Abstract
The interpretation of end points in azole antifungal drug susceptibility testing is problematic, in part due to incomplete growth inhibition of Candida species. Such trailing growth can cause the MICs of fluconazole for some isolates to be low (<1 μg/ml) after 24 h of growth but much higher (>64 μg/ml) after 48 h. Isolates having this type of growth have been described as having a low-high phenotype. Although these isolates would be considered resistant by current National Committee of Clinical Laboratory Standards definitions, growing evidence suggests that they are susceptible in vivo. To further characterize these isolates in vitro, microdilution susceptibility testing comparing the complex defined medium RPMI 1640 to a defined minimal medium (yeast nitrogen broth) was performed. Isolates having trailing growth in MOPS (morpholinepropanesulfonic acid)-buffered RPMI 1640 (pH 7.0) were found to have clear end points in the minimal medium at its native pH of 4.5. The pH of the medium influenced the low-high phenotype, as these same isolates trailed in minimal medium adjusted to a pH of ≥6.0 but did not trail in RPMI 1640 adjusted to a pH of ≤5.0. This pH effect was independent of the medium buffering capacity, as trailing was decreased in both minimal medium and RPMI 1640 (pH 4.5) buffered in citrate. Adjustment in the pH of MOPS-buffered RPMI 1640 reduced trailing in multiple strains of Candida albicans without affecting the MICs for isolates having known susceptible (low-low) and resistant (high-high) phenotypes. Adjustment of the medium pH could be considered to eliminate trailing in azole drug susceptibility testing.
Antifungal drug resistance has become increasingly important as yeasts have become a leading cause of nosocomial bloodstream infection in the United States (7). The development of a standardized method for antifungal susceptibility testing of yeasts by the National Committee for Clinical Laboratory Standards (NCCLS) (5) was a major advance which has facilitated both the development of interpretive breakpoints for in vivo resistance and epidemiological and molecular studies of drug resistance (11, 12, 16).
The approved-level version of the NCCLS document for antifungal susceptibility testing, document M27-A, includes broth macrodilution and microdilution methods for the determination of azole susceptibility. Although these methods have been found to be reproducible in many laboratories, the optimal end point turbidity and duration of incubation have yet to be established (10). Concern has arisen over the phenomenon of trailing growth in serial dilution testing, in which reduced but persistent growth of Candida species in high concentrations of an azole antifungal agent confuses end point determination. The NCCLS M27-A document addresses this problem by defining resistant isolates as those not achieving an 80% reduction in growth relative to the growth control in macrodilution testing and those not achieving a “prominent reduction in turbidity” in microdilution testing after 48 h in the presence of specified high concentrations of azole antifungal agents (5). For some isolates, however, trailing growth is so significant that the MICs for these isolates will appear to be low after 24 h but much higher after 48 h. For example, in the case of fluconazole, isolates for which the MICs are <4 μg/ml at 24 h and >64 μg/ml at 48 h have been described (10). These MICs are so discordant that they place the isolate into different MIC interpretive categories at the two time points, and we refer the isolates as having a low-high MIC phenotype. While the true relevance of such discordant MICs is as yet unclear, current evidence suggests that the lower MIC correlates most closely with the outcome in vivo. Isolates with the low-high phenotype obtained from the mouths of HIV-infected patients with oropharyngeal candidiasis appeared to respond clinically to fluconazole administration in the same fashion as susceptible yeasts (8). In another study, mice were infected with susceptible, resistant, and low-high-phenotype isolates. The low-high-phenotype isolates responded to fluconazole therapy in the same manner as the susceptible isolates, despite the fact that the NCCLS M27-A MIC of fluconazole for those isolates was >64 μg/ml (10).
The cause(s) of trailing growth has not been determined. Previous studies have documented that inoculum size, incubation time, medium pH, and buffer concentration can significantly alter susceptibility results, but studies have not specifically addressed trailing growth (14). In this study, we examined the effects of different media on the trailing behavior of clinical isolates that have been found to trail in vitro but that act as susceptible isolates in an animal model (10).
