Abstract
Penicillin allergies are reported by 8–15% of the US population, but up to 95% of these allergies do not correspond with a true allergy when tested. Recent studies have demonstrated that having a penicillin allergy label (PAL) results in a 50% increased odds of SSI among patients reporting a penicillin allergy entirely attributable to the use of a beta-lactam alternative antibiotic (primarily clindamycin or vancomycin). This study provides a review of the prevalence of PAL, the cross reactivity with cefazolin, immunogenic components of cefazolin and penicillin, and current guidelines for preoperative antibiotic selection in patients with PAL. Upon understanding these principles, a new set of guidelines and a risk stratification tool are proposed for assessing allergies and determining appropriate antibiotic choice, dosage, and timing in the orthopaedic preoperative setting.
Keywords: penicillin, allergy, antibiotics
Introduction:
Penicillin allergies are reported by 8–15% of the US population, but up to 95% of these allergies do not correspond with a true allergy when tested.1 With this emerging knowledge, the new term coined to refer to a penicillin allergy that has not yet been evaluated is penicillin allergy label (PAL).1,2 Having a PAL for a patient undergoing surgical intervention is not benign. A compelling article published in November 2019 correlates the risk of having a PAL with developing surgical site infection (SSI).3 With over 8,000 penicillin-allergic patients reviewed and over half of those patients having orthopaedic conditions, the authors found a 50% increased odds of SSI among patients reporting a penicillin allergy entirely attributable to the use of a beta-lactam alternative antibiotic (primarily clindamycin or vancomycin).3 In orthopaedic surgery, a PAL is the primary driver for choosing an alternative perioperative antibiotic, with clindamycin being utilized most frequently (OR 34.6, 95% CI 29.9–30.1, P<.005).4 The use of these second-line antibiotics leads to increased healthcare-associated infections including a 23% increased odds of Clostridium difficile, a 14% increased odds of methicillin-resistant Staphylococcus aureus (MRSA), and a 30% increased odds of vancomycin-resistant Enterococcus (VRE).5,6,7 In another multi-site, prospective cohort study, beta-lactam allergic patients who received a non-beta-lactam antibiotic as opposed to a beta-lactam antibiotic had significantly more adverse events reported in the form of hospital re-admissions for the same infection, acute kidney injury, C. difficile, or other drug-related events (OR 3.2, 95% CI 1.28–7.89).8 In addition to causing significant morbidity and mortality, SSIs make up 34% of total healthcare costs related to health-care associated infections, with an estimated attributable cost of $20,000 per case.8,9,10,11 Orthopaedic procedures are at particular risk given implanted material that results in both an increased propensity for developing infection and a more difficult process of eradicating infection once it occurs. In this review, the prevalence of PAL is discussed as well as the basis of concern related to penicillin cross reactivity with cefazolin, the immunogenic components of cefazolin and penicillin, and current guidelines for preoperative antibiotic selection in the setting of a PAL. Upon understanding these principles, new guidelines are proposed for assessing allergies and determining appropriate antibiotic choices in the preoperative setting.
Understanding Penicillin Allergy and Cross Reactivity:
Penicillin allergy is reported in approximately 8–15% of the general public.1 More than 95% of these reported allergies do not have an IgE-mediated or immediate hypersensitivity response when skin testing is performed.12 This might be because patients never had an allergy in the first place or the allergy diminished; in fact, approximately 80% of patients with IgE-mediated penicillin allergy lose sensitivity after a 10-year period.9,13,14
