Abstract
Studies on copper(II) tetrafluorenyl porphyrinate (CuTFP) and copper(II) tetraphenyl porphyrinate (CuTPP) have been focused on the charge carrier transport in their solid films and electroluminescence of their composites. In the dye layers deposited by resistive thermal evaporation, the mobilities of holes and electrons are on the order of 10–5 and 10–6 cm2 V–1 s–1 for the charge transport under the influence of traps, and the charge mobility reaches the order of 10–3 cm2 V–1 s–1 at space-charge-limited current in the nontrapping mode. For the dye molecules, the correlation between the mobility of charge carriers and the distribution of the electron density on the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), which serve as hopping sites for holes and electrons, respectively, is considered. Organic light-emitting diodes incorporating the dye molecules as emitting dopants demonstrate electroluminescence in the near-infrared (IR) range.
Introduction
Porphyrins are of a class of organic tetrapyrrole compounds with high light absorption in the visible range of electromagnetic radiation and properties of organic semiconductors. These properties allow the use of porphyrins of various structures as active components of electronic devices, such as various types of solar cells,1−5 organic light-emitting diodes (OLEDs),6−9 and organic field-effect transistors (OFETs).10,11 Their optical and electrical properties can be fine-tuned by synthetic modifications of the peripheral moieties.12−14 In turn, varying the metal atom gives the high intensity of phosphorescence observed in some synthetic and semisynthetic porphyrinates of palladium, platinum, iridium, and other metals. However, as it was shown earlier, not only metal porphyrinates with a pronounced effect of the “ heavy atom” can possess a high intensity of phosphorescence.15 A high intensity of phosphorescence can also be observed in porphyrinates of light metals—chromium(III), copper(II), and vanadium(IV)—containing an unpaired electron in the electron shell of the metal cation. Cu(II) porphyrinates, in addition to a high phosphorescence intensity, also show a strong change in the phosphorescence lifetime with an increase in temperature from 77 to 298 K. For example, for Cu(II) tetrafluorenyl porphyrinate in a polystyrene matrix, the phosphorescence lifetime decreases from 344 μs at 77 K to 5 μs at 298 K. The well-known Cu(II) tetraphenyl porphyrinate possesses similar properties. Pd, Pt, and Ir porphyrinates were previously widely studied as emitters in the red visible range in OLED compositions. Porphyrinates with heavy metals, in addition to the role of emitters in the red and near-IR ranges, also convert the energy of triplet excited states of polymers into light radiation by the mechanism of triplet–triplet energy transfer; the conversion was significantly enhanced due to the effect of the “heavy atom” of porphyrinates. However, similar studies for Cu(II) tetraphenyl and tetrafluorenyl porphyrinates (CuTPP and CuTFP, respectively) in composites for OLEDs have not been performed previously. At the same time, there has recently been an increased interest in porphyrins with reasonable charge carrier mobility for use as charge-transporting layers for perovskite solar cells.16−21 Additionally, it is also important to note that porphyrins with substituted fluorene-terminated groups can form smooth and pinhole-free morphology films.21
The aim of the present work is to clarify the prospects of using the CuTPP and CuTFP dyes (Figure 1) as an active material in electronic devices (light-emitting diodes, etc.). We investigate the optical properties of the dye molecules, electrical and electroluminescent characteristics of solid layers, and composite films with CuTPP and CuTFP, including measurements of the mobility of charge carriers.
Figure 1.
Structures of (a) copper(II) tetrafluorenyl porphyrinate (CuTFP) and (b) copper(II) tetraphenyl porphyrinate (CuTPP).
Results and Discussion
CuTFP and CuTPP Solid Layers
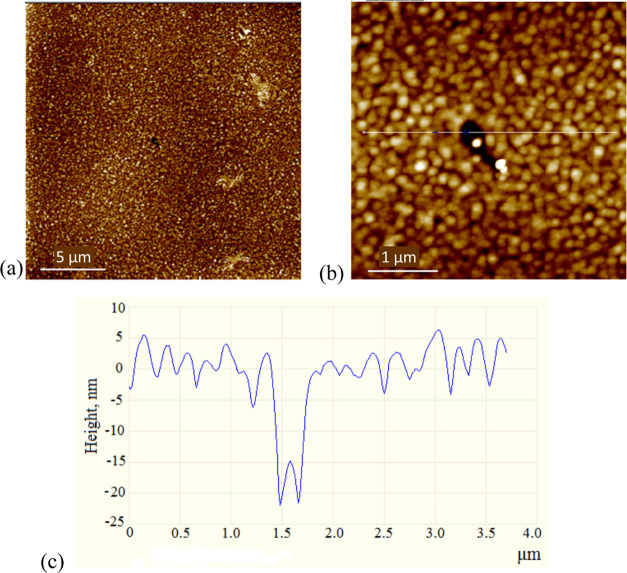
For the use of synthesized substances in applied research, the ability to form homogeneous amorphous or polycrystalline layers of a given thickness is important. By the resistive thermal evaporation (RTE) method, CuTPP and CuTFP layers, of uniform area, were formed on glass substrates. Atomic force microscopy (AFM) images of the topography are shown in Figures 2 and 3, respectively.
