Abstract
Background:
For estrogen receptor–positive (ER+)/human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC), the current standard first-line treatment includes an aromatase inhibitor in combination with a cyclin-dependent kinase 4/6 inhibitor. When resistance occurs, often related to the occurrence of ESR1 mutations, selective estrogen receptor modulators or degraders (SERDs) may be used, alone or in combination regimens. Amcenestrant (SAR439859), an optimized oral SERD, has shown clinical antitumor activity in combination with palbociclib in patients with ER+/HER2– ABC and, as monotherapy, in patients with and without ESR1 mutations. Here, we describe the study design of AMEERA-5, an ongoing, prospective, phase 3, randomized, double-blind, multinational study comparing the efficacy and safety of amcenestrant plus palbociclib versus letrozole plus palbociclib in patients with advanced (locoregional recurrent or metastatic) ER+/HER2– breast cancer.
Methods:
Patients are pre-/postmenopausal women and men with no prior systemic therapy for ABC. The planned enrollment is 1066 patients. Patients are randomized 1:1 to either amcenestrant 200 mg plus palbociclib 125 mg or letrozole 2.5 mg plus palbociclib 125 mg. Amcenestrant, letrozole, and their matching placebos are taken once daily continuously; palbociclib is taken once daily for 21 days, followed by 7 days off-treatment for a 28-day cycle. Treatment continues until disease progression, unacceptable toxicity, or decision to stop treatment. Pre-/perimenopausal women and men receive goserelin subcutaneously. Randomization is stratified by de novo metastatic disease, menopausal status, and visceral metastases. The primary endpoint is progression-free survival. The key secondary endpoint is overall survival; others are safety, pharmacokinetics, and quality of life.
Conclusions:
AMEERA-5 is evaluating the efficacy and safety of amcenestrant in combination with palbociclib as first-line therapy in pre-/postmenopausal women and men with ER+/HER2– ABC.
ClinicalTrials Identifier:
Keywords: amcenestrant, endocrine therapy, ER-positive/HER2-negative, metastatic breast cancer, selective estrogen receptor degrader
Introduction
Globally, breast cancer is the most common cancer and the primary cause of cancer death in females. 1 In 2020, more than 2.2 million women in the world were diagnosed with breast cancer. Although rare, breast cancer also occurs in men; compared with 276,480 women, an estimated 2,620 men were diagnosed with invasive breast cancer in 2020 in the United States. 2 The majority of breast cancers are hormone receptor positive (HR+), including both estrogen receptor–positive (ER+) cancers that account for approximately 75% of all breast cancers and progesterone receptor–positive cancers; the most common subtype is ER+/human epidermal growth factor receptor 2 negative (HER2–).3,4
Patients with metastatic breast cancer (MBC) have poor clinical outcomes, with a 5-year survival rate of approximately 28% across all MBC subtypes in the United States from 2010 to 2016. 1 In a French cohort study, patients with HR+/HER2− MBC had median overall survival (OS) of 42.9 months [95% confidence interval (CI) = 42.1–43.8], with a 5-year survival rate of 35.7% (95% CI = 34.8–36.6%) from 2008 to 2016, with no improvement in OS over time (43.4 versus 44.8 months with a diagnosis in 2008 versus 2016, respectively). 5 Treatment goals for patients with MBC include improving survival and quality of life (QoL), which places emphasis on the need for agents with minimal toxicity.6–8
Although cancer recurrence and mortality for patients with early-stage ER+ breast cancer are reduced by adjuvant endocrine therapy (ET), many patients still do relapse and experience disease progression. 9 Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors (e.g. palbociclib, ribociclib, abemaciclib) have transformed the treatment of metastatic ER+ breast cancer. 10 Of CDK4/6 inhibitors, palbociclib was the first to be approved for ER+/HER2– MBC, and its effectiveness is supported by many real-world evidence studies. 11 Currently, the preferred first-line therapy for metastatic ER+/HER2– breast cancer in postmenopausal women or in premenopausal women receiving ovarian ablation/suppression is now an aromatase inhibitor (AI) (e.g. letrozole, anastrozole, exemestane) in combination with a CDK4/6 inhibitor.7,8
This recommendation is based on several studies showing that for first-line therapy the addition of a CDK4/6 inhibitor to an AI significantly improves progression-free survival (PFS) compared with that with AI treatment alone in postmenopausal women.6,10,12–15 A number of ongoing and completed trials specifically, however, have included pre-/perimenopausal women (who also received treatment to suppress ovarian function, in accordance with current treatment guidelines pertaining to hormonal therapy in pre/perimenopausal women).6,12,16–18 In MONALEESA-7, which included only pre-/perimenopausal women, the addition of ribociclib to a nonsteroidal AI or tamoxifen (plus goserelin) improved PFS (hazard ratio = 0.55, 95% CI = 0.44–0.69, p < 0.0001) to a median of 23.8 months for the combination of ribociclib plus ET compared with a median of 13.0 months for ET alone, as well as OS (hazard ratio = 0.76, 95% CI = 0.61–0.96) with median OS 58.7 months versus 48.0 months, respectively.17,18
