UCL links the ophthalmic data from MEH with HES data from NHS Digital using the study ID. NHS, National Health Service.
Statistical analysis
Imaging-based studies within the AlzEye Study are generally planned to take the form of nested case–control studies. To improve efficiency, controls may be matched with cases, using conditional logistic regression for statistical modelling of binary outcomes and survival analysis for time-to-event data (eg, Cox proportional hazard modelling).34 In cases where the competing risk of death is prominent, subdistribution HRs with 95% CIs will be estimated as a sensitivity analysis.35 Alternative high-dimensional modelling approaches, such as vision transformers, will also be explored. Prior to receipt of HES data from NHS Digital, sample size calculations were undertaken. Specifically, we evaluated the association between OCT-derived peripapillary retinal nerve fibre layer and macular ganglion cell-inner plexiform layer thicknesses and dementia. Given an OR of 1.4 with an alpha of 5% and a power of 90% on a 1:1 matched study design, a total sample size of 2106 is required.36
Figures for this report were designed in R V.4.1.0 (R core team, 2021. R foundation for statistical computing, Vienna, Austria).
Findings to date
Extraction of unique patients attending MEH outpatient clinics between 1 January 2008 and 1 April 2018 generated a cohort of 353 157 unique patients. A breakdown of sociodemographic details by category of the cohort are provided in table 1. Of the cohort, 190 494 were female (53.9%) and the mean age was 68.4 (±13.9) years. Of the 3 53 157 patients, 186 651 had a total of 1 337 711 HES episodes in the study period. NHS Digital performs a hierarchical stepwise linkage approach providing a ‘Match Rank’ for each HES episode.37 Among the 1 337 711 HES episodes matched, Match Rank was two for 1 337 482 episodes (exact NHS number, exact date of birth and exact sex linked), four for 46 episodes (exact NHS number, exact sex and partial date of birth) and eight for 183 episodes (exact NHS number).
Table 1.
Baseline sociodemographic characteristics of the AlzEye cohort
| Characteristic | N (%) | |
| All | 353 157 | |
| Sex | Female | 190 494 (53.9) |
| Male | 162 663 (46.1) | |
| Age group (years)* | 40–49 | 35 262 (10.0) |
| 50–59 | 66 101 (18.7) | |
| 60–69 | 79 018 (22.4) | |
| 70–79 | 84 942 (24.1) | |
| 80+ | 87 834 (24.9) | |
| Ethnicity | Black | 31 614 (9.0) |
| White | 135 743 (38.4) | |
| South Asian | 48 119 (13.6) | |
| Other/Unknown | 137 681 (39.0) | |
| Index of multiple deprivation decile | 1 (most deprived) | 18 194 (5.2) |
| 2 | 50 443 (14.3) | |
| 3 | 50 869 (14.4) | |
| 4 | 42 603 (12.1) | |
| 5 | 38 964 (11.0) | |
| 6 | 36 906 (10.5) | |
| 7 | 31 317 (8.9) | |
| 8 | 28 180 (8.0) | |
| 9 | 29 906 (8.5) | |
| 10 (least deprived) | 24 610 (7.0) | |
| Unknown | 1165 (0.3) |
Data are shown as n(%).
*Age is taken as that of 1 April 2018.
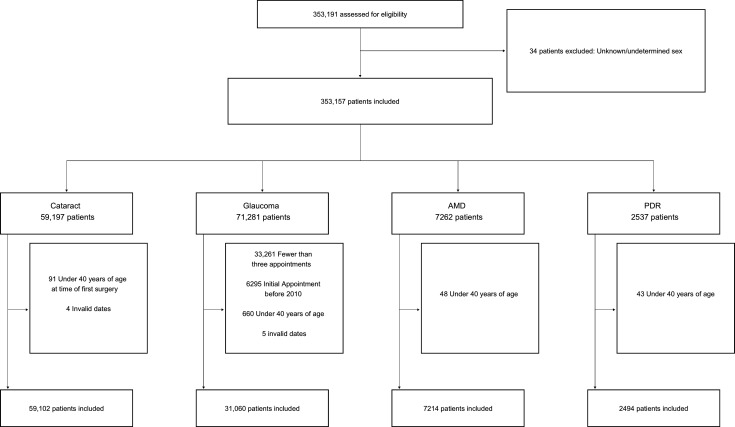
An illustration of the major common ophthalmic diseases within the cohort is shown in a Consolidated Standards of Reporting Trials style diagram in figure 4. Following the case definition and exclusion of invalid dates, a total of 59 102 patients had first eye cataract surgery, 31 060 glaucoma, 7214 neovascular AMD and 2494 PDR.
