Abstract
Objective
To date, there is no standard diagnostic practice to identify the underlying disease-causing mechanism for paediatric patients suffering from chronic fever without any specific diagnosis, which is one of the leading causes of death in paediatric patients. Therefore, we aimed this retrospective study to analyse medical records of paediatric patients with fever of unknown origin (FUO) to provide a preliminary basis for improving the diagnostic categories and facilitate the treatment outcomes.
Design
A retrospective study.
Setting
Beijing Children’s Hospital.
Participants
Clinical data were collected from 1288 children between 1 month and 18 years of age diagnosed with FUO at Beijing Children’s Hospital between January 2010 and December 2017.
Interventions
According to the aetiological composition, age, duration of fever and laboratory examination results, the diagnostic strategies were analysed and formulated.
Primary and secondary outcome measures
The statistical analyses were carried out using SPSS V.24.0 platform along with the χ2 test and analysis of variance (p<0.05).
Results
The duration of fever ranged from 2 weeks to 2 years, with an average of 6 weeks. There were 656 cases (50.9%) of infectious diseases, 63 cases (4.9%) of non-infectious inflammatory diseases (NIIDs), 86 cases (6.7%) of neoplastic diseases, 343 cases (26.6%) caused by miscellaneous diseases and 140 cases (10.9%) were undiagnosed. With increasing age, the proportion of FUO from infectious diseases gradually decreased from 73.53% to 44.21%. NIID was more common in children over 3 years old, and neoplastic diseases mainly occurred from 1 to 6 years of age. Among miscellaneous diseases, the age distribution was mainly in school-aged children over 6 years. Respiratory tract infection was the most common cause of FUO in children, followed by bloodstream infections. Bacterial infection was the most common cause in children with less than 1 year old, while the virus was the main pathogen in children over 1 year old.
Conclusions
The diagnosis of neoplastic diseases and miscellaneous diseases-related diseases still depends mainly on invasive examination. According to our clinical experience, the diagnostic process was formulated based on fever duration and the type of disease. This process can provide a guide for the diagnosis and treatment of paediatric FUO in the future.
Keywords: community child health, child protection, paediatric infectious disease & immunisation, paediatric pathology, diagnostic microbiology
Strengths and limitations of this study.
This study summarises the clinical data from a large cohort of 1288 children diagnosed with fever of unknown origin (FUO) between 2010 and 2017 in Beijing Children’s Hospital and discusses the aetiology distribution, clinical characteristics and diagnostic strategies of paediatric FUO.
Our study found that infectious diseases still ranked first (50.90%) based on aetiology distribution in each year from 2010 to 2017, which was consistent with published reports.
Based on this study, a practical stepwise approach to FUO diagnosis was constructed and could be very helpful in the clinical assessment of FUO.
A limitation of the study is its retrospective nature, meaning some data were missing.
Furthermore, because of the large sample size, we could collect follow-up data on patients’ health and whether there was any recurrent fever in those patients.
Introduction
Fever is a common symptomatic manifestation of a number of underlying diseases, including infections as well as a variety of inflammatory, neoplastic and rheumatological conditions.1 Usually fever due to viral infections is frequent in children without an apparent cause.2 Blood and nasopharyngeal secretion examinations often can be helpful in identifying the responsible virus. This kind of fever does not require any long-term treatment and disappears on its own without any sequelae. Hyperpyrexia, indicative of bacterial infection, can also be diagnosed quickly by checking the patient’s medical history, physical examination and basic laboratory tests.3–5 However, some children suffer from long-term fevers with temperature higher than 38.3°C that last for months or even years without any clear diagnosis. Such fevers are termed ‘fever of unknown origin’ (FUO).
To date, there are no standard diagnostic criteria for FUO. The classic definition of FUO was summarised by reviewing the case reports of 100 paediatric patients by Petersdorf and Beeson in 1961.6 Diagnostic criteria included periodic fever, a fever duration of more than 3 weeks and a weekly hospitalisation.7 Most of these patients exhibited intermittent self-limited viral infection without any definitive diagnosis.7 Subsequently, Durack and Street augmented the classification for special populations, such as immunodeficient patients.8 In recent studies, the definition of FUO in children often refers to unexplained fevers that last more than 1 or 2 weeks.9–11
Currently in China, the diagnostic criteria for paediatric FUO are derived from classic paediatric professional books: the eighth edition of Zhu Futang Practice of Pediatrics edited by Jiang Zaifang, Shen Kunling and Shen Ying, as an unexplained fever ≥2 weeks and a body temperature of >37.5℃. So, at present, FUO in Chinese children can be diagnosed according to this standard. Although Zhu Futang’s guidelines for FUO suggest to consider temperature, even varying, but maintaining at >37.5°C for more than 2 weeks, in order to avoid missing out those patients who might not show as high as 38.3°C body temperature all the time, but might have other diagnostic indications with body temperature >37.5°C for FUO. We followed the criteria for febrile body temperature of ≥38.3°C either occasionally or persistently for more than 2 weeks to maintain the study in agreement with the previous reports.12 The aetiology of FUO in children mainly includes infectious diseases, non-infectious inflammatory diseases (NIIDs), neoplastic diseases, miscellaneous diseases and undiagnosed diseases.13 14 It has been shown that infectious diseases account for 23%, rheumatic diseases 58%, neoplastic diseases 8% and other diseases for 15%. Among the infectious diseases, viral infection, non-tuberculous mycobacterial infection and cat scratch disease occupy the top three. In a Japanese study of 256 cases, it has been shown that among the infectious diseases, HIV/AIDS and tuberculosis (TB) were the main causes, and the final diagnosis accounted for the first place, exceeding 20%.15 While in some cases, rare pathogen-induced diseases, such as Q fever, are also one of the causes of FUO in children.16 In rheumatic diseases, juvenile idiopathic arthritis (JIA), polyarteritis, inflammatory bowel disease and systemic lupus erythematosus (SLE) are more common. In the other diseases group, histocytic necrotising lymphadenitis (HNL) and haematopoietic syndrome (HLH) are the top two.12 However, in case of FUO in adults, the most common causes account for quite a different disease phenotype, where infectious disease account for only 17% of cases and adult Still’s disease is the most common one.17 In this context, it is worth mentioning that 30 years ago, infectious diseases occupied the first place in the distribution of causes in FUO. However, with the change of time, there was a significant change in the distribution of causes.18 Interestingly, the proportion of infectious diseases in the aetiological distribution of FUO gradually decreases, while the proportion of undiagnosed diseases is on the rise.19
Due to the recent advances in diagnostic technology and the increase in global mixing of population with respect to geographical region and ethnicity, the categorical distribution of diagnoses of FUO in paediatric patients has significantly shifted compared with the previously published reports.20 Notably, the proportions of FUO caused by infectious diseases have gradually decreased, while the proportion of FUO due to rheumatic diseases, malignant tumours and miscellaneous diseases has gradually increased. Because of the quite complex and indefinite aetiopathology, diagnostic identification of FUO has remain challenging. In this context, development of fluorine-18 fluorodeoxyglucose-based positron emission tomography (FDG-PET) and in combination with CT (FDG-PET/CT) has been serving not only as a valuable imaging technique to stratify different types of FUO21–23 but also to monitor the treatment of infectious and inflammation-associated FUO that cannot reliably be diagnosed by conventional approaches.24 25 This diagnostic method has been proven to be effective in case of adult onset giant-cell arteritis in association with FUO.26
So far, there are few studies on children’s FUO, and most of the reported studies suffer from the limitation of statistically small sample sizes (49–290 cases), which render them less reliable for drawing any definite conclusion.11 27 28As far as we know, there has not been a large-scale systematic analysis of children’s FUO causes, aetiology and clinical characteristics in the last 20 years.
In order to fully understand the changes in the cause, aetiology and clinical characteristics of FUOs in children in recent years, we aimed to analyse the medical records of a large cohort of 1288 patients admitted to Beijing Children’s Hospital between January 2010 and December 2017 who met the diagnostic criteria for FUO in children. The clinical data were discussed regarding the cause, aetiology, clinical characteristics and diagnosis for establishing effective strategies to improve the early diagnosis rate of paediatric FUO.