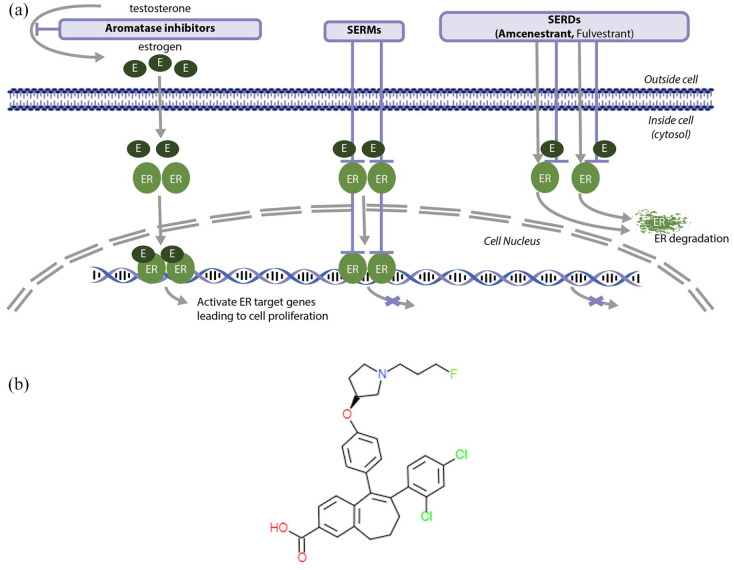
Although the addition of targeted agents (such as CDK4/6 inhibitors) can successfully prolong endocrine sensitivity and thus delay chemotherapy and its associated toxicity in patients with MBC, resistance to ET will ultimately occur.6,9 Mutations in the ligand-binding domain of ESR1, most commonly Y537 and D538, are a major mechanism leading to resistance to AIs and decreased sensitivity to tamoxifen and fulvestrant. 9 Strategies to prolong endocrine sensitivity include the sequential use of available agents (and combinations of agents) with different mechanisms of action, such as a selective ER degrader (SERD; e.g. fulvestrant) and selective ER modulators (e.g. tamoxifen, toremifene, raloxifene); breast cancer that is resistant to one class of ET may be sensitive to another (Figure 1(a)).6,19
Figure 1.
(a) Amcenestrant, an oral SERD, antagonizes and degrades the ER, resulting in inhibition of the ER signaling pathway and (b) amcenestrant structure.
E, estrogen; ER, estrogen receptor; SERD, selective estrogen receptor degrader; SERM, selective estrogen receptor modulator.
The only SERD approved to date, fulvestrant, has shown clinical benefits as initial monotherapy or in combination with ribociclib in postmenopausal patients with ER+ MBC.20,21 Furthermore, in women of any menopause status (including premenopausal women) with disease progression while on prior ET, fulvestrant, in combination with either palbociclib or abemaciclib, also has demonstrated clinical benefit.22–24 The pharmacokinetics of fulvestrant, however, require it to be administered by intramuscular injection.25–27
The current first-line standard treatments of an AI plus a CDK4/6 inhibitor for patients with ER+/HER2– MBC allow for a median PFS of around 20–28 months.6,13,15,28,29 For patients with no prior history of CKD4/6 inhibitor usage receiving fulvestrant plus CDK4/6 inhibitor as second-line therapy, the median PFS is 10–16 months.6,22,24 Because ER pathway–dependent targeting remains the major cornerstone therapy of ER+/HER2– MBC, there is a need for more effective ER-targeted therapies that could increase PFS and OS in patients with MBC. The development of new oral SERDs that can potentially achieve greater bioavailability may improve outcomes for patients with advanced/metastatic ER+/HER2– breast cancer, including in those with ESR1 mutations.30–35
Amcenestrant is an optimized oral SERD with potent dual activity that antagonizes and degrades the ER, resulting in inhibition of the ER signaling pathway (Figure 1(a)). 25 The fluoroalkylamine side chain of amcenestrant allows optimal ER binding (Figure 1(b)).25,36 Amcenestrant has demonstrated broader and superior ER antagonist and degrader activities compared with those of other SERDs having a cinnamic acid side chain, as well as antitumor activity in ER+ breast cancer cells, including tamoxifen-resistant lines and those with or without ESR1 mutations. 25 In terms of ER antagonism, degradation, target gene signature, and inhibition of tumor cell proliferation, the in vitro biological profile of amcenestrant was similar to that of fulvestrant. Amcenestrant, however, achieved tumor regression in an HCI013 patient-derived xenograft model harboring the Y537S ESR1 mutation, in contrast to fulvestrant at an exposure eightfold higher than the human equivalent dose, which resulted in partial antitumor activity in HCI013 tumors. 25
In the ongoing, multipart, phase 1/2 first-in-human dose-escalation and dose-expansion study (AMEERA-1) in postmenopausal women with pretreated ER+/HER2– breast cancer, amcenestrant ⩾150 mg showed encouraging antitumor activity, irrespective of ESR1 mutation status.30,37 Among patients treated with amcenestrant monotherapy at doses ⩾150 mg who were included in the evaluation of response (n = 59), there were five confirmed partial responses (PRs; 8.5%), 24 patients (40.7%) who had stable disease (SD), and 30 patients (50.8%) whose disease progressed. 30 The clinical benefit rate [CBR; complete response (CR) + PR + SD ⩾ 24 weeks] was 33.9%. 30 In AMEERA-1 cohorts that received the recommended phase 2 dose of amcenestrant 200 mg plus palbociclib 125 mg, among response-evaluable patients (n = 35) with no prior mammalian target of rapamycin inhibitor or CKD4/6 inhibitor treatment, the objective response rate was 34.3% with no CR and 12 PRs, and the CBR was 74.3%. 38 No clinically significant cardiac or ocular safety findings occurred in either set of cohorts.30,38
These promising results have led to the design and initiation of several further trials, including the study outlined in this article, AMEERA-5.