Figure 2.
AFM images of the CuTPP film with a scanning scale of (a) 5 μm and (b) 1 μm; (c) height profile along the indicated white line shown in panel (b). Grains of ca. 150 nm size are seen in panel (b) and the root mean square (rms) of the surface is 1.84 nm. The film thickness is 45 nm.
Figure 3.

(a) AFM image of the CuTFP layer with a scanning scale of 5 μm and (b) height profile along the indicated white line shown in panel (a). The surface consists of flat irregularly shaped islands with a height of ca. 20 nm, the space between which is filled with separate grains with a height of several tens of nanometers. The rms of the surface is 10.3 nm and the layer thickness is 150 nm.
The layers were completely amorphous, as can be seen from the X-ray diffraction spectra (Figure S1). The layers were impurity-free since the pressure in the vacuum chamber during their deposition remained unchanged and no impurities were detected by X-ray fluorescence spectroscopy (Figure S2).
UV–Vis Spectroscopy
UV–vis absorption spectra of CuTFP and CuTPP solid layers deposited by the RTE method are presented in Figure 4a,b. The luminescence excitation spectra of these layers (Figure 4c,d) closely correspond to their absorption spectra (Figure 4a,b): they both contain a Soret band in the range of 400–450 nm and a Q-band in the range of 500–650 nm. No signs of degradation of the dyes are seen in the spectra: they completely retained the characteristic absorption bands (Soret and Q-bands) after the sublimation process, which indicates their thermal stability. Thus, the RTE method allows the formation of the CuTFP and CuTPP solid layers with good surface uniformity, which are reasonable for application in thin-film devices.
Figure 4.
Absorption spectra (a, b—solid curves) and excitation spectra (c, d) of CuTFP (a, c) and CuTPP (b, d) solid layers formed by the RTE method. Absorption spectra of the dyes in chlorobenzene (a, b—dashed curves) recorded in 1 cm cuvettes.
The UV–vis absorption spectra of CuTPP and CuTFP in chlorobenzene shown in Figure 4a,b are quite similar to the corresponding spectra of the solid layers. They also exhibit the typical Soret band (400–450 nm) and Q-band (500–600 nm). In solutions, the shoulder at 400 nm is typical of porphyrins, so it cannot be unambiguously addressed to H-aggregates. The Soret band of J-aggregates of porphyrins is usually red-shifted with respect to nonaggregated molecules and should be registered at 450–500 nm. However, it is not seen in the solution spectra. As for the RTE solid layers, we cannot claim the absence of H- and J-aggregates, since the Soret band in the solid layers is more widespread than in the solutions and strongly deviates from the Gaussian shape.
The photoluminescence spectra of CuTFP and CuTPP in the RTE solid layers (Figure 5) exhibit luminescence in the red visible and near-IR range, so one may expect their activity in electroluminescence in these ranges as well.
Figure 5.
Photoluminescence spectra of (a) CuTFP and (b) CuTPP solid layers in the red visible and near-IR range.
Electrical Characterization
The electrical conductivity of a material is determined by two key parameters: the concentration and mobility of free charge carriers. The study of the electron transport in solid layers of the Cu(II) porphyrinates was carried out by measuring the mobility of charge carriers under two current conditions.
Current–voltage characteristics of the steady-state current obey the power law j ∼ Un in organic insulators (see, for example, ref (22)). Diodes based on both Cu(II) porphyrinates showed such a power-law dependence. As an example, Figure 6 shows the j–V curve in a ITO/CuTFP/Al diode. At low voltage, the dependence follows Ohm’s law. With increasing voltage, U > 0.7 V, the exponent increases to n = 3.5, and then takes the value n = 2 at U > 3.3 V. Once the traps are completely filled with the injected charge carriers, the space-charge-limited current (SCLC) flows in the nontrapping mode, for which the Mott–Gurney equation is valid23
where μSCLC is the mobility of the charge carriers, d is the thickness of the dye layer, ε0 is the vacuum permittivity, and ε is the relative dielectric constant of the dye layer. The constant ε = 3.5 was used for the Cu(II) porphyrinates studied.
Figure 6.

j–V curve of the ITO/CuTFP/Al diode with a 160-nm-thick CuTFP layer. The inset shows the j–V curve in the TFL region; VTFL = 3.2 V.