Historically it has been reported that cephalosporins (of which cefazolin is a first generation) have up to a 10% cross reactivity with penicillin. This is problematic in the surgical setting as level 1 evidence exists that demonstrates cefazolin to be the optimal antibiotic agent for preoperative prophylaxis due to its selectivity for skin flora, bactericidal mechanism, fast penetrance, and bioavailability at the surgical site.3,15,16 The oft quoted 10% cross-reactivity rate between penicillin and cephalosporin is based on a mere handful of early studies that have now been debunked in the light of more recent higher-level data.17,18,19 Among these reports are two studies from the 1970s;20,21 it is now known that cephalosporins introduced prior to 1980 had trace amounts of penicillin as contaminants,1 and results of these studies are inconclusive in light of this fact. Additionally, the nature and severity of reactions reported are not well documented. Another early study that reported a similar rate of 8% of any type of allergic reaction from administering cephalosporin in patients with a listed penicillin allergy was based on self-reported patient survey.22 In the past several years, new studies have found the true cross-reactivity rate to be much lower. One study in 2015 administered a first-generation cephalosporin (83% cefazolin) to 153 patients with a PAL that resulted in only one minor hypersensitivity response (urticaria). In fact, the rate of adverse reactions from administration of clindamycin proved to be higher (1.5%).22 Another study reviewed 413 patients with PAL who were all given cefazolin, and only one of these patients had a possible reaction. Although there was no documentation of an actual reaction, this was inferred from a note in the intra-operative anesthesia record that showed administration of diphenhydramine at the beginning of a case, which may reasonably have been given prophylactically.23 Finally, a recent study published in 2021 demonstrated that in a series of 452 patients with a PAL that underwent allergy testing, 0 out of 452 patients had a positive skin test when tested specifically for cefazolin or ceftriaxone.13
Reviewing the three-dimensional chemical structures and immunogenic components of both cefazolin and penicillin may be helpful in understanding why the true rate of cross reactivity may actually be close to zero as these recent studies suggest. Cephalosporins and penicillin are both considered beta lactams because they share a core beta lactam ring (Figure 1).13,24,25 They differ in that penicillin has a five-membered thiazolidine ring that is replaced by a six-membered dihydrothiazine ring in cephalosporins.26,27 After breakdown, penicillin retains its thiazolidine ring whereas cephalosporins degrade both their beta-lactam and dihydrothiazine ring.26 These breakdown products form the immunogenic components of these molecules, and the differences in the degradation process may explain the low cross-reactivity rate. Furthermore, cefazolin has two side chains (R1 and R2) whose structures do not match those of any other beta lactams including penicillin (Figure 1). True allergies to cefazolin are extremely rare (<1%) and are usually specific for its side chains, not the shared core ring.13,24
Figure 1.
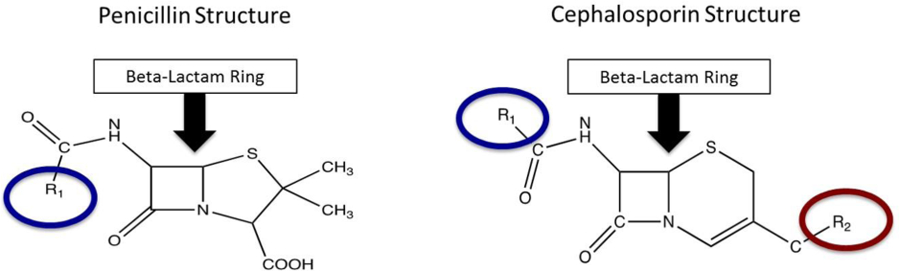
Comparison of cephalosporin and penicillin structures. Cephalosporins and penicillin both contain a core beta-lactam ring (yellow arrow). Cefazolin is a first-generation cephalosporin whose R1 and R2 side chains make up its primary immunogenic components which are unique and not shared with other beta lactams.
New Proposed Risk Stratification Tool:
A first-generation cephalosporin, and, in particular, cefazolin, is the preferred first-line agent based on the highest level of evidence for preoperative antibiotics in orthopaedics.15 The American Academy of Orthopaedic Surgeons (AAOS) guidelines on antibiotic prophylaxis reflect this, stating that a first-generation beta lactam should be used first as a prophylactic antibiotic.28 With regard to beta-lactam allergy, it is noted that alternatives for patients with a true penicillin allergy include clindamycin and vancomycin without further elaboration.29 Currently there are no other guidelines or risk stratification tools to guide surgeons on safe preoperative antibiotic choice. One recent study based on allergy and immunology data developed a validated risk stratification for patients in an intensive care unit (ICU) setting who had PALs.30 Patients were categorized into risk categories depending on their reported allergic reactions. Of the 184 patients who were considered “low risk” based on their listed reaction, no patient tested positive to penicillin on skin test, and none were symptomatic after receiving a dose of a first-generation cephalosporin. This led to a second phase of the study in which low-risk patients were given a cephalosporin without skin testing; of the 57 consecutive patients meeting this criteria, all tolerated the antibiotic without any reactions.30
We, therefore, propose an adapted version of this validated tool for orthopaedic surgeons (Table 1) to consider when determining their choice of preoperative antibiotic. Antibiotic selection can be made based on risk stratification of a patient’s reported index reaction to penicillin.
Table 1.