Methods and design
Study design
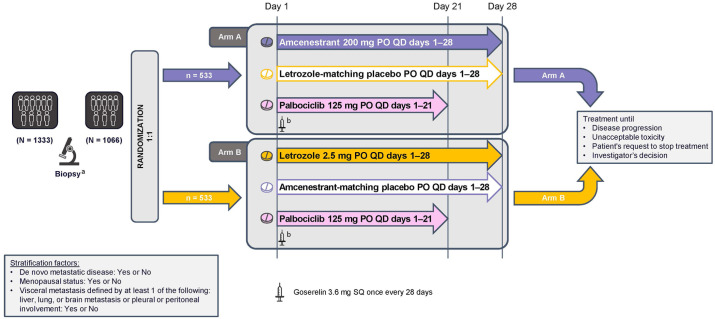
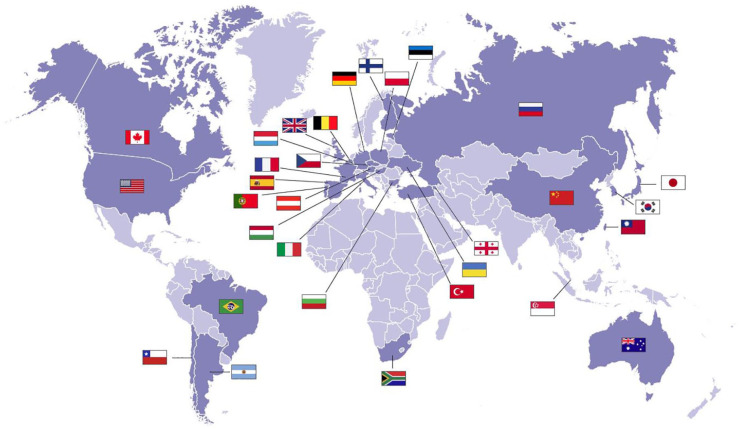
AMEERA-5 (NCT04478266) is a prospective, multinational, randomized, double-blind, double-dummy phase 3 trial that is designed to compare the efficacy and safety of amcenestrant plus palbociclib with that of letrozole plus palbociclib in patients with advanced, locoregional recurrent or metastatic ER+/HER2– breast cancer who have not received prior systemic therapy for their advanced disease (Figure 2). The study consists of a screening period (up to 28 days before randomization), an active treatment period (in 28-day cycles) to continue until disease progression, unacceptable toxicity, or the decision to stop treatment and follow-up (until death or final study cutoff date, whichever comes first). The planned enrollment is 1066 patients at 306 study sites in 31 countries around the world (Figure 3).
Figure 2.
AMEERA-5 study design. Randomization will be stratified by de novo metastatic disease (yes or no), menopausal status (yes or no), and visceral metastasis defined by at least one of the following: liver, lung, or brain metastasis or pleural or peritoneal involvement (yes or no).
PO, oral; QD, once daily; SQ, subcutaneous.
aArchived tissue or fresh sample obtained between screening and cycle 1 day 1.
bPre-/perimenopausal women and men will receive a subcutaneous goserelin implant (3.6 mg) on day 1 of every 28-day cycle.
Figure 3.
Countries with AMEERA-5 planned enrollment sites.
Ethical considerations
AMEERA-5 will be conducted in accordance with principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, as well as applicable International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice Guidelines, laws, and regulations. The protocol, subsequent amendments, and other relevant documents have been approved by the Institutional Review Board/Independent Ethics Committee at each study site (Supplementary Materials). Written informed consent will be obtained from all patients prior to enrollment.
Eligibility criteria
The study includes pre-/perimenopausal women, postmenopausal women, and men. In addition to not having received prior systemic therapy for advanced disease and Eastern Cooperative Oncology Group (ECOG) performance status 0–2, other key inclusion criteria are shown in Table 1. Pre-/perimenopausal women and men with no prior bilateral orchiectomy are recommended to receive a gonadotropin-releasing hormone agonist for ⩾4 weeks prior to randomization. Exclusion criteria include prior (neo)adjuvant treatment with another SERD and disease recurrence while on, or within 12 months of completion of, (neo)adjuvant ET (Table 1).