Methods
Selection criteria
This retrospective study analysed the medical records of children aged between 1 month and 18 years old admitted with a body temperature of >37.5°C for ≥2 weeks without an apparent source after preliminary investigations at Beijing Children’s Hospital from January 2010 to December 2017. Children who met the diagnostic criteria of fever according to ‘Zhu Futang Practice of Pediatrics’ were only included in this study. In all of these cases, inclusion of the study subjects closely followed the FUO definition and diagnosis guidelines proposed by Durack and Street.8
Diseases group
All the included cases were divided into five groups:infectious diseases, non-infectious inflammatory diseases, neoplastic diseases, miscellaneous diseases and undiagnosed diseases. All timelines summarised were based on the hospital discharge as the endpoint. Infectious diseases include respiratory infection, bloodstream infection, lymphadenitis, central nervous system (CNS) infections, urinary tract infections (UTI) and abdominal cavity infections. Non-infectious inflammatory diseases consisted of JIA, SLE, vasculitis, Behcet’s disease and so on. Haematological and neoplastic diseases included various leukaemias, lymphomas, solid tumours, Langerhans cell histiocytosis (LCH) and haematopoietic syndrome. Miscellaneous diseases included histiocytic necrotising lymphadenitis and pseudo-fever.
Disease diagnosis
The paediatric FUO diagnostic strategy was formulated by the FUO Research Group of the Infectious Diseases Department by comprehensively analysing domestic and foreign research reports and combining the results of the examinations and the diagnosed medical conditions.
The standard diagnosis of respiratory tract infections, including upper and lower respiratory tract infections, was performed based on the clinical characteristics of symptoms, combined with lung imaging, but no other infection sites were examined at that time. For bloodstream infections, positive pathogenic bacteria obtained from blood culture were used as diagnostic criteria. Aetiology diagnosis was based on whether there were infectious lesions, or the infectious pathogens were confirmed by laboratory inspection (serology, blood culture, blood smear, PCR, next-generation sequencing, etc).
Bacterial infection samples were obtained through the lesion site, body fluids, etc. Group A Streptococcus infections were all indicated by positive blood culture. In others, the diagnosis was based on the progressive increase in antistreptolysin O (ASO) titres. For Streptococcus angina-mediated suppurative pericarditis, the diagnosis was based on the positive culture of pericardiocentesis. Streptococcus sanguis infection was diagnosed for infective endocarditis by a positive blood culture test. Suppurative thyroiditis was confirmed by a positive pus puncture culture.
Mycoplasma infection was based on the blood mycoplasma antibody titres of ≥1:160. Brucella infection was confirmed by Brucella microagglutination test along with positive blood and bone marrow culture. The positive rate of TB infection was low. In addition to the positive antacid staining of the TB, a purified protein derivative (PPD) test, T-spot and chest imaging were performed for the identification of Mycobacterium tuberculosis infection.
For identification of viral infectious agents like Epstein-Barr virus (EBV), the diagnosis was performed by measuring viral DNA load, plus serological EBV antibodies to determine infection status, including previous infection, primary infection and reactivation of infections. Cytomegalovirus (CMV) infection diagnosis was performed by determining the titres of serological IgM and IgG antibodies, as well as viral DNA loads. Viral-PCR quantitative detection was used to diagnose human herpesvirus (HHV) types 6, 7 and 8 combined with the clinical manifestations of the child. In case of fungal infections, the diagnosis method was mainly based on the fungal culture. Cryptococcus infections were diagnosed based on the ink staining and bacterial antigen detection.
Imaging analysis
Once the patient undergoes initial rounds of checking up medical history, physical examinations and laboratory results, the imaging examinations can provide complementary results to conclude the diagnosis, because in most cases the goal of the examinations is to find the potentially diagnostic clues. Although in case of infectious and focal inflammatory processes can be detected by radiological examinations, such as abdominal ultrasound, MRI and CT scan. But the identification of the lesions may not be possible through these techniques, especially in case of FUO where there are no definitive diagnostic criteria. While FDG-PET scanning serves as the improved imaging method, since the FDG uptake relies on the rate of glycolysis in the targeted cell types. Moreover, it has higher resolution and sensitivity even in detecting chronic low-grade inflammations/infections, compared with conventional radiolabelling methods involving Gallium 67-citrate, which poses higher radiation burden due to longer half-life of the injected probe.29
Statistical analysis
All experimental data were expressed as mean±SD. SPSS V.24.0 software was used for statistical analysis. Data were analysed by the χ2 test or analysis of variance test. A p<0.05 was considered statistically significant.
Routine blood testing included a statistical analysis of white cell (WC) count, C-reactive protein (CRP), dynamic erythrocyte sedimentation rate (ESR) and procalcitonin (PCT). Youden Index was used to analyse the sensitivity and specificity of the disease diagnosis.
Results
Patient characteristics
From January 2010 to December 2017, a total of 3184 children were admitted to our hospital as ‘fever pending’, of which 1288 patients met the inclusion criteria. There were 786 males (61.0%) and 502 females (38.9%). Ages ranged from 1 month to 18 years old, with an average age of 7±4.1 years. The range of fever duration was 2 weeks to 2 years, with an average duration of 6 weeks.
Aetiology distribution
All children with FUO were divided into five groups based on their initial diagnostic outcomes: infectious disease, NIID, neoplastic disease, miscellaneous diseases and undiagnosed disease. The number of cases and the average age of children in each group are shown in table 1. We found that infectious disease (50.9%) was the leading cause of FUO, followed by miscellaneous diseases (26.6%), neoplastic disease (6.7%), NIID (4.9%) and undiagnosed disease (10.9%).
Table 1.
Diseases and age distribution of 1288 cases of FUO
| Diseases | Cases, n (%) | Ages (mean±SD, years) |
| Infectious diseases | 656 (50.9%) | 6.4±4.8 |
| Non-infectious inflammatory diseases | 63 (4.9%) | 8.7±3.8 |
| Neoplastic diseases | 86 (6.7%) | 4.0±3.6 |
| Miscellaneous diseases | 343 (26.6%) | 9.2±3.9 |
| Undiagnosed diseases | 140 (10.9%) | 6.9±4.6 |
FUO, fever of unknown origin.
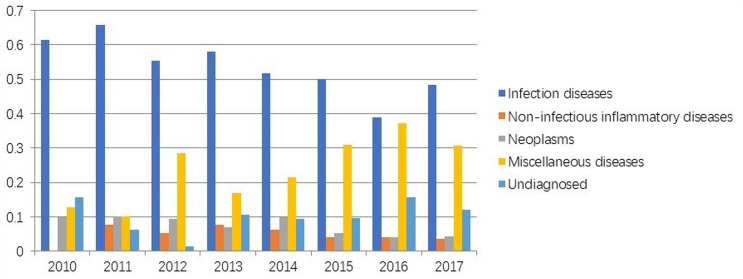
Figure 1 shows that between 2010 and 2017, the aetiology distribution was different; however, infectious diseases held the rank 1 in all years. Interestingly, we could see the increasing contribution of miscellaneous diseases towards recent years along with the rising number of patients with undiagnosed fever (figure 1). Furthermore, interaction study between patient age and plausible cause of FUO showed that the younger the children, the higher was the contribution of infectious diseases (table 2). FUO due to haematological diseases mainly occurred in children under 6 years of age. The contributions of NIID and undiagnosed disease were comparable among different age groups. Miscellaneous disorders were scattered in children over 6 years of age (table 2).
Figure 1.
The distribution of aetiology in 1288 children with fever of unknown origin in different years.
Table 2.
