Abstract
Objective This retrospective study aimed to investigate the clinical features of lung cancer patients with leptomeningeal metastasis (LM) and explore the clinical efficacy and tolerance of intrathecal pemetrexed (IP) combined with systemic antitumor therapy. Methods Thirty-four lung cancer patients (11 men, 23 women) with LM receiving IP at our hospital were retrospectively reviewed between August 2018 and December 2019. Identified cases showed either positive cerebrospinal fluid cytology or typical findings (leptomeningeal enhancement or ventricle broadening) upon imaging examination. Results Before the diagnosis of LM, 24 (70.6%) patients received EGFR-TKI therapy with or without other agents (antivascular therapy, or chemotherapy), 5 (14.7%) patients received chemotherapy, 1 (2.9%) patient received antivascular therapy, and 3 (8.8%) patients received ALK inhibitors. Fourteen (41.2%) patients did not change the systematic regimen at the beginning of IP, while 20 (58.8%) patients changed to antitumor agents. IP was administered for a median of 3 times (range, 1-12 times). The IP dose was 15, 20, 25, 30, and 40 mg in 8 (23.5%), 21 (58.8%), 2 (5.9%), 2 (5.9%), and 1 (5.9%) patient, respectively. In all IP dose levels, the major adverse events were myelosuppression and elevation of hepatic aminotransferases (EHA). Grade 1/2 myelosuppression occurred in 4 (11.8%) patients. Grade 1/2 EHA also occurred in 4 (11.8%) patients. Grades 3/4 adverse events were not observed. After IP and systematic therapy, the clinical manifestations related to LM in 26 (76.5%) patients improved. In the whole cohort, the median overall survival was 20 months. The median time from the initial IP administration until death or the last follow-up was 3.5 months. Conclusions IP showed controllable toxicity and good efficacy, prolonged the survival time, and improved the quality of life when combined with tailored systemic antitumor therapy in lung cancer patients.
Keywords: leptomeningeal metastasis, non-small-cell lung cancer, intrathecal pemetrexed, systemic therapy, quality of life
Introduction
As the survival times of lung cancer patients, especially those with non-small-cell lung cancer (NSCLC), have been significantly prolonged in recent years, an increase in the occurrence of leptomeningeal metastasis (LM) in these patients has been observed.1 LM in lung cancer can be a devastating complication, and the prognosis remains poor despite advances in systemic and local approaches.2,3 Treatment of LM is multidisciplinary, and the goal of treatment in patients with LM is to improve or stabilize the neurologic status of the patient and maintain or regain the quality of life to prolong survival.2 The optimal treatment strategy involves a multidisciplinary approach.4,5 Limited data are available to establish treatment recommendations in the management of LM; no randomized trials have shown a survival benefit from a specific treatment modality, and the optimal strategy is still poorly defined.2 Systemic therapies, such as pemetrexed,6 bevacizumab,7 new-generation epidermal growth factor receptor (EGFR)5,8 or anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs),9,10 and intrathecal therapy11 (or a combination of these modalities) are traditionally considered, with new innovative immunotherapies on the horizon.12
Intrathecal administration is the most common method of delivering chemotherapeutic agents in nonnodular and nonbulky LM of solid tumors, although its efficacy compared to systemic administration and choice of regimen is poorly understood due to limited randomized controlled trials.4 Three agents are commonly prescribed for the intrathecal treatment of LM: methotrexate, cytarabine, and thiotepa.11,13 Several schedules have been proposed, without agreement on the optimal dose, frequency of administration, or optimal treatment duration. Pemetrexed is a newer-generation multitargeted anti-folate agent and, when compared with methotrexate, has better tolerability and possesses fewer drug to drug interactions.4 Pemetrexed combined with platinum is considered as one of the first-line treatment options for advanced NSCLC with or without brain metastases (BMs).6,14 This implies that pemetrexed has the potential capacity to overcome central nervous system (CNS) involvement. The study of ChiCTR1800016615 has demonstrated that 50 mg pemetrexed of intrathecal administration as recommended dose results in few adverse events (AEs) and a good response rate of 84.6% for patients with EGFR-mutant NSCLC-LM who had failed on TKI.15 However, till now little additional research or clinical trials on intrathecal pemetrexed (IP) have been reported. Therefore, we collected the data of 34 patients with LM receiving IP to evaluate the clinical characteristics of these patients and to explore a rational, effective, and safe method for the administration of IP injections.
