Abstract
Introduction
Few interventions exist to address the high burden of stillbirths in apparently healthy pregnant women in low- and middle-income countries (LMICs). To establish whether a trial on the impact of routine Doppler screening in a low-risk obstetric population is warranted, we determined the prevalence of abnormal fetal umbilical artery resistance indices among low-risk pregnant women using a low-cost Doppler device in five LMICs.
Methods
We conducted a multicentre, prospective cohort study in Ghana, India, Kenya, Rwanda and South Africa. Trained nurses or midwives performed a single, continuous-wave Doppler screening using the Umbiflow device for low-risk pregnant women (according to local guidelines) between 28 and 34 weeks’ gestation. We assessed the prevalence of abnormal (raised) resistance index (RI), including absent end diastolic flow (AEDF), and compared pregnancy and health service utilisation outcomes between women with abnormal RI versus those with normal RI.
Results
Of 7151 women screened, 495 (6.9%) had an abnormal RI, including 14 (0.2%) with AEDF. Caesarean section (40.8% vs 28.1%), labour induction (20.5% vs 9.0%) and low birth weight (<2500 g) (15.0% vs 6.8%) were significantly more frequent among women with abnormal RI compared with women with normal RI. Abnormal RI was associated with lower birth weights across all weight centiles. Stillbirth and perinatal mortality rates were similar between women with normal and abnormal RI.
Conclusion
A single Doppler screening of low-risk pregnant women in LMICs using the Umbiflow device can detect a large number of fetuses at risk of growth restriction and consequent adverse perinatal outcomes. Many perinatal deaths could potentially be averted with appropriate intervention strategies.
Trial registration number
CTRI/2018/07/01486.
Keywords: primary care, public health, obstetrics
Strengths and limitations of this study.
This the first multicountry study assessing the prevalence of abnormal resistance index (RI) of the fetal umbilical artery in low-risk pregnant women in low-income and middle-income countries.
All research staff who applied Umbiflow underwent a standardised training; all Doppler recordings were independently reviewed for quality assurance and the lost to follow-up in the study was low.
To reflect usual obstetric practice at each site, the definition of low-risk pregnant women was based on local guidelines, so some conditions (such as a previous caesarean section or HIV) were considered differently across sites.
The prevalence of absent end diastolic flow might be underestimated as, despite our best efforts, 64 women with abnormal RI did not attend their referral visit.
Introduction
Nearly 2 million babies are stillborn annually, and 98% of these stillbirths occur in low- and middle-income countries (LMICs).1 It is estimated that up to 50% of antepartum stillbirths can be attributed to fetal growth restriction (FGR), a pathological inhibition of fetal growth that prevents the fetus from attaining its genetic growth potential.2 FGR increases the risk of stillbirth by eightfold, and is associated with neonatal death, perinatal morbidity and non-communicable diseases into adulthood.2–7 Placental insufficiency is the leading cause of FGR, and occurs mostly as a consequence of poor uteroplacental blood flow, placental thrombi and infarctions.8 9
Despite the adverse fetal and neonatal health outcomes associated with FGR, it is not adequately detected during routine antenatal care. An estimated 74% of babies with a birth weight below the 10th centile are not detected antenatally and in low-risk pregnancies, where there is a lower threshold of suspicion, the detection rate of FGR is even lower.10–13 There is a 5-fold increase in attributable risk for stillbirth if FGR was not detected antenatally.2 Clinical techniques such as history taking and serial physical assessments for identification of growth restricted fetuses have poor predictive values and have not been shown to reduce stillbirth or perinatal mortality.14–16 Doppler ultrasound can be used to assess blood flow in fetal umbilical vessels to identify placental insufficiency, and abnormal umbilical artery flow indices (such as a raised resistance index (RI)) are correlated with FGR and adverse fetal and neonatal outcomes.17 18
Cochrane review evidence shows that the use of Doppler to detect placental insufficiency in high-risk pregnancies, in conjunction with appropriate follow-up and care, reduces perinatal mortality.19 However, there is insufficient evidence to support the routine use of Doppler ultrasound in low-risk or unselected-risk pregnant women.20
In many LMICs, antenatal care for apparently healthy, low-risk women is often delivered in settings without access to Doppler ultrasound. Umbiflow, a mobile, continuous-wave Doppler ultrasound device which can be used by midwives and nurses is one method to deliver Doppler ultrasound service where expertise for conventional ultrasound is lacking (figure 1).21 Umbiflow has been validated against pulsed-wave Doppler in commercial ultrasound systems for the detection of fetal umbilical flow abnormalities in a South African population.22
Figure 1.

The Umbiflow device (Credit: Council for Scientific and Industrial Research/South African Medical Research Council)
The prevalence of abnormal umbilical blood flow in low-risk pregnant women in LMICs, and therefore the potential benefit of the use of Doppler and detection of FGR, is unknown. A study using Umbiflow in a low-risk population of pregnant women in Mamelodi, Pretoria, South Africa reported a higher than expected prevalence of fetal umbilical flow abnormalities—11.7% of women screened had an abnormal RI and 1.5% of the women had absent end diastolic flow (AEDF).23 Women with abnormal RI were referred and managed at a referral hospital using a standardised management protocol, which resulted in 42% risk reduction in perinatal mortality. These findings have prompted the need for further observational research into the prevalence of umbilical flow abnormalities in low-risk populations in other LMIC settings.
The WHO does not currently recommend the routine use of Doppler velocimetry for low-risk antenatal populations.24 However, the WHO antenatal care guideline panel remarked that the value of routine application of single Doppler ultrasound examination of fetal blood vessels in the third trimester needs rigorous research, particularly in LMICs. To address this need, WHO embarked on an international study to determine whether the high prevalence of abnormal fetal Doppler findings reported in the South African study is present in similar populations in other LMIC settings, to establish whether a trial on the impact of routine Doppler screening in low-risk obstetric population in LMICs is warranted.