Figure 4.
Consolidated Standards of Reporting Trials style flow chart illustrating the distribution of cataract, glaucoma, neovascular age-related macular degeneration (AMD) and proliferative diabetic retinopathy (PDR) within the AlzEye dataset.
Among the 1 87 811 patients with recorded HES episodes, 12 022 patients had episodes with coded myocardial infarction, 11 735 patients with all-cause stroke and 13 363 with dementia. Within the dementia group, 4487 patients had codes that were specific for Alzheimer’s dementia and 3381 for vascular dementia (table 2).
Table 2.
Number of patients by selected examples of specified 10th revision of International Classification of Diseases (ICD) codes relating to diabetes mellitus, cardiovascular and neurodegenerative diseases
| Group | Disease | ICD code(s) | Number of patients |
| Cardiovascular | Acute coronary syndrome | I21, I22 | 12 022 |
| Heart failure | I50 | 24 034 | |
| Atrial fibrillation | I48 | 32 848 | |
| Hypertension | I10, I15 | 151 937 | |
| Subarachnoid haemorrhage | I60 | 642 | |
| Intracerebral haemorrhage | I61 | 1865 | |
| Ischaemic stroke | I63-I64 | 9996 | |
| All stroke | I60, I61, I63, I64 | 11 735 | |
| Neurodegenerative | Alzheimer’s disease | F00, G30 | 4487 |
| Vascular dementia | F01 | 3381 | |
| Parkinson’s disease | G20 | 3211 | |
| All-cause dementia | E12, F00, F01, F02, F03, F106, F107, G30, G310 | 13 363 | |
| Other | Diabetes mellitus (types 1 and 2) | E10, E11 | 71 570 |
Imaging
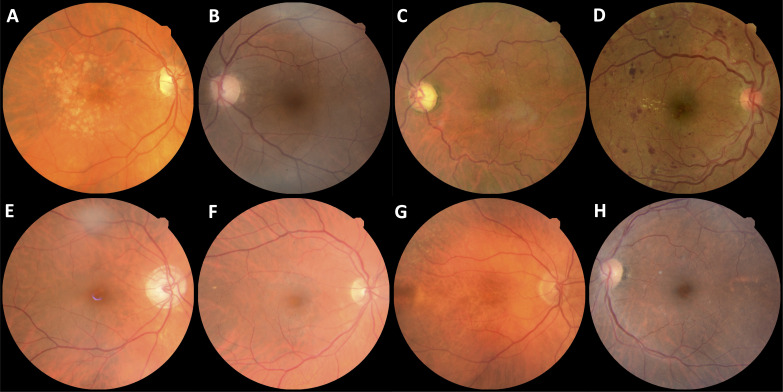
During the study period, a total of 6 261 931 images were acquired from 1 54 830 patients. The two leading image modalities were colour retinal photographs (n=1 874 175) and OCT (n=1 567 358). The distribution of imaging modalities across the three vendors used for retinal imaging at MEH—Topcon (Topcon Corp, Tokyo, Japan), Heidelberg (Heidelberg Engineering, Heidelberg, Germany) and Optos (Dunfermline, UK)—are shown in figure 5 and table 3. Most images were acquired on the Topcon system (n=5 553 826, 88.7%). Number of images by year is shown in figure 6. During the study period, annual imaging acquisition increased from 229 868 scans in 2008 to 1 021 904 in 2017. For 2018, collection stopped on 1 April precluding a complete annual figure. Example images of the major ophthalmic and systemic disease outcomes are shown in figure 7.
Figure 5.
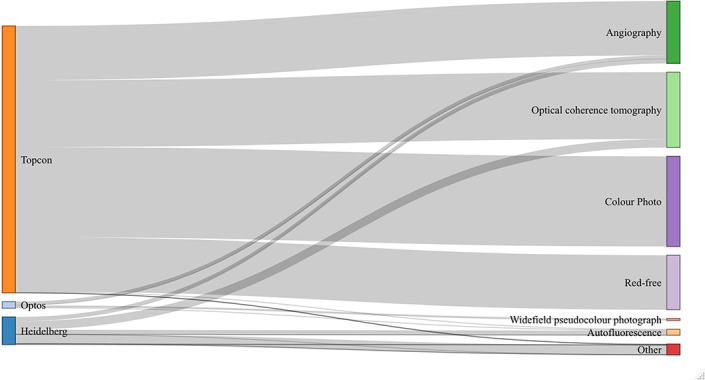
Parallel sets diagram illustrating the imaging modality across vendors within AlzEye. The majority of images were acquired on the Topcon system and the most frequent modalities were colour photography and optical coherence tomography. Designed using the networkD3 package.