Materials and Methods
Patients
Lung cancer patients who received IP with cytologically or radiographically proven LM were selectively collected at our hospital from August 2018 to December 2019 from electronic medical record database of our hospital retrospectively. Among the 34 patients, the median age was 54 years (range between 26 and 72 years). In addition, 31 (91.2%) patients were diagnosed with adenocarcinoma. Besides, 2 patients for whom biopsy examination could not be done, and primary lung cancer was highly suspected clinically. The inclusion criteria were as follows: (1) patients were pathologically proven to have lung cancer; (2) patients were diagnosed with LM positive by cerebrospinal fluid (CSF) cytology (malignant cells) and/or typical findings (leptomeningeal enhancement or ventricle broadening) upon imaging; (3) patients received IP during hospitalization. Exclusion criteria were as follows: (1) patients with serious CNS disorders including severe cerebral hernia or coma; (2) patients with lethal or uncontrollable systemic diseases including uncontrollable hypertension, diabetes, or severe cardiovascular diseases. This study was approved by the Ethics Committee of our hospital (approval no. KY-2021-00847). All patients provided written informed consent prior to enrollment in the study. This study was conformed to STROBE guidelines.16
Data Collection
The patients’ clinical data were obtained from electronic medical record database, including their demographic data, clinical characteristics, tumor-related features, treatment modalities, and clinical outcomes. Clinical characteristics included the age, gender, smoking status, and Karnofsky performance status (KPS) at LM diagnosis. Tumor-related features comprised of lung cancer histological types, EGFR/ALK mutation status, treatments before the diagnosis of LM, the presence of prior or concurrent BMs at LM diagnosis, enhanced MRI findings, CSF cytological results, date of LM diagnosis, and date of death or last follow-up. All treatment modalities were recorded, including administration of EGFR/ALK-TKIs, chemotherapy, angiogenesis inhibitor, and intrathecal chemotherapy.
Treatment Regimen of IP
Pemetrexed was administered by intrathecal injection via lumbar puncture. Pemetrexed lyophilized powder (100 mg) was dissolved in 0.9% sodium chloride solution (20 mL). The drug concentration of the solution was 5 mg pemetrexed per mL, and 3-, 4-, 5-, 6-, or 8-mL of the solution was used for intrathecal injection. The scheduled protocol included administration of IP twice per week, 3 to 4 days for a one-time administration, and 2 to 4 times for successive administrations every month. The times of IP were determined by the degree of alleviation of LM-related clinical symptoms. Before intrathecal injection, 1 mg vitamin B12 by intramuscular injection, 5 mg dexamethasone by intramuscular injection every month, and continuous folic acid supplementation of 400 μg QD were given to alleviate the adverse reaction of pemetrexed. After intrathecal injection, calcium folinate 100 mg was applied every 6 h for 2 days to reduce the adverse reaction of pemetrexed such as abnormal liver function and myelosuppression. The complete blood count and multichannel biochemical profiles were monitored before each IP administration.
Statistical Analysis
Descriptive statistics summarized patient characteristics including the median, frequency, and percentage for categorical variables. Overall survival (OSLM) was defined as the time from LM diagnosis to the time of death or last follow-up and was estimated using the Kaplan–Meier method. And differences between groups were analyzed by the log-rank test. Prognostic factors associated with survival were evaluated using a Cox proportional hazard model. Statistical analyses were performed using SPSS® software, version 25 (IBM Corp), and the values of P<.05 were defined as statistically significant.
Results
Patient Characteristics
A total of 34 lung cancer patients (11 men, 23 women) who met the inclusion criteria with LM from lung cancer were enrolled in this study. The median age was 54 years (range between 26 and 72 years). Among which, 31 (91.2%) patients were diagnosed adenocarcinoma. The mutational status of EGFR or ALK at the beginning of the tumor treatment was evaluated in 33 patients. The mutation was not detected in one patient since no specimen could be obtained. Of these patients, 27 (79.4%) were confirmed to have EGFR mutations. ALK rearrangement was detected in 4 patients. Two patients harbored no sensitive mutation. Neurological clinical manifestation was observed in all patients including headache (73.5%), dizziness (50.0%), nausea, and vomiting (38.2%). Before the diagnosis of LM, 24 (70.6%) patients received EGFR-TKI therapy with or without other agents (antivascular therapy, or chemotherapy), 5 (14.7%) patients received chemotherapy, 1 (2.9%) patient received antivascular therapy, and 3 (8.8%) patients received ALK inhibitors. The median KPS score was 40. The clinical characteristics and manifestation of these patients were detailed in Tables 1 and 2, respectively.
Table 1.
Patients’ Characteristics (n = 34).
| No. of patients (%)patients (%) | |
|---|---|
| Age at the time of LM diagnosis (years) | |
| Median (range) | 54 (26-72) |
| <60 | 26 (76.5%) |
| ≥60 | 8 (23.5%) |
| KPS at the time of LM diagnosis | |
| Median (range) | 40 (20-70) |
| ≥60 | 5 (14.7%) |
| <60 | 29 (85.3%) |
| Gender | |
| Male | 11 (32.4%) |
| Female | 23 (67.6%) |
| Histologic subtype | |
| Adenocarcinoma | 31 (91.2%) |
| Composite carcinoma | 1 (3.0%) |
| Undefined pathology | 2 (5.8%) |
| Smoking status | |
| Current/former smoker | 5 (14.7%) |
| Nonsmoker | 29 (85.3%) |
| Gene mutation | |
| EGFR mutation | 27 (79.4%) |
| EGFR 19Del | 11 (32.4%) |
| EGFR L858R | 10 (29.4%) |
| EGFR L833V H835L | 1 (2.9%) |
| EGFR exon 20 insertions | 2 (5.9%) |
| Details unknown of EGFR | 3 (8.8%) |
| ALK mutation | 4 (11.8%) |
| No sensitive mutation | 2 (5.9%) |
| Unknown | 1 (2.9%) |
Abbreviations: KPS, Karnofsky performance status; LM, leptomeningeal metastasis; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
Table 2.