The primary objective of this study was to determine the prevalence of abnormal (raised) umbilical artery RI, including AEDF, in low-risk pregnant women between 28 and 34 weeks’ gestation in LMICs, using a single screening with the Umbiflow device. The secondary objectives were to assess the prevalence of abnormal RI by gestational age (GA); determine the pregnancy outcomes of women screened; assess the distribution of RI in women with abnormal results; and assess the effects of Doppler screening on health service utilisation outcomes.
Methods
Study design
We conducted a multicountry, multicentre, facility-based, prospective cohort study using predefined eligibility criteria in Ghana, India, Kenya, Rwanda and South Africa. This design was used because it minimised selection and reporting bias to the greatest extent possible, allowed accurate determination of both the point and period prevalence of the primary outcomes of interest (abnormal RI, including AEDF) and involved diverse women and antenatal care settings. The design also allowed the follow-up of enrolled women to achieve the secondary objectives of the study. Findings have been reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement.25.
Setting
Across five participating countries, 11 primary healthcare facilities were purposively selected to participate (three sites in India, two sites in each of the other countries). All facilities normally offer routine antenatal care to low-risk pregnant women provided by midwives. All countries used an 8-visit antenatal care model, except for Kenya which used a 4-visit antenatal care model. Each facility was provided with an Umbiflow device, a laptop computer (with Umbiflow software preinstalled) and a printer.
Study participants
The population of interest were pregnant women who received antenatal care at participating facilities during the study period. Women were eligible if they were at low risk of pregnancy complications according to local antenatal care guidelines, had an estimated GA between 28 weeks 0 days and 34 weeks 0 days (according to the best obstetric estimate),26 had a live, singleton pregnancy, were expected to deliver at the recruiting facility or within the catchment area and were willing and able to give informed consent. Local antenatal care guidelines were very similar across all study sites: women with pre-existing medical conditions (eg, type 1 or type 2 diabetes mellitus, hypertension, renal disease or other such conditions), poor obstetric history, pregnancy complications (eg, vaginal bleeding, infection, severe anaemia) or a fetus with a known congenital anomaly (chromosomal or structural) were considered high-risk and were not eligible. Pregnant women with advanced maternal age or teenagers are considered high-risk across all study sites, though age definitions vary slightly. Antenatal care guidelines in India are more stringent than the other four countries—a pregnant woman who is rhesus negative, HIV-infected or who had a previous caesarean section was considered high-risk in India, whereas in the other four countries a woman with any one of these was considered low risk.
During the recruitment period, all women attending participating antenatal clinics who were between 28 and 34 weeks’ gestation (ie, potentially eligible women) were approached by research staff and formally screened for eligibility. In higher volume facilities, where the number of potentially eligible women exceeded capacity of the research team, a random sampling method was used to approach, screen and counsel women for recruitment in order to minimise selection bias. Eligible women were counselled about the study and written informed consent was obtained prior to recruitment. Women were screened and recruited until the target sample size for the country was reached.
Patient and public involvement
Patients were not involved in the development of the protocol. During site visits, participants in the study were informally asked about their experience with the study.
Doppler assessment with Umbiflow
The Umbiflow device consists of a handheld continuous-wave Doppler probe with a universal serial bus cable that connects to a Windows-based platform (eg, laptop computer, tablet or smartphone) on which the Doppler analysis software is installed (figure 1).22 A trained research nurse or midwife performed a single Umbiflow assessment for all recruited women during their antenatal clinic visit between 28 and 34 weeks’ gestation. Training of the research staff was conducted by an expert trainer according to a standardised manual of operations in a 3-day curriculum. Based on a woman’s history and estimated due date, the Umbiflow software automatically calculates the GA. During the examination, the Umbiflow software displays the fetal umbilical artery waveform and produces an audible signal. The software automatically calculates the three routinely used and highly correlated indices (RI, pulsatility index and systolic/diastolic ratio), as well as the fetal heart rate, and plots the obtained RI against the GA as the software has RI centiles built-in.27 28
An abnormal RI was defined as RI≥75th centile for the GA of the fetus. This cut-off centile was chosen for Umbiflow based on the best correlation with perinatal mortality in a cohort of South African women with pregnancies classified as high risk.21 Women with a normal RI (ie, <75th centile for the GA) continued with their usual antenatal care. Women who had an abnormal RI, or where a RI reading could not be obtained after two separate unsuccessful attempts, were immediately referred to a higher level facility for further obstetric evaluation, including fetal growth and pulsed-wave Doppler ultrasound assessment. Women were managed according to local antenatal care policies; clinical care was not standardised across sites as the primary objective of the study was solely to determine the prevalence of abnormal Doppler. However, due to the nature of the test and its results, there was an intrinsic ethical responsibility to refer and further manage women with abnormal results. Digital recordings of all Umbiflow assessments were saved electronically and independently reviewed for quality by a clinical expert.
Primary and secondary outcomes
Primary outcomes included the prevalence of abnormal RI of the fetal umbilical artery as obtained with Umbiflow, including the prevalence of AEDF (confirmed on pulsed-wave Doppler ultrasound). Secondary outcomes included pregnancy outcomes, and health service utilisation outcomes following the Umbiflow assessment.
Data collection
All women were followed from time of recruitment until 7 days postpartum or hospital discharge after giving birth (whichever came first). Participant information, including sociodemographic characteristics, nutritional status, behavioural factors and medical and obstetric history, was obtained at recruitment through interview and medical record review. The findings of the Umbiflow assessment were documented and digital recordings saved in real time. Birth and perinatal outcomes were obtained from medical records. All data were collected using paper-based case report forms and later double-entered into a REDCap database. All data were non-identifiable, using unique, sequential participant numbers.