Disease distribution of children with FUO at different ages
| Age (years) | Diseases, n (%) | |||||
| Infectious diseases | Non-infectious inflammatory diseases | Neoplastic diseases | Miscellaneous diseases | Undiagnosed | Total | |
| <1 | 125 (73.53) | 2 (1.18) | 12 (7.06) | 15 (8.82) | 16 (9.41) | 170 (100%) |
| 1–3 | 114 (57.87) | 5 (2.54) | 35 (17.77) | 23 (11.68) | 20 (10.15) | 197 (100%) |
| 3–6 | 76 (47.50) | 11 (6.88) | 18 (11.25) | 24 (15.00) | 31 (19.38) | 160 (100%) |
| 6–12 | 239 (45.06) | 33 (6.27) | 16 (3.04) | 192 (36.50) | 48 (9.13) | 528 (100%) |
| >12 | 103 (44.21) | 14 (6.01) | 5 (2.15) | 86 (36.91) | 25 (10.73) | 233 (100%) |
| The total | 657 | 65 | 86 | 340 | 140 | 1288 |
χ2, p<0.05
FUO, fever of unknown origin.
We next compared the duration of unknown fever suspected to develop from different diagnosed aetiologies (table 3). There were significant differences in the fever duration of different aetiologies. The fever duration with the infectious disease was under 8 weeks (76.06%). There was significant number of patients with NIID (88.89%), neoplastic disease (81.40%), miscellaneous diseases (70.55%) and undiagnosed diseases (85.00%) whose fever lasted longer than 4 weeks. Table 3 shows the breakdown of number of patients who had fever duration 4–8 weeks and more than 8 weeks. Taken together, these results suggest that although infectious disease may be the leading cause of paediatric FUO, but, FUO due to undiagnosed diseases has the most the severe effect on the patients with long lasting fever.
Table 3.
Comparison of the fever time from different diseases
| Diseases | Cases, n (%) | ||
| <4 weeks | 4–8 weeks | >8 weeks | |
| Infectious diseases | 248 (37.80) | 251 (38.26) | 157 (23.93) |
| Non-infectious inflammatory diseases | 7 (11.11) | 30 (47.62) | 26 (41.27) |
| Neoplastic diseases | 16 (18.60) | 42 (48.84) | 28 (32.56) |
| Miscellaneous diseases | 101 (29.45) | 158 (46.06) | 84 (24.49) |
| Undiagnosed | 21 (15.00) | 58 (41.43) | 61 (43.57) |
χ2, p<0.05
Pathogen distribution
Next, we compared the children’s age group and the most predominant pathogen causing FUO in that age group (table 4). We found that bacterial infection (61.7%) was predominant in children of less than 1 year of age and gradually decreased with age. The contribution of viral infection-induced FUO was highest between 1 and 6 years of age (~50%), and slightly reduced with age (~46%). Interestingly, TB infection was significantly high at ≥6 year of age. And mycoplasma infection was more common in children from 3 (pre-school) to 12 years (school) of age.
Table 4.
Distribution of pathogens in children with FUO at different ages
| Ages (years) | Pathogens, n (%) | ||||
| Virus | Bacteria | Tuberculosis | Mycoplasma | The total | |
| <1 | 29 (30.85) | 58 (61.7) | 6 (6.39) | 1 (1.06) | 94 |
| 1–3 | 43 (50.59) | 38 (44.71) | 2 (2.35) | 2 (2.35) | 85 |
| 3–6 | 26 (50.00) | 19 (36.54) | 1 (1.92) | 6 (11.54) | 52 |
| 6–12 | 74 (45.68) | 43 (26.54) | 25 (15.43) | 20 (12.35) | 162 |
| >12 | 31 (46.97) | 16 (24.24) | 16 (24.24) | 3 (4.55) | 66 |
χ2, p<0.05
FUO, fever of unknown origin.
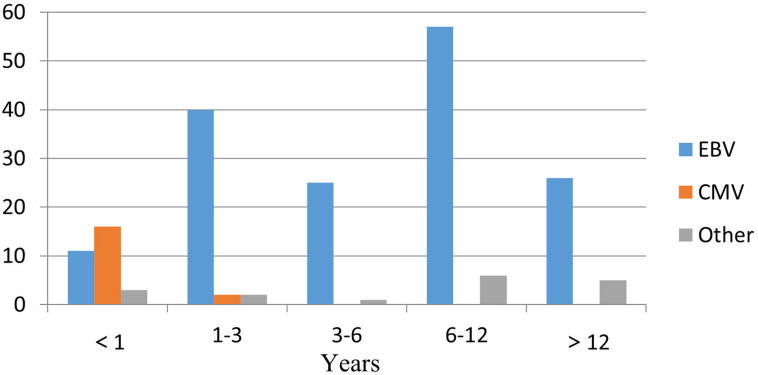
Viral infection was the most common pathogen in infectious diseases, with a total of 203 cases (30.95%) including all age groups. Among them, there were 163 children (80.30%) with confirmed viral pathogens. The primary EBV infection with reactivation was the most common in 132 cases (80.98%). The second highest EBV-related disease was haemophagocytic lymphohistiocytosis (EBV-HLH) in 18 cases (11.04%) followed by chronic active EBV in 13 patients (7.98%) in this cohort. There were only seven cases (5.30%) of typical EBV infectious mononucleosis, while other EBV infections showed common clinical manifestations. CMV infection accounted for 18 cases (10.80%), and human herpesvirus type 6 and 7 infections were found in 7 cases (4.20%). There were seven cases with mixed infections (4.20%), including two cases of EBV+CMV and five cases of EBV+HHV type 6 or 7. Other viral infections included parvovirus B19 infection in 2 cases (1.20%). At different ages, the proportion of viral infections was also different. CMV infection was mainly in infants <1 year of age, while EBV was primarily found in children 6–12 years of age (figure 2).
Figure 2.
Viral composition in children with fever of unknown origin at different ages. CMV, cytomegalovirus; EBV, Epstein-Barr virus.
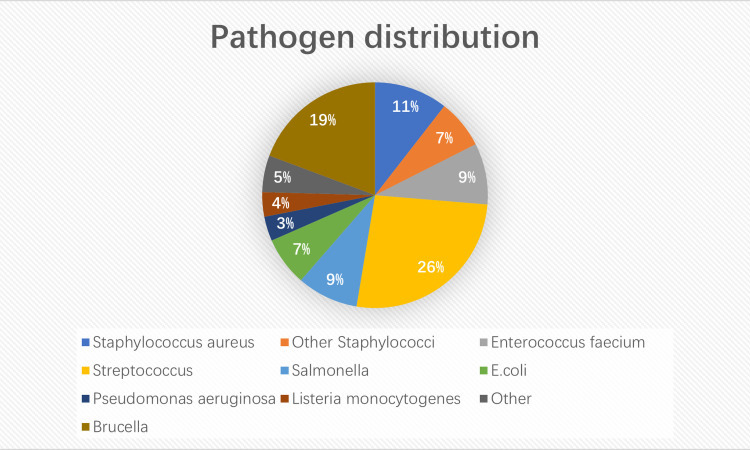
A total of 57 cases of bacterial infection (except tuberculosis) were identified. The detailed pathogen distribution is shown in figure 3. The leading one was Streptococcus sp, mainly Group A haemolytic streptococcus, with a total of 15 cases (26.30%). Streptococcal TSS and skin and soft tissue infections had the highest prevalence (13.30%) in two patients each. Septicaemia was detected in one patient (6.67%), while major proportion of the patients (40%) exhibited other streptococcal infections. Other streptococcal bacteria found in this cohort of patients, included Streptococcus anginosus, Streptococcus sanguis, Streptococcus mitis and Streptococcus constellatus affecting one patient (6.67%) in each of the cases. Among other bacterial pathogens, Brucella counted as the second highest, with 11 cases (19.30%). Brucella infection was clinically characterised by recurrent fever, and a small number of children showed recurrent fevers, along with joint involvement and sweating as the main manifestations. In six cases (10.53%), patients were infected with Staphylococcus aureus leading to suppurative lymphadenitis (50.00%). The main pathogen of UTI was Escherichia coli, followed by Enterococcus faecium and Enterococcus faecalis.
Figure 3.
Composition of bacterial infection in children with fever of unknown origin.