MATERIALS AND METHODS
Strains and media.
The strains used in this study are listed in Table 1. All are clinical isolates, from both patients with oropharyngeal candidiasis and patients with candidemia (10). Many of the isolates were previously used in a study examining the low-high phenotype in an animal model (10). The isolates were stored at −70°C and subcultured on yeast extract peptone dextrose agar (10 g of yeast extract, 20 g of peptone, 20 g of glucose, 20 g of agar per liter) prior to antifungal susceptibility testing. All isolates had previously undergone fluconazole susceptibility testing by the M27-A microdilution procedure and were classified as having a low-low, high-high, or low-high fluconazole MIC phenotype, according to the definitions described below.
TABLE 1.
Results of MIC testing of multiple Candida speciesa
| Isolate | Species | 24-/48-h MIC
|
MIC phenotype
|
||
|---|---|---|---|---|---|
| YAD | RPMI | YAD | RPMI | ||
| 707-15 | C. albicans | 0.5/4 | 0.5/>64 | L/L | L/H |
| 630-11 | C. albicans | 2/4 | 2/>64 | L/L | L/H |
| 623-11 | C. albicans | 2/2 | 2/>64 | L/L | L/H |
| 578-8 | C. albicans | 2/2 | 2/>64 | L/L | L/H |
| 572-10 | C. albicans | 4/8 | 2/>64 | L/L | L/H |
| 34-504-040 | C. albicans | 2/2 | 2/64 | L/L | L/H |
| 34-028-117 | C. albicans | 2/2 | 1/>64 | L/L | L/H |
| 630-20 | C. albicans | 2/4 | 2/>64 | L/L | L/H |
| 34-023-096 | C. albicans | 2/2 | 1/>64 | L/L | L/H |
| 34-013-027 | Candida parapsilosis | 8/16 | 2/8 | L/L | L/L |
| 572-9 | C. parapsilosis | 16/8 | 64/>64 | L/L | H/H |
| 34-013-0129 | C. parapsilosis | 1/4 | 64/>64 | L/L | H/H |
| 572-36 | C. tropicalis | 0.5/8 | 0.25/64 | L/L | L/H |
| 572-35.1 | C. tropicalis | 2/2 | 4/>64 | L/L | L/H |
| A9763 | Saccharomyces cerevisiae | 16/16 | 8/16 | L/L | L/L |
| 707-9 | Candida lipolytica | 64/>64 | 64/>64 | H/H | H/H |
| Y533 | C. lusitaniae | 4/4 | 1/>64 | L/L | L/H |
| 34-013-028 | C. lusitaniae | 0.25/4 | 64/>64 | L/L | H/H |
| 559-7.1 | C. lusitaniae | 8/16 | 4/8 | L/L | L/L |
| 625-4 | Candida glabrata | 3/8 | 64/>64 | L/L | H/H |
| 625-4 | C. glabrata | 64/>64 | 16/64 | H/H | H/H |
| 572-32 | C. glabrata | 64/>64 | 16/64 | H/H | H/H |
| 630-10 | Candida krusei | 64/>64 | 64/64 | H/H | H/H |
| A6258 | C. krusei | 64/>64 | 64/>64 | H/H | H/H |
| 9013-8 | C. krusei | 64/64 | 64/>64 | H/H | H/H |
Shown are 24- and 48-h MICs and the MIC phenotype (as defined in Materials and Methods) in unbuffered YAD (pH 4.5) and MOPS-buffered RPMI 1640 (pH 7.0). Note that all isolates having a low-high (L/H) phenotype in RPMI 1640 had a low-low (L/L) phenotype in YAD. Isolate 707-15, used in many of the experiments in this study, was tested in an animal model and was found to respond as a fluconazole-susceptible isolate (10). Isolates having MICs that resulted in discrepant susceptibility interpretations (L/L versus high-high [H/H] phenotypes) are highlighted in boldface.