Sample size
We estimated that 1266 women were needed per country to detect a prevalence of 1.2% of AEDF in fetuses of women undergoing Umbiflow assessment, based on preliminary findings of Nkosi et al. in South Africa.23 With 10% loss to follow-up, about 1407 women per country were required. With five countries, the target study sample size was 7035 women.
Statistical analysis
Analysis was primarily descriptive and based on participants with outcome data available. The Shapiro-Wilk test was used to test for normality. To assess differences between women with abnormal and normal RI, the non-parametric Mann-Whitney U test was used for numerical variables and the χ2 test was used for categorical variables. The two-proportions z-test was used for cases where only certain categories were compared. The WHO multinational fetal growth charts were used for categorising birth weights according to percentiles, corrected for GA and sex.29 When comparing the cumulative percentage of birth weights according to centiles in neonates of woman with normal and abnormal RI, the two-sample Kolmogorov-Smirnov test was used. All tests were performed at a 5% level of significance.
Results
Recruitment
Between 15 October 2018 and 20 January 2020, 9191 women were screened for eligibility (figure 2). A total of 7151 women were recruited and underwent an Umbiflow assessment: 6656 women (93.1%) had a normal RI and 495 women (6.9%) had an abnormal RI. The majority of women with abnormal RI (415, 83.8%) attended their referral and underwent further obstetric evaluation, including pulsed-wave Doppler ultrasound assessment. A total of 206 recruited women (2.9%) were lost to follow-up after Umbiflow assessment (ie, pregnancy outcomes could not be obtained).
Figure 2.
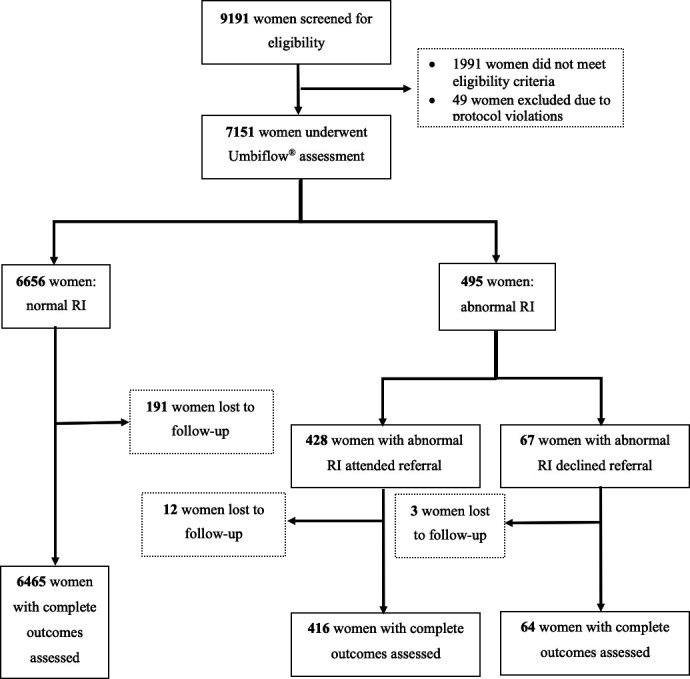
Recruitment flowchart. RI, resistance index.
Characteristics of women screened with Umbiflow
The mean maternal age was 27.4 years and one-third of the women were nulliparous (table 1). Most women (82.2%) were married or cohabitating, and 32.4% were employed at time of recruitment. Most women were on folic acid and iron supplementation; 4.4% had moderate or severe anaemia based on the most recent haemoglobin level. Overall HIV prevalence was 5.7%, largely due to the high HIV prevalence among women recruited in South Africa (20.8%). In 61.5% of the women, last menstrual period was used to estimate the GA at the time of Umbiflow assessment.
Table 1.
Characteristics of women assessed with Umbiflow
| N=7151 | |
| Woman’s age (years) mean (SD) | 27.4±5.5 |
| Marital status N (%) | |
| Married/cohabitating | 5879 (82.2) |
| Single/separated/divorced/widowed | 1262 (17.6) |
| Unknown | 10 (0.1) |
| Currently gainfully employed N (%) | 2318 (32.4) |
| Height (cm) N, mean (SD) | 5505, 157.9±6.7 |
| Weight at this visit (kg) N, mean (SD) | 6427, 66.5±13.8 |
| Mid upper arm circumference (cm) N, mean (SD) | 6513, 27.7±4.2 |
| Presence of anaemia in pregnancy based on most recent haemoglobin level N (%) | |
| Normal haemoglobin level | 3365 (58.9) |
| Mild anaemia | 2095 (36.7) |
| Moderate anaemia | 242 (4.2) |
| Severe anaemia | 11 (0.2) |
| Parity N (%) | |
| 0 | 2541 (35.5) |
| 1–2 | 3824 (53.5) |
| 3+ | 786 (11.0) |
| Gestational age at time of recruitment N (%) | |
| 28 weeks 0–28 weeks 6 days | 1083 (15.1) |
| 29 weeks 0–29 weeks 6 days | 1351 (18.9) |
| 30 weeks 0–30 weeks 6 days | 1508 (21.1) |
| 31 weeks 0–31 weeks 6 days | 1112 (15.6) |
| 32 weeks 0–32 weeks 6 days | 1044 (14.6) |
| 33 weeks 0–34 weeks 0 days | 1053 (14.7) |
| Method used to estimate gestational age N (%) | |
| Certain last menstrual period | 4396 (61.5) |
| First trimester ultrasound (up until 13 weeks 6 days) | 775 (10.8) |
| Second trimester ultrasound (14 and 27 weeks 6 days) | 1326 (18.5) |
| Third trimester ultrasound (28 weeks 0 days and beyond) | 597 (8.3) |
| Symphysis-fundal height measurement | 57 (0.8) |
| HIV status N (%) | |
| Test negative | 6690 (93.6) |
| Test positive, not on HIV medication | 21 (0.3) |
| Test positive, on HIV medication | 386 (5.4) |
| Test not done | 24 (0.3) |
| Unknown | 30 (0.4) |
Primary outcome
Of 7151 women who underwent Umbiflow assessment, 495 women had an abnormal RI giving an overall prevalence of 6.9%. The highest country-level prevalence was observed in Ghana (9.9%) and Rwanda (8.3%), and the lowest in Kenya (4.6%) (table 2). The overall prevalence of AEDF was 0.2% (14 of 7151 women). All countries had a prevalence of AEDF less than 0.2% except South Africa (0.7%). No cases of reversed end diastolic flow were identified.