In addition, mycoplasma was one of the main pathogens causing FUO, with a total of 32 cases (4.88%) (table 4). The diagnosis was based on a mycoplasma antibody test of ≥1:160. Among them, 24 cases (75.00%) caused respiratory tract infection, 3 cases (9.40%) CNS infection and 5 cases (15.60%) mixed infection. The occurrence of Mycobacterium tuberculosis infection was detected in 50 patients. TB infection was the most common among them, affecting 34 patients (68%). In five cases, patients were diagnosed with lymph node TB (10%). Primary complex and tuberculous meningitis were detected in three cases (6%) each. Pulmonary TB was diagnosed in two patients (4%), and one patient in each was found with symptoms for TB of intestines, urinary TB and systemic disseminated TB. Three patients were considered to have a possible primary immunodeficiency disease, of which one was diagnosed with chronic granulomatous disease.
Among other rare pathogenic fungal infections, seven cases were Cryptococcus neoformans (63.60%) and two cases were Candida albicans (18.20%). Additionally, four cases (57.10%) of a new type of cryptococcal infections manifested as systemic disseminated cryptococcosis (age 2–4 years). Among them, one case involved an 11-year-old boy with a new type of cryptococcal meningitis. At the early stage, he had a recurrent fever and joint pain, which was considered connective tissue diseases(CTDS) in early diagnosis, but at the later stage, he developed headache symptoms. Cerebrospinal fluid (CSF) ink staining and the presence of cryptococcal antigens suggested that the infection was due to a new type of cryptococcal meningitis. Other rare pathogens included three cases of black fever caused by Leishmania spp. The disease mainly manifests as fever, hepatosplenomegaly and changes in the blood system, which meets the clinical criteria for haemophagocytic syndrome. In addition, one case was infected with H. barbadensis and three cases were infected with Babesia spp.
Clinical characteristics
Infectious diseases
There were 375 cases identified with infectious lesions, as shown in table 5. Respiratory infections were the most common and accounted for 55.70%, while the second most common bloodstream infections accounted for 13.60%. Abdominal abscesses were the most common in abdominal infections with 23 cases (95.80%), including spleen abscesses (74.00%), liver abscesses in 2 cases (8.70%), multiple abdominal abscesses in 2 cases (8.70%), iliopsoas abscess in 1 case (4.30%) and appendix abscess with 1 case (4.30%). Additionally, 18 cases (4.80%) of UTI were diagnosed, of which 14 cases (77.78%) were in infants of less than 3 years age, 12 cases were female (66.67%), 6 cases had basic urinary system diseases (33.33%), including bladder ureter regurgitation in 5 cases (83.33%) and 1 case (16.67%) occurred after hypospadias. Please see table 8 for all other minor forms of infections.
Table 5.
Distribution of 375 FUO with identified different lesion infections
| Lesion location | Cases (n) | Rate (%) |
| Respiratory tract | 209 | 55.7 |
| Bloodstream infection | 51 | 13.6 |
| Lymphadenitis | 41 | 10.9 |
| Coeliac infection | 24 | 6.4 |
| Central nervous system infection | 23 | 6.1 |
| Urinary system infection | 18 | 4.8 |
| Septic arthritis | 4 | 1.0 |
| Infective endocarditis | 2 | 1.0 |
| Osteomyelitis | 3 | 1.0 |
FUO, fever of unknown origin.
Non-infectious inflammatory diseases
Among NIIDs, JIA was the most common with 41 cases (65.00%). Children with JIA were characterised by repetitive exaggerated fever and fever-related rashes at different stages of the disease, accompanied by a significant increase in inflammatory indicators. Systemic lupus erythematosus (SLE) occurred in a total of 11 cases (16.90%), including 7 females (63.60%) and 4 males (36.40%). The clinical manifestations were recurrent fever and no other typical signs of SLE, and they were eventually diagnosed by positive autoantibodies. Laboratory tests revealed that 9 of 11 children (82.00%) with decreased white cell (WC) concentration of <4×109/L, with an average of 3.6×109/L. Other forms of NIID included Behcet’s syndrome in two cases (3.20%) and vasculitis in three cases (4.80%). In two cases of Takayasu arteritis, the youngest patient was 2 months old and was admitted with the symptom of ‘intermittent fever for 20 days’. After admission, the blood leucocyte count was 12×109 /L, neutrophils were 80%, and CRP was 111 mg/L. The body temperature was improved after anti-infective treatment, but inflammatory indicators were remained significantly increased and were ultimately diagnosed as Takayasu arteritis by vascular ultrasound.
Neoplastic diseases
Among haematological diseases, there were 36 cases (41.90%) of HLH (including 18 cases (50.00%) of EBV-related haematopoietic syndrome), 10 cases of LCH (11.60%), 17 cases of leukaemia (19.80%), 15 cases of lymphoma (17.40%) (6 cases non-Hodgkin’s lymphoma and 3 Hodgkin’s lymphoma) and 8 other cases (9.30%). In LCH, the age ranged from 2 months to 5 years, and most were between 1 and 3 years of age, characterised by rash, hepatosplenomegaly and lymphadenopathy. Among the children with leukaemia, 10 were acute lymphoblastic leukaemia (ALL) (66.70%) and other types included acute myelogenous leukaemia and juvenile myelomonocytic leukaemia.
Miscellaneous diseases
Among miscellaneous diseases, histiocytic necrotising lymphadenitis (HNL) was the most common, with a total of 217 cases (63.30%), followed by 55 cases of pseudo-fever (14.60%). The detailed disease composition is shown in table 6. In addition to fever, the typical clinical manifestations of HNL were non-purulent enlargement of the cervical lymph nodes with tenderness and a decrease in WC count. The average WC count in HNL cases was 3.75×109 /L. None of the children had evidence of SLE.
Table 6.
Miscellaneous diseases
| Diseases | Case (n, %) |
| Histocytic necrotising lymphadenitis | 217 (63.27%) |
| Pseudo FUO | 55 (16.03) |
| Intracranial demyelinating disease | 15 (4.37%) |
| Kawasaki disease | 9 (2.62%) |
| Drug fever | 7 (2.04%) |
| Summer fever and disorders of temperature regulation | 7 (2.04%) |
| Ectodermal dysplasia | 5 (1.46%) |
| Inflammatory bowel disease | 3 (0.87%) |
| Viral infection-related proliferative diseases | 3 (0.87%) |
| Infection-associated vasculitis | 3 (0.87%) |
| Optic neuromyelitis | 2 (0.58%) |
| Periodic fever | 2 (0.58%) |
| Autoinflammatory disease | 2 (0.58%) |
| Diabetes insipidus | 1 (0.29%) |
| Other | 12 (3.5%) |
FUO, fever of unknown origin.
In nine children with Kawasaki disease, six (66.60%) were atypical Kawasaki disease with coronary dilation, one had Kawasaki disease accompanied by arthritis and two had typical Kawasaki disease. There were five cases of ectodermal dysplasia, two cases of drug fever, five cases of drug hypersensitivity syndrome, including three cases from cephalosporins and two cases from antiepileptic drugs. In addition, 15 cases of intracranial immune demyelinating lesions showed long-term fever, of which 6 cases were accompanied by convulsions, and four cases showed fever only.
The undiagnosed diseases
Among undiagnosed diseases, 100 cases improved (71.43%) after treatment. Based on the diagnosis, the proportion of the infectious disease was highest, with a total of 75 cases (53.00%).
Significance of routine blood tests (CRP, ESR and PCT) in the diagnosis of FUO
The inflammatory indicators WC, CRP, dynamic ESR and PCT were compared between different aetiology and pathogen groups. The results are shown in tables 7 and 8. Variance analysis between NIID and non-NIID groups showed that WC, CPR, ESR and PCT in the NIID group were significantly higher than those in other groups (p<0.05). The Youden Index is defined as the sum of sensitivity and specificity minus 1, indicating that the larger the index of the screening method, the better the effect of the screening experiment and the greater the authenticity. In the analysis of inflammatory indicators of rheumatic immune diseases, the relevant sensitivity and specificity were analysed. According to the Youden Index, rheumatoid immune diseases should be considered when routine blood testing showed WC>15.7×109/L, CRP >33.5 mg/L and ESR>36.5 mm/hour. In the infectious disease group, comparing bacterial infections with non-bacterial infectious diseases, the variance analysis showed that WC, CPR, ESR and PCT were significantly higher in the bacterial infection group than in the other groups (p<0.05).