Susceptibility testing was performed in RPMI 1640 (supplemented with glutamine [American Bioorganics, Niagara Falls, N.Y.]) and yeast nitrogen base with ammonium and dextrose (YAD; 1.7 g of yeast nitrogen base without ammonium sulfate or amino acids, 5 g of ammonium sulfate, 5.4 g of dextrose per liter). RPMI 1640 and YAD were adjusted to pH values ranging from 4.0 to 7.0, using NaOH or HCl. As noted in the text, buffering was achieved with morpholinepropanesulfonic acid (MOPS; 0.165 M) and, in specific experiments, with 10 mM citrate (Sigma, St. Louis, Mo.).
Susceptibility testing and definitions.
Fluconazole powder of known potency was obtained from Pfizer (New York City, N.Y.). Susceptibility to fluconazole was tested by the NCCLS broth macrodilution and microdilution methods (5). In microdilution testing, growth was measured with an automated microtiter reader (EIA Autoreader, Bio-Tek Instruments) at a wavelength of 530 nm after 24 and 48 h of growth. The optical density of the drug- and fungus-free control well was subtracted from all test readings, and the ratio of growth with the drug to growth without the drug was computed at each drug concentration. The MIC was computed as the lowest concentration of fluconazole to cause an 80% inhibition of growth relative to that of the drug-free control (MIC80).
Isolates for which the fluconazole MICs at 24 and 48 h were ≤16 μg/ml were defined as having a low-low phenotype. Isolates for which the MICs at 24 and 48 h were >8 and ≥32 μg/ml, respectively, were defined as having a high-high phenotype. Isolates for which the 24-h MIC was ≤8 μg/ml and the 48-h MIC was ≥32 μg/ml were defined as having the low-high phenotype. This low-high phenotype thus most accurately describes isolates that have trailing end points, or discordant MIC interpretations, after 24 and 48 h of growth.
RESULTS
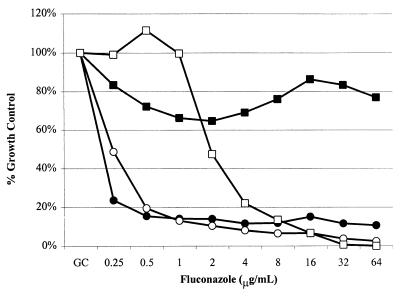
Susceptibility behavior was tested for the trailing isolate 707-15 in MOPS-buffered RPMI 1640 at pH 7.0 and in YAD lacking supplemental buffer unbuffered at pH 4.5. Isolate 707-15 was previously tested in an animal model and found to respond as a susceptible isolate (10). As is seen in Fig. 1, this isolate has a low-high phenotype in MOPS-buffered RPMI 1640 but it has a low-low phenotype in unbuffered YAD.
FIG. 1.
Effect of medium on trailing. The susceptibility of isolate 707-15 to fluconazole was tested in RPMI 1640 (solid symbols) and YAD (open symbols), using the microtiter version of the M27-A method, as described in the text. Relative growth (y axis) is calculated as the percentage of growth (optical density at 530 nm) relative to growth in a drug-free well (Growth Control) for serial dilutions of fluconazole (x axis). The squares represent 48-h readings, and the circles represent 24-h readings of the same plate. The results represent the means from experiments performed in triplicate.