Table 3.
Retinal imaging within the AlzEye dataset by vendor and imaging modality
| Vendor | Modality | Number of images | Number of patients |
| Topcon | Angiography | 1 128 723 | 21 225 |
| Autofluorescence | 11 761 | 2078 | |
| Colour photography | 1 874 175 | 139 307 | |
| Red-free | 1 146 854 | 122 453 | |
| OCT | 1 391 826 | 138 911 | |
| Other | 487 | 48 | |
| Heidelberg | Angiography | 89 264 | 4061 |
| Autofluorescence | 94 533 | 16 863 | |
| Infrared | 192 634 | 21 676 | |
| OCT | 175 532 | 21 191 | |
| Other | 19 781 | 2439 | |
| Optos | Angiography | 77 813 | 2215 |
| Autofluorescence | 18 590 | 5666 | |
| Pseudocolour photography | 39 958 | 6887 |
Angiography refers to dye-based techniques (fluorescein and indocyanine green).
OCT, optical coherence tomography.
Figure 6.
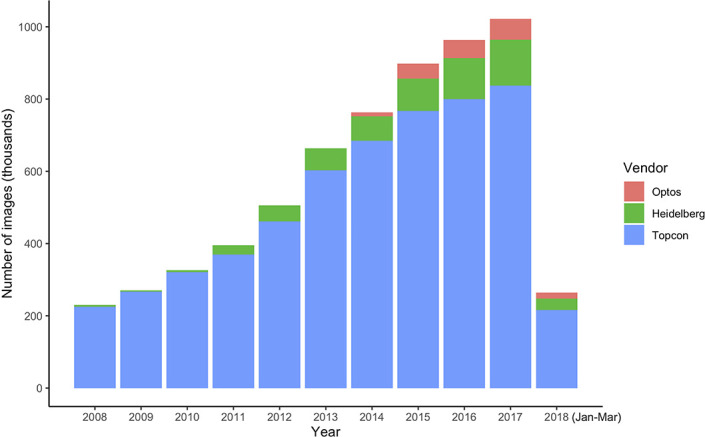
Stacked bar chart of the annual number of images acquired during the study period for the three leading device vendors at Moorfields Eye Hospital. Data for 2018 represents 3 months only prior to the study end date.
Figure 7.
Example colour retinal photographs of patients with ophthalmic and systemic diseases within AlzEye. (A) Age-related macular degeneration. (B) Cataract. (C) Glaucoma. (D) Proliferative diabetic retinopathy. (E) Prevalent Alzheimer’s disease. (F) Incident ischaemic stroke. (G) Incident myocardial infarction. (H) Prevalent vascular dementia.
Strengths and limitations
To our knowledge, we have created the world’s largest retinal imaging research dataset available presently, linking secondary healthcare ophthalmic data from 3 53 157 patients seen over a 10-year period with information on general health and key systemic diseases, as captured through admissions to any hospital within the NHS of England. This comprises 6 261 931 images, obtained using seven different modalities from three different manufacturers, in 1 54 830 patients. The current large-scale UK cohort, UKBB, provides useful context for AlzEye. Cross-sectional data are available in UKBB with two retinal imaging modalities (colour retinal photography and OCT) obtained using technology from one manufacturer (Topcon) and at a single time point in 67 321 people. Notwithstanding the recognised limitations (see ‘Limitations of the AlzEye cohort’ section) of real-world datasets and the coding within the HES database, AlzEye provides some distinct advantages beyond purely scale. Imaging data are longitudinal, highly multimodal and pertain to an ethnically and socioeconomically diverse cohort representative of the adult population with eye disease. Moreover, AlzEye has demonstrated relatively low cost. The study is funded through a charity small grant award and NIHR BRC support amounting to £20 000.