Charge carrier mobility was also measured by charge extraction by the linear increasing voltage (CELIV) technique.24 The characteristic point on the transient curve (Figure S3) is the time at which the conduction current reaches its maximum value tmax. The CELIV mobility μ is calculated according to the equation
where A = U/t is the voltage ramp, J(0) is the capacitance current, and ΔJ is the conduction current at time tmax. Table 1 lists the mobility of charge carriers in CuTFP and CuTPP layers with thicknesses of 160 and 100 nm, respectively.
Table 1. Charge Carrier Mobility in Cu(II) Porphyrinate Solid Layersa.
| mobility (cm2 V–1 s–1) | |||
|---|---|---|---|
| SCLC | CELIV |
||
| dye | holes and electrons | holes | electrons |
| CuTFP | 5.6 × 10–3 | 1.6 × 10–5 | 3.6 × 10–6 |
| CuTPP | 1.0 × 10–3 | 1.9 × 10–6 | 8.7 × 10–6 |
Calculated from 10 replicates, the confidence interval is 95%.
For the CuTFP and CuTPP layers, different μSCLC values can originate from the difference in (1) the density of traps of charge carriers in the bulk of the layers and (2) the rate of electron transfer between adjacent molecules. For considering the former, the trap density, Nt, was estimated from the expression Nt = 2εε0VTFL/ed2, where VTFL is the trap-filled limit voltage (Figure 6) and e is the elementary charge.25 On this basis, the Nt values for the CuTFP and CuTPP layers were obtained to be equal to 4.3 × 1016 and 5.1 × 1016 cm–3, respectively, and the difference in the trap density is negligible. Indeed, the calculations of the electron density distribution in molecules, as shown below, suggest that the rate of hole transfer between the dye molecules in the CuTFP layer is higher than in CuTPP.
Generally, in organic molecular glasses, the charge transport can be considered as a chain of redox processes,26 with electron transfer from a radical anion (LUMO + 1) to a neutral molecule (LUMO) for the electron transport and from a neutral molecule (HOMO) to a radical cation (HOMO – 1) for the hole transport. Obviously, the probability of electron transfer depends significantly on the overlap of the boundary orbitals of radical ions and neighboring neutral molecules.
From the quantum-chemical calculations (Supporting Information), for CuTPP, when density functional theory (DFT) simulates the overlapping of orbitals between radical ions and neutral molecules for CuTPP, the overlapping of the frontier orbitals is, on average, greater for the electron transport than for the hole transport. For CuTFP, the overlap values of the frontier orbitals are, on average, greater for the hole transport than for the electron transport. The HOMO and LUMO levels of CuTFP (Table S3) are slightly higher than those of CuTPP (Table S1), while the difference of the HOMO level (0.08 eV) is greater than that of the LUMO level (0.01 eV). However, in the case of the cation, the difference increases: the LUMO level of the CuTFP radical cation (Table S4) is higher than that of CuTPP (Table S2) by as large as 0.18 eV. Therefore, the CuTFP radical anion is only slightly more difficult to form than the CuTPP anion, while the CuTFP radical cation, on the contrary, is noticeably easier than the CuTPP cation. Additionally, the reorganization energy also has a significant effect on the charge transport. For CuTPP, the electron reorganization energy (0.39 eV) is ca. 1.5 times less than the hole reorganization energy (0.25 eV). For CuTFP, the hole reorganization energy (0.17 eV) is more than two times less than the electron reorganization energy (0.38 eV). Thus, the hole transport prevails for CuTFP compared to CuTPP. The lower reorganization energy of CuTFP, compared to CuTPP, also explains its relatively larger SCLC mobility.
Electroluminescence
The HOMO–LUMO levels in CuTFP and CuTPP (Figure 7, Table 2) match reasonably well with those of PEDOT:PSS, a typical hole-transporting material, and TPBi, an electron-transporting (hole blocking) material (Figure S8). For this reason, PVK:PBD with Cu(II) porphyrinate composites were prepared, where PVK served as the film-forming matrix and PBD (Figure S8) served as an electron conductive dopant. For OLEDs (Figure 8), electroluminescence (EL) spectra were recorded (Figure 9). CuTPP and CuTFP produce EL emissions with maxima at 786 and 795 nm, respectively, which are absent on the EL spectrum of the Cu(II) porphyrinate-free OLED (Figure S9). Both PVK and the dye can contribute to UV emission.
Figure 7.
Cyclic voltammograms of (a) CuTFP and (b) CuTPP. The red lines show the tangents to the curves. The accuracy of the CV experiments is ±0.02 V.
Table 2. Energy of the HOMO and LUMO Levels Calculated from CVA Data (Figure 7) and Calculated with DFT (Tables S1 and S3).
| HOMO
(eV) |
LUMO
(eV) |
|||
|---|---|---|---|---|
| dye | CVA | DFT | CVA | DFT |
| CuTFP | –4.93 | –5.07 | –3.24 | –2.59 |
| CuTPP | –5.21 | –5.15 | –3.07 | –2.60 |
Figure 8.