Patients’ Clinical Manifestation (n = 34).
| Clinical manifestation | No. of patients (%) |
|---|---|
| Headache | 25 (73.5%) |
| Dizzy | 17 (50.0%) |
| Nausea and vomiting | 13 (38.2%) |
| Disorders of consciousness | 6 (17.6%) |
| Expression disorder | 6 (17.6%) |
| Optic nerve involvement | 6 (17.6%) |
| Walking instability | 5 (14.7%) |
| Auditory nerve involvement | 2 (5.9%) |
| Epilepsy or convulsion | 2 (5.9%) |
| Defecation difficulty | 1 (2.9%) |
Patterns and Clinical Presentation of LM
All patients underwent lumbar puncture at LM diagnosis, and 29 (85.3%) patients displayed malignant cells on CSF examination. Twelve (35.3%) patients showed typical findings (leptomeningeal enhancement or ventricle broadening) on enhanced MRI of the entire neuraxis (brain with/without the whole spine). LM was detected in 3 (8.8%) patients at the initial diagnosis of NSCLC, and 31 (91.2%) patients developed LM during the course of disease. The response to treatments of an extracranial disease at the time of LM diagnosis was a partial response (PR) or stable disease (SD) in 23 (67.6%) patients, progressive disease (PD) in 3 patients (Table 3). Seven (20.6%) patients were diagnosed with concurrent LM and BM; prior BM was noted in 16 (47.1%) patients. Eight (23.5%) patients were not diagnosed with BM when LM was diagnosed. The median time of diagnosis from tumor to LM is 12 months (range between 0 and 76 months).
Table 3.
Patterns and Clinical Presentations of LM (n = 34).
| No. of patients (%) | |
|---|---|
| Brain metastases upon LM diagnosis | |
| Brain metastases before LM | 16 (47.1%) |
| Concurrent LM and brain metastases | 7 (20.65) |
| LM only | 8 (23.5%) |
| Unknown | 3 (8.8%) |
| Presentation of LM | |
| At the initial diagnosis of lung cancer | 3 (8.8%) |
| During treatment | 31 (91.2%) |
| Status of extracranial disease at LM diagnosis | |
| PR/SD | 23 (67.6%) |
| PD | 3 (8.8%) |
| Not evaluated | 3 (8.8%) |
| Unknown | 2 (5.9%) |
| The modality of LM diagnosis | |
| MRI − /cytology + | 21 (61.8%) |
| MRI + /cytology + | 8 (23.5%) |
| MRI + /cytology − | 5 (14.7%) |
Abbreviations: LM, leptomeningeal metastasis; MRI, magnetic resonance imaging; PR, partial response; SD, stable disease; PD, progressive disease.
Treatments
The treatment modalities at the start of IP administration were summarized in Table 4. Of note, 14 patients did not change the systematic regimen, and 20 patients changed to antitumor agents depending on the condition of the disease. IP was administered for a median of 3 times (range between 1 and 12 times). The dose of IP was 15 mg in 8 patients, 20 mg in 21 patients, 25 mg in 2 patients, 30 mg in 2 patients, and 40 mg in 1 patient (Table 4).
Table 4.
Treatment
| Patient no. | Gene mutation | Prior systematic treatment | Systematic treatment during IP | Dose of IP | No. of IP | Neurological symptoms assessment |
|---|---|---|---|---|---|---|
| P1 | EGFR L858R | Gefitinib | Gefitinib | 15 mg | 1 | Worse |
| P2 | EGFR L833V H835L | Osimertinib | Osimertinib | 15 mg | 5 | Worse |
| P3 | Details unknown of EGFR | Osimertinib | Osimertinib | 15 mg | 1 | Stable |
| P4 | EGFR 19Del | Afatinib | Afatinib | 20 mg | 2 | Improved |
| P5 | EGFR 19Del | Osimertinib | Osimertinib | 20 mg | 2 | Improved |
| P6 | EGFR 19Del | Osimertinib | Osimertinib | 20 mg | 5 | Improved |
| P7 | EGFR 19Del | Osimertinib + Lenvatinib | Osimertinib + Lenvatinib | 20 mg | 7 | Improved |
| P8 | EGFR L858R | Osimertinib | Osimertinib | 20 mg | 1 | Worse |
| P9 | EGFR L858R | Osimertinib + Bevacizumab | Osimertinib + Bevacizumab | 20 mg | 3 | Improved |
| P10 | Details unknown of EGFR | Osimertinib | Osimertinib | 20 mg | 2 | Worse |
| P11 | Details unknown of EGFR | Osimertinib | Osimertinib | 20 mg | 3 | Improved |
| P12 | EGFR L858R | Pemetrexed + Platinum | Pemetrexed + Platinum | 20 mg | 2 | Improved |
| P13 | EGFR 20Ins | Pemetrexed + Platinum | Pemetrexed + Platinum | 20 mg | 5 | Improved |
| P14 | EGFR 19Del | Osimertinib + Bevacizumab | Osimertinib + Bevacizumab | 25 mg | 3 | Improved |
| P15 | EGFR 19Del | Icotinib + Fotemustine | Osimertinib + Anlotinib | 15 mg | 4 | Improved |
| P16 | EGFR L858R | Icotinib | Osimertinib | 15 mg | 4 | Improved |
| P17 | EGFR 19Del | Gefitinib + Pemetrexed + Platinum | Osimertinib | 20 mg | 4 | Improved |
