Abstract
Background: Ferric chloride is widely utilized in inducing thrombosis in small vessels of experimental animals. However, the lack of its application in large blood vessels of experimental animals and inconsistent concentration has limited its application. Therefore, we systematically tested the most suitable concentration and reliable induction time in the experiment of using ferric chloride to induce rat carotid artery thrombosis. Methods: In this study, we selected the common carotid artery of 59 Sprague–Dawley rats as the target vessel. The exploration process was divided into three stages. First, to determine the optimum induction concentration, we compared the effects of 30–60% ferric chloride on thrombus formation within 24 h. Second, to confirm the handling time, we tested different induction times from 3 min to 10 min. Lastly, we used the thrombolytic drug rt-PA to detect whether the formed thrombus can be lysed. Doppler blood flow imaging and H–E staining were employed to estimate the blood flow and thrombus. The ATP levels in the brain were measured using a bioluminescence ATP assay kit. Results: We found that the application of 50% ferric chloride for 10 min was enough to successfully induce thrombosis in the rat carotid artery and without spontaneous thrombolysis after 24 h. It is better than other concentrations and will lead to the decline of the ATP content in the ischemic hemisphere. Conclusions: Our results indicate that the rat carotid artery thrombosis model induced by 50% ferric chloride for 10 min is stable and reliable.
1. Introduction
Stroke has become one of the pathologies posing tremendous socioeconomic burden, with the highest collective morbidity, disability, and death rate.1,2 Ischemic stroke accounts for more than half of all stroke cases, and it has become the main cause of death and disability, with no effective treatment. Currently, the main treatment approach encompasses the use of cerebral protective agents and thrombolytic drugs.3 Among the thrombolytic drugs, urokinase, alteplase (rt-PA), and tenecteplase have proven to be effective, with rt-PA being the most commonly used drug in clinical practice.4 It is currently believed that thrombolytic therapy within 4.5 h after the onset of stroke symptoms is relatively effective.5 Regrettably, less than 10% of the ischemic patients could benefit from rt-PA administration owing to its strict time window. Nowadays, increasing studies focus on how to extend the time window of rt-PA and discover novel thrombolytic drugs, which require credible vascular thrombosis animal models.
Preclinical thrombolytic drug development requires thrombolytic drug experiments in small animal models and subsequently in nonhuman primate models. At present, vascular thrombosis induction methods employed in thrombolytic drug experiments mainly include the injection of autologous thrombus,6 artery–vein bypass thrombosis,7 vascular ligation,8 electrolysis-induced vascular injury,9 photochemically induced thrombotic occlusion,10 and ferric chloride thrombotic induction.11 As a method recently discovered in the last twenty years, ferric chloride has been widely used in small animal vessels, especially in the mouse carotid artery and middle cerebral artery (MCA) models.12,13 Research has found that the main mechanism behind ferric chloride-induced thrombosis involves causing local vascular endothelial cell detachment and exposing basement membrane components to circulating blood cells; meanwhile, large amounts of iron ions accumulate on endothelial cells and then encourage platelets and the tissue factor to attach to the surface, forming aggregates. This process leads to the thrombin reaction and eventually induces thrombosis.14 Studies have also demonstrated that the thrombus induced by ferric chloride is sensitive to anticoagulants and antiplatelet drugs.15,16
In order to meet the thrombolytic needs after embolization of large vessels, researchers attempted to focus on the MCA and the common carotid artery (CCA) of rats. However, larger arterial diameters required higher ferric chloride concentrations and longer induction times. Calculating the induction time was usually used to judge success or failure in establishing thrombosis, with the application of a Doppler flowmeter to detect blood flow or measure changes in blood vessels under direct observation with intravital microscopy.11 However, there are some problems. Researchers usually ignore the change in thrombosis after removing the ferric chloride solution or filter paper. Most of the researchers only observed the effect of thrombosis during the induction time. Other problems include the induction time ranging from 5 min to 1 h, as well as different induction methods (direct dripping of the ferric chloride solution and paper sticking), solution concentration from 30% to 60%, and so forth.17−22 These factors have led to a uniform standard for ferric chloride-induced carotid artery thrombosis, which makes it difficult to compare the results between different trials and the efficacy of thrombolytic drugs or antithrombotic drugs.
In order to minimize the influence of these different factors and take into account the follow-up effect after the induction of ferric chloride, it is necessary to improve the method and systematically test the influence of different concentrations and induction times. In this study, to set up a stable, reproducible, and consistent model without the autolysis of the thrombus in a shorter induction time, we improved the model of ferric chloride-induced CCA thrombosis so that the thrombosis could be highly reproducible. We verified the effect of the thrombus induced by different concentrations at different induction times through a series of comparative experiments for a long term and selected the optimal concentration and induction time. Meanwhile, fibrinolytic ability within the thrombolytic time window was evaluated by the intravenous injection of rt-PA and the nature of the thrombus was assessed by hematoxylin–eosin (H–E) staining. Changes in the adenosine triphosphate (ATP) content in the brain after CCA embolization were detected using an ATP assay kit.
2. Materials and Methods
2.1. Animals and Groups
All Sprague–Dawley (SD) rats weighing 350–450 g were purchased from Slaccas Experimental Animal Limited Liability Company (China, Shanghai), with free access to water and food. All rats were kept in a temperature-controlled cage, with a 12 h light/dark cycle. All animals used in the study, experimental protocols, and procedures were approved and were in accordance with guidelines set by the animal Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Permit number 2021,0193), following the National Institutes of Health regulations for laboratory animal use.