Energy diagram for the OLEDs studied. The architecture of the OLEDs is ITO/PEDOT:PSS(30 nm)/PVK:PBD/dye(70 nm)/TPBi(20 nm)/LiF(1 nm)/Al(80 nm), where ITO and LiF/Al are the anode and cathode, respectively.
Figure 9.

EL spectra of the OLEDs based on the PVK :PBD:dye composite consisting of (a) CuTFP and (b) CuTPP with concentrations of (1) 1 wt % and (2) 5 wt % at an applied voltage of 15 V.
The ratio of EL emission intensities in the near IR (780–800 nm) to UV (390–415 nm) in OLEDs with CuTPP is higher than that in OLEDs with CuTFP (Table 3 and Figure 9). In our opinion, this is due to the fact that PVK can absorb part of the UV electroluminescence and transfer the excitation energy to the dye molecules, which emit photoluminescence in the IR region. Indeed, the transfer of excitation energy from PVK to CuTPP molecules is more efficient than to CuTFP molecules, as follows from the reduced photoluminescence in the UV region of PVK:CuTPP compared to PVK:CuTPP upon excitation in the UV absorption band of PVK (Figure 10).
Table 3. Maximum Intensity of EL Emission in the UV and Near-IR Ranges in OLEDs Based on CuTFP and CuTPP Doped in PVK.
| dye content (wt %) | emission wavelength in UV (nm)/IR (nm) | corresponding intensity (μW cm–2 nm–1) | intensity ratio |
|---|---|---|---|
| CuTFP, 1% | 410/795 | 7.44/1.22 | 6.1/1 |
| CuTFP, 5% | 410/795 | 10.13/2.91 | 3.5/1 |
| CuTPP, 1% | 395/786 | 1.95/3.51 | 1/1.8 |
| CuTPP, 5% | 395/786 | 2.51/3.78 | 1/1.5 |
Figure 10.
Photoluminescence spectra of (a) CuTPP:PVK and (b) CuTFP:PVK layers of 70 nm thickness. The excitation wavelength is 345 nm.
The excitation spectra of CuTPP and CuTFP in the PVK matrix presented in Figures S10–S13 (measured at 770 nm position of the emission monochromator) contain the Soret and Q-bands characteristic of metals. The spectra confirm that the red visible and near-IR emission belongs to CuTPP and CuTFP molecules. The band with low intensity at 385 nm corresponds to the harmonics of the 770 nm signal and does not belong to the composite films.
We believe that the higher intensity of EL OLED with CuTPP than CuTFP is due to the fluorenyl peripheral fragments in the latter. The fluorenyl structure is very similar to that of the carbazolyl moiety in PVK; therefore, hole transfer in the PVK:PBD:CuTFP film can be more efficient than in PVK:PBD:CuTPP, despite some difference in the HOMO levels of PVK and CuTFP. In addition, the hole mobility value and imbalance of hole and electron mobilities in the CuTFP solid layer are also higher than those in the CuTPP layer (Table 1). As a consequence, in PVK:PBD:CuTFP-based OLEDs, the high mobility of charge carriers decreases, confining them within the device emissive region and leading to the probability of bimolecular recombination.
CuTFP- and CuTPP-incorporated OLEDs exhibit EL bands in the near-IR spectral range with a maximum at around 800 nm, whereas OLEDs of the same diode architecture and based on the same PVK:PBD matrix with various metal-free and Zn atoms consisting of porphyrins demonstrate the EL band at 650–660 nm only.9 Earlier, we reported27 about OLEDs based on J-aggregates of cyanine dyes, which also emit at a wavelength of 800 nm. Nevertheless, the simplicity of the synthesis of the Cu(II) porphyrinates and the formation of the light-emitting films in comparison with those of cyanine dyes are the advantage of the studied OLEDs.
Conclusions
The mobility of charge carriers in copper(II) porphyrinate, CuTFP and CuTPP, solid layers has been measured for the first time. Both materials possess high SCLC mobility of the order of 10–3 cm2 V–1 s–1. Since the operating current ranges in organic electronic devices are around and in the SCLC range, the studied Cu(II) porphyrinates are promising materials for modern optoelectronics.
According to electrical and electroluminescence characterization, the hole and electron mobilities have a greater imbalance in the CuTFP solid layers and PVK:PBD:CuTFP composite films than in those with CuTPP. The CuTFP solid layers demonstrate a high SCLC mobility of charge carriers compared to that of CuTPP solid layers. The DFT quantum-chemical calculations of the energy levels of frontier molecular orbitals, intermolecular overlap of the orbitals, and reorganization energy of electronic states are in good agreement with the data on the electron and hole mobilities in the studied porphyrinates. In the near-IR range, the EL intensity in OLEDs based on the PVK:PBD:CuTFP composite is lower than that in OLEDs based on the PVK:PBD:CuTPP composite, which also correlates with a higher mobility of charge carriers in CuTFP.