| P18 | EGFR L858R | Erlotinib + Bevacizumab | Osimertinib + Pemetrexed | 20 mg | 6 | Improved |
| P19 | EGFR 19Del | Icotinib | Osimertinib | 20 mg | 3 | Improved |
| P20 | EGFR L858R | Icotinib | Osimertinib | 20 mg | 1 | Stable |
| P21 | EGFR L858R | Docetaxel + Platinum + Temozolomide | Osimertinib | 20 mg | 5 | Improved |
| P22 | EGFR 19Del | Osimertinib | Pemetrexed + Platinum + Anlotinib | 20 mg | 2 | Improved |
| P23 | EGFR L858R | Osimertinib | Osimertinib + Anlotinib | 20 mg | 5 | Improved |
| P24 | EGFR L858R | Osimertinib | Osimertinib + Anlotinib | 20 mg | 9 | Improved |
| P25 | EGFR 19Del | Pemetrexed | Pemetrexed + Bevacizumab | 20 mg | 6 | Improved |
| P26 | EGFR 20Ins | Afatinib | Poziotinib | 30 mg | 8 | Improved |
| P27 | EGFR 19Del | Osimertinib | Osimertinib + Bevacizumab | 40 mg | 3 | Improved |
| P28 | ALK | Crizotinib | Brigatinib | 15 mg | 12 | Improved |
| P29 | ALK | Lorlatinib | Lorlatinib + Pemetrexed + Bevacizumab | 15 mg | 2 | Stable |
| P30 | ALK | None | Alectinib | 25 mg | 5 | Improved |
| P31 | ALK | Crizotinib | Alectinib | 30 mg | 9 | Improved |
| P32 | No sensitive mutation | Pemetrexed | Pemetrexed | 15 mg | 2 | Worse |
| P33 | No sensitive mutation | Anlotinib | Osimertinib | 20 mg | 3 | Improved |
| P34 | Unknown | Gefitinib | Osimertinib | 20 mg | 2 | Improved |
Abbreviations: EGFR, epidermal growth factor receptor; IP, intrathecal pemetrexed; ALK, anaplastic lymphoma kinase.
Clinical Response Evaluation and Adverse Events
After administration of IP and systematic therapy, the clinical manifestations related to LM improved in 26 (76.5%) patients, the LM-related symptoms remained stable for 3 patients, and worsened for 5 patients. The median KPS score was elevated to have increased to 70 from 40 (range between 20 and 90) after IP administration. In all dose levels of IP, the major AEs were grade 1/2 of myelosuppression or elevation of hepatic aminotransferases (EHA) in 8 patients. Other AEs were not detected in these patients. No grade 3 or grade 4 AEs occurred. The AEs improved after symptomatic treatment, including administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF) and drugs with hepatoprotective efficacy such as glutathione, monoammonium glycyrrhizinate, or bicyclo (Table 5).
Table 5.
Toxicities and Adverse Events (AEs).
| Patient no. | Dose | AE | Grade of CTCAE | Time point of AE | Management |
|---|---|---|---|---|---|
| P15 | 15 mg | Myelosuppression | II | After the second IP | rhG-CSF |
| P2 | 15 mg | Myelosuppression | II | After the first IP | rhG-CSF |
| P28 | 15 mg | EHA | I | After the 11th IP | Glutathione, monoammonium glycyrrhizinate |
| P17 | 20 mg | EHA | I | After the first IP | Glutathione, monoammonium glycyrrhizinate |
| P21 | 20 mg | Myelosuppression | I | After the second IP | rhG-CSF |
| P24 | 20 mg | Myelosuppression | II | After the third IP | rhG-CSF |
| P26 | 30 mg | EHA | II | After the fourth IP | Glutathione, monoammonium glycyrrhizinate, bicyclo |
| P31 | 30 mg | EHA | I | After the second IP | Glutathione, monoammonium glycyrrhizinate, bicyclo |
Abbreviations: AE, adverse event; IP, intrathecal pemetrexed; EHA, elevation of hepatic aminotransferases; rhG-CSF, recombinant human granulocyte colony-stimulating factor.
Typical Case
Here we presented the treatment of Patient 2, who harbored a rare complex mutation of L833V and H835L in EGFR, detected by next-generation sequencing, while real-time polymerase chain reaction was negative. Osimertinib was selected as the fourth-line therapy. During the period of treatment with osimertinib, neuroimaging indicated LM with ventricle broadening and leptomeningeal enhancement. As no superior therapy to osimertinib was available, and with no neurological symptoms, osimertinib was continued. Following this course of treatment, the leptomeningeal enhancement had abated and the patient's disease condition was relatively stable for 31 months. Due to the development of neurological symptoms beginning July 2019, including intolerable headache, dizziness, nausea, vomiting, and hearing loss, the patient was switched to 3 courses of IP of 15 mg every 3 to 4 days. After the dose, her neurological symptoms were alleviated. KPS improved from 40 to 80. No severe AEs occurred, and the neurological progression-free survival time was 4 weeks.