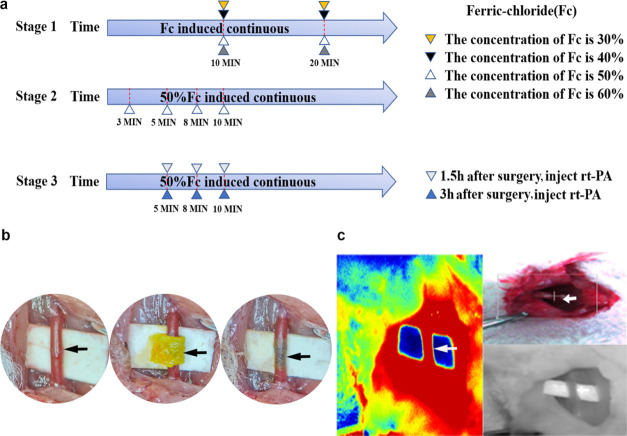
54 SD rats (male) were randomly reared in 18 different groups. Five more SD rats (male) were selected to detect the ATP content in the brain tissue after induction of the carotid artery thrombus with selected optimal conditions. The experiment schedule was divided into three stages (Figure 1a). The first stage compared the thrombus induction efficiency among ferric chloride concentrations ranging from 30 to 60%. The comparison was set as follows: (A1) 30% ferric chloride solution for 10 min, (A2) 30% ferric chloride solution for 20 min, (A3) 40% ferric chloride solution for 10 min, (A4) 40% ferric chloride solution for 20 min, (A5) 50% ferric chloride solution for 10 min, (A6) 50% ferric chloride solution for 20 min, (A7) 60% ferric chloride solution for 10 min, and (A8) 60% ferric chloride solution for 20 min. The second stage focused on the incubation time. The time course was set as follows: (B1) 3, (B2) 5, (B3) 8, and (B4) 10 min. The third stage involved detecting whether the ferric chloride-induced thrombus can be dissolved using rt-PA and the thrombolysis time window postoperation. The comparison was divided as follows: (C1) 5 min group postoperation 1.5 h thrombolysis, (C2) 8 min group postoperation 1.5 h thrombolysis, (C3) 10 min group postoperation 1.5 h thrombolysis, (D1) 5 min group postoperation 3 h thrombolysis, (D2) 8 min group postoperation 3 h thrombolysis, (D3) 10 min group postoperation 3 h thrombolysis.
Figure 1.
(a) Schematic diagram of the exploratory experiment: the experiment was divided into three stages. The first stage compared the thrombus induction efficiency of 30, 40, 50, and 60% ferric chloride-soaked filter wrapped around the right carotid artery for 10 and 20 min. The second stage compared the efficiency of thrombus induction when 50% ferric chloride was exposed to the right carotid artery for 3, 5, 8, and 10 min. The third stage investigated the thrombolytic effect of rt-PA injected at 1.5 and 3 h after the induction of thrombosis for different exposure times. (b) shows the rat carotid artery as viewed under the microscope. The black arrow indicates the dissected carotid artery; a ferric chloride-soaked filter paper wrapped around the carotid artery after operation. (c) Doppler blood flow imaging, camera image, and laser speckle images.
2.2. Induction of CCA Thrombosis in Rats
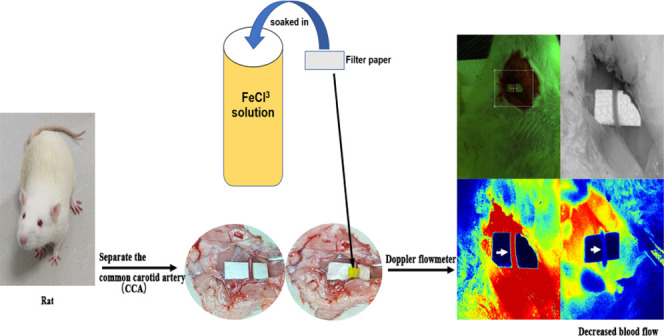
To explore the optimal treatment concentration and the most suitable time to form a stable thrombus by induction with the ferric chloride solution, the SD rats were anesthetized with 2% isoflurane and fixed in the supine position. The skin, subcutaneous fascia, and muscles were cut through a median neck incision (3 cm), and the sternocleidomastoid muscle was exposed; then, the right CCA was bluntly dissected away from surrounding fascia and tissue for full exposure. A filter paper with a width of 2 * 4 mm was soaked in ferric chloride (m/v, Sigma, 236489, USA, dissolved in deionized water) solutions of different concentrations for 1 min, and excess solution was scraped off the surface. After placing a small piece of nonabsorbent paper (or plastic wrap) under the blood vessel to protect the surrounding tissue, the soaked filter paper was wrapped around the carotid artery (wrapping a circle is more adequate than attaching the side wall of blood vessel or dripped solution on surface) for the required time (Figure 1b). After removing the filter paper, the incision was closed, sutured, and disinfected with iodophor. After operation, the rats were returned to the cage for resuscitation.
2.3. CCA Thrombolysis Test
To detect whether the ferric chloride-induced carotid thrombus could be dissolved by a thrombolytic drug, we injected a recombinant tissue plasminogen activator (rt-PA, Actilyse, Boehringer Ingelheim, Germany) through the tail vein at a dose of 10 mg/kg. 10% of the total dose per rat was given as a rapid bolus within 1 min, and the remaining 90% was maintained for 30 min through a microinjection pump. The dose of rt-PA and the method of administration of the drugs to treat cerebral ischemia are similar to clinical medication.23
2.4. CCA Blood Flow Detection
Blood flow was detected using PeriCam PSI (Sweden), which employs a near infrared laser to detect and at the same time visualize blood flow in real time (Figure 1c). We detected the carotid blood flow of the rat carotid artery at 1, 2, 3, and 24 h after operation (after wrapping with a ferric chloride-soaked filter). Meanwhile, the carotid blood flow in the rt-PA injection group was recorded at 1 h (rt-PA 1 h), 2 h (rt-PA 2 h), and 24 h after injection. The blood flow rates at each time point were recorded. Taking into account the anesthesia condition, the detection time point was slightly different. In this experiment, we considered a successful arterial occlusion (TTO) as the postoperative blood flow/preoperative blood flow ratio was less than 10%.
2.5. H–E Staining of Blood Vessels
24 h after carotid artery thrombosis, the rats were euthanized after routine anesthesia. The postoperative right carotid artery was separated, both ends ligated, and then cut at a length of 0.5 cm. After that, the vessel was soaked in 4% paraformaldehyde for 24 h then dehydrated in different ethanol gradients every other day, and finally embedded in paraffin. Also, the carotid artery was cut at a thickness of 20 μm using a paraffin microtome (Leica, Germany). H–E staining was performed on blood vessel slices. The images were obtained using a scanning fluorescence microscope (Leica DMi8, Germany).