The results show that CuTPP is a promising material for light-emitting layers of OLEDs, whereas, due to the imbalance in the mobility of holes and electrons, CuTFP is a promising material for the hole-transport layer. The latter is relevant for the development of perovskite solar cells in which such dyes can be used in charge-transport layers.
Experimental Section
Synthesis of Compounds
Details on the syntheses of CuTPP and CuTFP are presented in the Supporting Information.
Formation of CuTFP and CuTPP Solid Layers by Resistive Thermal Evaporation (RTE)
Solid layers of the dyes were deposited by the resistive thermal evaporation (RTE) method in a vacuum thermal evaporator MB Evap with control of the thickness and the deposition rate of the layers at a vacuum of 2 × 10–6 mbar. The resolution for the deposition rate was 0.05 Å/s. The evaporator was conjugated to an MBraun glovebox.
The CuTPP compound was deposited onto the substrates at the rate of 0.17 Å/s at a crucible temperature of 345 °C. The residual pressure in the vacuum chamber increased slightly from 7 × 10–6 to 9 × 10–6 mbar, which indicated the absence of impurities in the layers and the thermal stability of the compound.
The CuTFP compound was deposited onto the substrates at the rate of 0.60 Å/s at a crucible temperature of 430 °C, and the residual pressure in the vacuum chamber remained constant, which indicated the absence of impurities in the layers and the thermal stability of the compound.
X-ray Fluorescence Spectroscopy
XRF spectroscopy was carried out with a micro-XRF analyzer XGT-7200 (Horiba Ltd.) for elemental analysis of the evaporated porphyrins. A porphyrin layer obtained by the RTE method was scraped off the glass substrate. For measurements, the porphyrin powder was placed on a scotch film.
X-ray Diffraction Spectra
X-ray diffraction spectra were recorded by an Empyrean X-ray diffractometer (Malvern Panalytical Ltd.) for the characterization of the porphyrin layers deposited on glass substrates by the RTE method.
Atomic Force Microscopy (AFM)
The surface topology of the solid layers prepared by RTE was studied using an EnviroScope atomic force microscope with a Nanoscope-V controller (Veeco). The elastic constants of standard cantilevers were in the range of 5–40 N/m and the resonance frequencies were in the range of 150–350 kHz. The samples were scanned at small cantilever vibration amplitudes in the range of 2–10 nm. Small scanning amplitudes and the radius of curvature of the probes provided a small effect on the surface from the side of the probe, which is necessary for high-precision AFM measurements of heights. For AFM measurements of limiting resolution, ultrasharp cantilevers from Nanotuning (Russia) with carbon whiskers with a radius of curvature of several nanometers, grown on the tip of standard probes, were used.
Spectroscopy
UV–vis absorption spectra were recorded with a Shimadzu UV-3101PC spectrophotometer. The photoluminescence spectra of the layers were recorded with a Horiba–Jobin Yvon–S.A.S. modular spectrofluorimeter. A 450 W xenon lamp was used as the source of exciting light; an R928 photomultiplier tube and an InGaAs-based near-IR semiconductor detector were used as recording detectors. The angle between the film surface and the excitation beam was 30°.
Cyclic Voltammetry (CVA)
In thin layers of the porphyrinates, HOMO/LUMO energy levels were determined by CVA, as described earlier.28 The CVA experiment was carried out at a scan rate of 20 mV/s in a three-electrode, three-compartment electrochemical cell in the glovebox with a dry argon atmosphere. Platinum sheets served as working and counter electrodes. A 0.2 M solution of tetrabutylammonium hexafluorophosphate (NBu4PF6, Fluka) in acetonitrile (ACN) was used as an electrolyte. An Ag wire immersed into the electrolyte solution with the addition of 0.1 M AgNO3 was used as a pseudo reference electrode (Ag/Ag+). It was calibrated against the ferrocene/ferricenium couple (0.039 V vs Ag/Ag+) and its potential was recalculated to the energy scale using an energy value of 4.988 eV for Fc/Fc+ in ACN. Thus, the energy level of Ag/Ag+ in the present work is 4.95 eV. The substances investigated (0.4 mg) were dissolved in 0.2 M solution of NBu4PF6 in ACN and placed into the working compartment of the electrochemical cell. The values of potentials corresponding to the HOMO and LUMO levels were determined by applying a tangent to the onset of anodic and cathodic currents.
Electrical Measurements
The charge carrier mobility in 100–160 nm solid films of CuTFP and CuTPP prepared by vacuum thermal deposition was calculated from the data of the current–voltage (j–V) curves and transient currents of the CELIV (charge extraction by linear increasing voltage) technique.24 The details of the j–V curves and CELIV measurements were described earlier.22,28,29 The measurements were carried out in a MBraun glovebox with argon gas at a residual concentration of [H2O] < 1 ppm and [O2] < 1 ppm.