Survival and Prognostic Factors
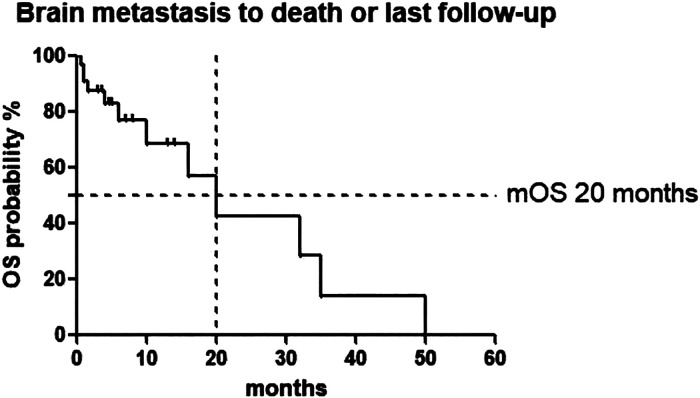
Follow-up was completed in all 34 patients until December 2019. At the end of follow-up, 22 patients were still alive, whereas 12 (35.3%) patients had died at the last follow-up. No patients were lost during follow-up. In the whole cohort, the median time of LM diagnosis until death or last follow-up (the median OSLM) was 20 months. The median time between the initial IP administration and death or the last follow-up was 3.5 months. Table 6 showed the results of the univariate analyses of prognostic factors associated with survival in patients with LM. All these factors including age, KPS score, smoking status, and presentation of LM were not associated with prolonged survival in the univariate analysis (P > .05) (see Figure 1).
Table 6.
Prognostic Factor Analysis for Survival in Patients With LM.
| Parameter | Media OS (months) | Univariate P value |
|---|---|---|
| Age at the time of LM diagnosis (years) | ||
| <60 | 20.0 | .493 |
| ≥60 | - | |
| KPS at the time of LM diagnosis | ||
| ≥60 | 10.0 | .219 |
| <60 | 20.0 | |
| Gender | ||
| Male | 6.0 | .282 |
| Female | 20.0 | |
| Smoking status | ||
| Current/former smoker | 35.0 | .608 |
| Nonsmoker | 20.0 | |
| Presentation of LM | ||
| At the initial diagnosis of lung cancer | 4.0 | .366 |
| During treatment | 20.0 | |
| Status of extracranial disease at LM diagnosis | ||
| PR/SD | 32.0 | .158 |
| PD | 16.0 | |
Abbreviations: OS, overall survival; LM, leptomeningeal metastasis; PR, partial response; SD, stable disease; PD, progressive disease.
Figure 1.
Leptomeningeal metastasis to death or last follow-up. Abbreviation: OS, overall survival.
Discussion
This retrospective study focused on lung cancer patients with LM. NSCLC is characterized by a high incidence of CNS metastasis, with approximately 3.8% of all NSCLC patients developing LM in the course of their disease, which is prevalent in patients harboring EGFR mutations (9.4%).17,18 In this study of 34 patients, 31 (92.1%) patients harbored EGFR or ALK mutations, with the EGFR mutation being more notably present in 27 patients. The high frequency of gene mutation among these patients may be due to the advancement of antitumor drugs especially molecular targeted drugs such as TKI, prolonging the survival time of patients with advanced tumors significantly.19,20 Meanwhile, due to the limited ability of some antitumor drugs, such as first-generation TKIs, in crossing the blood–brain barrier (BBB) and penetrating the CNS,21,22 the incidence rate of LM increased in those patients after therapies with an improved survival.23
In 34 patients, an increased occurrence of headache (73.5%) was observed, which was much higher than Palma's report (22%).24 And the occurrence of nausea and vomiting was 38.2% in the 34 patients. Clinical manifestations of intracranial hypertension remained dominant in these patients and seriously affected patients’ quality of life. Thus, we should improve LM identification methods. Till now, LM diagnosis mostly depends on clinical signs and symptoms, complete contrast-enhanced neuraxis MRI, or CSF cytology.25 Finding tumor cells in CSF is still the “gold standard” for LM diagnosis.4 Out of 34 patients, less than half patients showed typical findings on MRI examination of the entire neuraxis, and 29 displayed malignant cells on CSF cytology. Thus, the diagnosis of LM is still hampered by the low sensitivities of its diagnostic tools: MRI examination of the brain and/or spine and CSF cytology. More sensitive and precise techniques, such as CSF-circulating tumor cell, are needed to improve LM diagnosis and monitoring.26
Most patients with meningeal metastasis are in a critical condition with life threatening, and only intrathecal chemotherapy combined with rational systemic therapy could control the disease better. As for treatment, we tailor the patients’ antitumor treatment to their individual condition in order to effectively and quickly relieve the disease, mainly in terms of the drugs that can penetrate the BBB. Among oncogenic drivers, activated EGFR mutations are found in 79.4% of patients at a high proportion. Osimertinib, as a third-generation EGFR-TKI, is proved to have great penetration of the BBB. It shows impressive efficacy in controlling both systemic and CNS diseases, and is considered to be the standard of care for EGFR-mutated advanced NSCLC.27 NSCLC patients with LM treated with osimertinib experienced an LM objective response rate of 55% to 62% and median progression-free survival (PFS) of 8 to 11 months.8,28,29 Based on the above-mentioned evidence, we chose osimertinib for patients with LM when the standard doses of icotinib or gefitinib failed or maintain osimertinib for some patients when they developed LM. In addition, we explored the efficacy of almonertinib, another third-generation TKI, in patients with LM from EGFR mutant NSCLC sponsored by Hansen Pharma of China. “Pulsatile” high-dose weekly erlotinib or high-dose erlotinib could also be a choice for refractory LMs of NSCLC after failure of standard dose EGFR TKIs.30,31