Table 2.
Prevalence of abnormal resistance index by country
| Abnormal resistance index—N (%, 95% CI) | Absent end-diastolic flow—N (%) | |
| Ghana (N=1534) | 152 (9.91, CI 8.41 to 11.40) | 0 (0.00) |
| India (N=1408) | 79 (5.61, CI 4.41 to 6.81) | 1 (0.07) |
| Kenya (N=1407) | 64 (4.55, CI 3.46 to 5.64) | 1 (0.07) |
| Rwanda (N=1403) | 117 (8.33, CI 6.89 to 9.79) | 2 (0.14) |
| South Africa (N=1399) | 83 (5.93, CI 4.69 to 7.17) | 10 (0.71) |
| All (N=7151) | 495 (6.92, CI 6.33 to 7.51) | 14 (0.20) |
Secondary outcomes
Prevalence of abnormal RI by GA
The prevalence of abnormal RI by GA at time of screening varied between 5.9% and 7.9%, with no clear peak or optimal GA for identification of abnormal RI (p=0.36) (figure 3).
Figure 3.
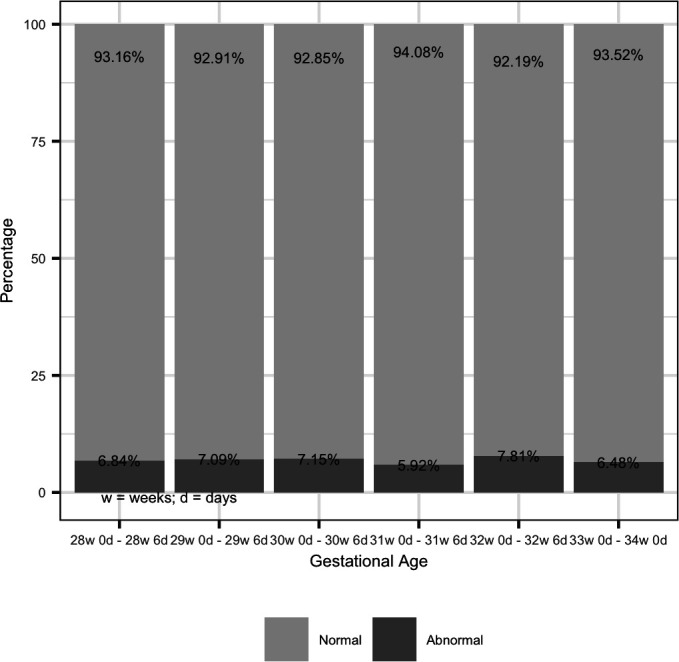
Prevalence of abnormal resistance index by gestational age.
Pregnancy outcomes
Birth outcomes were obtained for 6945 women recruited into the study: 480 women with an abnormal RI and 6465 women with a normal RI (table 3). A total of 5854 (84.3%) women experienced labour, of whom the majority had a spontaneous onset (5284, 90.3%) and 569 (9.7%) were induced. The overall caesarean section rate was 28.9%. Three women died (all of whom had a normal RI)—two were due to obstetric haemorrhage and for one woman the cause of death was unknown.
Table 3.
Birth outcomes following Doppler assessment with Umbiflow
| All women assessed N=6945 |
Abnormal RI N=480 |
Normal RI N=6465 |
P value | |
| Woman experienced labour N (%) | 5854 (84.3) | 366 (76.2) | 5488 (84.9) | <0.01 |
| Mode of onset of labour N (%) | ||||
| Spontaneous | 5284 (90.3) | 291 (79.5) | 4993 (91.0) | <0.01* |
| Induced | 569 (9.7) | 75 (20.5) | 494 (9.0) | |
| Unknown | 1 (0.0) | 0 (0.0) | 1 (0.0) | – |
| Final mode of birth N (%) | ||||
| Cephalic vaginal birth | 4793 (69.0) | 274 (57.1) | 4519 (69.9) | <0.01* |
| Breech vaginal birth | 38 (0.5) | 4 (0.8) | 34 (0.5) | – |
| Vacuum or forceps vaginal birth | 104 (1.5) | 6 (1.3) | 98 (1.5) | |
| Caesarean section | 2010 (28.9) | 196 (40.8) | 1814 (28.1) | |
| Experienced maternal complications† N (%) | 202 (2.9) | 16 (3.3) | 186 (2.9) | 0.66 |
| Admission to intensive care or special care unit N (%) | 26 (0.4) | 2 (0.4) | 24 (0.4) | – |
| Maternal death during pregnancy until 7 days postpartum N (%) | 3 (0.04) | 0 (0.00) | 3 (0.05) | – |
| Gestational age at birth | ||||
| Under 34 weeks | 118 (1.7) | 20 (4.2) | 99 (1.5) | <0.01 |
| 34 weeks up to 37 weeks | 458 (6.6) | 21 (4.4) | 437 (6.8) | 0.05 |
| 37 weeks up to 42 weeks | 5991 (86.2) | 404 (84.2) | 5587 (86.4) | 0.18 |
| 42 weeks and above | 375 (5.4) | 35 (7.3) | 341 (5.3) | 0.07 |
| Unknown | 1 (0.0) | 0 (0.0) | 1 (0.0) | – |
| Stillbirth | 65 (0.9) | 8 (1.7) | 57 (0.9) | 0.14 |
| Neonatal sex | ||||
| Male | 3655 (52.6) | 221 (46.1) | 3434 (53.1) | <0.01* |
| Female | 3286 (47.3) | 259 (54.0) | 3027 (46.8) | |
| Unknown | 4 (0.1) | 0 (0.0) | 4 (0.1) | – |
| Apgar score below 7 at 5 min | 166 (2.7) | 14 (3.4) | 152 (2.7) | 0.46 |
| Birth weight (g) | ||||
| N, mean (SD) | 6901, 3095±491 | 474, 2913±514 | 6427, 3108±486 | <0.01 |
| <2500 | 506 (7.3) | 71 (15.0) | 435 (6.8) | <0.01* |
| ≥2500 | 6395 (92.7) | 403 (85.0) | 5992 (93.2) | |
| Unknown | 44 (0.6) | 6 (1.3) | 38 (0.6) | – |