Comparison with other resources
UKBB is the major comparator for AlzEye, being the largest of the prospective epidemiological cohort datasets which provides cross-sectional retinal imaging in association with systemic disease variables.38 One of the limitations of UKBB is that, unlike AlzEye, it provides minimal longitudinal retinal images. Another prospective cohort study, the Rotterdam Study, does collect longitudinal retinal imaging data from approximately 15 000 participants, of which 5065 participants were eligible for OCT scanning in 2017.39 The Rotterdam Study has uncovered several landmark findings, particularly in regard to causal determinants, but its cohort remains relatively small in comparison to UKBB and AlzEye with the majority of participants recruited from one district within Rotterdam, the Netherlands.7 40 The Singapore Epidemiology of Eye Disease is one longitudinal multimodal retinal imaging initiative which is underway, in which 10 033 participants of Chinese, Indian and Malay ethnicity have been recruited to undergo six yearly retinal imaging.41 A recent review of ophthalmic imaging datasets did not reveal any additional relevant publicly available datasets that included linked systemic health data.42 Additionally, our own review of the literature has not identified any examples of large-scale linked real-world datasets (ie, including those with restricted access) which include linked systemic health data. The scarcity of such resources suggests that the construction of such datasets is challenging to undertake, presumably due to factors such as cost, required duration and delayed output, retention of participants and concerns over technological redundancy. The AlzEye approach is an important alternative model in this context.
Potential research impact from the novel AlzEye cohort
Several epidemiological opportunities arise with AlzEye. First, it provides a real-world snapshot of ophthalmic secondary care use, representing approximately 1.2% of the UK population aged 40 years and above (27 858 459 in 2011).43 This is a powerful tool for informing public health and policymaking in eye services and is exceptional in characterising the potential impact that may arise from the intersect between disabling diseases such as stroke and PDR.
Second, it allows the identification and exploration of relationships between newly diagnosed ophthalmic disease (or newly referred to hospital eye services) and emerging systemic events and accruing multimorbidity. Patients tend to respond early to issues with their sight and an understanding of how an ophthalmic presentation is linked to an increased likelihood of serious systemic disease may provide an opportunity for earlier intervention in those diseases.44
Third, nested case–control studies evaluating retinal-based oculomic biomarkers in those with systemic diseases (eg, dementia) can provide insight into their value in either static or dynamic risk prediction. Newer modelling approaches have highlighted the potential utility of the retina in screening for and risk stratification of cardiovascular, neurodegenerative, renal, hepatic and haematological diseases.45–51
Finally, by its magnitude and wealth of high-quality labels, both ophthalmic and systemic, AlzEye provides a powerful catalyst for high-dimensional model development, echoing that of ImageNet, a database currently exceeding 14 million images, which propelled deep learning and computer vision research forward a decade ago.52
Lessons learnt from the AlzEye approach
AlzEye highlights an opportunity for maximising the value of routinely collected data to support research for patient benefit. However, there are a number of governance and technical challenges when undertaking large-scale investigator-led data linkage.53 In AlzEye, early dialogue between experts in information governance, IT and data protection at both institutional parties (MEH and UCL) as well as the data production team at NHS Digital established a privacy-by-design linkage approach, which enhanced privacy preservation while maintaining computational feasibility.30 54 At its worst, an intrusion of the identifiable data during the development of AlzEye would have informed the violator that a given individual had visited MEH at some point between 2008 and 2018. Due to the novel approach of AlzEye within our centre, the greatest governance hurdle was securing study sponsorship, a process which took nearly 8 months. Once approved, permissions from the bodies of the HRA and overall approval were given within 8 weeks. Linking with high-dimensional imaging data also posed several technical obstacles. As highlighted recently by the American Academy of Ophthalmology, ophthalmic imaging technologies suffer from limited interoperability and low compliance to standardised formats, such as DICOM.55 A key undertaking within AlzEye was thus the secure and robust but efficient fully automated processing of raw ophthalmic imaging data from its proprietary file format with associated metadata to standard DICOM form with the identifiers stripped. Fortunately, while this operation requires significant technical and engineering input, most medical imaging modalities already benefit from standardisation among vendors obviating this step for other researchers seeking to emulate our approach. Finally, a key objective of AlzEye is the development of clinical prediction models using deep learning approaches, which require significant computing capacity. Provisions for graphics processing units (GPU) housed within UCL enable this step; however, others may consider recent guidance on the safeguards required for locating health data within cloud environments and the implications this brings for accessing virtual GPUs.56
Limitations of the AlzEye cohort
Despite the opportunities afforded by AlzEye, there are several limitations to this kind of approach and potential sources of bias. First, caution must be paid to the validity of HES diagnostic coding.57 Although previous validation studies have concluded that discharge coding within HES is sufficiently robust for research purposes,31 58 sizeable proportions of cases may be missed when using individual sources.59 For example, recent work linking the EHRs of 54.4 million people in England showed that HES captured 80.5% and 65% of myocardial infarctions and stroke/transient ischaemic attacks, respectively, when compared with linkage additionally incorporating death registry and primary care records.60 One mitigation strategy for this source of bias for real-world data is therefore linking to multiple sources. In terms of selection bias, as a hospital-attending cohort, the individuals within the AlzEye cohort are likely to have greater medical comorbidity than the general population, limiting the external validity of any findings. In addition, by the very nature of the dataset, patients within the AlzEye cohort will have definite or suspected ophthalmic disease, particularly among those with repeated retinal imaging. The risk of under-recording of potentially important variables such as smoking may also lead to residual confounding.