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | |
|---|---|
| 1 | Adult (⩾18 years) women of any menopausal status or men with histologically or cytologically proven locoregionally recurrent or metastatic breast cancer not amenable to curative treatment (as assessed by the study investigator), and for whom chemotherapy is not indicated |
| 2 | ER+ /HER2– in both primary tumor and locoregionally recurrent or metastatic sites (as assessed by IHC or FISH) |
| 3 | No previous systemic anti-cancer treatment for their locoregionally recurrent or metastatic disease |
| 4 | In pre-/perimenopausal women and men with no prior bilateral orchiectomy, GnRH agonist inhibition is recommended ⩾ 4 weeks prior to randomization |
| 5 | Measurable disease evaluable per RECIST v.1.1, 39 or nonmeasurable bone only disease with at least one predominant lytic bone lesion or mixed lytic-blastic lesion a |
| 6 | ECOG performance status 0–2 |
| 7 | Willing and able to provide tumor tissue |
| 8 | Capable of giving informed consent |
| Exclusion criteria | |
| 1 | Known active brain metastases |
| 2 | Diagnosis of any other malignancy (except adequately treated basal or squamous cell cancer or in situ cervical cancer) within 3 years prior to randomization |
| 3 | Prior (neo)adjuvant treatment with another SERD |
| 4 | Disease recurrence while on, or within 12 months of completion of (neo)adjuvant ET ± CDK4/6 inhibitors |
| 5 | Unrecovered acute toxic effects (grade > 1) of prior anti-cancer therapy or surgical procedures |
| 6 | Advanced, symptomatic visceral spread; at risk of life-threatening complications in the short term |
| 7 | Significant concomitant illness that would adversely affect participation in the study |
| 8 | Inadequate hematological, renal, coagulation, or hepatic function |
| 9 | Unwilling to use recommended contraception methods, where applicable |
| 10 | Participation in any other clinical study within 4 weeks before randomization |
| 11 | Major surgery or radiotherapy within 4 weeks before randomization |
| 12 | Medical history or ongoing gastrointestinal disorders that may affect the absorption of amcenestrant, letrozole, or palbociclib |
| 13 | Treatment with drugs that • Are known to prolong the QT interval (premenopausal and male participants) • Are sensitive substrates of P-glycoprotein or breast cancer resistance protein • Are strong CYP3A inhibitors or inducers (within 2 weeks before first study treatment administration or five elimination half-lives, whichever is longest) • Have the potential to inhibit UGT (within 2 weeks before first study treatment administration or five elimination half-lives, whichever is longest) • Have a narrow therapeutic window and are metabolized by CYP3A |
| 14 | Known sensitivity or contraindications to any of the study treatments or their excipients |
CDK4/6, cyclin-dependent kinase 4 and 6; CYP, cytochrome P450; ECOG, Eastern Cooperative Oncology Group; ER+, estrogen receptor positive; ET, endocrine therapy; FISH, fluorescent in situ hybridization; GnRH, gonadotropin-releasing hormone; HER2–, human epidermal growth factor receptor 2 negative; IHC, immunohistochemistry; RECIST, Response Evaluation Criteria in Solid Tumors; SERD, selective estrogen receptor degrader; UGT, uridine 5′-diphosphoglucuronosyltransferase.
Patients with nonmeasurable mixed metastatic (bony-visceral) disease were allowed entry into the study prior to the December 2020 protocol amendment.
Randomization and treatment
Eligible patients are randomized 1:1 to oral treatment with either amcenestrant, palbociclib, and letrozole-matching placebo, or letrozole, palbociclib, and amcenestrant-matching placebo in 28-day cycles (Figure 2). Randomization is stratified by de novo metastatic disease (yes or no), menopausal status (yes or no), and visceral metastasis involving at least one of the following: liver, lung, or brain metastasis or pleural or peritoneal involvement (yes or no). Randomization is performed centrally by an interactive response technology. All participants, investigators, study site pharmacists, the study sponsor, and all stakeholders, except for the data monitoring committee and independent statistician, will remain blinded to study treatment throughout the study period (i.e. until the date of the last visit or scheduled procedure for the last patient in the study), unless the investigator deems unblinding is warranted in the case of an adverse event (AE) or serious AE, in which case the patient must withdraw from study medication.
Amcenestrant (200 mg), letrozole (2.5 mg), and their matching placebos will be taken once daily continuously either with or without food at approximately the same time each day. Palbociclib (125 mg) will be taken once daily for 21 days, followed by 7 days off treatment. In addition, pre-/perimenopausal women and men will receive a subcutaneous goserelin implant (3.6 mg) on day 1 of every 28-day cycle.
No dose reductions are permitted for letrozole or amcenestrant; however, dose omissions are permitted in the case of severe toxicity. Palbociclib dosing may be omitted or delayed and/or reduced in the event of significant treatment-related toxicity. In such instance and if an imbalance occurs between the two arms, an adjusted analysis for the palbociclib relative dose intensity would be discussed with the steering committee as a future exploratory objective. Study treatment will continue until disease progression, unacceptable toxicity, or withdrawal at the patient’s request or investigator’s decision. At the discretion of the investigator, and if in the best interests of the patient, a patient may continue study treatment beyond disease progression provided no new anti-cancer treatment is initiated. If palbociclib is prematurely discontinued, a patient may continue on the active treatment phase (at the investigator’s discretion); however, if amcenestrant, letrozole, or their matching placebos are prematurely discontinued because of toxicity, the patient will be discontinued from the active treatment phase of the study and enter the follow-up phase.
During the study, no investigational or anti-cancer agents (i.e. chemotherapy, immunotherapy, targeted therapy, ET) are allowed other than study medication and no herbal medications or food supplements are allowed. No concurrent radiotherapy (unless palliative use in a lesion that was not used for response assessment) or cancer-related surgery is allowed. Concomitant medications are allowed for preexisting medical conditions, including treatments for bone stabilization and anemia, and for treatment-emergent neutropenia.