| Neonate required resuscitation at birth | 586 (8.4) | 38 (7.9) | 548 (8.5) | 0.72 |
| During the first 7 days of life, the neonate was diagnosed with a medical condition | 431 (6.2) | 41 (8.5) | 390 (6.0) | 0.02 |
| Congenital abnormality | 30 (0.4) | 4 (0.8) | 26 (0.4) | – |
| Neonate admitted to an intensive care unit or special care unit | 377 (5.4) | 44 (9.2) | 333 (5.2) | <0.01 |
| Neonatal death at 7 days or at discharge | 93 (1.3) | 9 (1.9) | 84 (1.3) | 0.43 |
*χ2 p value for this variable reported over all categories.
†Maternal complications after birth included any of the following: postpartum haemorrhage, postpartum preeclampsia/eclampsia, anaemia requiring blood transfusion, postpartum endometritis, infection of caesarean incision site or perineal laceration site, respiratory tract infection, urinary tract infection, mastitis, postpartum psychosis, deep vein thrombosis, pulmonary embolism, peripartum cardiomyopathy; percentages in parentheses.
RI, resistance index.
The majority of babies were born at term (86.2%), 8.3% were preterm (<37 weeks’ gestation) and 5.4% were post term (>42 weeks). The mean birth weight was 3095 g; 7.3% of babies were <2500 g. There were 93 perinatal deaths: 65 stillbirths and 28 early neonatal deaths (stillbirth rate of 9.4/1000 births and early neonatal death rate of 4.1/1000 live births).
Comparison of pregnancy outcomes between women with an abnormal and normal RI shows similarities in several outcomes, including frequencies of women with complications after birth, term births, Apgar score <7 at 5 min, neonatal resuscitation at birth, stillbirths and perinatal deaths. However, women with an abnormal RI were significantly more likely to give birth via caesarean section (40.8% vs 28.1%, p<0.01), have induced labours (20.5% vs 9.0%, p<0.01) and were more likely to have an early preterm birth <34 weeks’ gestation (4.2% vs 1.5%, p<0.01) than women with a normal RI. The leading indications for caesarean section in women with an abnormal RI were suspected or confirmed FGR (20.4%) and fetal distress (17.9%) (abnormal RI alone was not an indication for caesarean section across study sites), whereas in women with normal RI the leading indications were previous caesarean section (34.3%) and fetal distress (16.0%) (data not shown).
Babies of women with abnormal RI were more likely to be admitted to an intensive care or special care unit (9.2% vs 5.2%, p<0.01) but the duration of admission did not differ between the two groups. The mean birth weight was significantly lower in women with an abnormal RI (2913 g vs 3108 g, p<0.01); low birth weight (<2500 g) was significantly more frequent among women with abnormal RI compared with women with normal RI (15.0% vs 6.8%, p<0.01). Even after correction for GA at birth and neonatal sex, abnormal RI was associated with lower birth weights across all weight centiles (p<0.0001) (figure 4).
Figure 4.
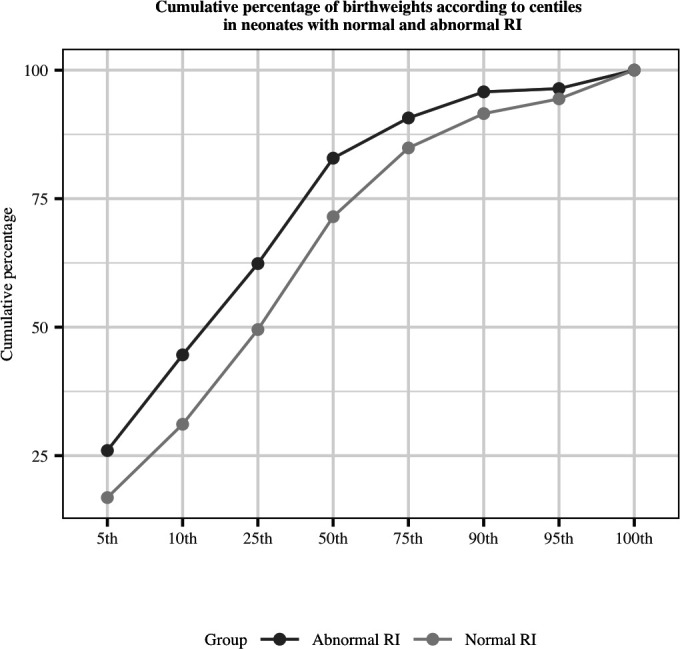
Cumulative percentage of birth weights according to centiles in neonates of women with normal and abnormal resistance index (RI).
RI thresholds for identifying fetuses at increased risk of perinatal mortality
We were unable to identify a specific RI threshold associated with increased risk of perinatal mortality due to few events.