2.6. Measurements of ATP in the Rat Brain
The ATP levels in the cerebral hemisphere of the carotid artery embolization side and contralateral cerebral hemisphere of the rat brain were measured using a bioluminescence ATP assay kit (Beyotime, China). In brief, the rats were routinely sacrificed and the brain was quickly collected. The brain tissue was placed in liquid nitrogen to rapidly cool down. Both cerebral hemispheres were weighed separately and lysed using the lysis buffer (200 μL per 20 mg) on ice. The brain tissue was then homogenized, and the supernatant was collected by centrifugation at 12,000g at 4 °C for 5 min. Next, 20 μL of the collected supernatant and 100 μL of luciferase reagent were mixed in a black 96-well plate. Luminescence was recorded using a microplate reader (SpectraMax iD5, Molecular Devices, USA) to calculate the ATP content.
2.7. Statistical Analyses
The statistical analysis of the experimental data was performed using SPSS 22.0 software and Prism 7.0. All time-related results were expressed as means ± standard deviation (SD). One-way ANOVA and two-way ANOVA were used in blood flow analysis. The T-test was used for ATP content analysis. The results were only considered to be statistically significant at P < 0.05.
3. Results
3.1. Thrombogenic Effect of 50% Ferric Chloride Is Better than 30% and 40% Ferric Chloride
Through Doppler blood flow imaging, we found that thrombi induced by 30 and 40% ferric chloride showed postoperative thrombus autolysis. The results showed that part of the thrombus was autolyzed after 24 h of operation, leading to the recovery of blood flow (Figures 2 and 3). However, exposure to 50 and 60% ferric chloride solutions was more effective and produced stable thrombi compared with those induced by exposure to 30 and 40% ferric chloride at the same induction time. We also tested the blood flow changes among 30, 40, 50, and 60% ferric chloride solutions at different induction times. The result showed that wrapping the carotid artery for 20 min with a 40, 50, or 60% soaked ferric chloride filter paper all resulted in the formation of stable thrombi after 24 h without recanalization (Figures 2 and 3), while in the 30% group, the thrombus was partially autolyzed 24 h after the operation with the recovery of blood flow. Moreover, exposure to 50 or 60% ferric chloride reduced blood flow to at least 10% without autolysis. By comparing the thrombus formation results of exposure to 40% ferric chloride for 20 min and 50% ferric chloride for 10 min, we observed that 50% ferric chloride reduced the blood flow to less than 10% within a shorter induction time, and no significant difference was observed at 10 or 20 min induction time (not marked in the figure but statistically significant differences). A shorter induction time meant less time under anesthesia, thus reducing anesthetic burden on experimental animals. Therefore, we opted for 50% ferric chloride as the optimum concentration to induce thrombosis in the subsequent experiments.
Figure 2.
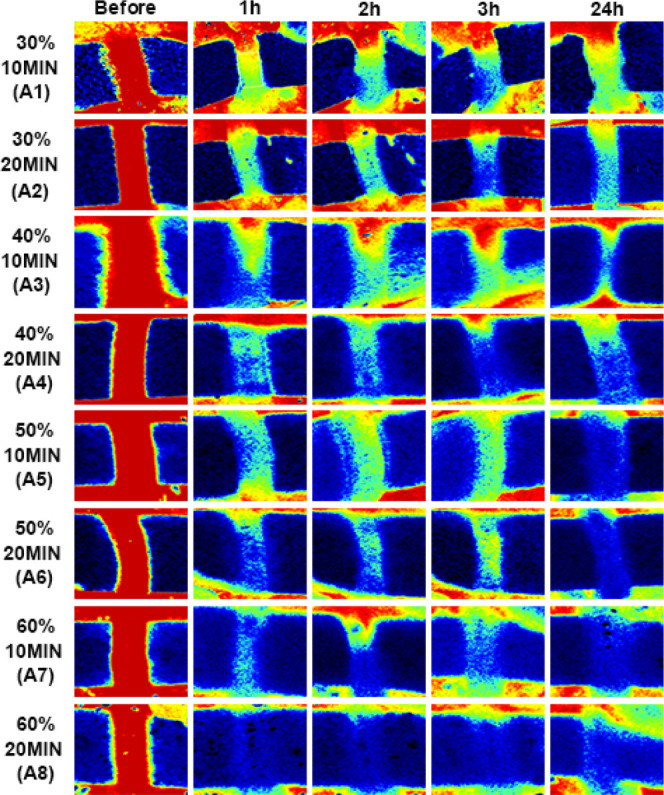
Serial Doppler blood flow images after successful carotid artery thrombosis induction using ferric chloride: (A1) 30% 10 min: 30% ferric chloride for 10 min. (A2) 30% 20 min: 30% ferric chloride for 20 min. (A3) 40% 10 min: 40% ferric chloride for 10 min. (A4) 40% 20 min: 40% ferric chloride for 20 min. (A5) 50% 10 min: 50% ferric chloride for 10 min. (A6) 50% 20 min: 50% ferric chloride for 20 min. (A7) 60% 10 min: 50% ferric chloride for 10 min. (A8) 60% 20 min: 60% ferric chloride for 20 min. The carotid artery is red during the states of high blood flow, green in moderate blood flow, and blue in low blood flow or no blood flow.
Figure 3.
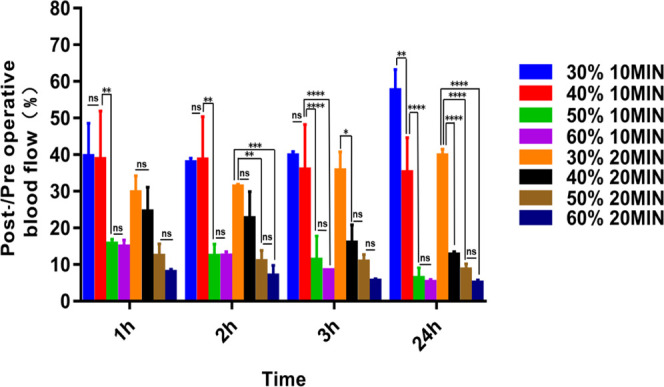
Post-/preoperative blood flow ratios of the different groups (A1–A8); statistical results showed significant differences (****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05; ns = not significant, n = 3). One-way ANOVA and two-way ANOVA.