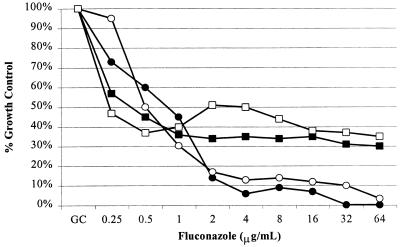
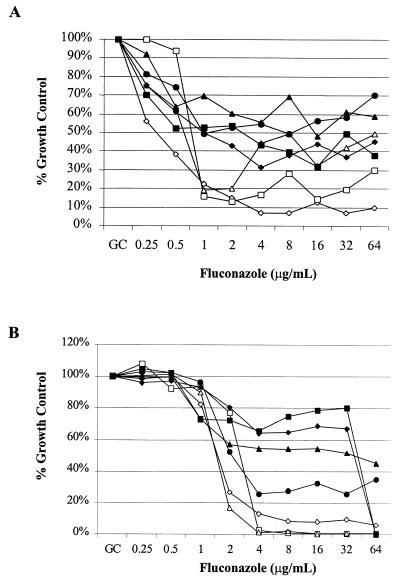
To determine if a different pH was the cause of the change in phenotype, the susceptibility behavior of the Candida albicans isolate (707-15) was determined in MOPS-buffered RPMI 1640 adjusted to a pH of 4.0 and in unbuffered YAD adjusted to a pH of 7.0 (Fig. 2). The low-low phenotype was seen in both MOPS-buffered RPMI 1640 and unbuffered YAD with a pH of 4.0, and the low-high phenotype occurred in both media at a pH of 7.0. The effect of the medium pH was further explored by performing susceptibility measurements in MOPS-buffered RPMI 1640 and unbuffered YAD at a range of pH values (Fig. 3). Figure 3 shows the behavior of the trailing isolate 707-15 in the two different medium pH arrays. In these studies, isolate 707-15 clearly has less inhibition of growth after 48 h in medium having a high pH. The low-high phenotype was observed at a pH of ≥5.0 in RPMI 1640 (Fig. 3A) and ≥5.5 in YAD (Fig. 3B).
FIG. 2.
Effect of medium pH on trailing. C. albicans isolate 707-15 was grown for 48 h in MOPS-buffered RPMI 1640 (solid symbols) and unbuffered YAD (open symbols), as described in the text. Relative growth (y axis) is calculated as the percentage of growth (optical density at 530 nm) relative to growth in a drug-free well (Growth Control) for serial dilutions of fluconazole (x axis). Significant trailing was seen with both media adjusted to a pH of 7.0 (squares) but not in media adjusted to a pH of 4.0 (circles).
FIG. 3.
pH arrays in RPMI 1640 and YAD. C. albicans trailing isolate 707-15 was used to assess trailing in RPMI 1640 (A) and YAD (B) adjusted to pHs 7 (solid squares), 6.5 (solid diamonds), 6.0 (solid triangles), 5.5 (solid circles), 5.0 (open triangles), 4.5 (open squares), and 4.0 (open diamonds). All media were buffered in MOPS (0.165 M) and incubated with shaking at 33°C. Relative growth (y axis) is calculated as the percentage of growth (optical density at 530 nm) relative to growth in a drug-free well (Growth Control), for serial dilutions of fluconazole (x axis). Shown are the results of growth after 48 h.
The susceptibility to fluconazole was determined for multiple C. albicans and non-albicans Candida species, using both unbuffered YAD at its native pH of 4.5 and MOPS-buffered RPMI 1640 at pH 7.0 (Table 1). The low-high phenotype of all C. albicans and Candida tropicalis isolates was eliminated in unbuffered YAD at a pH of 4.5. The fluconazole MICs determined in the two media were similar for most isolates, with the exceptions shown.
Since the elimination of trailing was observed in media that lacked supplemental buffer and with a low pKa, it was possible that the change in trailing behavior merely reflected a lack of buffering capacity in the medium. A decrease in medium pH after 24 h of growth in unbuffered medium might have been the cause of the subsequent growth inhibition. This hypothesis was tested by buffering YAD and RPMI 1640 with 10 mM citrate, a buffer with a pKa of 4.77, and adjusting the pHs of both media to 4.5. The behavior of the isolate did not change relative to that in medium lacking citrate buffer. In MOPS-buffered RPMI 1640 at pH 4.5, the MIC for 707-15 after 48 h of growth was 1.0 (Fig. 3A), and in citrate-buffered RPMI 1640 at pH 4.5, the MIC was 2 (data not shown). The 48-h MIC for 707-15 was 4.0 in both MOPS-buffered YAD at pH 4.5 (Fig. 3B) and YAD buffered with citrate at pH 4.5 (data not shown). As was noted in Fig. 3, the 48-h MICs for the same isolate in both media at a pH of 7.0 was greater than 64 μg/ml. Thus, the inhibition of trailing growth in media having low pH is consistent and independent of buffer and buffering capacity.