The effect of the screening with the Umbiflow device on utilisation of health service
Women in the abnormal RI group were more likely to have antenatal investigations—such as additional ultrasounds, blood tests or cardiotocography—following Umbiflow screening. 79.5% of these women had 4 or more investigations versus 65.3% of women with a normal RI (p<0.01) (table 4). The median number of antenatal investigations per woman in the abnormal RI group was 6 versus 5 in the normal RI group (p<0.01). Women with an abnormal RI had more antenatal visits than women with a normal RI: 3 versus 2, respectively (p<0.01).
Table 4.
Health service utilisation outcomes
| All N=6945 |
Abnormal RI N=480 |
Normal RI N=6465 |
P value | |
| Number of antenatal investigations* per woman after Umbiflow assessment median (IQR) | 5 (3, 7) | 6 (4, 9) | 5 (3, 7) | <0.01 |
| four or more antenatal investigations* after Umbiflow assessment N (%) | 4494 (66.3) | 381 (79.5) | 4113 (65.3) | <0.01 |
| Number of antenatal care visits per woman since Umbifow assessment N, median (IQR) | 6746, 2 (1, 3) | 472, 3 (2, 4) | 6274, 1 (1, 3) | <0.01 |
*Antenatal investigations included any of the following: full blood count, blood type, haemoglobin electrophoresis, urinalysis, urine culture, rubella test, syphilis test, HIV test, hepatitis B test, hepatitis C test, glucose tolerance test, ultrasound examination, full biophysical profile, amniocentesis, antenatal cardiotocography, labour admission cardiotocography, continuous cardiotocography during labour.
RI, resistance index.
Discussion
Key findings
In this multicountry prospective cohort study of low-risk pregnant women in five LMICs, we found a 6.9% prevalence of abnormal RI of the fetal umbilical artery, and an overall AEDF prevalence of 0.2%. All countries in this study had a prevalence of AEDF below 0.2%, except South Africa with an AEDF prevalence of 0.7%. The prevalence of abnormal RI was reasonably equally distributed across 28–34 weeks’ gestation. Women with abnormal RI were more likely to receive obstetric interventions such as caesarean section and labour induction, and had a higher frequency of antenatal investigations and clinic visits. While stillbirth and perinatal mortality rates were similar between women with abnormal and normal RI, we found that abnormal RI was associated with lower birth weights across all weight centiles, after correcting for neonatal sex and GA at birth.
Interpretation
The prevalence of abnormal RI in this study was slightly lower than expected compared with previous South African data as reported by Nkosi et al.23 Another study using Umbiflow in 9 centres in South Africa by Hlongwane et al. found a 12.5% prevalence of abnormal RI, including AEDF prevalence of 1.2%.30 The reason for the higher prevalence in pregnant women in South Africa is not yet known; however, it is possible that the higher HIV prevalence in this setting may play a role.
Even though this study did not find a high prevalence of AEDF, we did detect nearly 500 fetuses with placental insufficiency at risk of FGR and therefore at risk of adverse perinatal outcomes. These fetuses were smaller at birth, irrespective of the GA at which they were born. The leading indications for caesarean section in women with abnormal RI were FGR and fetal distress, both of which are suggestive of underlying placental insufficiency. Abnormal RI alone was not an indication for caesarean section across study sites; however, women who had an abnormal RI were referred to a higher level of care where they received further intervention such as ultrasound. Thus, it was not surprising there were more investigations and interventions in the group with an abnormal RI, and these interventions might have prevented perinatal deaths.
Using conventional ultrasound, an estimated fetal weight below the 10th centile for the GA is generally used to diagnose FGR. However, this approach does not identify fetuses who are appropriate for GA, but did not reach their genetic growth potential. Furthermore, to diagnose FGR using ultrasound criteria, serial ultrasound examinations may be required, and we need to acknowledge that in LMICs, low-risk healthy pregnant women often do not have access to conventional imaging ultrasound (either single or serial ultrasound examinations).31 Previous research has also demonstrated that even when conventional ultrasound is made available in LMICs, stillbirth or neonatal mortality rates will not necessarily improve.32 These findings suggest that Umbiflow can help detect those fetuses with placental insufficiency at risk of FGR (across all weight centiles) and not just fetuses with an estimated fetal weight below the 10th centile. It can therefore assist in differentiating between the truly growth restricted and not growth restricted fetus, rather than the ‘small’ and ‘not-small’ fetus. Umbiflow can be implemented at primary healthcare facilities, and be done by healthcare workers of all levels as it does not require advanced obstetric ultrasound expertise.
Strengths and limitations
To our knowledge, this is the first multicountry study assessing the prevalence of abnormal RI of the fetal umbilical artery in low-risk pregnant women in LMICs. All research staff who applied Umbiflow underwent a standardised training, and all Doppler recordings were independently reviewed for quality assurance. Overall, the lost to follow-up in the study was low (2.9%). Nonetheless, our study has some limitations. First, the definition of low-risk pregnant women was based on local guidelines; we did not mandate a specific risk screening protocol across all sites. While this was done to be pragmatic and reflect usual obstetric practice at each site, some conditions (such as a previous caesarean section or HIV) were considered differently across sites. Second, the prevalence of AEDF might be underestimated as, despite our best efforts, 64 women with abnormal RI did not attend their referral visit. The 75th centile cut-off was chosen as it was the best predictor of perinatal morbidity and mortality in a referral hospital and in a low-risk population this cut-off detected approximately 10% of fetuses.23 However, secondary analyses are planned to investigate different cut-offs. Last, we acknowledge that FGR and Doppler abnormalities can arise beyond 34 weeks’ gestation. For this study, a single screening was chosen to determine the prevalence and guide further research. The screening time was selected between 28 and 34 weeks’ gestation because there were insufficient neonatal services in the countries to manage neonates under 28 weeks’ gestation if delivery was required immediately; and the peak incidence of small-for-gestational-age stillbirths was 34–37 weeks’ gestation, allowing time to intervene prior to a stillbirth.33
Implications for policy, practice and research
This study demonstrates that a single Doppler screening with Umbiflow between 28 and 34 weeks’ gestation in low-risk pregnant women in LMICs can detect a large number of fetuses who are at risk of FGR and adverse perinatal outcomes that may otherwise not have been detected. The Umbiflow device is inexpensive and can be used by healthcare providers at lower levels of care and thus can be used to screen pregnant populations on a large scale to identify previously undetected FGR. Randomised trials that embed intervention strategies with Doppler screening in low-risk women in LMICs are urgently needed to assess impact on priority outcomes, and to inform clinical practice.