3.2. Exposure to 50% Ferric Chloride for 10 min Is the Best for Inducing Thrombosis
In order to select the reliability and appropriate induction time of the thrombus induced by 50% ferric chloride, we compared the thrombogenic effect of 50% ferric chloride exposed for 3, 5, 8, and 10 min. The carotid blood flow imaging showed that the thrombus formed in the 3 min group was unstable and that blood flow was still high (more than 30%) after 24 h (Figure 4a,b). The 5, 8, and 10 min groups resulted in blood flow reduction to less than 20% within 3 h after surgery, but the blood flow of the 5 min group and 8 min group was partially restored at 24 h, while in the 10 min group, there was no evidence of blood flow recovery 24 h postinduction, indicating that induction with 50% ferric chloride for 10 min is optimal for thrombus model construction.
Figure 4.
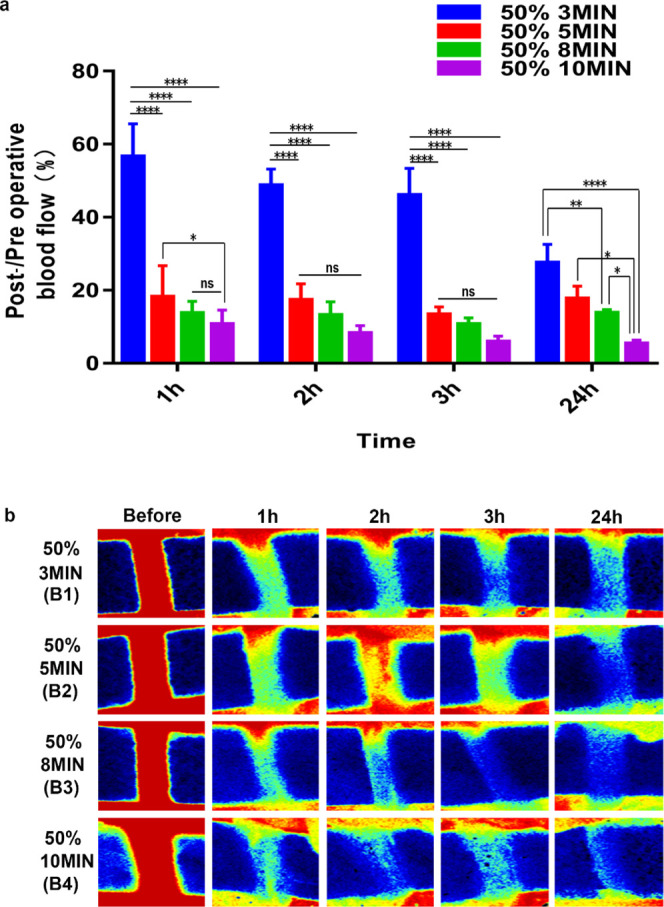
(a) Post-/preoperative blood flow ratios in the B1–B4 groups. Statistical results showed significant differences. (b) shows serial Doppler blood flow images after exposure to 50% ferric chloride for 3, 5, 8, and 10 min (****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05; ns = not significant, n = 3). One-way ANOVA and two-way ANOVA.
3.3. rt-PA Administration 1.5 h after Surgery Can Partially Dissolve the Thrombus Induced by Ferric Chloride
To determine whether the thrombus formed following exposure to 50% ferric chloride for 10 min can be dissolved by thrombolytic drugs, we used the most clinically applied thrombolytic drug rt-PA to conduct thrombolytic tests 1.5 h after successful induction. According to the blood flow results following rt-PA administration 1.5 h after surgery, we found that in the 5 min group, blood flow recovered after 24 h (Figure 5a,b), while in the 8 and 10 min groups, there was only partial blood flow restoration at 10–20% at 24 h after thrombosis induction.
Figure 5.
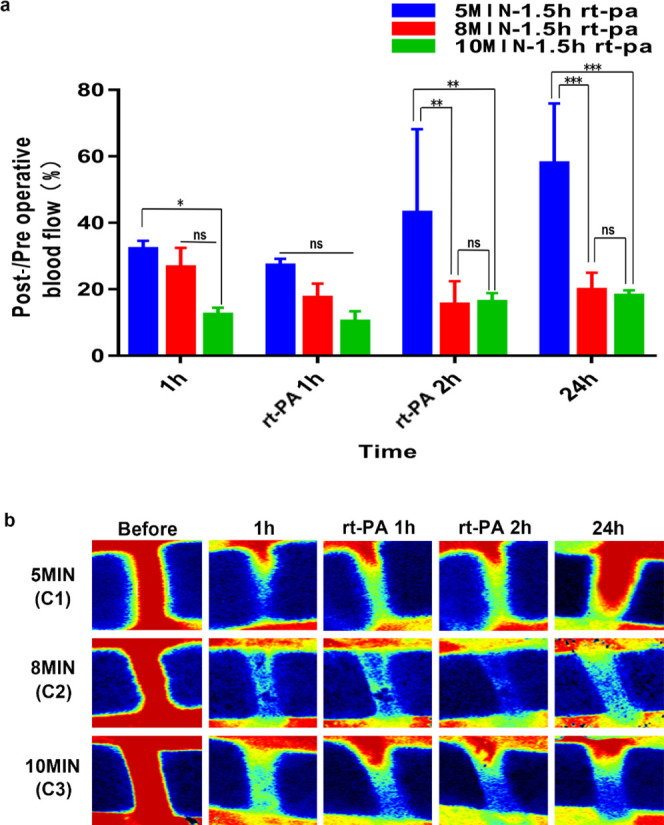
(a) Post-/preoperative blood flow ratios in the C1–C3 groups. (b) shows serial Doppler blood flow images after exposure to 50% ferric chloride for 5, 8, and 10 min with rt-PA intravenously administered 1.5 h after operation. (****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05; ns = not significant, n = 3). One-way ANOVA and two-way ANOVA.