Table 7.
Comparison of inflammatory indicators from different aetiologies in children with FUO
| Aetiology | WC | CRP | ESR | PCT | ||||||||
| Case, n | WC (mean±SD, ×109/L) | P value | Case, n | CRP (mean±SD, mg/L) | P value | Case, n | ESR (mean±SD, mm/hour) | P value | Case, n | PCT (mean±SD, μg/L) | P value | |
| NIID | 63 | 14.40±8.81 | 0.000 | 63 | 62.78±54.47 | 0.000 | 63 | 57.94±32.14 | 0.000 | 51 | 1.33±3.37 | 0.019 |
| Infectious diseases | 656 | 9.71±7.24 | 653 | 27.06±42.00 | 585 | 26.15±27.12 | 408 | 0.78±2.60 | ||||
| Miscellaneous diseases | 340 | 5.75±4.07 | 342 | 14.38±21.42 | 311 | 25.77±22.39 | 232 | 0.34±1.14 | ||||
| Undiagnosed | 140 | 10.10±8.83 | 139 | 39.29±47.58 | 129 | 35.47±28.05 | 101 | 0.83±2.40 | ||||
| Neoplastic diseases | 86 | 9.35±11.98 | 86 | 42.33±45.08 | 75 | 42.95±37.83 | 61 | 1.16±3.12 | ||||
| Total | 1285 | 8.91±7.58 | 1283 | 27.78±40.96 | 1163 | 29.89±28.37 | 853 | 0.73±2.39 | ||||
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; NIID, non-infectious inflammatory disease; PCT, procalcitonin; WC, white cell count.
Table 8.
Comparison of different pathogenic inflammatory indicators in children with FUO
| Pathogens | WC | CRP | ESR | PCT | ||||||||
| Cases (n) |
WC (mean±SD, ×109/L) |
P value | Cases (n) | CRP (mean±SD, mg/L) | P value | Cases (n) |
ESR(mean±SD, mm/hour) | P value | Cases (n) |
PCT (mean±SD, μg/L) | P value | |
| Other | 825 | 8.23±7.26 | 0.000 | 824 | 26.41±37.83 | 0.000 | 753 | 31.38±29.33 | 0.000 | 559 | 0.63±2.01 | 0.000 |
| Virus | 204 | 8.01±4.73 | 204 | 9.38±12.07 | 180 | 19.99±19.64 | 115 | 0.47±1.18 | ||||
| Tuberculosis | 50 | 7.91±3.57 | 50 | 17.38±31.30 | 45 | 25.07±32.73 | 32 | 0.24±0.45 | ||||
| Bacteria | 174 | 13.79±10.74 | 173 | 61.83±59.04 | 158 | 36.8±28.42 | 129 | 1.57±4.27 | ||||
| Mycoplasma | 32 | 7.46±3.42 | 32 | 12.70±20.21 | 27 | 21.74±24.76 | 18 | 0.26±0.36 | ||||
| Total | 1285 | 8.91±7.58 | 1283 | 27.78±40.96 | 1163 | 29.89±28.37 | 853 | 0.73±2.39 | ||||
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FUO, fever of unknown origin; PCT, procalcitonin; WBC, white cell count.
Discussion
This study summarises the clinical data from 1288 children diagnosed with FUO between 2010 and 2017 in Beijing Children’s Hospital and discusses the aetiology distribution, clinical characteristics and diagnostic strategies of paediatric FUO.
Infectious diseases
Our study found that infectious diseases still ranked first (50.90%) based on aetiology distribution in each year from 2010 to 2017, which was consistent with published reports. Riganter analysed 15 studies of FUO, which included between 49 and 290 cases from 1961 to 2011, and the proportion of infectious disease was 16%–69%.11 27 28Our study showed that the proportion of infectious diseases decreased while the proportion of miscellaneous diseases increased significantly compared with the previous studies, which might be related to the change of aetiology distribution and the continuous innovation of clinical diagnostic methods in recent years.
In this study, the most common pathogen for infectious diseases was virus. Viral infections were the most common pathogens in children over 1 year of age, of which EBV was the most common of all viruses. Notably, CMV infection was the most common in infants younger than 1 year, and other important viruses included herpes viruses HHV-6 and HHV-7.30 31 Among the diseases caused by EBV infection, classic EBV-associated infectious mononucleosis accounted for a small proportion, and the clinical symptoms of most children were not typical. In addition, the proportion of cases with EBV-associated HLH, chronic active EBV infection, or other EBV-related vasculitis also significantly increased over time, with a clinical manifestation of long-term fever.
It has been reported that UTI is the most common among infectious diseases31; however, there were only 18 cases of UTI in this study. This might be due to routine urine examinations at the early stage of infection, which would exclude most of these cases. Of these, 14 cases were young children with less than 3 years old (12 females, 2 males), suggesting that young females are still vulnerable to urinary tract infections. In the infant group, bacterial infections were the most common (61.70%). UTIs were the common causes of bloodstream and CNS infections. The pathogens of UTI in this study were mainly Enterococcus spp (E. faecalis and E. faecium), followed by E. coli and Pseudomonas aeruginosa. These results were consistent with previous studies.32
We found that bloodstream infections ranked second and were more common in infants. Some studies have shown that bloodstream infections account for about 10% of FUO in newborns and 5% in children of less than 3 months of age.33 In addition, CNS diseases with infectious lesions, such as tuberculous meningitis, fungal meningitis and Listeria monocytogenes meningitis, accounted for 6.10% of cases and often caused long-term fever as it was difficult to identify the specific pathogen during diagnosis and treatment. Recently, a child with a liver abscess was found to have a Klebsiella pneumoniae infection by next-generation sequencing of the peritoneal effusion. This suggests that next-generation sequencing can provide better technical support for the diagnosis of pathogenic FUO in the future.34
Among the bacterial infections, Streptococcus spp was the primary pathogen. Group A haemolytic streptococcus, which causes respiratory infections, invasive infections and sepsis, accounted for over 66.7% in this study. In particular, invasive infections, which is characterised by low blood culture positive rates and the diagnosis of which depend on ASO values, leads to multiorgan involvement and long-term fever during this process. Brucella infection is also a common cause of FUO in children, especially in developing countries.35 It has been shown that commonly used antibiotics often fail to eliminate the infections from rare pathogens, leading to poor diagnosis and treatment outcomes in paediatric FUO.36 37 Salmonella often causes occult bloodstream infection and easily leads to FUO in children.32 Some studies have shown that although tests for bacteria were negative in the first blood culture, about 10% of the cases obtained positive results in subsequent blood culture tests.38 TB infection, which is characterised clinically by the lack of specificity, limited diagnostic methods, and low positive rates, can also lead to an FUO diagnosis. Therefore, in some instances, repeated blood culture tests are necessary for proper diagnosis in children with FUO.
In this study, Cryptococcus neoformans infection accounted for 7 cases of the 11 children with fungal infections. Cryptococcal infections, characterised by no obvious symptom and systemic dissemination, cause FUO and affect multiple organs including the CNS, lymph nodes, liver, spleen and lungs. Unlike the common CNS or intracranial infections caused by Cryptococcus, the initial symptoms in our study were fever and joint pain, suggesting rheumatic diseases; however, this was followed by a headache at the late stage. In these patients, CSF samples were examined, and Cryptococcus infection was confirmed by ink staining and Cryptococcus antigen positivity. These results suggest that when children have multiple organ involvement, attention should be paid to the possibility of Cryptococcus infection. Routine Cryptococcal antigen testing can be helpful in the diagnosis during early examinations.
Non-infectious inflammatory diseases
The proportion of cases with NIID was not high in our study. At only 4.90%, it ranked fifth among the five types of diseases. This result was different from that of published studies, which reported about 10%–30%.39 40 The reason could be that those cases were screened based on the diagnosis of ‘fever pending’ on admission and did not involve children with joint symptoms.