Susceptibility testing was repeated for the low-high-phenotype isolate (707-15) by the macrodilution method. Growth inhibition was clearly much more visible “by the naked eye” when the testing was performed in citrate-buffered RPMI 1640 (pH 4.5) than when it was performed in MOPS-buffered RPMI 1640 (pH 7.0), and the end points were consistent with those found by the microdilution method (data not shown).
DISCUSSION
Although the development of a standardized method for antifungal susceptibility testing and the establishment of breakpoints for fluconazole susceptibility mark major advances in the field of medical mycology, the complexities of susceptibility testing are numerous and optimization of the methodology is ongoing. Although the standardized NCCLS method has been found to have good interlaboratory reproducibility, many of the variables (time, medium, and end point) were adopted arbitrarily (9). The trailing phenomenon demonstrates an in vitro-in vivo discrepancy that might be eliminated by subtle alterations in the susceptibility-testing protocol. Previous authors have verified that isolates having a low-high phenotype are susceptible in vivo despite the fact that the 48-h fluconazole MIC for these isolates is >64 μg/ml. Revankar et al. found that episodes of oropharyngeal candidiasis caused by such C. albicans isolates in patients with HIV infection respond to low doses (100 mg/day) of fluconazole (8). In a mouse model, low-high-phenotype C. albicans isolates respond to fluconazole administration in a manner that is equivalent to those having a low-low MIC phenotype (10). In this study, these same isolates were found to have clear susceptible MIC end points when testing was performed in the minimal medium YAD. Further experiments showed that the critical factor changing this behavior is medium pH. This observation not only has potential importance in the interpretation and development of antifungal susceptibility testing, it also yields important information pertaining to azole susceptibility mechanisms and Candida physiology.
The complexity of antifungal susceptibility testing is illustrated by the observations that inoculum size, incubation time, temperature, presence of shaking, and choice of medium can alter susceptibility results (1, 9). Previous studies have found that the medium pH can alter the azole MICs for Candida species. An acidic pH has been noted to increase the MICs of fluconazole for selected Candida species in several studies (3, 6, 13). In this study, few isolates had a minimal (twofold) increase in MIC under acidic conditions, but these findings did not change the classification of the isolates from susceptible to resistant. Of interest is the fact that this observation appears to be repeated in specific Candida species. For instance, several isolates of Candida lusitaniae behaved in this manner in this study and in that by Peng and Galgiani (6). The slight increase in MIC in YAD may be the result of the difference in medium composition. In contrast, other isolates in this study were found to have increased MICs in RPMI 1640 compared to those in YAD, which appeared to be unrelated to the trailing effect (Table 1). Whether these isolates are in fact susceptible or resistant to fluconazole in vivo, or why there appears to be a difference between Candida species, is as yet unknown.
The results of these experiments suggest that acidic conditions decrease the trailing effect in all isolates, independent of the medium tested or the buffering capacity. Multiple previous studies have found that high concentrations of buffer (MOPS) result in increased MICs (2, 14, 15). These findings are consistent with our study in that low concentrations of buffer may result in changes in pH during incubation, thus decreasing the trailing effect at a 48-h MIC reading.
Although buffered medium at a pH of 7.0 has been accepted as standard, it is not yet clear that this pH is optimal with regard to clinical relevance. McIntyre and Galgiani previously found that cilofungin MICs were highly variable in medium having a pH of 7.0 and much less varied when tested at a pH of 3. Animal studies subsequently suggested that the lower pH more accurately predicted the clinical outcome (4). The finding that trailing can be decreased under acidic conditions supports this concept for fluconazole as well, although more studies are necessary to document whether isolates with differing MICs are in fact susceptible or resistant in vivo. Also, it should be noted that this study does not address the effect of acidic medium pH on the susceptibility testing of other antifungal drugs. Further studies are necessary in order to determine if modifications (changes in medium or duration of incubation) should be made in our current antifungal susceptibility testing methods.