Endpoints
The primary endpoint is PFS (Table 2). A random sample-audit blinded independent review committee will be used to provide assurance of the PFS determination based on investigator assessment. The key secondary endpoint is OS. Other secondary endpoints are objective response rate, duration of response, CBR, PFS on the next line of therapy (PFS2), pharmacokinetics of amcenestrant and palbociclib, QoL, time to first chemotherapy, and safety (Table 2).
Table 2.
Primary and secondary endpoints and definitions.
| Primary endpoint |
| Progression-free survival (PFS): time from randomization to the earlier of first documented tumor progression based on Response Evaluation Criteria in Solid Tumors (RECIST 1.1) 39 as assessed by the investigator or radiologist, or death from any cause |
| Key secondary endpoint |
| Overall Survival (OS): time from randomization to death |
| Secondary efficacy endpoints |
| Objective response rate: proportion of patients who have a confirmed partial or complete response as the best overall response determined by RECIST 1.1 from randomization to the earliest of disease progression, death, cutoff date, or initiation of post-treatment anti-cancer therapy |
| Duration of response: time from CR or PR until progressive disease or death |
| Clinical benefit rate: proportion of patients with confirmed CR, PR, or SD for at least 24 weeks from the date of randomization until disease progression, death, study cutoff date, or initiation of post treatment anti-cancer therapy |
| PFS on next line of therapy (PFS2): time from the date of randomization to the date of first documentation of progressive disease on the next systemic anti-cancer therapy |
| Time to first chemotherapy: the time interval from the date of randomization to the start date of the first chemotherapy after study treatment discontinuation |
| Other secondary endpoints |
| Pharmacokinetics of amcenestrant and palbociclib |
| Health-related QoL, as evaluated by EORTC QLQ-C30, QLQ-BR23/BR45 and EQ-5D-5L |
| Safety, evaluated through AEs, serious AEs, laboratory abnormalities |
AEs, adverse events; CR, complete response; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer core quality of life questionnaire; EQ-5D-5L, EuroQoL questionnaire with 5 dimensions and 5 levels per dimension; PR, partial response; QLQ-BR23/BR45, EORTC QLQ breast cancer–specific module; QoL, quality of life; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Patient-reported QoL outcomes will be assessed electronically via the European Organization for Research and Treatment of Cancer (EORTC) core quality of life questionnaire (QLQ-C30) and breast cancer–specific module (QLQ-BR23/BR45) and the EuroQoL questionnaire with five dimensions and five levels per dimension (EQ-5D-5L).
Assessment schedule
At screening, patients will undergo a comprehensive physical examination, including vital signs, ECOG performance status, triplicate 12-lead electrocardiogram (ECG), and laboratory assessments. With the exception of ECG, which will be measured again within 30 days after the end of treatment, these assessments will also be performed during treatment on day 1 (±3 days) of each treatment cycle and then within 30 days after the end of treatment. Laboratory assessments will also be performed on day 15 (±1 day) of cycles 1 and 2 and as clinically indicated.
Tumor assessments (using computed tomography/magnetic resonance imaging scans) will be performed during screening and then every 12 weeks (±7 days) during treatment (and during follow-up for patients who discontinued treatment without documented progressive disease). After disease progression, patients will have follow-up visits every 24 weeks (±7 days) for documentation of survival status and post-study anti-cancer treatment and responses. Patients with bone lesions at baseline will also have bone scans performed every 24 weeks (±7 days) from randomization for the first 18 months, and then every 12 weeks (±7 days).
QoL questionnaires will be completed on day 1 of cycles 1, 2, 3, and 4 and then every three cycles starting with cycle 6, as well as at the end of treatment and at the first follow-up visit.
Blood samples for pharmacokinetic analysis of amcenestrant and palbociclib will be collected on days 1 and 15 of cycles 1 and 2 and then on day 1 of cycles 3, 4, 7, and 10.
AEs will be recorded throughout the treatment period and until at least 30 days after the end of treatment; severity will be graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Statistical analyses
Efficacy analyses will be performed using the intent-to-treat population, defined as participants who were assigned to a randomized intervention regardless of whether the intervention was received. Patients will be analyzed according to the treatment arm assigned at randomization. The primary (PFS) and key secondary (OS) endpoints will be compared between treatment arms using a stratified log-rank test, with stratification factors as entered in the interactive response technology system, with a one-sided type I error rate of 2.5%. A hierarchical testing strategy will be used, such that an OS comparison will be performed only if the primary analysis of PFS is statistically significant. The hazard ratio estimates and corresponding 95% two-sided CIs will be provided using the Cox proportional hazard model. The Kaplan–Meier method will be used for time-to-event efficacy endpoints; quantiles and probabilities of being event-free at different time points, along with corresponding 95% CIs, will be presented by treatment arm. Other efficacy endpoints will be reported using descriptive statistics by treatment arm.
Safety analyses will be summarized utilizing descriptive statistics in the safety population, defined as participants who were randomized and received at least one dose of study medication. Participants will be analyzed according to the treatment arm they actually received.