The enrichment of multimodal health data acquired as part of a patient’s routine clinical care with nationally held databases provides a powerful foundation for discovery science and epidemiological research. We highlight key considerations and challenges for those seeking to link high-dimensional data sources, from high-resolution imaging to waveform data, with locally held specialist data. Additionally, we provide the cohort profile for AlzEye, a powerful platform for oculomic discovery, specifically evaluating the association between retinal morphology and both cardiovascular diseases and dementia. Beyond discovery, the AlzEye cohort is anticipated to become an important resource for the development and validation of deep learning-based clinical prediction models that may enable earlier intervention for patients at risk of these life-threatening conditions.
Collaboration
National and international collaborations are welcomed though restrictions on access to the cohort mean that only the AlzEye researchers can directly analyse individual-level systemic health data. Interested researchers should contact the Chief Investigator at p.keane@ucl.ac.uk.
Supplementary Material
Acknowledgments
The authors thank Karen Bonstein and Andi Skilton for support with patient and public involvement and engagement, Menachem Katz, Ben Ward, Ross Green, Maxim Daniline and Simon St John-Green for information technology support, Llinos Bradley and Declan Flanagan for information governance expertise, Anthony Peacock for advice on use of trusted research environments and Antonio de la Plaza Larrea and Richard Macmillan for legal guidance on data sharing agreements. The authors are also grateful to Anthony Khawaja and Cathie Sudlow for feedback on study design.
Footnotes
Twitter: @sktywagner, @fintanhughes, @cortina_borja, @s_e_petersen, @konbalaskas, @pearsekeane
Contributors: SKW, AKD and PAK wrote the first draft of the manuscript, which was critically revised by FH, MCB, RS, NP, XL, HM, DCA, ET, SEP, KB, JH, AP and JSR. Authors SKW, FH, NP, HM, JH, AP, JSR, AKD and PAK were involved in the original design of the study. RS, NP and DCA: computer science expertise. JH: information governance. MCB, AP, JSR and AKD provided statistical and epidemiological guidance. All authors have approved the final version of this manuscript. The Chief Investigator (PAK) accepts full responsibility, as guarantor, for the finished work, the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This study was funded through a small grant awarded by Fight for Sight (grant reference: 24AZ171). This research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests: SKW is funded through a Medical Research Council Clinical Research Training Fellowship (MR/TR000953/1). NP is funded by a Moorfields Eye Charity Career Development Award (R190031A). HM is supported by the National Institute for Health Research’s (NIHR) Comprehensive Biomedical Research Centre (BRC) at University College London Hospitals. SEP receives support from the NIHR BRC at Barts. KB has received speaker fees from Novartis, Bayer, Alimera, Allergan, Roche and Heidelberg; meeting or travel fees from Novartis and Bayer; compensation for being on an advisory board from Novartis and Bayer; consulting fees from Novartis and Roche and research support from Apellis, Novartis and Bayer. AP receives financial support from the NIHR BRC based at Moorfields Eye Hospital (MEH) NHS Foundation Trust and UCL Institute of Ophthalmology; is part of the Steering Committee of the Advanced Nerve and Glaucoma Imaging (ANGI) network which is sponsored by ZEISS and Steering Committee of the OCTiMS Study which is sponsored by Novartis and reports speaker fees from Heidelberg Engineering. JSR receives support from the NIHR as a senior investigator and via the NIHR BRCs at MEH and Great Ormond Street Hospital. AKD is director of INSIGHT, the HDRUK Health Data Research Hub for Eye Health. PAK is supported by a Moorfields Eye Charity Career Development Award (R190028A) and a UK Research & Innovation Future Leaders Fellowship (MR/T019050/1); receives research support from Apellis; is a consultant for DeepMind, Roche, Novartis, Apellis and Bitfount; is an equity owner in Big Picture Medical and has received speaker fees from Heidelberg Engineering, Topcon, Allergan, Roche and Bayer, meeting or travel fees from Novartis and Bayer and compensation for being on an advisory board from Novartis and Bayer.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Cohort Description section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No additional data are available. The data are subject to the contractual restrictions of the data sharing agreements between National Health Service Digital, Moorfields Eye Hospital and University College London and are therefore not available for access beyond the AlzEye research team.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the research ethics committee (18/LO/1163, approved 01/08/2018) and the the Confidential Advisory Group for Section 251 support (18/CAG/0111, approved 13/09/2018). The National Health Service Health Research Authority gave final approval on 13 September 2018. Approval for Section 251 was obtained.