The mechanism by which pH affects trailing has not yet been determined. Since the isolates appeared to grow equally well in the absence of fluconazole in RPMI 1640 at pH 4.0 to 7.0 (data not shown), the explanation likely involves more than an alteration of the optical density of the growth control. Other possible explanations include a pH effect on cellular morphology and changes in the expression of genes involved in drug resistance. It is possible that fluconazole has a more “cidal” action at a low pH, as has been noted with the azole D0870 against Cryptococcus neoformans (17). Further investigations to determine the mechanism of this observation are being performed.
ACKNOWLEDGMENTS
This research was supported in part by NIH Adult Leukemia Research Center Core Grant CA18029 22 (Fred Hutchinson Cancer Research Center) and in part by NIH R01 DE11367 to T.C.W. K.A.M. is the recipient of the 1998 National Foundation of Infectious Diseases John P. Utz Medical Mycology Fellowship. T.C.W. is supported by a New Investigator Award from the M. J. Murdock Charitable Trust and is the recipient of a New Investigator Award from the Burroughs Wellcome Fund.
REFERENCES
- 1.Anaissie E, Paetznick V, Bodey G P. Fluconazole susceptibility testing of Candida albicans: microtiter method that is independent of inoculum size, temperature, and time of reading. Antimicrob Agents Chemother. 1991;35:1641–1646. doi: 10.1128/aac.35.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacek D M, Noskin G A, Trakas K, Peterson L R. Initial use of a broth microdilution method suitable for in vitro testing of fungal isolates in a clinical microbiology laboratory. J Clin Microbiol. 1995;33:1884–1889. doi: 10.1128/jcm.33.7.1884-1889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre K A, Galgiani J N. In vitro susceptibilities of yeasts to a new antifungal triazole, SCH 3904: effects of test conditions and relation to in vivo efficacy. Antimicrob Agents Chemother. 1989;33:1095–1100. doi: 10.1128/aac.33.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre K A, Galgiani J N. pH and other effects on the antifungal activity of cilofungin (LY 121019) Antimicrob Agents Chemother. 1989;33:731–735. doi: 10.1128/aac.33.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 6.Peng T, Galgiani J N. In vitro studies of a new antifungal triazole, D0870, against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob Agents Chemother. 1993;37:2126–2131. doi: 10.1128/aac.37.10.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller M A, Jones R N, Doern G V, Sader H S, Hollis R J, Messer S A. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY program. J Clin Microbiol. 1998;36:1886–1889. doi: 10.1128/jcm.36.7.1886-1889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revankar S G, Kirkpatrick W R, McAtee R K, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol. 1998;36:153–156. doi: 10.1128/jcm.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rex J H, Pfaller M A, Rinaldi M G, Polak A, Galgiani J N. Antifungal susceptibility testing. Clin Microbiol Rev. 1993;6:367–381. doi: 10.1128/cmr.6.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex J H, Nelson P W, Paetznick V L, Lozano-Chiu M, Espinel-Ingroff A, Anaissie E J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob Agents Chemother. 1998;42:129–134. doi: 10.1128/aac.42.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro/in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 12.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers T E, Galgiani J N. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob Agents Chemother. 1986;30:418–422. doi: 10.1128/aac.30.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tornatore M A, Noskin G A, Hacek D M, Obias A A, Peterson L R. Effects of incubation time and buffer concentration on in vitro activities of antifungal agents against Candida albicans. J Clin Microbiol. 1997;35:1473–1476. doi: 10.1128/jcm.35.6.1473-1476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner E, Seibold M, Antweiler E. Susceptibility testing of Candida species for fluconazole: the role of buffering in the agar dilution assay. Mycoses. 1993;36:125–130. doi: 10.1111/j.1439-0507.1993.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 16.White T C, Marr K A, Bowden R A. Clinical, cellular and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada H, Watanabe T, Kato K, Mochizuki H. Fungicidal mechanism of action of D0870 against Cryptococcus neoformans under acidic conditions. Antimicrob Agents Chemother. 1997;41:2710–2713. doi: 10.1128/aac.41.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]