Systemic chemotherapy is the preferred treatment of choice in patients with LM from NSCLC that have no actionable mutations or failed on EGFR-TKI treatment. A platinum-based combination is the main treatment in advanced NSCLC with BM or LM NSCLC at diagnosis without oncogenic driver mutations or programmed death-ligand 1 tumor proportion score values ≥50%.2 A standard of care regimen has not been established. The role of newer agents, such as bevacizumab, pemetrexed, and anlotinib, is yet to be defined.4 Pemetrexed is a compound currently approved both in combination with platinum in first-line setting and as a single agent in maintenance or second-line setting for the treatment of nonsquamous NSCLC.2 Although CNS penetration of pemetrexed is less than 5%,32 it demonstrated a consistent activity against BM from NSCLC with an intracranial response rate of about 40%.6,33 Bevacizumab is a recombinant humanized monoclonal antibody against vascular endothelial growth factor (VEGF), and animal studies have shown that VEGF prolongs the survival of mice with LM.34 The BRAIN study demonstrates encouraging efficacy of bevacizumab with first-line paclitaxel and carboplatin in patients of asymptomatic, untreated BMs with NSCLC.35 The response rate of BMs in this study was as high as 61.2%.35 Anlotinib as a novel multitargeted TKI has a broad spectrum of inhibitory action on tumor angiogenesis. Clinical trial ALTER-0303 demonstrated anlotinib could prolong the PFS and OS with or without BM with pretreated NSCLC.36 As for ICIs, in the pooled retrospective analysis of KEYNOTE-021, -189, and -407, no matter with stable BM or without BM, pembrolizumab plus platinum-based histology-specific chemotherapy improved clinical outcomes versus chemotherapy alone across all programmed death ligand 1 subgroups.37 It indicates this regimen is a standard treatment option for treatment-naive patients with advanced NSCLC, including patients with stable BMs. While data on the efficacy of immunotherapy for LM are currently limited, because of the exclusion of these patients from clinical trials. In Lizza's study, most patients with NSCLC having LM do not benefit from ICI treatment while median PFS on ICIs was 2.0 months and median OS from the start of ICIs was 3.7 months.12 Thus, further studies are needed to explore the efficacy of ICIs in NSCLC of LM.
As for ALK rearrangements, LM is found in about 5% of ALK-positive cases and usually presents as a late complication.38 Because of the low penetration of crizotinib in the CNS, the CNS is a frequent relapse site for 40% patients treated with crizotinib.39 However, alectinib, which is a second-generation ALK/RET inhibitor with excellent CNS penetration, has impressive systemic and CNS efficacy in patients with ALK-rearranged NSCLC, and can be considered for LM patients.39 Brigatinib, as a potent ALK/ROS/EGFR inhibitor, also has shown impressive intracranial responses of 50% to 67% and a median intracranial PFS of longer than a year in patients with ALK-positive NSCLC40 ALK-positive patients with NSCLC showed substantial intracranial responses to Lorlatinib than crizotinib (66% vs 20%).41 In the phase 2 ASCEND-7 study of ceritinib, mPFS and OS was 5.2 months and 7.2 months in patients with ALK-positive NSCLC-LM. With the excellent CNS penetration of these ALK inhibitors, they can be considered as a treatment of choice for LM patients with ALK rearrangement NSCLC. However, investigations regarding the potential anti-LM activity of these ALK inhibitors are few in number.4
In 34 patients, the dose level of pemetrexed was higher as compared with NCT03101579, wherein the maximum tolerated dose for pemetrexed was 10 mg.42 However, no grade 3 and 4 EHA occurred, and grade 1/2 EHA or myelosuppression happened only in a few patients. Other AEs, such as radiculitis, were not observed in this study. We considered that the increased dose level and obviously decreased adverse reaction were due to the innovatively supplementation of vitamin B12 and folic acid and the rescue therapy of calcium folinate after intrathecal injection. Compared with IP administration at 15 mg, the incidence of myelosuppression and EHA did not increase in patients receiving IP at 20 mg. In another study of ChiCTR1800016615, the recommended dose of pemetrexed observed in the phase 1 study was 50 mg.15 Grade 3-4 myelosuppression, physical pain, or headache was observed in this study with the supplementation of vitamin B12 and folic acid. Since in this study, only 5 patients received IP at more than 20 mg, confirming whether an increased dose of pemetrexed will increase the incidence of AE requires more evidence.