Discussion
Amcenestrant is an optimized, oral SERD with demonstrated potent dual activity, which antagonizes and degrades the ER, resulting in inhibition of the ER signaling pathway and degradation activities in preclinical studies. 25 Preliminary results from an ongoing, first-in-human phase 1/2 trial (AMEERA-1) showed that amcenestrant has promising antitumor activity as monotherapy and in combination with the CDK4/6 inhibitor palbociclib and that no clinically significant cardiac or ocular safety findings occurred.30,37,38
Despite encouraging results in the FALCON study showing that first-line fulvestrant monotherapy significantly improved PFS compared with that with anastrozole monotherapy (median PFS 16.6 months versus 13.8 months, respectively; hazard ratio = 0.797, 95% CI = 0.637–0.000, p = 0.0486), combining fulvestrant plus palbociclib in the PARSIFAL study showed no difference in efficacy compared with the combination of letrozole plus palbociclib (median PFS 27.9 months versus 32.8 months, respectively; hazard ratio = 1.1, 95% CI = 0.9–1.5, p = 0.321).20,40 Thus, there is a need to explore whether amcenestrant, an optimized SERD, plus a CDK4/6 inhibitor could improve PFS compared with that of an AI in combination with the same CDK4/6 inhibitor.
AMEERA-5 is a prospective, multinational, randomized, double-blind, double-dummy phase 3 trial that is designed to compare the efficacy and safety of amcenestrant plus palbociclib with that of letrozole plus palbociclib in patients (pre-/peri- and postmenopausal women and men) with advanced, locoregionally recurrent or metastatic ER+/HER2– breast cancer, who have not received prior systemic therapy for their advanced disease. The study was initiated on 14 October 2020. As of 21 June 2021, 415 patients have been enrolled. The planned enrollment is 1066 patients from 31 countries. This currently ongoing study will demonstrate whether amcenestrant, a new oral SERD, in combination with palbociclib reduces the risk of tumor progression or death, which was not demonstrated with fulvestrant plus palbociclib.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221083956 for AMEERA-5: a randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2– advanced breast cancer by Aditya Bardia, Javier Cortes, Sara A. Hurvitz, Suzette Delaloge, Hiroji Iwata, Zhi-Ming Shao, Dheepak Kanagavel, Patrick Cohen, Qianying Liu, Sylvaine Cartot-Cotton, Vasiliki Pelekanou and Joyce O’Shaughnessy in Therapeutic Advances in Medical Oncology
Acknowledgments
Editorial support was provided by Michelle Daniels and Elizabeth Strickland, inScience Communications (Philadelphia, PA, USA), and funded by Sanofi. Jim Trinh, inScience Communications (Philadelphia, PA, USA), provided assistance with the manuscript submission process on behalf of the authors.
Footnotes
Author contributions: Aditya Bardia: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Javier Cortes: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Sara A. Hurvitz: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Suzette Delaloge: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Hiroji Iwata: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Zhi-Ming Shao: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Dheepak Kanagavel: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Patrick Cohen: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Qianying Liu: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Sylvaine Cartot-Cotton: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Vasiliki Pelekanou: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Joyce O’Shaughnessy: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AB reports consulting or advisory roles at Biotheranostics, Daiichi Sankyo/AstraZeneca, Foundation Medicine, Genentech, Immunomedics, Merck, Novartis, Pfizer, Philips, Puma Biotechnology, Radius Health, Sanofi, and Spectrum Pharmaceuticals; consulting or advisory roles (to his institution) with Genentech/Roche, Immunomedics, Innocrin Pharma, Novartis, Pfizer, and Radius Health; and research funding to his institution from AstraZeneca/Daiichi Sankyo, Genentech, Immunomedics, Merck, Novartis, Pfizer, Radius Health, and Sanofi.
JC reports stock/ownership interest at MedSIR; honoraria from Celgene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung; consulting or advisory role at AstraZeneca, Athenex, Bioasis, Biothera, Boehringer Ingelheim, Celgene, Cellestia Biotech, Clovis Oncology, Daiichi Sankyo, ERYTECH Pharma, GlaxoSmithKline, Kyowa Kyrin, Leuko, Lilly, Merck Sharp & Dohme, Merus, Polyphor, Roche, Seattle Genetics, and SERVIER; and research funding to his institution from ARIAD, AstraZeneca, Baxalta, Bayer, Eisai, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur, Puma Biotechnology, Queen Mary University of London, and Roche; and travel/accommodations/expenses from Daiichi Sankyo, Eisai, Novartis, Pfizer, and Roche.
SAH reports stock/ownership interests at Ideal Implant and ROM Tech; research funding to her institution from Ambryx, Amgen, Arvinas, Bayer, Biomarin, Cascadian Therapeutics, Daiichi Sankyo, Dignitana, Genentech/Roche, Gilead Sciences, GlaxoSmithKline, Immunomedics, Lilly, Macrogenics, Merrimack, Novartis, OBI Pharma, Pfizer, Phoenix Molecular Designs, Pieris Pharmaceuticals, Puma Biotechnology, Radius Health, Sanofi, Seattle Genetics, and Zymeworks; and travel/accommodations/expenses from Lilly; and other relationships with Pfizer and Roche.