References
- 1.Munevar S. Unlocking big data for better health. Nat Biotechnol 2017;35:684–6. 10.1038/nbt.3918 [DOI] [PubMed] [Google Scholar]
- 2.Shilo S, Rossman H, Segal E. Axes of a revolution: challenges and promises of big data in healthcare. Nat Med 2020;26:29–38. 10.1038/s41591-019-0727-5 [DOI] [PubMed] [Google Scholar]
- 3.Obermeyer Z, Emanuel EJ. Predicting the future — big data, machine learning, and clinical medicine. N Engl J Med Overseas Ed 2016;375:1216–9. 10.1056/NEJMp1606181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj R, Sethi A, Nambiar R. Big data in genomics: an overview. 2014 IEEE International Conference on Big Data (Big Data), IEEE, 2014. [Google Scholar]
- 5.He KY, Ge D, He MM. Big data analytics for genomic medicine. Int J Mol Sci 2017;18:412. 10.3390/ijms18020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016;278:563–77. 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofman A, Breteler MMB, van Duijn CM, et al. The Rotterdam study: objectives and design update. Eur J Epidemiol 2007;22:819–29. 10.1007/s10654-007-9199-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riboli E, Hunt KJ, Slimani N, et al. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr 2002;5:1113–24. 10.1079/PHN2002394 [DOI] [PubMed] [Google Scholar]
- 9.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–9. 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–34. 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry Z, Mannan F, Gibson-White A, et al. Research Outputs of England’s Hospital Episode Statistics (HES) Database: Bibliometric Analysis. J Innov Health Inform 2017;24:329. 10.14236/jhi.v24i4.949 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Matsuyama Y, Ohashi Y, et al. When to conduct probabilistic linkage vs. deterministic linkage? A simulation study. J Biomed Inform 2015;56:80–6. 10.1016/j.jbi.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 13.Hospital information boosts UK Biobank resource. Available: https://www.ukbiobank.ac.uk/2013/09/20000-participants-return-for-a-repeat-assessment/ [Accessed 13 Oct 2020].
- 14.Luben R, Hayat S, Khawaja A, et al. Residential area deprivation and risk of subsequent hospital admission in a British population: the EPIC-Norfolk cohort. BMJ Open 2019;9:e031251. 10.1136/bmjopen-2019-031251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Large P. Annual mid-year population estimates, UK - Office for National Statistics, 2014. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/2014-06-26 [Accessed 23 Jul 2021].
- 16.Ministry of Housing, Communities & Local Government . English indices of deprivation, 2015. 2015. Available: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015 [Accessed 18 Sep 2020].
- 17.Great Britain . National health service act 2006. The Stationery Office, 2006. [Google Scholar]
- 18.Data access Request service (DARS). Available: https://digital.nhs.uk/services/data-access-request-service-dars [Accessed 7 Jul 2021].
- 19.Independent group advising on the release of data. Available: https://webarchive.nationalarchives.gov.uk/20200706184649/https://digital.nhs.uk/about-nhs-digital/corporate-information-and-documents/independent-group-advising-on-the-release-of-data [Accessed 7 Jul 2021].