In ITO/dye/Al devices, j–V curves of the steady-state injection currents were measured under forward bias at a scan rate of 0.05 V/s using a Keithley 2401 source meter unit.
The MIS-CELIV technique involves the measurement of transient currents at a linear increasing voltage applied to the sample of a metal–insulator–semiconductor (MIS) structure. The architecture of the samples was ITO/SiO2(70 nm)/dye/Al(80 nm). A SiO2 insulator layer was deposited onto the ITO/glass substrate by conventional magnetron sputtering. The SiO2 insulator layer blocks the injection of charge carriers between the ITO electrode and the dye layer. For hole (electron) mobility measurements, a positive (negative) bias was applied to the Al electrode. As a result, holes (electrons) were accumulated at the SiO2 interface; then, applying an opposite bias of increasing voltage, these charge carriers were extracted from the device.
Ternary Composite Films
Solutions of ternary mixtures of PVK (poly(vinylcarbazole)):PBD (2-(4-biphenyl)-5-(4-t-butyl-phenyl)-1,3,4-oxadiazole):Cu(II) porphyrinate in a weight ratio of (70 – x):30:x, where x was 0, 1, and 5, were prepared in chloroform. PVK and PBD were purchased from Lumtec (Luminescence Technology Co.) and used without further purification. The composite films were prepared from the solutions by spin-coating at 2000 rpm and dried at 60 °C for 1 h.
Fabrication and Characterization of Organic Light-Emitting Diodes (OLEDs)
The fabricated OLEDs were of the architecture of ITO/PEDOT:PSS (30 nm)/PVK:PBD:Cu(II) porphyrinate (70 nm)/TPBi (20 nm)/LiF (1 nm)/Al (80 nm), where poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) was used as a hole injection and transporting layer, PVK as a hole-transporting material, and PBD as an electron-transporting material doped with a Cu(II) porphyrinate compound all as an emissive layer, and TPBi (2,2′,2′-(1,3,5-benzinetriyl)-tris(1-phenyl-1-H-benzimidazole)) was used as an electron injection and transporting layer. ITO/glass substrates (Kintec) were thoroughly cleaned and treated with O2 plasma. On the ITO/glass substrate, the PEDOT:PSS (CLEVIOS P VP AI 4083) layer was deposited from an aqueous solution by spin-coating at 2000 rpm in air and then dried in an Ar atmosphere at 110 °C for 20 min. The light-emitting films of the ternary blends were prepared from the solutions by spin-coating at 2000 rpm and dried at 60 °C for 1 h. The TBi layer was deposited at a rate of 0.6 Å/s at a crucible temperature of 192 °C at vacuum of 2 × 10–6 mbar. Electroluminescence (EL) spectra of the OLEDs were recorded using an Avantes 2048 fiber-optic spectrofluorimeter in an Ar atmosphere with O2 and H2O contents <10 ppm at room temperature. The current–voltage–brightness characteristics were measured using a Keithley 2601 source meter unit, a Keithley 6485 picoammeter, and a TKA-04/3 light meter-brightness meter.
Theoretical Calculations
Quantum-chemical calculations were performed using the Gaussian 0930 software package with the density functional theory (DFT) method by implementing the hybrid correlation–exchange functional B3LYP.31 The 6-31G(d) basis set was used for geometry optimizations, the 6-31G(d,p) basis set was used for molecular orbitals and energy calculations, and electrons of the copper atom were rendered by the LaNL2DZ basis set with an effective potential for internal electrons. The solvent effects were accounted for by the polarizable continuum model (PCM) with dielectric constant ε = 3.5 and dynamic dielectric constant ε∞ = 2.5, which are close to those of the Cu(II) porphyrinate films under study. The geometry of the molecules was fully optimized, and the absence of imaginary frequencies confirmed their stationary character. The electron wave functions were tested for stability. Intermolecular orbital overlap was calculated based on the relative position of the molecules taken from the structure of the CuTPP crystal.32
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of Russia (grant agreement no. 075-15-2020-779).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06557.