Our study found that JIA accounted for 41 cases (65.00%). Based on the exclusive method, it is challenging to diagnose JIA. Due to a lack of obvious clinical manifestations, the diagnosis of JIA is based on recurring high-grade fevers and the appearance of a fever-related rash. In addition, imaging examinations and inflammatory indicators may give some indication of arthritic manifestations. The current study suggests that when inflammatory indicators are significantly increased (WC>15.7×109/L, CRP >33.5 mg/L, ESR >36.5 mm/hour and PCT>0.225 ng/mL), the possibility of rheumatic immune disease should be considered.
Our study also suggests that for children younger than 1-year-old, clinicians should pay more attention to the possibility of systemic vasculitis. In this group, the youngest child with systemic vasculitis was 2 months old, which was similar to that reported in the previous literatures.41
Neoplastic diseases
Regarding blood diseases, HLH was secondary in children with FUO, especially EBV-HLH, which accounted for 50.00%. This was consistent with the previous study.42 Some sensitive indicators such as ferritin (SF), soluble interleukin (IL)-2 receptor, plasma EBV-DNA, IFN-γ and IL-10 were used to monitor HLH disease activity. This study suggests a poor prognosis with an absolute value of neutrophils <0.5×109/L, SF >2000 µg/L, plasma EBV-DNA >1×105/mL and non-recovery of blood counts after 2 weeks of chemotherapy.39 In our studies, five of the six children who died were diagnosed with HLH, suggesting that the disease is fatal and the prognosis is poor. Therefore, it should be detected and treated as early as possible. In addition, in children infected with secondary HLH, attention needs to be paid to black fever caused by Leishmania,42–44 which is one of the causes of FUO and HLH.
Children with leukaemia that manifests as FUO may complain of bone and muscle pain along with the decrease of WC and platelet counts, suggesting the possibility of leukaemia.45 In that case, bone marrow cytology should be performed as soon as possible. ALK-positive large interstitial lymphomas are difficult to distinguish from infectious diseases based on clinical FUO manifestations. A case of ALK-positive large-cell anaplastic lymphoma with recurrent fever, hepatosplenomegaly, lung disease and multiple lymph node involvement was finally confirmed by a right inguinal lymph node biopsy. In addition, in children with long-term recurrent fever along with haemorrhagic, thorny rash and hepatosplenomegaly, the possibility of LCH should be considered. These two diseases are the most difficult to distinguish from infectious diseases. Pathological biopsies of relevant tissues are helpful to assist in diagnosis, such as biopsies of skin, lymph nodes, liver or lungs.
Miscellaneous diseases
Other diseases accounted for 26.6% of all FUO cases. HNL, the most common in this group, is one of the causes of FUO.46 47 Typical characteristics include fever, non-purulent painful lymphadenopathy and leucocytopenia. School-aged children were predominant in this group, with fever lasting an average of 38 days. Among them, 121 cases were confirmed by pathological biopsy (55.80%). There is a possibility of recurrence and SLE development, although the disease is mostly self-limiting. Therefore, children need to be followed up with for a long time. The aetiology of this disease is still not clear, and it may be related to infection and immune factors.48 49 Hormone therapy has previously been considered effective.
The proportion of cases with Kawasaki disease in this study was not high, at only 2.62%, which was different from previous studies.11 This might be because typical Kawasaki disease could be diagnosed at an early stage. Atypical Kawasaki disease is characterised by fever, elevated inflammatory indicators and coronary artery dilatation.50 Therefore, for children with FUO accompanied by elevated inflammatory indicators, cardiac coronary artery ultrasound is essential. At the same time, it is necessary to pay attention to other causes of disease, such as the possibility of EBV infection and Mycoplasma pneumoniae infection.
It is worth noting that an increasing proportion of pseudo-fever was found in school-aged children. With the change of social environment, psychological problems gradually increase. Pseudo-fever has become one of the leading causes of paediatric FUO.51 In this study, 53 cases (96.36%) were over 8 years old with obvious self-conscious symptoms. Fever was a prominent feature, but the skin touch temperature and the rectal temperature were normal during the examination. Psychological problems were found in some children, such as being tired of school, stress, etc. Studies have suggested that these children may have Sjogren’s syndrome.52 To determine if this group of children can be diagnosed as Sjogren’s syndrome, long-term follow-up is still needed.
Drug fever (DF) is a type of adverse drug reaction. It is an unexpected increase in body temperature during medication and is often misdiagnosed in the clinic. Antibiotics like β-lactams and vancomycin are the most common cause. Previous studies have suggested that antibacterial drugs cause the highest proportion of drug fevers, between 30% and 60%.53 54 In drug hypersensitivity syndrome, reactivation of HHV-6 infection is the key to inducing such diseases. It is suggested that when considering the existence of such diseases in children, the detection of HHV-6 should be performed at the same time.
Paediatric FUO caused by ectodermal dysplasia has been gradually recognised in recent years. Congenital ectodermal dysplasia is a genetic disease characterised by defects in tissue development of ectodermal origin, such as sweat glands, hair and teeth, and is X-linked recessively inherited.55 For such diseases, it is helpful to carry out targeted examinations, such as skin biopsy and genetic testing. Studies have suggested that abnormalities in body temperature regulation may be related to hypoxic asphyxia in the perinatal period.1
Undiagnosed diseases
Although the current clinical diagnostic and treatment methods are continually changing and the early diagnosis rate of FUO has been improved, 10.90% of children still had unknown aetiology and need to be followed up for long-term observation. For these children, the results of clinical diagnosis suggest that they may have viral infections or atypical bacterial infections that are difficult to detect.1 28
Limitations
There were certain limitations in this study which should be considered carefully during the data interpretation and these include the following: (1) this was a single-centre study, which might not be able to fully explain the composition of the FUO of the entire group of children, needing multicentre studies in future to revalidate these results; (2) the cases reviewed in this study were recorded between 2010 and 2017, which were relatively widely distributed, and the clinical data contained in them have not been followed up to date. Therefore, it was out of the scope of the study to reconfirm the final diagnosis for some children whose initial diagnosis remained unknown. However, the follow-up investigation is currently going on for the paediatric patients with unknown aetiology at the time of discharge to supplement relevant information.
Conclusion
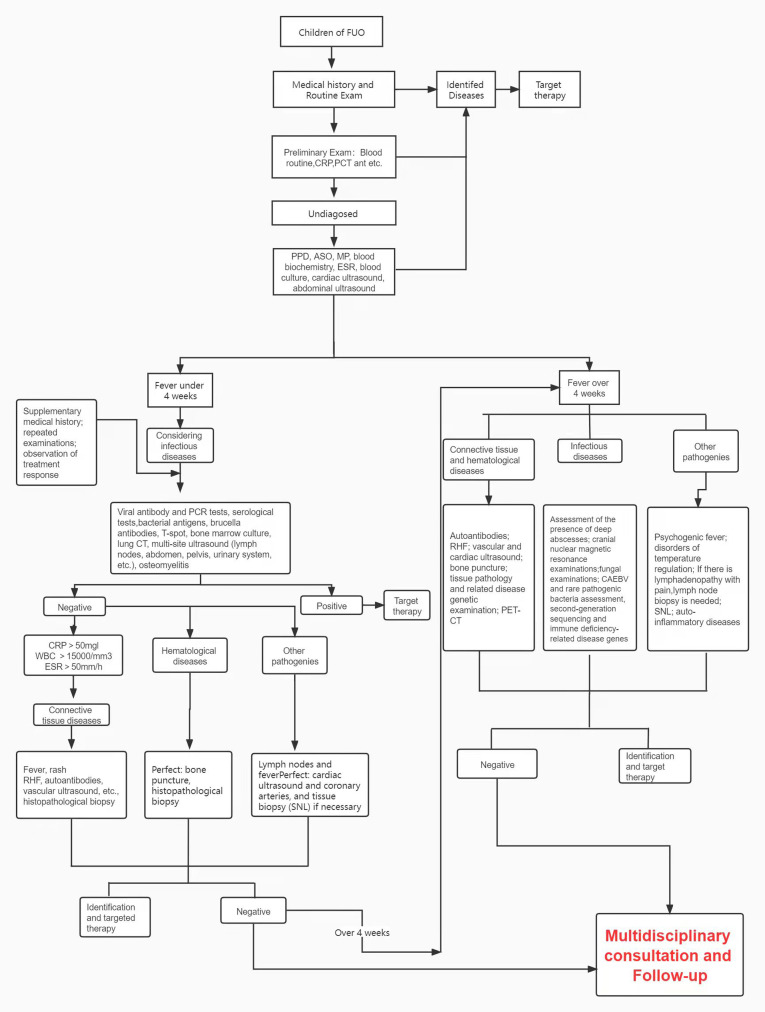
Due to the various causes that lead to FUO, routine blood examinations can be used as a means of preliminary aetiology diagnosis, including inflammatory indicators such as WC count, CRP, ESR and PCT, which can be helpful to distinguish between infectious and non-infectious diseases. Other examination methods are also being gradually applied in clinical screening, such as next-generation sequencing and PET–CT scans, all of which contribute to the identification of infectious lesions, tumorous diseases and other diseases types.35 56 57 Based on this study, a practical stepwise approach to FUO diagnosis was constructed (figure 4) and could be very helpful in the clinical assessment of FUO.