A modified risk stratification tool for preoperative antibiotic selection in the setting of a penicillin allergy label
| Low Risk | Moderate Risk | High Risk | |
|---|---|---|---|
| • Side effects/intolerances (GI symptoms, headache, muscle aches, psychiatric disturbances) • Limited hypersensitivity reactions (self-limited cutaneous rash, urticaria >5 years ago, itching) • Nonspecific (unknown reaction, remote childhood reaction) |
• Disseminated hypersensitivity or anaphylaxis (swelling of face or throat, angioedema, difficulty breathing, urticaria <5 years ago) | • Stevens Johnsons Syndrome or toxic epidermal necrolysis (diffuse ulceration, blistering, pustulosis) • Multiorgan hypersensitivity response (history of kidney or liver injury) |
|
| Plan: | Give cefazolin | Consideration for allergy testing referral in elective setting. Give clindamycin or vancomycin. | Give clindamycin or vancomycin |
Low risk - Patients who fall under a low-risk category include those whose index reactions are either side effects or intolerances (gastrointestinal symptoms, headache, musculoskeletal symptoms, or psychiatric disturbances), limited hypersensitivity response (self-limited cutaneous rash, urticaria more than 5 years ago, itching), or lacking information (unknown allergy or remote childhood reaction). These patients should receive cefazolin despite having a listed penicillin allergy. The majority of patients, approximately 60%, fall into this category based on initial screening.2,30
Moderate risk - Patients who fall under a moderate-risk category are those with prior history of more disseminated hypersensitivity reaction or anaphylaxis (swelling of face and throat, angioedema, difficulty breathing, urticaria less than 5 years ago). These patients in the elective setting should be considered for allergy testing. If patients have not been allergy tested to clear them of their PAL, then they should receive clindamycin or vancomycin.
Severe risk - Patients who fall under a severe-risk category are those whose index reactions are concerning for toxic epidermal necrolysis or Stevens Johnson Syndrome (mucosal or skin blistering, ulceration, or pustulosis), or those with concern for multiorgan involvement (history of kidney or renal injury). This occurs in <0.5% of patients. These patients should receive clindamycin or vancomycin.
Of note, it has been demonstrated in the literature that between 112 and 124 patients with reported penicillin allergy would need allergy evaluation to prevent one SSI.3 The orthopaedic literature has reproduced numbers comparable to this and demonstrate increased use of cefazolin after allergy testing.31 In a study done in patients with joint arthroplasty, all those with a reported beta-lactam allergy were referred preoperatively to a drug allergy clinic to optimize antimicrobial prophylaxis. Of those sent for penicillin skin testing, 99% of patients had no penicillin allergy, and all were given cefazolin at the time of surgery without any adverse reactions.32 Another study found 97% of patients cleared of their PAL after allergy testing, and that a beta-lactam alternative resulted in a significantly higher rate of prosthetic joint infection even when controlled for by other risk factors.33 Given the relatively low number needed to evaluate and the effectiveness of an allergy referral program to rid patient charts of PALs, referring a patient for allergy testing in a moderate-risk category appears to be a worthwhile endeavor. It also has been suggested that evaluating a penicillin allergy with skin testing may be cost-saving for orthopaedic procedures and the healthcare system,33,34,35,36,37 with an anticipated further reduction in costs to evaluate low-risk PAL patients who do not require skin testing.30,38
Clinical practice guidelines state that correct dosing for cefazolin is 2 g for adult patients or 3 grams when the weight is greater than 120 kg every 4 hours. Pediatric dosing is 30 mg/kg. Clindamycin dosing is 900 mg in adult patients every 6 hours. Pediatric dosing is 10 mg/kg. Vancomycin dosing is 15 mg/kg and needs to be administered 60 to 120 min prior to incision to achieve minimum inhibitory concentration.15 Timing of vancomycin is especially important; in the study by Blumenthal et al,3 97.5% of patients who received vancomycin as a preoperative antibiotic did not receive it in the recommended time frame; initiation occurred at a median of 24 minutes before induction, whereas only 1.7% of patients did not receive cefazolin in the recommended time frame.
Summary
A PAL has a significant deleterious effect on outcomes in patients undergoing surgery. When a PAL deters surgeons from using cefazolin, which is a first-line antibiotic agent, there is a significant increase in odds for SSI, drug-related adverse events from second-line options, increased readmissions, and increased costs. Patients undergoing orthopaedic procedures are at particular risk for developing SSI given the use of implanted material, and it is worth the effort to reconsider current guidelines, which are based on outdated, low-level evidence that overestimates the cross-reactivity rate of cefazolin and penicillin. We propose an adapted version of a validated risk stratification tool for orthopaedic use in the preoperative setting to help guide surgeons in antibiotic selection in the setting of a PAL. Implementing this tool simply requires knowledge of a patient’s specific reaction to penicillin when listed. This can be done as part of the patient intake sheet and requires very little extra time on the part of the surgeon, making it an evidence-based easy-to-use, no-cost, and high-value intervention.