- 20.Perez-Rovira A, MacGillivray T, Trucco E, et al. Vampire: vessel assessment and measurement platform for images of the retina. Annu Int Conf IEEE Eng Med Biol Soc 2011;2011:3391–4. 10.1109/IEMBS.2011.6090918 [DOI] [PubMed] [Google Scholar]
- 21.Liew G, Wang JJ, Cheung N, et al. The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology 2008;115:1951–6. 10.1016/j.ophtha.2008.05.029 [DOI] [PubMed] [Google Scholar]
- 22.Keane PA, Grossi CM, Foster PJ, et al. Optical Coherence Tomography in the UK Biobank Study - Rapid Automated Analysis of Retinal Thickness for Large Population-Based Studies. PLoS One 2016;11:e0164095. 10.1371/journal.pone.0164095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peate I. The NHS diabetic eye screening programme. British Journal of Healthcare Assistants 2019;13:596–9. 10.12968/bjha.2019.13.12.596 [DOI] [Google Scholar]
- 24.Fasler K, Fu DJ, Moraes G, et al. Moorfields AMD database report 2: fellow eye involvement with neovascular age-related macular degeneration. Br J Ophthalmol 2020;104:684–90. 10.1136/bjophthalmol-2019-314446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasler K, Moraes G, Wagner S, et al. One- and two-year visual outcomes from the Moorfields age-related macular degeneration database: a retrospective cohort study and an open science resource. BMJ Open 2019;9:e027441. 10.1136/bmjopen-2018-027441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ICD-10 version, 2010. Available: https://icd.who.int/browse10/2010/en [Accessed 22 Jul 2021].
- 27.Asaria P, Elliott P, Douglass M, et al. Acute myocardial infarction hospital admissions and deaths in England: a national follow-back and follow-forward record-linkage study. Lancet Public Health 2017;2:e191–201. 10.1016/S2468-2667(17)30032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalfe A, Neudam A, Forde S, et al. Case definitions for acute myocardial infarction in administrative databases and their impact on in-hospital mortality rates. Health Serv Res 2013;48:290–318. 10.1111/j.1475-6773.2012.01440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick N, Lacaille D, Bhole V, et al. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286. 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.UK Biobank Outcome Adjudication Group . Definitions of stroke, UK Biobank phase 1 outcomes adjudication. Available: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_stroke.pdf [Accessed 23 Aug 2021].
- 31.Brown A, Kirichek O, Balkwill A, et al. Comparison of dementia recorded in routinely collected hospital admission data in England with dementia recorded in primary care. Emerg Themes Epidemiol 2016;13:11. 10.1186/s12982-016-0053-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lea NC, Nicholls J, Dobbs C, et al. Data safe Havens and trust: toward a common understanding of trusted research platforms for governing secure and ethical health research. JMIR Med Inform 2016;4:e22. 10.2196/medinform.5571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Certificate client directory search results. Available: https://www.bsigroup.com/en-GB/our-services/certification/certificate-and-client-directory/search-results/?searchkey=licence%3dIS%2b612909%26company%3duniversity%2bcollege%2bLondon&licencenumber=IS%20612909 [Accessed 26 Jul 2021].
- 34.Cox DR. Regression models and Life-Tables. Journal of the Royal Statistical Society: Series B 1972;34:187–202. [Google Scholar]
- 35.Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 36.Mutlu U, Colijn JM, Ikram MA, et al. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol 2018;75:1256–63. 10.1001/jamaneurol.2018.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harper G. Linkage of maternity hospital episode statistics data to birth registration and notification records for births in England 2005–2014: quality assurance of linkage of routine data for singleton and multiple births. BMJ Open 2018;8:e017898. 10.1136/bmjopen-2017-017898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chua SYL, Thomas D, Allen N, et al. Cohort profile: design and methods in the eye and vision Consortium of UK Biobank. BMJ Open 2019;9:e025077. 10.1136/bmjopen-2018-025077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutlu U, Bonnemaijer PWM, Ikram MA, et al. Retinal neurodegeneration and brain MRI markers: the Rotterdam study. Neurobiol Aging 2017;60:183–91. 10.1016/j.neurobiolaging.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 40.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32:807–50. 10.1007/s10654-017-0321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majithia S, Tham Y-C, Chee M-L, et al. Cohort profile: the Singapore epidemiology of eye diseases study (seed). Int J Epidemiol 2021;50:41–52. 10.1093/ije/dyaa238 [DOI] [PubMed] [Google Scholar]
- 42.Khan SM, Liu X, Nath S, et al. A global review of publicly available datasets for Ophthalmological imaging: barriers to access, usability, and generalisability. Lancet Digit Health 2021;3:e51–66. 10.1016/S2589-7500(20)30240-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Age groups, 2018. Available: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/age-groups/latest#:~:text=by%20ethnicity%20Summary-,The%20data%20shows%20that%3A,aged%2060%20years%20and%20over [Accessed 15 Jul 2021].