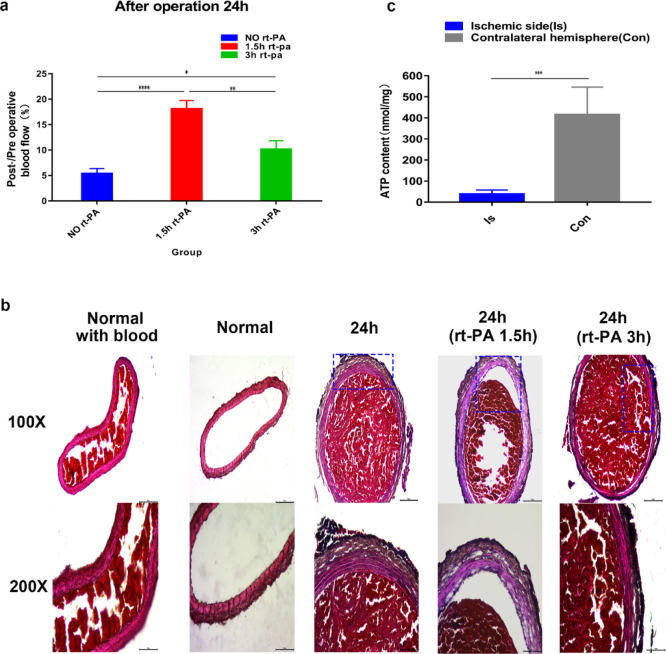
3.4. rt-PA Administration 3 h after Surgery Is Inefficient
Taking into account the actual clinical thrombolytic time, the rats were given the same dose of rt-PA 3 h after surgery. The results showed inferior thrombolytic benefits when compared to rt-PA administration 1.5 h after successful thrombosis induction. Through comparison of blood flow imaging, we found partial blood flow restoration 24 h after the operation in the 5 and 8 min groups, while in the 10 min group, rt-PA administered at 3 h after surgery showed only a short-term thrombolytic effect, and blood flow after 24 h was still about 10% of preoperative blood flow (Figure 6a,b). Comparisons of blood flow restoration after 24 h in the 50% ferric chloride-induced thrombosis group following rt-PA administration at 1.5 and 3 h postinduction showed that these two groups exhibited obvious differences (Figure 7a). The results showed that rt-PA administration 1.5 h after induction can effectively lyse part of the thrombus and significantly improve blood flow. Albeit evident thrombus dissolution, blood flow restoration benefits of rt-PA administration 3 h after surgery remained inferior to the observations in the 1.5 h group. This situation is similar to the real clinical situation, in which the thrombolytic effect of rt-PA decreases as time progresses.
Figure 6.
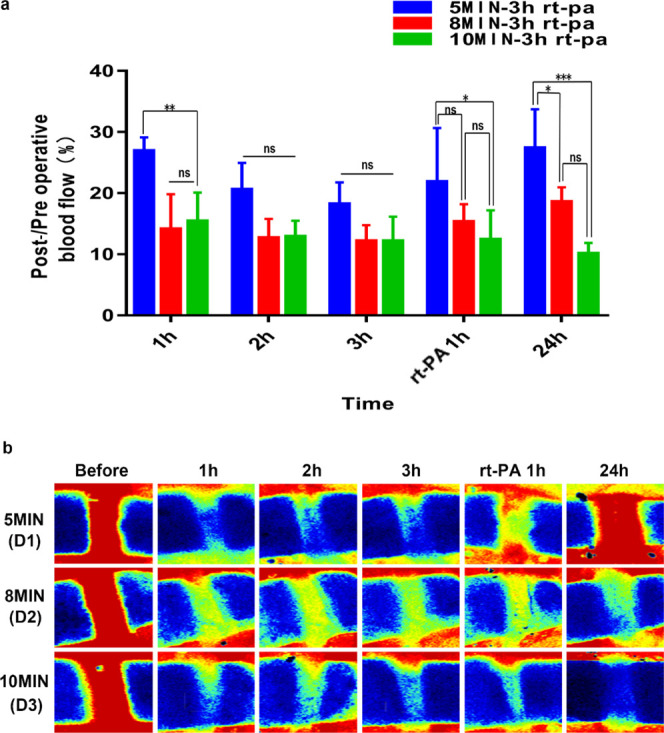
(a) Post-/preoperative blood flow ratios for D1–D3 groups; statistical results showed significant differences. (b) shows serial Doppler blood flow images after exposure to 50% ferric chloride for 5, 8, and 10 min with rt-PA intravenously administered 3 h after operation. (****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05; ns = not significant, n = 3). One-way ANOVA and two-way ANOVA.
Figure 7.
(a) Post-/preoperative blood flow ratios in the 50% 10 min group, 50% 10 min 1.5 h rt-PA group, and 50% 10 min 3 h rt-PA group. Statistical results showed significant differences. (b) H–E staining of the rat carotid artery. Normal with blood: rat carotid artery with blood. Normal: carotid artery without blood. 24 h: H–E staining of the rat carotid artery 24 h after exposure to 50% ferric chloride for 10 min rt-PA 24 h (1.5 h): H–E staining 24 h after exposure to a 50% ferric chloride solution for 10 min, followed by rt-PA administration 1.5 h after surgery. rt-PA 24 h (3 h): H–E staining 24 h after exposure to the 50% ferric chloride solution for 10 min and rt-PA administered 3 h after surgery (scale bar: 100 μm). (c) Detection of ATP content in the brain. Ischemic side: the blood supply of the cerebral hemisphere originates from the carotid artery with the embolized side. Contralateral hemisphere (Con): normal blood supply (n = 5). (****P < 0.0001, ***P < 0.001, **P < 0.01, and *P < 0.05; ns = not significant, n = 3). One-way ANOVA and T-test.
3.5. Carotid Artery Thrombosis Induced by Ferric Chloride Is Stable and Dense
The normal rat CCA (one specimen each with and without blood), a thrombosed carotid artery specimen following 10 min exposure to 50% ferric chloride, and carotid arteries following rt-PA thrombolysis (administration time: 1.5 and 3 h after thrombus induction) of a 0.5 cm length each were cut and fixed for H–E staining. We found that 50% ferric chloride induced uniform and dense thrombosis in the carotid arteries within 24 h after surgery (Figure 7b). After the administration of rt-PA, the thrombus in the lumen was partially dissolved both in the 1.5 h group and the 3 h group; at the same time, exposure to ferric chloride damaged the adventitia of the carotid artery, but the intima and media remained intact.
3.6. ATP Levels in the Ischemic Side of the Brain Decrease
To determine whether CCA embolization affects the ATP content of one side of the cerebral hemisphere, we collected brain tissues after the induction of thrombosis in the CCA by 50% ferric chloride for 10 min and detected the ATP content of the brain. The results showed that the content of ATP in the cerebral hemisphere with blood supply from the carotid artery thrombosis induced by ferric chloride was significantly lower than that in the opposite side (Figure 7c).