The combination of IP and rational systemic treatment showed good efficacy in treating patients with LM. The clinical response rate of LM was inspiring of 76.5%, but lower than Fan’s report of 84.6%,15 maybe due to lower dose of pemetrexed in this study. Among the 13 patients with EGFR mutation who did not change their systematic regimen, no LM-related clinical manifestations were relieved after intrathecal injection in patients receiving IP at 15 mg, while LM-related clinical manifestations improved after intrathecal injection in most patients (9/10) who received 20 or 25 mg IP. The efficacy of IP may be related to the increased dose of pemetrexed. As for the 14 patients with EGFR mutation who opted to change to antitumor agents, the clinical manifestation related to LM improved after IP and individual systemic treatments in 12 patients, as well as in patients with ALK rearrangement. For patients with no sensitive mutation or unknown mutation, this treatment modality may just be an undertaking. In the entire cohort, the median OSLM was 20 months, which was longer than previous studies having 9.8 to 18.8 months of the median survival.1,6,29 The result is inspiring, especially for NSCLC patients with LM. The application of a novel intrathecal drug like pemetrexed, combined with an appropriate systemic treatment, could be considered for the treatment of LM patients with NSCLC. In the univariate analyses of prognostic factors, no factors were associated with prolonged survival in this study. While in Yu’s retrospective study, a good ECOG PS (≤2) at the time of LM diagnosis, a prior history of brain radiation, and administration of EGFR TKI after LM diagnosis were associated with prolonged survival in the univariate analysis.1 The median time of the initial IP administration until death or last follow-up was 3.5 months, which was similar to the survival time of 3.8 months in the NCT03101579 trial.42 Thus, whether the increased dose and frequency of IP will increase its efficacy or AEs in LM patients still needs to be explored. Meanwhile, more LM patients are attracted to our center to receive IP. We hope and believe that our exploration of the rational and effective intrathecal chemotherapy will benefit more LM patients.
There are some limitations in our study. Due to the small number of patients with LM, only limited 34 patients were enrolled in this retrospective study. Although the combination of IP and systemic treatment showed preliminary efficacy and safety in treating lung patients with LM, larger and prospective investigations are needed to explore the optimal dose, frequency of administration, or optimal treatment duration of IP. In addition, majority of patients harbored EGFR mutations and more investigations are needed in wild-type NSCLC or other driving mutations NSCLC.
Conclusions
IP showed controllable toxicity and good efficacy, prolonged the survival time, and improved the quality of life when combined with tailored systemic antitumor therapy in lung cancer patients. Further studies are necessary to explore a more rational and effective method of administration.
Abbreviations
- AEs
adverse events
- ALK
anaplastic lymphoma kinase
- BBB
blood–brain barrier
- BM
brain metastasis
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CSF-CTC
CSF-circulating tumor cell
- EGFR
epidermal growth factor receptor
- EHA
elevation of hepatic aminotransferases
- IP
intrathecal pemetrexed
- KPS
Karnofsky performance status
- LM
leptomeningeal metastasis
- NSCLC
non-small-cell lung cancer
- OSLM
overall survival
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- rhG-CSF
recombinant human granulocyte colony-stimulating factor
- SD
stable disease
- TKIs
tyrosine kinase inhibitors
- VEGF
vascular endothelial growth factor
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors declared the following financial support with respect to the research, authorship, and/or publication of this article: This study was supported by a grant from the Foundation of Chinese Society of Clinical Oncology (Y-HS2019/2- 008).
Ethical Approval: Our study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (approval no. KY-2021-00847). All patients provided written informed consent prior to enrollment in the study.
ORCID iD: Xingya Li https://orcid.org/0000-0001-9121-6613
References
- 1.Yan W, Jing W, An N, et al. The clinical characteristic and prognostic factors of leptomeningeal metastasis in patients with non-small-cell lung cancer-a retrospective study from one single cancer institute. Cancer Med. 2019;8:2769‐2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turkaj A, Morelli AM, Vavalà T, Novello S. Management of leptomeningeal metastases in non-oncogene addicted non-small cell lung cancer. Front Oncol. 2018;8:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brower JV, Saha S, Rosenberg SA, Hullett CR, Ian Robins H. Management of leptomeningeal metastases: prognostic factors and associated outcomes. J Clin Neurosci. 2016;27:130‐137. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43‐e55. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Lee EM. A retrospective analysis of the clinical outcomes of leptomeningeal metastasis in patients with solid tumors. Brain Tumor Res Treat. 2018;6:54‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol. 2011;22:2466‐2470. [DOI] [PubMed] [Google Scholar]
- 7.Lu ZQ, Cai J, Wang X, et al. Osimertinib combined with bevacizumab for leptomeningeal metastasis from EGFR-mutation non-small cell lung cancer: a phase II single-arm prospective clinical trial. Thorac Cancer. 2021;12:172‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JCH, Kim SW, Kim DW, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38:538‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geraud A, Mezquita L, Bigot F, et al. Prolonged leptomeningeal responses with Brigatinib in two heavily pretreated ALK-rearranged non-small cell lung cancer patients. J Thorac Oncol. 2018;13:e215‐e217. [DOI] [PubMed] [Google Scholar]