Sources of Support or Funding:
None
Footnotes
Conflicts of Interest: The authors report no conflicts of interest in regard to this work.
References
- 1.Stone CA, Trubiano J, Coleman DT, Rukasin CRF, Phillips EJ. The challenge of de-labeling penicillin allergy. Allergy 2020; 75(2):273–288. Doi: 10.1111/all.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman DT, Stone CA Jr, Wei WQ, Phillips EJ. Readiness for penicillin allergy testing: perception of allergy label (PEN-PAL) survey. J Allergy Clin Immunol Pract 2020; 8(9):3180–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal KG, Ryan EE, Li Y, Lee H, Kuhlen JL, Shenoy ES. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis 2018; 66(3):329–336. Doi: 10.1093/cid/cix794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman DT, Stone CA, Wei WQ, Phillips EG. Penicillin allergy labels drive perioperative prophylactic antibiotic selection in orthopedic procedures. J Allergy Clin Immunol Pract 2020; 8(10):3634–3636. Doi: 10.1016/j.jaip.20202.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull AL, Worth LJ, Richards MJ. Impact of vancomycin surgical antibiotic prophylaxis on the development of methicillin-sensitive staphylococcus aureus surgical site infections: report from Australian Surveillance Data (VICNISS). Ann Surg 2012; 256:1089–1092. Doi: 10.1097/SLA.0b013e31825fa398. [DOI] [PubMed] [Google Scholar]
- 6.Berríos-Torres SI, Yi SH, Bratzler DW, et al. Activity of commonly used anti-microbial prophylaxis regimens against pathogens causing coronary artery bypass graft and arthroplasty surgical site infections in the United States, 2006–2009. Infect Control Hosp Epidemiol 2014; 35:231–239. Doi: 10.1086/675289. [DOI] [PubMed] [Google Scholar]
- 7.Yee J, Dixon CM, McLean AP, Meakins JL. Clostridium difficile disease in a department of surgery. The significance of prophylactic antibiotics. Arch Surg 1991; 126:241–246. Doi: 10.1001/archsurg.1991.091410260131019. [DOI] [PubMed] [Google Scholar]
- 8.MacFadden DR, DaDelfa A, Leen J, et al. Impact of reported beta-lactam allergy on inpatient outcomes: a multicenter prospective cohort study, Clin Infect Dis 2016;63:904–910. Doi: 10.1093/cid/ciw462. [DOI] [PubMed] [Google Scholar]
- 9.Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 1999; 103:918–924. Doi: 10.1016/s0091-6749(99)70439-2. [DOI] [PubMed] [Google Scholar]
- 10.McDanel J, Perncevich E, Diekema D, et al. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin Infect Dis 2015;61:361–367. Doi: 10.1093/cid/civ308. [DOI] [PubMed] [Google Scholar]
- 11.Charneski L, Deshpande G, Smith SW. Impact of an antibiotic allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy 2011; 31:742–747. Doi: 10.1592/phco.31.8.742. [DOI] [PubMed] [Google Scholar]
- 12.Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA 2019;321(2):188–199. Doi: 10.1001/jama.2018.19283. [DOI] [PubMed] [Google Scholar]
- 13.Stone CA, Trubiano JA, Phillips EJ. Testing strategies and predictors for evaluating immediate and delayed reactions to cephalosporins. J Allergy Clin Immunol Pract 2021; 9(1):435–444. Doi: 10.1016/j.jaip.2020.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105(4):259–273. Doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 15.ASHP Therapeutic Guidelines: Clinical practice guidelines for antimicrobial prophylaxis in surgery. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery - therapeutic-guidelines-antimicrobial-prophylaxis-surgery.ashx Accessed January 20, 2021.