4. Conclusions
Through establishing carotid artery thrombosis rat models, we proved that a 10 min exposure of 50% ferric chloride is effective in inducing a stable and uniform thrombus in a rat carotid artery without the evidence of autolysis after 24 h. rt-PA can partially offset the thrombogenic effect of ferric chloride, and its efficacy is related to the time of administration after the onset of thrombosis. The ATP content in the brain tissue of the CCA-embolized side decreased significantly. We believe that thrombosis induced by this concentration and time of exposure is the most suitable and closer to the clinical situation.
5. Discussion
In recent years, ferric chloride has been widely applied in constructing experimental animal models of cerebral ischemia or vascular embolism. However, most researchers only use ferric chloride to induce arterial thrombosis in mice.12,24 Some researchers use ferric chloride to induce thrombosis in rat common carotid arteries, without an established standard concentration or induction time. Thus, our experiment established a modified rat CCA thrombosis model, which was induced by the optimal concentration and induction time of ferric chloride.
In this experiment, a strip of ferric chloride-soaked filter paper was wrapped around the carotid artery to induce thrombosis. Compared with previous methods, this method ensures that ferric chloride was absorbed via the circumferential adventitia, which results in thrombosis, occluding most of the vascular wall. When compared to the thrombus resulting from contact with only a fraction of the vascular wall or dripped solution on the surface, it can also speed up the formation of blood clots. Ferric chloride can induce the thrombus in a short period of time; however, the thrombus could undergo autolysis partly over time. Most studies only monitor changes in the blood flow within a few hours after thrombosis, which is different from the clinical situation. When the human body produces a thrombus, most of the smaller thrombi and unstable thrombi will autolyze themselves without obvious abnormal symptoms. Therefore, we also measured the blood flow 24 h after the operation. Our experiments show the evidence of autolysis in thrombi formed from exposure to 30 and 40% ferric chloride solutions for 10 min, but no autolysis was observed in the 50 and 60% groups 24 h after operation. In order to compare the stability of thrombus formation at different ferric chloride concentrations and the relationship between concentration and time, we tested the thrombus formation efficiency of different ferric chloride concentrations for 20 min and obtained similar results to 10 min of induction. These results prove that the low-concentration (less than 50%) ferric chloride solution-induced thrombus is amenable to autolysis and requires a longer induction time to significantly reduce the blood flow. Therefore, during the construction of a rat thrombus model, we should take into account both of the possibility of autolysis and induction time. Li’s study used 30% ferric chloride solution for more than 30 min so that the blood flow less than 90%.25 This significantly prolongs the operation time, and spontaneous thrombolysis may occur 24 h after surgery. Qin’s research used low-concentration (less than 30%) ferric chloride to establish thrombus models for comparing the antithrombotic effects of different drugs 24 h after operation that may lead to large bias and autolysis.18
By comparing the blood flow in the 50 and 60% groups 24 h after operation, we found no significant evidence of autolysis; therefore, we believed that exposure to 50% ferric chloride is sufficient for constructing a reliable rat CCA model. In an attempt to seek an optimum induction time, we found that wrapping the carotid artery with a 50% ferric chloride-soaked filter paper for 3–5 min can induce the thrombus but without complete vascular occlusion. Extended exposure times were inversely proportional to blood flow and directly related to the degree of vascular occlusion. As the induction time approached 10 min, blood flow decreased by more than 90%, indicating total vascular occlusion, which clinically is adequate to cause severe ischemia or infarction.26
An exposure time of 10 min is enough to induce a stable thrombus while simultaneously keeping the animal’s duration under anesthesia to a minimum and reducing operative difficulty. In an attempt to determine the antithrombotic effect of orally administered Mozuku in a rat carotid artery thrombosis model, Toshinori and colleagues applied ferric chloride for 30 min, an extended induction time based on our research.27 Also, Sudo et al. aimed to test whether 40% ferric chloride could induce arterial occlusion and explore differences in platelet aggregation and thrombus formation among laboratory rats. In their research, rats were anesthetized with sodium pentobarbital for 60 min, which immensely increased risks associated with anesthesia.28 Sodium pentobarbital led to a significant decrease in HR, LVSP, and +dp/dt in isolated hearts29 and cardiotoxic effects that make it unfitting for long-term animal anesthesia; therefore, it is necessary to reduce the induction time to allow substitution for a safer anesthetic.
Our goal is to establish a reproducible carotid artery thrombosis model for testing the efficacy of thrombolytic drugs. For this reason, we used rt-PA intravenous injection after successful carotid artery thrombosis induction in rats. It is worth mentioning that we found that rt-PA administration within a certain period of time after establishing thrombosis can greatly increase the efficiency of thrombolysis (rt-PA administration 1.5 h after successful model construction was better than that at 3 h), a pattern similar to the observed time window of thrombolysis in clinical stroke patients. Thus, it provides a relatively reliable model for developing or testing thrombolytic drugs with better curative effect. At the same time, H–E staining confirmed that damage was only limited to the adventitia, sparing the media and intima, and there was no rupture observed.
The ischemia or hypoperfusion state changes the anaerobic metabolic process, and the content of ATP in the tissue significantly reduces.30 Cerebral blood flow decreases to less than 15% of the baseline within the core, which leads to reductions in the ATP levels to 25% of baseline.31 Our experiments confirmed that after 50% ferric chloride-induced carotid artery embolization, blood flow in the cerebral hemisphere began to decrease, and the results showed that the ATP content of the embolized side less than 25% of the contralateral normal cerebral hemisphere, reflecting the high efficiency of thrombus formation at this concentration and induction time, can significantly reduce blood flow in this side. This proves that our model is stable and reliable and can also be used to study energy metabolism in the hypoperfused state of the brain after carotid artery embolization.
We believe that in the future, this method can be applied on comparable vascular diameters of larger animals, such as cerebral arteries of nonhuman primate species. Actually, we have begun to use ferric chloride to embolize the cerebral arteries of nonhuman primates(macaque) with initial evidence of success based on our experimental results. The physiological structure of nonhuman primate experimental animals is more similar to that of human beings, making it an important experimental animal in verifying the therapeutic effect of novel drugs on human ischemic stroke. Through analysis of previously published data, we found that the current methods that exist for establishing nonhuman primate cerebral ischemia models mainly include MCA clamping and local microinjection of endothelin 1.32,33 Angiography-guided autologous thrombus or embolus injection into the MCA to induce cerebral ischemia is also another method.6,34 However, the nonhuman primate stroke models constructed by these methods are not quite suitable for thrombolysis experiments after cerebral infarction. Theoretically, since the diameter (1.0–1.5 mm) of the MCA in nonhuman primates is similar to that of the carotid artery in rats, ferric chloride-induced thrombosis should be applicable in inducing MCA thrombosis in nonhuman primates.