SD reports consulting or advisory roles (to her institution) with AstraZeneca and Pierre Fabre; research funding to her institution from AstraZeneca, Exact Sciences, Lilly, Novartis, Pfizer, Puma Biotechnology, Roche/Genentech, and Sanofi; and travel/accommodations/expenses from AstraZeneca, Pfizer, and Roche.
HI reports honoraria from AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kirin, Lilly Japan, Pfizer, and Taiho Pharmaceutical; consulting or advisory roles with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly Japan, Novartis, and Pfizer; and research funding to his institution from AstraZeneca, Bayer, Boehringer Ingelheim, Chugai Pharma, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly Japan, MSD, Nihonkayaku, Novartis, Pfizer, and Sanofi.
ZMS reports no disclosures.
DK, PC, QL, and SCC are employees of Sanofi and may hold shares and/or stock options in the company.
VP is a former employee of Sanofi and a current employee of Bayer.
JO discloses honoraria from AbbVie, Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Genentech, Genomic Health, GRAIL, HERON, Immunomedics, Ipsen, Jounce Therapeutics, Lilly, Merck, Myriad Pharmaceuticals, Novartis, Odonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Samsung, Sanofi, Seattle Genetics, and Syndax; consulting or advisory roles with AbbVie, Agendia, Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Eisai, Genentech, Genomic Health, GRAIL, HERON, Immunomedics, Ipsen, Jounce Therapeutics, Lilly, Merck, Myriad Pharmaceuticals, Novartis, Odonate Therapeutics, Pfizer, Puma Biotechnology, Roche, Samsung, Sanofi, Seattle Genetics, and Syndax; speakers’ bureau fees from AstraZeneca, Lilly, Novartis, and Pfizer; research funding to her institution from Seattle Genetics; and travel/accommodations/expenses from AbbVie, Agendia, Amgen, AstraZeneca, Celgene, Eisai, Genomic Health, GRAIL, Ipsen, Jounce Therapeutics, Lilly, Myriad Pharmaceuticals, Novartis, Pfizer, Puma Biotechnology, Roche, Sanofi, and Seattle Genetics.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored and funded by Sanofi.
ORCID iDs: Aditya Bardia  https://orcid.org/0000-0003-4885-1157
https://orcid.org/0000-0003-4885-1157
Javier Cortes  https://orcid.org/0000-0001-7623-1583
https://orcid.org/0000-0001-7623-1583
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Aditya Bardia, Oncology/Hematology, Massachusetts General Hospital, Harvard Medical School, BHX-237, 55 Fruit Street, Boston, MA 02114, USA.
Javier Cortes, Oncology Department, International Breast Cancer Center (IBCC), Barcelona, Spain.
Sara A. Hurvitz, Breast Medical Oncology, University of California Los Angeles Jonsson Comprehensive Cancer Center, Los Angeles, CA, USA
Suzette Delaloge, Medical Oncology, Institute Gustave Roussy, Villejuif, France.
Hiroji Iwata, Breast Oncology, Aichi Cancer Center Hospital, Nagoya, Japan.
Zhi-Ming Shao, Oncology/Surgery, Fudan University, Shanghai, China.
Dheepak Kanagavel, Research and Development, Sanofi, Vitry-sur-Seine, France.
Patrick Cohen, Research and Development, Sanofi, Vitry-sur-Seine, France.
Qianying Liu, Research and Development, Sanofi, Cambridge, MA, USA.
Sylvaine Cartot-Cotton, Research and Development, Sanofi, Vitry-sur-Seine, France.
Vasiliki Pelekanou, Research and Development, Sanofi, Cambridge, MA, USA.
Joyce O’Shaughnessy, Oncology/Internal Medicine, Baylor University Medical Center, Texas Oncology, US Oncology, Dallas, TX, USA.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. American Society of Clinical Oncology (ASCO). Breast cancer: statistics, https://www.cancer.net/cancer-types/breast-cancer/statistics (2021, accessed 8 October 2021).