Synthesis of dyes; X-ray diffraction spectra; X-ray fluorescence data; CELIV measurements; DFT calculations, and electroluminescence spectra of OLED and excitation spectra of PVK:dye composites (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kalyanasundaram K.; Vlachopoulos N.; Krishnan V.; Monnier A.; Grätzel M. Sensitization of titanium dioxide in the visible light region using zinc porphyrins. J. Phys. Chem. A 1987, 91, 2342–2347. 10.1021/j100293a027. [DOI] [Google Scholar]
- Belcher W. J.; Wagner K. I.; Dastoor P. C. The effect of porphyrin inclusion on the spectral response of ternary P3HT:porphyrin:PCBM bulk heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 447–452. 10.1016/j.solmat.2006.09.007. [DOI] [Google Scholar]
- Yella A.; Lee H.-W.; Tsao H. N.; Yi C.; Chandiran A. K.; Nazeeruddin M. K.; Diau E. W.-G.; Yeh C.-Y.; Zakeeruddin S. M.; Grätzel M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. 10.1126/science.1209688. [DOI] [PubMed] [Google Scholar]
- Yella A.; Mai C.-L.; Zakeeruddin S. M.; Chang S.-N.; Hsieh C.-H.; Yeh C.-Y.; Grätzel M. Molecular Engineering of Push–Pull Porphyrin Dyes for Highly Efficient Dye-Sensitized Solar Cells: The Role of Benzene Spacers. Angew. Chem., Int. Ed. 2014, 53, 2973–2977. 10.1002/anie.201309343. [DOI] [PubMed] [Google Scholar]
- Zervaki G. E.; Roy M. S.; Panda M. K.; Angaridis P. A.; Chrissos E.; Sharma G. D.; Coutsolelos A. G. Efficient Sensitization of Dye-Sensitized Solar Cells by Novel Triazine-Bridged Porphyrin–Porphyrin Dyads. Inorg. Chem. 2013, 52, 9813–9825. 10.1021/ic400774p. [DOI] [PubMed] [Google Scholar]
- Zhu L.-J.; Wang J.; Reng T.-G.; Li C.-Y.; Guo D.-C.; Guo C.-C. Effect of substituent groups of porphyrins on the electroluminescent properties of porphyrin-doped OLED devices. J. Phys. Org. Chem. 2010, 23, 190–194. 10.1002/poc.1592. [DOI] [Google Scholar]
- Baldo M. A.; O’Brien D. F.; You Y.; Shoustikov A.; Sibley S.; Thompson M. E.; Forrest S. R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. 10.1038/25954. [DOI] [Google Scholar]
- Graham K. R.; Yang Y.; Sommer J. R.; Shelton A. H.; Schanze K. S.; Xue J.; Reynolds J. R. Extended Conjugation Platinum(II) Porphyrins for use in Near-Infrared Emitting Organic Light Emitting Diodes. Chem. Mater. 2011, 23, 5305–5312. 10.1021/cm202242x. [DOI] [Google Scholar]
- Razavi H.; Mohajerani E.; Safari N.; Shahroosvand H.; Khabbazi A. Electroluminescence and photoluminescence in porphyrin derived compounds in a new polymeric PVK:PBD host. J. Porphyrins Phthalocyanines 2012, 16, 396–402. 10.1142/S1088424612500642. [DOI] [Google Scholar]
- Noh Y. Y.; Kim J. J.; Yase K.; Nagamatsu S. Organic field-effect transistors by a wet-transferring method. Appl. Phys. Lett. 2003, 83, 1243–1245. 10.1063/1.1600518. [DOI] [Google Scholar]
- Hoang M. H.; Kim Y.; Kim M.; Kim K. H.; Lee T. W.; Nguyen D. N.; Kim S.-J.; Lee K.; Lee S. J.; Choi D. H. Unusually High-Performing Organic Field-Effect Transistors Based on π-Extended Semiconducting Porphyrins. Adv. Mater. 2012, 24, 5363–5367. 10.1002/adma.201202148. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Sun Z.; Richy N.; Mongin O.; Paul F.; Blanchard-Desce M.; Paul-Roth C. O. Synthesis, characterization and optical properties of new tetrafluorenyl-porphyrins peripherally functionalized with conjugated 2-fluorenone groups. New J. Chem. 2021, 45, 15053–15062. 10.1039/D1NJ01410B. [DOI] [Google Scholar]
- Wong W.-Y.; Ho C.-L. Heavy metal organometallic electrophosphors derived from multi-component chromophores. Coord. Chem. Rev. 2009, 253, 1709–1758. 10.1016/j.ccr.2009.01.013. [DOI] [Google Scholar]
- Tameev A. R.; Yusupov A. R.; Vannikov A. V.; Tedoradze M. G.; Enakieva Y. Y.; Gorbunova Y. G.; Tsivadze A. Y. The effect of phosphoryl–substituted porphyrins on mobility of charge carriers in p3ht polymer photoconductor. Prot. Met. Phys. Chem. Surf. 2018, 54, 1076–1080. 10.1134/S2070205118060230. [DOI] [Google Scholar]