In summary, our experimental results confirmed that good thrombosis stability at 24 h postoperation can be attained by 50% ferric chloride for 10 min. This model can be used as a highly reliable tool for preclinical verification and research on anticoagulants and thrombolytic drugs. Meanwhile, it can also be used to study the ATP changes in the cerebral hypoperfusion state after complete carotid artery occlusion with a promising potential for application in other blood vessels in the future.
Acknowledgments
This research was funded by grants from the National Natural Science Foundation of China (81820108011 and 81771262), Zhejiang Provincial Natural Science Foundation (2017C03027 and 2020C03022), and Major Science and Technology Project of the Wenzhou Science and Technology Bureau (ZS2017007).
Author Contributions
Experimental design, X.L. and Q.Z.; manuscript writing, X.L., L.A.B., and Q.Z.; review, K.J. and F.S.-D.; establishment of animal models, X.L. and P.Z.. detection of blood flow, P.Z. and S.Y.; analysis, J.C. and L.A.B.; H–E staining, X.L. and Z.L.
The authors declare no competing financial interest.
References
- Feigin V. L.; Krishnamurthi R. V.; Parmar P.; Norrving B.; Mensah G. A.; Bennett D. A.; Barker-Collo S.; Moran A. E.; Sacco R. L.; Truelsen T.; Davis S.; Pandian J. D.; Naghavi M.; Forouzanfar M. H.; Nguyen G.; Johnson C. O.; Vos T.; Meretoja A.; Murray C. J. L.; Roth G. A.; Group G. B. D. W.; Group G. B. D. S. P. E. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehndiratta M. M.; Khan M.; Mehndiratta P.; Wasay M. Stroke in Asia: geographical variations and temporal trends. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1308–1312. 10.1136/jnnp-2013-306992. [DOI] [PubMed] [Google Scholar]
- Jr H. P. A., Stroke a vascular pathology with inadequate management. J. Hypertens. 2003, 21 (). [DOI] [PubMed] [Google Scholar]
- Bao X.; Wei J.; Feng M.; Lu S.; Li G.; Dou W.; Ma W.; Ma S.; An Y.; Qin C.; Zhao R. C.; Wang R. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011, 1367, 103–113. 10.1016/j.brainres.2010.10.063. [DOI] [PubMed] [Google Scholar]
- Lansberg M. G.; Bluhmki E.; Thijs V. N. Efficacy and Safety of Tissue Plasminogen Activator 3 to 4.5 Hours After Acute Ischemic Stroke. Stroke 2009, 40, 2438–2441. 10.1161/strokeaha.109.552547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Wu D.; Chen J.; Chen C.; Yao T.; He X.; Ma Y.; Zhi X.; Liu R.; Ji X. Intranasal salvinorin A improves neurological outcome in rhesus monkey ischemic stroke model using autologous blood clot. J. Cerebr. Blood Flow Metabol. 2021, 41, 723. 10.1177/0271678x20938137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B.; Xu J. Antithrombotic effect of a novel protein fromFusariumsp. CPCC 480097 in a rat model of artery-vein bypass thrombosis. Pharmaceut. Biol. 2012, 50, 866–870. 10.3109/13880209.2011.641023. [DOI] [PubMed] [Google Scholar]
- Payne H.; Brill A. Stenosis of the Inferior Vena Cava: A Murine Model of Deep Vein Thrombosis. JoVE 2017, e56697. 10.3791/56697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J. A.; Wrobleski S. K.; Hawley A. E.; Lucchesi B. R.; Wakefield T. W.; Myers D. D. Electrolytic Inferior Vena Cava Model (EIM) of Venous Thrombosis. JoVE 2011, e2737. 10.3791/2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa A.; Ohno K.; Jakubowski J. A.; Mizuno M.; Sugidachi A. Prasugrel reduces ischaemic infarct volume and ameliorates neurological deficits in a non-human primate model of middle cerebral artery thrombosis. Thromb. Res. 2015, 136, 1224–1230. 10.1016/j.thromres.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Bonnard T.; Hagemeyer C. E. Ferric Chloride-induced Thrombosis Mouse Model on Carotid Artery and Mesentery Vessel. JoVE 2015, e52838. 10.3791/52838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeara N.; Alamri F. F.; Jayaraman S.; Lee P.; Karamyan S. T.; Arumugam T. V.; Karamyan V. T. Motor deficit in the mouse ferric chloride-induced distal middle cerebral artery occlusion model of stroke. Behav. Brain Res. 2020, 380, 112418. 10.1016/j.bbr.2019.112418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. W.; Schiering N.; Melkko S.; Ewert S.; Salter J.; Zhang Y.; McCormack P.; Yu J.; Huang X.; Chiu Y.-H.; Chen Z.; Schleeger S.; Horny G.; DiPetrillo K.; Muller L.; Hein A.; Villard F.; Scharenberg M.; Ramage P.; Hassiepen U.; Côté S.; DeGagne J.; Krantz C.; Eder J.; Stoll B.; Kulmatycki K.; Feldman D. L.; Hoffmann P.; Basson C. T.; Frost R. J. A.; Khder Y. MAA868, a novel FXI antibody with a unique binding mode, shows durable effects on markers of anticoagulation in humans. Blood 2019, 133, 1507–1516. 10.1182/blood-2018-10-880849. [DOI] [PubMed] [Google Scholar]
- Eckly A.; Hechler B.; Freund M.; Zerr M.; Cazenave J.-P.; Lanza F.; Mangin P. H.; Gachet C. Mechanisms underlying FeCl3-induced arterial thrombosis. J. Thromb. Haemostasis 2011, 9, 779–789. 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- Robertson J. O.; Li W.; Silverstein R. L.; Topol E. J.; Smith J. D. Deficiency of LRP8 in mice is associated with altered platelet function and prolonged time for in vivo thrombosis. Thromb. Res. 2009, 123, 644–652. 10.1016/j.thromres.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinides S. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial__thrombi after vascular injury in mice. Circulation 2001, 103, 576–583. [DOI] [PubMed] [Google Scholar]