Conclusion
This study shows that screening a low-risk pregnant population with Umbiflow detects a large number of fetuses with placental insufficiency and who were at risk of FGR. This high prevalence warrants further research into large-scale implementation so, with appropriate referral and intervention, perinatal mortality and morbidity could potentially drastically be decreased, especially in LMICs.
Supplementary Material
Acknowledgments
We would like to thank all women who participated in the study. We also thank all research assistants, nurses and midwives for the successful conduct of the study (Ghana: Mabel Osei-Wusu, Maame Akosua Asante, Constance Nkansah, Lilian Nkonu, Dorcas Agbeke, Lydia Anku, Sarah Darko, Zuleihatu Nakobu, Gertrude Ashong, Bridget Vida Kodzo, Nancy Otabil, Christopher Debrah Alpha; India: Jyoti Patil, Mariya Nadakatti, Laxmibai Teli, Renuka Dombar, Kamala Hugar, Mallamma Talikoti; Kenya: Paschalia Ndolo, Amina Hassan, Brenda Yator, Wilfred Brunei, Maureen Achieng; Rwanda: Gerald Kaberuka, Jean Bosco Karangwa; South Africa: Suzan Mogale, Agnes Sefatjana). We thank all country data managers and their data management and data entry teams for their contributions to high quality data: Chris Guure (Ghana), Johan Adriaan Pretorius (South Africa), Amit Revankar (India), Mark Sigei (Kenya), Louange Gutabarwa Twahirwa (Rwanda). We thank Dr Padmaja Walvekar, Dr Sphoorthi Mastiholi and Dr Manjunath Somannavar for their valuable contributions to study implementation in India. Thanks to all obstetricians who reviewed and followed up the women with abnormal Umbiflow results. Special thanks to Dr Abiodun Adanikin (WHO consultant) for his support in general study oversight, Dr Chrystelle Wedi for preparing the first draft of the protocol with OTO, the SAMRC for their support in UmbiflowTM research and the CSIR for the providing of the UmbiflowTM devices and technical support.
Footnotes
Contributors: This study was conceived by OTO. OTO and JPV coordinated the writing of the study protocol, with input from the country principal investigators. VV prepared the statistical analysis plan and led statistical analysis with TB. All country principal investigators (RA, EM, SSG, YP, AK, UC, ZPQ, AO, GG, SR, RCP, VV) were part of the Umbiflow International Study steering group and led the study with support from the co-investigators in each country. The Umbiflow International Study steering group reviewed and interpreted the final data at a workshop convened by WHO. The first draft of the manuscript was prepared by VV, with substantial input from JPV, RCP and OTO. All authors reviewed and revised the manuscript draft critically for intellectual content and approved the final manuscript for publication. VV, JPV, RCP and OTO are the guarantors of this study. The manuscript represents the views of the named authors only.
Funding: The study was funded by the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored programme executed by the WHO.
Competing interests: The South African Medical Research Council (SAMRC)/University of Pretoria (UP) Maternal and Infant Healthcare Strategies Unit (VV, RCP) has previously received funding from SAMRC and the Council for Scientific and Industrial Research (CSIR) for Umbiflow research done by Nkosi et al. and Hlongwane et al. The CSIR provided the Umbiflow Doppler probes and Umbiflow software used in this study. As a satellite research unit, the SAMRC Maternal and Infant Healthcare Strategies Unit receives research funding from the SAMRC.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Request for access to these data can be made to the WHO through srhmph@who.int. Data sharing with any individual or organisation will be subject to WHO data sharing policy.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was reviewed and approved by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) Research Projects Review Panel (RP2) (A65924) and the WHO Ethics Review Committee (ERC0003034). The study was further approved by the following institutional ethics committees in participating countries: Ghana Health Service Ethics Review Committee, KLE Academy of Higher Education and Research Institutional Ethics Committee, Indian Council of Medical Research (Health Ministry’s Screening Committee), Kenyatta National Hospital – University of Nairobi Ethics and Research Committee, Rwanda National Ethics Committee and University of Pretoria Faculty of Health Sciences Research Ethics Committee. Participants gave informed consent to participate in the study before taking part. All activities were conducted conform the Declaration of Helsinki.