- 3. Stravodimou A, Voutsadakis IA. The future of ER+/HER2- metastatic breast cancer therapy: beyond PI3K inhibitors. Anticancer Res 2020; 40: 4829–4841. [DOI] [PubMed] [Google Scholar]
- 4. Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther 2018; 186: 1–24. [DOI] [PubMed] [Google Scholar]
- 5. Grinda T, Antoine A, Jacot W, et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort. ESMO Open 2021; 6: 100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frassoldati A, Biganzoli L, Bordonaro R, et al. Endocrine therapy for hormone receptor-positive, HER2-negative metastatic breast cancer: extending endocrine sensitivity. Future Oncol 2020; 16: 129–145. [DOI] [PubMed] [Google Scholar]
- 7. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020; 31: 1623–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020; 18: 452–478. [DOI] [PubMed] [Google Scholar]
- 9. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell 2020; 37: 496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spring LM, Wander SA, Andre F, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 2020; 395: 817–827. [DOI] [PubMed] [Google Scholar]
- 11. Harbeck N, Bartlett M, Spurden D, et al. CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol 2021; 17: 2107–2122. [DOI] [PubMed] [Google Scholar]
- 12. Shah M, Nunes MR, Stearns V. CDK4/6 inhibitors: game changers in the management of hormone receptor-positive advanced breast cancer? Oncology 2018; 32: 216–222. [PMC free article] [PubMed] [Google Scholar]
- 13. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 14. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 15. Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Boer R, Hui R, Lim E, et al. Optimizing care for younger women with hormone receptor-positive, HER2-negative metastatic breast cancer. Asia Pac J Clin Oncol 2020; 16(Suppl. 5): 3–14. [DOI] [PubMed] [Google Scholar]
- 17. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019; 381: 307–316. [DOI] [PubMed] [Google Scholar]
- 18. Tripathy D, Im S-A, Colleoni M, et al. Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib. Cancer Res 2021; 81(4, Suppl.): PD2-04. [Google Scholar]
- 19. Brufsky AM, Dickler MN. Estrogen receptor-positive breast cancer: exploiting signaling pathways implicated in endocrine resistance. Oncologist 2018; 23: 528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016; 388: 2997–3005. [DOI] [PubMed] [Google Scholar]
- 21. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 22. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 23. Loibl S, Turner NC, Ro J, et al. Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist 2017; 22: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 25. Shomali M, Cheng J, Sun F, et al. SAR439859, a novel selective estrogen receptor degrader (SERD), demonstrates effective and broad antitumor activity in wild-type and mutant ER-positive breast cancer models. Mol Cancer Ther 2021; 20: 250–262. [DOI] [PubMed] [Google Scholar]
- 26. Lu Y, Liu W. Selective estrogen receptor degraders (SERDs): a promising strategy for estrogen receptor positive endocrine-resistant breast cancer. J Med Chem 2020; 63: 15094–15114. [DOI] [PubMed] [Google Scholar]
- 27. AstraZeneca. Faslodex (prescribing information). Wilmington, DE: AstraZeneca Pharmaceuticals LP, 2019. [Google Scholar]
- 28. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018; 29: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 29. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 30. Linden HM, Campone M, Bardia A, et al. A phase 1/2 study of SAR439859, an oral selective estrogen receptor (ER) degrader (SERD), as monotherapy and in combination with other anti-cancer therapies in postmenopausal women with ER-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC): AMEERA-1. Cancer Res 2021; 81(4, Suppl.): PD8-08. [Google Scholar]
- 31. Bardia A, Kaklamani V, Wilks S, et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-positive, HER2-negative advanced breast cancer. J Clin Oncol 2021; 39: 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maglakelidze M, Bulat I, Ryspayeva D, et al. Rintodestrant (G1T48), an oral selective estrogen receptor degrader, in combination with palbociclib for ER+/HER2– advanced breast cancer: Phase 1 results. J Clin Oncol 2021; 39(15, Suppl.): 1063. [Google Scholar]
- 33. Jhaveri KL, Boni V, Sohn J, et al. Safety and activity of single-agent giredestrant (GDC-9545) from a phase Ia/b study in patients (pts)) with estrogen receptor-positive (ER+), HER2-negative locally advanced/metastatic breast cancer (LA/mBC). J Clin Oncol 2021; 39(15, Suppl.): 1017. [Google Scholar]
- 34. Jhaveri KL, Lim E, Hamilton EP, et al. A first-in-human phase 1a/b trial of LY3484356, an oral selective estrogen receptor (ER) degrader (SERD) in ER+ advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): results from the EMBER study. J Clin Oncol 2021; 39(15, Suppl.): 1050. [Google Scholar]
- 35. Im S-A, Hamilton EP, Cussac AL, et al. SERENA-4: a phase 3 comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive, HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. J Clin Oncol 2021; 39(15, Suppl.): TPS1101. [Google Scholar]
- 36. Mottamal M, Kang B, Peng X, et al. From pure antagonists to pure degraders of the estrogen receptor: evolving strategies for the same target. ACS Omega 2021; 6: 9334–9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campone M, Bardia A, Ulaner GA, et al. Dose-escalation study of SAR439859, an oral selective estrogen receptor degrader, in postmenopausal women with estrogen receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer. Cancer Res 2020; 80(4, Suppl.): P5-11-02. [Google Scholar]
- 38. Chandarlapaty S, Linden HM, Neven P, et al. AMEERA-1: Phase 1/2 study of amcenestrant (SAR439859), an oral selective estrogen receptor (ER) degrader (SERD), in combination with palbociclib in postmenopausal women with ER+/human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer. In: Poster presented at: 2021 ASCO annual meeting, Virtual, 4–8 June 2021. [Google Scholar]
- 39. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 40. Llombart-Cussac A, Pérez-García JM, Bellet M, et al. PARSIFAL: a randomized, multicenter, open-label, phase II trial to evaluate palbociclib in combination with fulvestrant or letrozole in endocrine-sensitive patients with estrogen receptor (ER)[+]/HER2[-] metastatic breast cancer. J Clin Oncol 2020; 38(15, Suppl.): 1007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221083956 for AMEERA-5: a randomized, double-blind phase 3 study of amcenestrant plus palbociclib versus letrozole plus palbociclib for previously untreated ER+/HER2– advanced breast cancer by Aditya Bardia, Javier Cortes, Sara A. Hurvitz, Suzette Delaloge, Hiroji Iwata, Zhi-Ming Shao, Dheepak Kanagavel, Patrick Cohen, Qianying Liu, Sylvaine Cartot-Cotton, Vasiliki Pelekanou and Joyce O’Shaughnessy in Therapeutic Advances in Medical Oncology