- Maione F.; Parisi A.; Caiazzo E.; Morello S.; D’Acquisto F.; Mascolo N.; Cicala C. Interleukin-17A Exacerbates Ferric Chloride-Induced Arterial Thrombosis in Rat Carotid Artery. Int. J. Inflamm. 2014, 2014, 1–6. 10.1155/2014/247503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y.-R.; You S.-J.; Zhang Y.; Li Q.; Wang X.-H.; Wang F.; Hu L.-F.; Liu C.-F. Hydrogen sulfide attenuates ferric chloride-induced arterial thrombosis in rats. Free Radical Res. 2016, 50, 654–665. 10.3109/10715762.2016.1164311. [DOI] [PubMed] [Google Scholar]
- Surin W. R.; Prakash P.; Barthwal M. K.; Dikshit M. Optimization of ferric chloride induced thrombosis model in rats: Effect of anti-platelet and anti-coagulant drugs. J. Pharmacol. Toxicol. Methods 2010, 61, 287–291. 10.1016/j.vascn.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Lin L.; Zhang J.; Zhang Q.; Zhao N.; Wu L.; Chen J.; Wu Z.; Wu G.; Lin J.; Chen Y.; Xu Y. Thrombolytic and Antiplatelet Effects of a Novel Plasminogen Activator from the Venom of Gloydius Brevicaudus Viper. J. Atheroscler. Thromb. 2015, 22, 1080–1090. 10.5551/jat.27649. [DOI] [PubMed] [Google Scholar]
- Kurz K. D. RAT MODEL OF ARTERIAL THROMBOSIS INDUCED BY FERRIC CHLORIDE. Thromb. Res. 1990, 60, 269–280. 10.1016/0049-3848(90)90106-M. [DOI] [PubMed] [Google Scholar]
- Robinson M. A.; Welsh D. C.; Bickel D. J.; Lynch J. J.; Lyle E. A. Differential effects of sodium nitroprusside and hydralazine in a rat model of topical FeCl3-induced carotid artery thrombosis. Thromb. Res. 2003, 111, 59–64. 10.1016/j.thromres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Yin B.; Li D.-D.; Xu S.-Y.; Huang H.; Lin J.; Sheng H.-S.; Fang J.-H.; Song J.-N.; Zhang M. Simvastatin pretreatment ameliorates t-PA–induced hemorrhage transformation and MMP-9/TIMP-1 imbalance in thromboembolic cerebral ischemic rats. Neuropsychiatric Dis. Treat. 2019, 15, 1993–2002. 10.2147/ndt.s199371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.; Wang J.; Huang S.; Siaw-Debrah F.; Nyanzu M.; Zhuge Q. Polyacrylic acid-coated nanoparticles loaded with recombinant tissue plasminogen activator for the treatment of mice with ischemic stroke. Biochem. Biophys. Res. Commun. 2019, 516, 565–570. 10.1016/j.bbrc.2019.06.079. [DOI] [PubMed] [Google Scholar]
- Li P.-C.; Yang C.-C.; Hsu S.-P.; Chien C.-T. Repetitive progressive thermal preconditioning hinders thrombosis by reinforcing phosphatidylinositol 3-kinase/Akt-dependent heat-shock protein/endothelial nitric oxide synthase signaling. J. Vasc. Surg. 2012, 56, 159–170. 10.1016/j.jvs.2011.11.062. [DOI] [PubMed] [Google Scholar]
- Orrapin S.; Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst. Rev. 2017, 1–50. 10.1002/14651858.cd001081.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuzawa T.; Mima A.; Ueshima S. Antithrombotic Effect of Oral Administration of Mozuku (Cladosiphon okamuranus, Brown Seaweed) Extract in Rat. J. Nutr. Sci. Vitaminol. 2019, 65, 171–176. 10.3177/jnsv.65.171. [DOI] [PubMed] [Google Scholar]
- Yamada Y.; Toga K.; Nagamura Y.; Hayashi H.; Ito H.; Sudo T. Genetic strain differences in platelet aggregation and thrombus formation of laboratory rats. Thromb. Haemostasis 2017, 97, 665–672. 10.1160/TH06-05-0268. [DOI] [PubMed] [Google Scholar]
- Jiang X.; Gao L.; Zhang Y.; Wang G.; Liu Y.; Yan C.; Sun H. A comparison of the effects of ketamine, chloral hydrate and pentobarbital sodium anesthesia on isolated rat hearts and cardiomyocytes. J. Cardiovasc. Med. 2011, 12, 732–735. 10.2459/jcm.0b013e32834a6697. [DOI] [PubMed] [Google Scholar]
- Widgerow A. D. Ischemia-reperfusion injury: influencing the microcirculatory and cellular environment. Ann. Plast. Surg. 2014, 72, 253–260. 10.1097/sap.0b013e31825c089c. [DOI] [PubMed] [Google Scholar]
- Smith W. S. Pathophysiology of focal cerebral ischemia: a therapeutic perspective. J. Vasc. Intervent. Radiol. 2004, 15, S3–S12. 10.1097/01.rvi.0000108687.75691.0c. [DOI] [PubMed] [Google Scholar]
- Meloni B. P.; Chen Y.; Harrison K. A.; Nashed J. Y.; Blacker D. J.; South S. M.; Anderton R. S.; Mastaglia F. L.; Winterborn A.; Knuckey N. W.; Cook D. J. Poly-Arginine Peptide-18 (R18) Reduces Brain Injury and Improves Functional Outcomes in a Nonhuman Primate Stroke Model. Neurother. 2020, 17, 627–634. 10.1007/s13311-019-00809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P.; Huang H.; Zhang L.; He J.; Zhao X.; Yang F.; Zhao N.; Yang J.; Ge L.; Lin Y.; Yu H.; Wang J. A pilot study on transient ischemic stroke induced with endothelin-1 in the rhesus monkeys. Sci. Rep. 2017, 7, 45097. 10.1038/srep45097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F. C.; Zhang X.; Kempf D. J.; Yepes M. S.; Connor-Stroud F. R.; Zola S.; Howell L. An Enhanced Model of Middle Cerebral Artery Occlusion in Nonhuman Primates Using an Endovascular Trapping Technique. AJNR 2015, 36, 2354–2359. 10.3174/ajnr.a4448. [DOI] [PMC free article] [PubMed] [Google Scholar]