References
- 1.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME) . A neglected tragedy: the global burden of stillbirths. New York: United Nations Children’s Fund, 2020. [Google Scholar]
- 2.Gardosi J, Madurasinghe V, Williams M, et al. Maternal and fetal risk factors for stillbirth: population based study. BMJ 2013;346:f108. 10.1136/bmj.f108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz J, Lee AC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013;382:417–25. 10.1016/S0140-6736(13)60993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre S, Blair E, Badawi N, et al. Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstet Gynecol 2013;122:869–77. 10.1097/AOG.0b013e3182a265ab [DOI] [PubMed] [Google Scholar]
- 5.Bukowski R, Burgett AD, Gei A, et al. Impairment of fetal growth potential and neonatal encephalopathy. Am J Obstet Gynecol 2003;188:1011–5. 10.1067/mob.2003.233 [DOI] [PubMed] [Google Scholar]
- 6.Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Semin Perinatol 2008;32:213–8. 10.1053/j.semperi.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73. 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salafia CM, Minior VK, Pezzullo JC, et al. Intrauterine growth restriction in infants of less than thirty-two weeks' gestation: associated placental pathologic features. Am J Obstet Gynecol 1995;173:1049–57. 10.1016/0002-9378(95)91325-4 [DOI] [PubMed] [Google Scholar]
- 9.Alberry M, Soothill P. Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed 2007;92:F62–7. 10.1136/adc.2005.082297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sovio U, White IR, Dacey A, et al. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the pregnancy outcome prediction (POP) study: a prospective cohort study. Lancet 2015;386:2089–97. 10.1016/S0140-6736(15)00131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueras F, Gardosi J. Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 2011;204:288–300. 10.1016/j.ajog.2010.08.055 [DOI] [PubMed] [Google Scholar]
- 12.Hepburn M, Rosenberg K. An audit of the detection and management of small-for-gestational age babies. Br J Obstet Gynaecol 1986;93:212–6. 10.1111/j.1471-0528.1986.tb07895.x [DOI] [PubMed] [Google Scholar]
- 13.Backe B, Nakling J. Effectiveness of antenatal care: a population based study. Br J Obstet Gynaecol 1993;100:727–32. 10.1111/j.1471-0528.1993.tb14263.x [DOI] [PubMed] [Google Scholar]
- 14.Bais JMJ, Eskes M, Pel M, et al. Effectiveness of detection of intrauterine growth retardation by abdominal palpation as screening test in a low risk population: an observational study. Eur J Obstet Gynecol Reprod Biol 2004;116:164–9. 10.1016/j.ejogrb.2004.01.037 [DOI] [PubMed] [Google Scholar]
- 15.Mufenda J, Gebhardt S, van Rooyen R, et al. Introducing a Mobile-Connected umbilical Doppler device (UmbiFlow™) into a primary care maternity setting: does this reduce unnecessary referrals to specialised care? results of a pilot study in Kraaifontein, South Africa. PLoS One 2015;10:e0142743. 10.1371/journal.pone.0142743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert Peter J, Ho JJ, Valliapan J, et al. Symphysial fundal height (SFH) measurement in pregnancy for detecting abnormal fetal growth. Cochrane Database Syst Rev 2015;9:CD008136. 10.1002/14651858.CD008136.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society for Maternal-Fetal Medicine Publications Committee, Berkley E, Chauhan SP, et al. Doppler assessment of the fetus with intrauterine growth restriction. Am J Obstet Gynecol 2012;206:300–8. 10.1016/j.ajog.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 18.Salafia CM, Pezzullo JC, Minior VK, et al. Placental pathology of absent and reversed end-diastolic flow in growth-restricted fetuses. Obstet Gynecol 1997;90:830–6. 10.1016/S0029-7844(97)00473-0 [DOI] [PubMed] [Google Scholar]
- 19.Alfirevic Z, Stampalija T, Gyte GM. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev 2013;11:CD007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alfirevic Z, Stampalija T, Medley N. Fetal and umbilical Doppler ultrasound in normal pregnancy. Cochrane Database Syst Rev 2015;4:CD001450. 10.1002/14651858.CD001450.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugo EJC, Odendaal HJ, Grove D. Evaluation of the use of umbilical artery Doppler flow studies and outcome of pregnancies at a secondary Hospital. J Matern Fetal Neonatal Med 2007;20:233–9. 10.1080/14767050601134926 [DOI] [PubMed] [Google Scholar]
- 22.Theron GB, Theron AM, Odendaal HJ, et al. Comparison between a newly developed PC-based Doppler umbilical artery waveform analyser and a commercial unit. S Afr Med J 2005;95:62–4. [PubMed] [Google Scholar]
- 23.Nkosi S, Makin J, Hlongwane T, et al. Screening and managing a low-risk pregnant population using continuous-wave Doppler ultrasound in a low-income population: a cohort analytical study. S Afr Med J 2019;109:347–52. 10.7196/SAMJ.2019.v109i5.13611 [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization . WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, 2016. [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007;85:867–72. 10.2471/BLT.07.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee opinion no 700: methods for estimating the due date. Obstet Gynecol 2017;129:e150–4. 10.1097/AOG.0000000000002046 [DOI] [PubMed] [Google Scholar]
- 27.Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol 2013;41:233–9. 10.1002/uog.12371 [DOI] [PubMed] [Google Scholar]
- 28.Pattinson RC, Theron GB, Thompson ML, et al. Doppler ultrasonography of the fetoplacental circulation--normal reference values. S Afr Med J 1989;76:623–5. [PubMed] [Google Scholar]
- 29.Kiserud T, Piaggio G, Carroli G, et al. The world Health organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med 2017;14:e1002220. 10.1371/journal.pmed.1002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hlongwane T, Cronje T, Nkosi B, et al. The prevalence of abnormal Doppler's of the umbilical artery in a low-risk pregnant population in South Africa. EClinicalMedicine 2021;34:100792. 10.1016/j.eclinm.2021.100792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 2016;48:333–9. 10.1002/uog.15884 [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg RL, Nathan RO, Swanson D, et al. Routine antenatal ultrasound in low- and middle-income countries: first look - a cluster randomised trial. BJOG 2018;125:1591–9. 10.1111/1471-0528.15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavin T, Preen DB, Pattinson R. Timing and cause of perinatal mortality for small-for-gestational-age babies in South Africa: critical periods and challenges with detection. Matern Health Neonatol Perinatol 2016;2:11. 10.1186/s40748-016-0039-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Request for access to these data can be made to the WHO through srhmph@who.int. Data sharing with any individual or organisation will be subject to WHO data sharing policy.