- 10.Ou SH, Sommers KR, Azada MC, Garon EB. Alectinib induces a durable (>15 months) complete response in an ALK-positive non-small cell lung cancer patient who progressed on crizotinib with diffuse leptomeningeal carcinomatosis. Oncologist. 2015;20:224‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YL, Zhou L, Lu Y. Intrathecal chemotherapy as a treatment for leptomeningeal metastasis of non-small cell lung cancer: a pooled analysis. Oncol Lett. 2016;12:1301‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriks LEL, Bootsma G, Mourlanette J, et al. Survival of patients with non-small cell lung cancer having leptomeningeal metastases treated with immune checkpoint inhibitors. Eur J Cancer. 2019;116:182‐189. [DOI] [PubMed] [Google Scholar]
- 13.Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010;11:871‐879. [DOI] [PubMed] [Google Scholar]
- 14.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543‐3551. [DOI] [PubMed] [Google Scholar]
- 15.Fan C, Zhao Q, Li L, et al. Efficacy and safety of intrathecal pemetrexed combined with dexamethasone for treating tyrosine kinase inhibitor-failed leptomeningeal metastases from EGFR-mutant NSCLC-a prospective, open-label, single-arm phase 1/2 clinical trial (unique identifier: chiCTR1800016615). J Thorac Oncol. 2021;16:1359‐1368. [DOI] [PubMed] [Google Scholar]
- 16.Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962‐1969. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800‐804. [DOI] [PubMed] [Google Scholar]
- 18.Liao BC, Lee JH, Lin CC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 2015;10:1754‐1761. [DOI] [PubMed] [Google Scholar]
- 19.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239‐246. [DOI] [PubMed] [Google Scholar]
- 20.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121‐128. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Chen M, Zhong W, et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer. 2013;14:188‐193. [DOI] [PubMed] [Google Scholar]
- 22.Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399‐405. [DOI] [PubMed] [Google Scholar]
- 23.Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103:2344‐2348. [DOI] [PubMed] [Google Scholar]
- 24.Palma JA, Fernandez-Torron R, Esteve-Belloch P, et al. Leptomeningeal carcinomatosis: prognostic value of clinical, cerebrospinal fluid, and neuroimaging features. Clin Neurol Neurosurg. 2013;115:19‐25. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19:484‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang BY, Li YS, Guo WB, et al. Detection of driver and resistance mutations in leptomeningeal metastases of NSCLC by next-generation sequencing of cerebrospinal fluid circulating tumor cells. Clin Cancer Res. 2017;23:5480‐5488. [DOI] [PubMed] [Google Scholar]
- 27.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113‐125. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Choi Y, Han J, et al. Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol. 2020;15:1758‐1766. [DOI] [PubMed] [Google Scholar]
- 29.Ahn MJ, Chiu CH, Cheng Y, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15:637‐648. [DOI] [PubMed] [Google Scholar]
- 30.Grommes C, Oxnard GR, Kris MG, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura T, Hata A, Takeshita J, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol. 2015;75:1261‐1266. [DOI] [PubMed] [Google Scholar]
- 32.Kumthekar P, Grimm SA, Avram MJ, et al. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J Neurooncol. 2013;112:247‐255. [DOI] [PubMed] [Google Scholar]
- 33.Bailon O, Chouahnia K, Augier A, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol. 2012;14:491‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reijneveld JC, Taphoorn MJ, Kerckhaert OA, Drixler TA, Boogerd W, Voest EE. Angiostatin prolongs the survival of mice with leptomeningeal metastases. Eur J Clin Invest. 2003;33:76‐81. [DOI] [PubMed] [Google Scholar]
- 35.Besse B, Le Moulec S, Mazières J, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): a nonrandomized, phase II study. Clin Cancer Res. 2015;21:1896‐1903. [DOI] [PubMed] [Google Scholar]
- 36.Jiang S, Liang H, Liu Z, et al. The impact of anlotinib on brain metastases of non-small cell lung cancer: post hoc analysis of a phase III randomized control trial (ALTER0303). Oncologist. 2020;25:e870‐e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powell SF, Rodríguez-Abreu D, Langer CJ, et al. Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with NSCLC and stable brain metastases: pooled analysis of KEYNOTE-021, −189, and −407. J Thorac Oncol. 2021;16:1883‐1892. [DOI] [PubMed] [Google Scholar]
- 38.Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2013;8:1570‐1573. [DOI] [PubMed] [Google Scholar]
- 39.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829‐838. [DOI] [PubMed] [Google Scholar]
- 40.Huber RM, Hansen KH, Paz-Ares Rodríguez L, et al. Brigatinib in crizotinib-refractory ALK + NSCLC: 2-year follow-up on systemic and intracranial outcomes in the phase 2 ALTA trial. J Thorac Oncol. 2020;15:404‐415. [DOI] [PubMed] [Google Scholar]
- 41.Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018‐2029. [DOI] [PubMed] [Google Scholar]
- 42.Pan Z, Yang G, Cui J, et al. A pilot phase 1 study of intrathecal pemetrexed for refractory leptomeningeal metastases from non-small-cell lung cancer. Front Oncol. 2019;9:838. [DOI] [PMC free article] [PubMed] [Google Scholar]