Figure 4.
Flowchart illustrating FUO diagnosis. ASO, antistreptolysin O; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FUO, fever of unknown origin; PPD, purified protein derivative; WC, white cell count.
Supplementary Material
Footnotes
Contributors: BH and GL designed and conducted the study. BH responsible for the overall content as the guarantor. T-MC, S-PL, H-LH and S-YL extracted the data from hospital prescribing records. LG, H-YC and L-YG analysed, scrubbed and maintained research data. BH wrote the manuscript. S-PL reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding: The study was supported by the Special Fund of the Paediatric Medical Coordinated Development Centre of Beijing Hospitals Authority (No. XTCX201817), and the principal investigator of the grant (BH).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by the Medical Ethics Committee of the National Center for Children’s Health, Capital Medical University (No. 2020-Z-053). The name of Ethics Committee: Medical Ethics Committee of National Center for Children’s Health, Capital Medical University Reference number (ID): 2020-Z-053. Participants gave informed consent to participate in the study before taking part.
References
- 1.Chusid MJ. Fever of unknown origin in childhood. Pediatr Clin North Am 2017;64:205–30. 10.1016/j.pcl.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 2.Colvin JM, Muenzer JT, Jaffe DM, et al. Detection of viruses in young children with fever without an apparent source. Pediatrics 2012;130:e1455–62. 10.1542/peds.2012-1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfeld-Yehoshua N, Barkan S, Abu-Kishk I, et al. Hyperpyrexia and high fever as a predictor for serious bacterial infection (Sbi) in children-a systematic review. Eur J Pediatr 2018;177:337–44. 10.1007/s00431-018-3098-x [DOI] [PubMed] [Google Scholar]
- 4.Esposito S, Rinaldi VE, Argentiero A, et al. Approach to neonates and young infants with fever without a source who are at risk for severe bacterial infection. Mediators Inflamm 2018;2018:1–11. 10.1155/2018/4869329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trippella G, Galli L, De Martino M, et al. Procalcitonin performance in detecting serious and invasive bacterial infections in children with fever without apparent source: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 2017;15:1041–57. 10.1080/14787210.2017.1400907 [DOI] [PubMed] [Google Scholar]
- 6.Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine 1961;40:1–30. 10.1097/00005792-196102000-00001 [DOI] [PubMed] [Google Scholar]
- 7.Statler VA, Marshall GS. Characteristics of patients referred to a pediatric infectious diseases clinic with unexplained fever. J Pediatric Infect Dis Soc 2016;5:249–56. 10.1093/jpids/piv008 [DOI] [PubMed] [Google Scholar]
- 8.Durack DT, Street AC. Fever of unknown origin--reexamined and redefined. Curr Clin Top Infect Dis 1991;11:35–51. [PubMed] [Google Scholar]
- 9.Kim Y-S, Kim K-R, Kang J-M, et al. Etiology and clinical characteristics of fever of unknown origin in children: a 15-year experience in a single center. Korean J Pediatr 2017;60:77–85. 10.3345/kjp.2017.60.3.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow A, Robinson JL. Fever of unknown origin in children: a systematic review. World J Pediatr 2011;7:5–10. 10.1007/s12519-011-0240-5 [DOI] [PubMed] [Google Scholar]
- 11.Cho C-Y, Lai C-C, Lee M-L, et al. Clinical analysis of fever of unknown origin in children: a 10-year experience in a Northern Taiwan medical center. J Microbiol Immunol Infect 2017;50:40–5. 10.1016/j.jmii.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 12.Kasai K, Mori M, Hara R, et al. National survey of childhood febrile illness cases with fever of unknown origin in Japan. Pediatr Int 2011;53:421–5. 10.1111/j.1442-200X.2010.03296.x [DOI] [PubMed] [Google Scholar]
- 13.Tolan RW. Fever of unknown origin: a diagnostic approach to this vexing problem. Clin Pediatr 2010;49:207–13. 10.1177/0009922809347799 [DOI] [PubMed] [Google Scholar]
- 14.Chien Y-L, Huang F-L, Huang C-M, et al. Clinical approach to fever of unknown origin in children. J Microbiol Immunol Infect 2017;50:893–8. 10.1016/j.jmii.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 15.Yamanouchi M, Uehara Y, Yokokawa H, et al. Analysis of 256 cases of classic fever of unknown origin. Intern Med 2014;53:2471–5. 10.2169/internalmedicine.53.2218 [DOI] [PubMed] [Google Scholar]
- 16.Baymakova M, Popov GT, Andonova R, et al. Fever of unknown origin and Q-fever: a case series in a Bulgarian Hospital. Caspian J Intern Med 2019;10:102–6. 10.22088/cjim.10.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naito T, Tanei M, Ikeda N, et al. Key diagnostic characteristics of fever of unknown origin in Japanese patients: a prospective multicentre study. BMJ Open 2019;9:e032059. 10.1136/bmjopen-2019-032059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoji S, Imamura A, Imai Y, et al. Fever of unknown origin: a review of 80 patients from the Shin'etsu area of Japan from 1986-1992. Intern Med 1994;33:74–6. 10.2169/internalmedicine.33.74 [DOI] [PubMed] [Google Scholar]
- 19.Horowitz HW. Fever of unknown origin or fever of too many origins? N Engl J Med 2013;368:197–9. 10.1056/NEJMp1212725 [DOI] [PubMed] [Google Scholar]
- 20.Antoon JW, Peritz DC, Parsons MR, et al. Etiology and resource use of fever of unknown origin in hospitalized children. Hosp Pediatr 2018;8:135–40. 10.1542/hpeds.2017-0098 [DOI] [PubMed] [Google Scholar]
- 21.Blokhuis GJ, Bleeker-Rovers CP, Diender MG, et al. Diagnostic value of FDG-PET/(CT) in children with fever of unknown origin and unexplained fever during immune suppression. Eur J Nucl Med Mol Imaging 2014;41:1916–23. 10.1007/s00259-014-2801-z [DOI] [PubMed] [Google Scholar]
- 22.Bleeker-Rovers CP, Vos FJ, de Kleijn EMHA, et al. A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol. Medicine 2007;86:26–38. 10.1097/MD.0b013e31802fe858 [DOI] [PubMed] [Google Scholar]
- 23.Wright WF, Mulders-Manders CM, Auwaerter PG, et al. Fever of Unknown Origin (FUO) - A Call for New Research Standards and Updated Clinical Management. Am J Med 2022;135:173–8. 10.1016/j.amjmed.2021.07.038 [DOI] [PubMed] [Google Scholar]
- 24.Bleeker-Rovers CP, de Kleijn EMHA, Corstens FHM, et al. Clinical value of FDG PET in patients with fever of unknown origin and patients suspected of focal infection or inflammation. Eur J Nucl Med Mol Imaging 2004;31:29–37. 10.1007/s00259-003-1338-3 [DOI] [PubMed] [Google Scholar]
- 25.Bleeker-Rovers CP, Vos FJ, Mudde AH, et al. A prospective multi-centre study of the value of FDG-PET as part of a structured diagnostic protocol in patients with fever of unknown origin. Eur J Nucl Med Mol Imaging 2007;34:694–703. 10.1007/s00259-006-0295-z [DOI] [PubMed] [Google Scholar]