- Chernyad’ev A. Y.; Kotenev V. A.; Tsivadze A. Y. A New Tetrafluorene-Substituted Copper(II) Porphyrinate as a Promising Phosphorescent Temperature Sensor. Prot. Met. Phys. Chem. Surf. 2018, 54, 817–821. 10.1134/S2070205118050040. [DOI] [Google Scholar]
- Chou H.-H.; Chiang Y.-H.; Li M.-H.; Shen P.-S.; Wei H.-J.; Mai C.-L.; Chen P.; Yeh C.-Y. Zinc Porphyrin–Ethynylaniline Conjugates as Novel Hole-Transporting Materials for Perovskite Solar Cells with Power Conversion Efficiency of 16.6%. ACS Energy Lett. 2016, 1, 956–962. 10.1021/acsenergylett.6b00432. [DOI] [Google Scholar]
- Chiang Y.-H.; Chou H.-H.; Cheng W.-T.; Li Y.-R.; Yeh C.-Y.; Chen P. Porphyrin Dimers as Hole-Transporting Layers for High-Efficiency and Stable Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 1620–1626. 10.1021/acsenergylett.8b00607. [DOI] [Google Scholar]
- Kang S. H.; Lu C.; Zhou H.; Choi S.; Kim J.; Kim H. K. Novel π-extended porphyrin-based hole-transporting materials with triarylamine donor units for high performance perovskite solar cells. Dyes Pigm. 2019, 163, 734–739. 10.1016/j.dyepig.2018.12.065. [DOI] [Google Scholar]
- Si C.-D.; Lv X.-D.; Long S.-J. Perovskite solar cells employing copper(I/II) porphyrin hole-transport material with enhanced performance. Inorg. Chem. Commun. 2020, 112, 107701 10.1016/j.inoche.2019.107701. [DOI] [Google Scholar]
- Agresti A.; Berna B. B.; Pescetelli S.; Catini A.; Menchini F.; Di Natale C.; Paolesse R.; Di Carlo A. Copper-Based Corrole as Thermally Stable Hole Transporting Material for Perovskite Photovoltaics. Adv. Funct. Mater. 2020, 30, 2003790 10.1002/adfm.202003790. [DOI] [Google Scholar]
- Qin T.; Wu F.; Zhu L.; Chi W.; Zhang Y.; Yang Z.; Zhao J.; Chi Z. A hole-transporting material with substituted fluorene as end groups for high-performance perovskite solar cells. Org. Electron. 2022, 100, 106325 10.1016/j.orgel.2021.106325. [DOI] [Google Scholar]
- Tameev A. R.; Pereshivko L.Y.; Vannikov A. V. Electrophysical properties of poly(N -vinylcarbazole)-carbon nanotubes composite films. Polym. Sci., Ser. A 2009, 51, 182–186. 10.1134/S0965545X09020060. [DOI] [Google Scholar]
- Lampert M. A.; Mark P.. Current Injection in Solids; Academic: New York, 1970. [Google Scholar]
- Juška G.; Arlauskas K.; Viliu̅nas M.; Kočka J. Extraction Current Transients: New Method of Study of Charge Transport in Microcrystalline Silicon. Phys. Rev. Lett. 2000, 84, 4946–4949. 10.1103/PhysRevLett.84.4946. [DOI] [PubMed] [Google Scholar]
- Bube R. H. Trap density determination by space-charge-limited currents. J. Appl. Phys. 1962, 33, 1733–1737. 10.1063/1.1728818. [DOI] [Google Scholar]
- Tameev A. R.; He Z.; Milburn G. H. W.; Kozlov A. A.; Vannikov A. V.; Puchala A.; Rasala D. Electron drift mobility in polystyrene doped with bispyrazolopyridine derivatives. Appl. Phys. Lett. 2002, 81, 969–971. 10.1063/1.1497711. [DOI] [Google Scholar]
- Maltsev E. I.; Lypenko D. A.; Bobinkin V. V.; Tameev A. R.; Kirillov S. V.; Shapiro B. I.; Schoo H. F. M.; Vannikov A. V. Near-infrared Electroluminescence in Polymer Composites Based on Organic Nanocrystals. Appl. Phys. Lett. 2002, 81, 3088–3090. 10.1063/1.1515880. [DOI] [Google Scholar]
- Demina N. S.; Rasputin N. A.; Irgashev R. A.; Tameev A. R.; Nekrasova N. V.; Rusinov G. L.; Nunzi J. M.; Charushin V. N. Benzo[b]selenophene/Thieno[3,2-b]indole-Based N,S,Se-Heteroacenes for Hole-Transporting Layers. ACS Omega 2020, 5, 9377–9383. 10.1021/acsomega.0c00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malov V. V.; Tanwistha G.; Nair V. C.; Maslov M. M.; Katin K. P.; Unni K. N. N.; Tameev A. R. Hole mobility in thieno[3,2-b]thiophene oligomers. Mendeleev Commun. 2019, 29, 218–219. 10.1016/j.mencom.2019.03.035. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.. et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford CT, 2013.
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- He H.-S. A second polymorph of (5,10,15,20-tetraphenylporphyrinato)copper(II). Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, m976–m977. 10.1107/S1600536807008574. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.