- 44.Enoch J, McDonald L, Jones L, et al. Evaluating whether sight is the most valued sense. JAMA Ophthalmol 2019;137:1317–20. 10.1001/jamaophthalmol.2019.3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabanayagam C, Xu D, Ting DSW, et al. A deep learning algorithm to detect chronic kidney disease from retinal Photographs in community-based populations. Lancet Digit Health 2020;2:e295–302. 10.1016/S2589-7500(20)30063-7 [DOI] [PubMed] [Google Scholar]
- 46.Mitani A, Huang A, Venugopalan S, et al. Detection of anaemia from retinal fundus images via deep learning. Nat Biomed Eng 2020;4:18–27. 10.1038/s41551-019-0487-z [DOI] [PubMed] [Google Scholar]
- 47.Poplin R, Varadarajan AV, Blumer K. Prediction of cardiovascular risk factors from retinal fundus Photographs via deep learning. Nat Biomed Eng 2018;2:158–64. [DOI] [PubMed] [Google Scholar]
- 48.Wisely CE, Wang D, Henao R. Convolutional neural network to identify symptomatic Alzheimer’s disease using multimodal retinal imaging. Br J Ophthalmol (Published Online First: 26 November 2020). [DOI] [PubMed]
- 49.Cheung CY, Xu D, Cheng C-Y, et al. A deep-learning system for the assessment of cardiovascular disease risk via the measurement of retinal-vessel calibre. Nat Biomed Eng 2021;5:498–508. 10.1038/s41551-020-00626-4 [DOI] [PubMed] [Google Scholar]
- 50.Xiao W, Huang X, Wang JH, et al. Screening and identifying hepatobiliary diseases through deep learning using ocular images: a prospective, multicentre study. Lancet Digit Health 2021;3:e88–97. 10.1016/S2589-7500(20)30288-0 [DOI] [PubMed] [Google Scholar]
- 51.Wagner SK, Fu DJ, Faes L, et al. Insights into systemic disease through retinal imaging-based Oculomics. Transl Vis Sci Technol 2020;9:6. 10.1167/tvst.9.2.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng J, Dong W, Socher R. ImageNet: a large-scale hierarchical image database. 2009 IEEE Conference on Computer Vision and Pattern Recognition, 2009. [Google Scholar]
- 53.Harron K, Dibben C, Boyd J, et al. Challenges in administrative data linkage for research. Big Data Soc 2017;4:205395171774567. 10.1177/2053951717745678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Data protection by design and default. Available: https://ico.org.uk/for-organisations/guide-to-data-protection/guide-to-the-general-data-protection-regulation-gdpr/accountability-and-governance/data-protection-by-design-and-default/ [Accessed 13 Jul 2021].
- 55.Lee AY, Campbell JP, Hwang TS, et al. Recommendations for standardization of images in ophthalmology. Ophthalmology 2021;128:969–70. 10.1016/j.ophtha.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.NHS and social care data: off-shoring and the use of public cloud services. Available: https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/nhs-and-social-care-data-off-shoring-and-the-use-of-public-cloud-services [Accessed 13 Jul 2021].
- 57.Sinha S, Peach G, Poloniecki JD, et al. Studies using English administrative data (Hospital episode statistics) to assess health-care outcomes—systematic review and recommendations for reporting. Eur J Public Health 2013;23:86–92. 10.1093/eurpub/cks046 [DOI] [PubMed] [Google Scholar]
- 58.Burns EM, Rigby E, Mamidanna R, et al. Systematic review of discharge coding accuracy. J Public Health 2012;34:138–48. 10.1093/pubmed/fdr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013;346:f2350. 10.1136/bmj.f2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wood A, Denholm R, Hollings S, et al. Linked electronic health records for research on a nationwide cohort of more than 54 million people in England: data resource. BMJ 2021;373:n826. 10.1136/bmj.n826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data are available. The data are subject to the contractual restrictions of the data sharing agreements between National Health Service Digital, Moorfields Eye Hospital and University College London and are therefore not available for access beyond the AlzEye research team.