- 26.Baymakova M, Demirev A, Kostadinova I, et al. Giant-Cell arteritis without cranial manifestations presenting as fever of unknown origin: a diagnostic value of 18F-FDG PET/CT. Clin Ter 2018;169:e274–6. 10.7417/CT.2018.2092 [DOI] [PubMed] [Google Scholar]
- 27.Rigante D, Esposito S. A roadmap for fever of unknown origin in children. Int J Immunopathol Pharmacol 2013;26:315–26. 10.1177/039463201302600205 [DOI] [PubMed] [Google Scholar]
- 28.Antoon JW, Potisek NM, Lohr JA. Pediatric fever of unknown origin. Pediatr Rev 2015;36:380–91. 10.1542/pir.36.9.380 [DOI] [PubMed] [Google Scholar]
- 29.Kouijzer IJE, Mulders-Manders CM, Bleeker-Rovers CP, et al. Fever of unknown origin: the value of FDG-PET/CT. Semin Nucl Med 2018;48:100–7. 10.1053/j.semnuclmed.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, Tan X, Li Y, et al. Human herpes viruses are associated with classic fever of unknown origin (FUO) in Beijing patients. PLoS One 2014;9:e101619. 10.1371/journal.pone.0101619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoudi S, Mehrazmay A, Salesi M, et al. Fever of unknown origin: a retrospective study of 95 children in an Iranian referral hospital. Br J Biomed Sci 2014;71:40–2. 10.1080/09674845.2014.11669961 [DOI] [PubMed] [Google Scholar]
- 32.Arora R, Mahajan P. Evaluation of child with fever without source: review of literature and update. Pediatr Clin North Am 2013;60:1049–62. 10.1016/j.pcl.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 33.Niehues T. The febrile child: diagnosis and treatment. Dtsch Arztebl Int 2013;110:764–73. 10.3238/arztebl.2013.0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L-Y, Li Y-J, Liu L-L, et al. Detection of pediatric bacterial meningitis pathogens from cerebrospinal fluid by next-generation sequencing technology. J Infect 2019;78:323–37. 10.1016/j.jinf.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 35.Attard L, Tadolini M, De Rose DU, et al. Overview of fever of unknown origin in adult and paediatric patients. Clin Exp Rheumatol 2018;36 Suppl 110:10–24. [PubMed] [Google Scholar]
- 36.Castellazzi ML, Marchisio P, Bosis S. Listeria monocytogenes meningitis in immunocompetent and healthy children: a case report and a review of the literature. Ital J Pediatr 2018;44:152. 10.1186/s13052-018-0595-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wattier RL, Dvorak CC, Auerbach AD, et al. Repeat blood cultures in children with persistent fever and neutropenia: diagnostic and clinical implications. Pediatr Blood Cancer 2015;62:1421–6. 10.1002/pbc.25466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi X-chun, Liu X-qing, Zhou B-tong, et al. Major causes of fever of unknown origin at Peking Union medical college hospital in the past 26 years. Chin Med J 2013;126:808–12. [PubMed] [Google Scholar]
- 39.Chouchane S, Chouchane CH, Ben Meriem CH, et al. [Prolonged fever in children. Retrospective study of 67 cases]. Arch Pediatr 2004;11:1319–25. 10.1016/j.arcped.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 40.Seashore CJ, Lohr JA. Fever of unknown origin in children. Pediatr Ann 2011;40:26–30. 10.3928/00904481-20101214-07 [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Sun L, Upadhyaya RS, et al. Case report: Takayasu arteritis in a 3-month-old Chinese girl. Medicine 2018;97:e12637. 10.1097/MD.0000000000012637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scalzone M, Ruggiero A, Mastrangelo S, et al. Hemophagocytic lymphohistiocytosis and visceral leishmaniasis in children: case report and systematic review of literature. J Infect Dev Ctries 2016;10:103–8. 10.3855/jidc.6385 [DOI] [PubMed] [Google Scholar]
- 43.Rajagopala S, Dutta U, Chandra KSP, et al. Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis--case report and systematic review. J Infect 2008;56:381–8. 10.1016/j.jinf.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 44.Vanhinsbergh L, Mason A, Godfrey A. Visceral leishmaniasis presenting as haemophagocytic lymphohistiocytosis. BMJ Case Rep 2019;12:e232576. 10.1136/bcr-2019-232576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones OY, Spencer CH, Bowyer SL, et al. A multicenter case-control study on predictive factors distinguishing childhood leukemia from juvenile rheumatoid arthritis. Pediatrics 2006;117:e840–4. 10.1542/peds.2005-1515 [DOI] [PubMed] [Google Scholar]
- 46.Lamzaf L, Harmouche H, Maamar M, et al. Kikuchi-Fujimoto disease: report of 4 cases and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis 2014;131:329–32. 10.1016/j.anorl.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 47.Kang HM, Kim JY, Choi EH, et al. Clinical characteristics of severe histiocytic necrotizing lymphadenitis (Kikuchi-Fujimoto disease) in children. J Pediatr 2016;171:208–12. 10.1016/j.jpeds.2015.12.064 [DOI] [PubMed] [Google Scholar]
- 48.Lelii M, Senatore L, Amodeo I, et al. Kikuchi-Fujimoto disease in children: two case reports and a review of the literature. Ital J Pediatr 2018;44:83. 10.1186/s13052-018-0522-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry AM, Choi SM. Kikuchi-Fujimoto disease: a review. Arch Pathol Lab Med 2018;142:1341–6. 10.5858/arpa.2018-0219-RA [DOI] [PubMed] [Google Scholar]
- 50.Lynch J, Patra KP, Vijay C, et al. A case of fever of unknown origin. Hosp Pediatr 2015;5:452–5. 10.1542/hpeds.2014-0238 [DOI] [PubMed] [Google Scholar]
- 51.Hamilton JL, John SP. Evaluation of fever in infants and young children. Am Fam Physician 2013;87:254–60. [PubMed] [Google Scholar]
- 52.Wang H-C, Chang K, Lin C-Y, et al. Periodic fever as the manifestation of primary Sjogren's syndrome: a case report and literature review. Clin Rheumatol 2012;31:1517–9. 10.1007/s10067-012-2039-8 [DOI] [PubMed] [Google Scholar]
- 53.Sun H-Y, Chen Y-C, Wang Y-W, et al. A prospective study of antimicrobial-related adverse drug reactions in hospitalized patients. J Microbiol Immunol Infect 2008;41:151–9. [PubMed] [Google Scholar]
- 54.Walter EJ, Hanna-Jumma S, Carraretto M, et al. The pathophysiological basis and consequences of fever. Crit Care 2016;20:200. 10.1186/s13054-016-1375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deshmukh S, Prashanth S. Ectodermal dysplasia: a genetic review. Int J Clin Pediatr Dent 2012;5:197–202. 10.5005/jp-journals-10005-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q, Shan C, Wu P, et al. Clinical value of Dual-phase 18F-FDG SPECT with serum procalcitonin for identification of etiology in tumor patients with fever of unknown origin. Asian Pac J Cancer Prev 2014;15:683–6. 10.7314/APJCP.2014.15.2.683 [DOI] [PubMed] [Google Scholar]
- 57.Tokmak H, Ergonul O, Demirkol O, et al. Diagnostic contribution of (18)F-FDG-PET/CT in fever of unknown origin. Int J Infect Dis 2014;19:53–8. 10.1016/j.ijid.2013.10.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The datasets generated during the current study are available from the corresponding author on reasonable request.