- 16.Prokuski L Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg 2008;16:283–293. Doi: 10.5435/00124635-200805000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Herbert ME. Medical myth. Ten percent of patients who are allergic to penicillin will have serious reactions if exposed to cephalosporins. Culture and Medicine 2000;172:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano A, Mayorga C, Torres MJ, et al. Immediate allergic reactions to cephalosporins: cross-reactivity and selective responses. J Allergy Clin Immunol 2000; 106:177–183. Doi: 10.1067/mai.2000.111147. [DOI] [PubMed] [Google Scholar]
- 19.Romano A, Valluzzi RL, Caruso C, Maggioletti M, Quarantino D, Gaeta F. Cross-reactivity and tolerability of cephalosporins in patients with IgE-mediated hypersensitivity to penicillins. J Allergy Clin Immunol Pract 2018; 6(5);1662–1672. Doi: 10.1016/j.jaip.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Dash CH. Penicillin allergy and the cephalosporins. J Antimicrob Chemother 1975; 1 (3 suppl):107–118. [DOI] [PubMed] [Google Scholar]
- 21.Petz LD. Immunologic reactions of humans to cephalosporins. Postgrad Med J 1971; 47 suppl:64–69. [PubMed] [Google Scholar]
- 22.Beltran RJ, Kako H, Chovanec T, Ramsh A, Bissonnette B, Tobias JD. Penicillin allergy and surgical prophylaxis: cephalosporin cross-reactivity risk in a pediatric tertiary care center. J Pediatr Surg 2015; 50:856–859. Doi: 10.1016/j.jpedsurg.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Goodman EJ, Morgan MJ, Johnson PA, Nichols BA, Denk N, Gold BB. Cephalosporins can be given to penicillin-allergic patients who do not exhibit an anaphylactic response. J Clin Anesth 2001; 13:551–564. [DOI] [PubMed] [Google Scholar]
- 24.Trubiano JA, Stone CA, Grayson ML, et al. The 3 Cs of antibiotic allergy-classification, cross-reactivity, and collaboration. J Allergy Clin Immunol Pract 2017; 5(6):1532–1542. Doi: 10.1016/j.jaip.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solensky R: Penicillin allergy: immediate reactions. UpToDate https://www.uptodate.com/contents/penicillin-allergy-immediate-reactions?source=related_link#subscribeMessage. Accessed January 20, 2021.
- 26.Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005;115(4):1048–1049. Doi: 10.1542/peds.2004-1276. [DOI] [PubMed] [Google Scholar]
- 27.Khan DA, Banerji A, Berstein JA, et al. Cephalosporin allergy: current understanding and future challenges. J Allergy Clin Immunol Pract 2019;7(7):2105–2114. Doi: 10.1016/j.jaip.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AAOS. Appropriate use criteria for the management of patients with orthopaedic implants undergoing dental procedures https://www5.aaos.org/uploadedFiles/PreProduction/Quality/AUCs_and_Performance_Measures/appropriate_use/auc-patients-with-orthopaedic-implants-dental-procedures.pdf. Accessed January 20, 2021. [DOI] [PubMed]
- 29.AAOS. Perioperative appropriate antibiotics https://aaos.org/quality/quality-programs/quality-toolkits/perioperative-appropriate-antibiotics/. Accessed January 20, 2021.
- 30.Stone CA, Stollings JL, Lindsell CJ, et al. Risk-stratified management to remove low-risk penicillin allergy labels in the ICU. Am J Respir Crit Care Med 2020; 201(12):1572–157. Doi: 10.1164/rccm.202001-0089LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Markus P, Osmon D, Estes L, Gosselin V, Hanssen A. Reduction of vancomycin use in orthopedic patients with a history of antibiotic allergy. Mayo Clin Proc 2000; 75(9):902–6. Doi: 10.4065/75.9.902. [DOI] [PubMed] [Google Scholar]
- 32.McDanel DL, Azar AE, Dowden AM, et al. Screening for beta-lactam allergy in joint arthroplasty patients to improve surgical prophylaxis practice. J Arthroplasty 2017;32:S101–S108. Doi: 10.1016/j.arth.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Wyles C, Hevesi M, Osmon D, et al. 2019 John Charnley Award: Increased risk of prosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin: the value of allergy testing for antibiotic prophylaxis. Bone Joint J 2019; 101-B(6_Supple_B):9–15. Doi: 10.1302/0301-620X.101B6.BJJ-2018-1407.R1 [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal KG, Yu Li, Banerji A, Yun BJ, Long AA, Walensky RP . The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract 2018; 6(3):1019–1027. Doi: 10.1016/j.jaip.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sousa-Pinto B, Blumenthal KG, Macy E, et al. Penicillin allergy testing is cost-saving: an economic evaluation study. Clin Infect Dis 2020. Feb 28;ciaa194. Doi 10.1093/cid/ciaa194. [DOI] [PMC free article] [PubMed]
- 36.Lee RU. Penicillin allergy delabeling can decrease antibiotic resistance, reduce costs, and optimize patient outcomes. Fed Pract 2020. 37(10):460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagani NR, Moverman MA, Puzzitiello RN, Menendez ME, Barnes CL, Kavolus JJ. Preoperative allergy testing for patients reporting penicillin and cephalosporin allergies is cost-effective in preventing infection after total knee and hip arthroplasty. J Arthroplasty 2021; 36(2):700–704. Doi: 10.1016/j.arth.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 38.Trubiano JA, Vogrin S, Chua KYL, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med 2020;180(5):745–752. Doi: 10.1001/jamainternmed.2020.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]


