Abstract
Steal syndrome is a potential complication of surgically created arteriovenous fistulas that can result in sensory and/or motor deficits, or tissue loss in the affected limb. Several surgical techniques have been developed to treat steal syndrome, but all have potential drawbacks. We detail a novel, modified plication technique which involves sequential, longitudinal application of pledgets along the venous outflow to gradually narrow it, and consequently decrease flow. Its potential benefits include protection of the vein from bare suture, less turbulent flow, and thus lower risk of thrombosis. Implementation of this technique in two patients resulted in symptomatic relief and continuation of uninterrupted hemodialysis at 9- and 12-month follow-up, respectively.
Keywords: Arteriovenous fistula, hemodialysis, plication technique, steal syndrome
Introduction
First documented in 1955 by Emile Holman, steal syndrome (SS) is a complication that can occur in congenital, traumatic, or surgically created arteriovenous (AV) fistulas. 1 SS is a potential complication that can result in pain, numbness, muscle weakness, and even limb-threatening gangrene. 2 These symptoms may occur only during dialysis or be present continuously in severe cases. While up to 80% of patients with an AV fistula are estimated to have physiological steal that is well compensated for by arterial collaterals and distal vasodilation, 1%–4.6% of patients have true, symptomatic SS.2,3 The incidence of SS doubles in patients with diabetes mellitus (DM). 4
Treatment of SS has advanced from when simple ligation and loss of the fistula was the only option. Therapeutic modalities now include methods of narrowing the vein or allowing more flow to bypass the fistula, all of which are intended to increase arterial flow to the distal limb. These techniques vary in complexity, and which is chosen will depend on whether the patient is experiencing low-flow or high-flow SS. All available options, however, have potential drawbacks. We present a novel, modified plication technique for the treatment of high-flow SS and its outcome in two patients.
Case report
Two patients were chosen as potential candidates to undergo this novel surgical technique to treat their SS. The first patient is a 53-year-old Native American man with end-stage renal disease (ESRD) secondary to type 2 DM who developed right-hand ischemic symptoms inclusive of paresthesia and progressive digital paresis (weak grasp) 3 months after right brachiocephalic fistula creation. On exam, in comparison to the contralateral hand, his right hand was cool to the touch with pallor of the digits and diminished distal pulses which evidently were pronounced with compression of the fistula. Also, there was a noticeable weakness of his right hand. Doppler ultrasound (US) measured a flow rate of 3.9 L/min through the fistula which measured 1.1 cm in diameter. The second patient is an 83-year-old White woman with ESRD secondary to hypertensive nephrosclerosis who presented with progressive left-hand coolness, paresthesia, and paresis over 6 months following creation of a left brachiocephalic fistula and subsequent transposition. Exam findings were similar to Patient 1, except sensory and motor deficits were identified in the left hand. Doppler US measured a flow rate of 4.45 L/min through the fistula which measured 1.3 cm in diameter.
The patients consented to undergo the procedure and were brought to the operating room. A 5-cm longitudinal skin incision, under the effect of single injection peripheral nerve block and locally infiltrated 0.25% marcaine, made superficial to the palpable thrill of an AV fistula and distal to the anastomosis exposes the vein. The flow within the vein to be plicated is controlled proximally and distally with vessel loops. An angled-jaw vascular clamp placed longitudinally along approximately 4 cm of the vein “roof” apposes the “roof sides” to achieve gradual narrowing and flow reduction (Figure 1). The initial 6-0 polypropylene pledgeted suture is placed below the clamp at an angle to ensure a gradual narrowing of the vein. Additional interrupted, horizontal mattress, 6-0 polypropylene pledgeted sutures are placed sequentially along the length of the isolated segment, thereby plicating the vein and narrowing the lumen (Figure 1). Diameter of the isolated vein segment is reduced to less than 1 cm, but no less than 8 mm. Patency of the fistula is ascertained and a flow rate, measured by Doppler US, goal reduction of at least 25%, or no less than 600 mL/min. The wound is then closed using 3-0 polyglactin and 4-0 poliglecaprone to approximate the subdermal and subcuticular layers, respectively.
Figure 1.
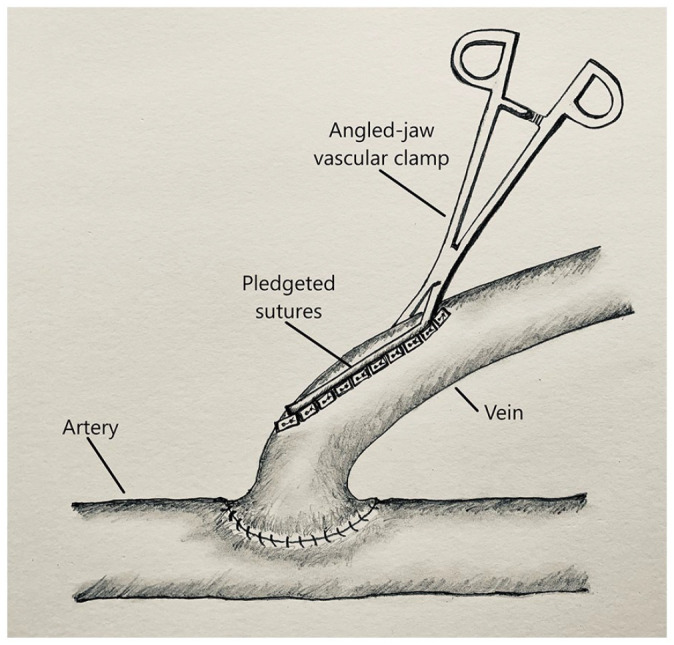
Illustration of sequential pledgeted plication of the venous outflow of an arteriovenous fistula using an angled-jaw vascular clamp.
Implementation of the aforementioned surgical technique in both patients achieved reduction of the venous limb diameter to under 1 cm for approximately 4–5 cm just distal to the anastomosis. Flow rate, measured intra-operatively with a Doppler US, was reduced to 2.7 and 2.05 L/min in Patients 1 and 2, respectively. Both patients experienced good incisional healing. Except for mild coolness of Patient 2’s left hand, complete resolution of sensory and motor deficits occurred in both patients. Both Patients 1 and 2 continued to undergo thrice-a-week uninterrupted hemodialysis at 9 and 12 months following pledgeted plication of the AV fistula, respectively.
Ethical approval to report this case was obtained from The Avera Institutional Review Board. Written informed consent was obtained from the patients for their anonymized information to be published in this article.
Discussion
SS following AV fistula creation results from altered flow through the fistula due to low resistance within the vein. If the resistance is low enough, flow may even reverse in the artery distal to the anastomosis. 5 Whether there is high-flow or low-flow through the fistula, the steal phenomenon can threaten perfusion of the distal limb if there is insufficient collateral circulation. 6 Treatment options will vary, however, depending on this classification as high-flow or low-flow. Treatment options for steal syndrome include simple techniques of ligation, banding and plication of the fistula, 5 and complex procedures including distal revascularization with interval ligation (DRIL), 7 the “extension technique,” 4 and inter-position of a loop graft 6 (summarized in Table 1).
Table 1.
Descriptive summary of flow reduction and flow restoration surgical techniques for the treatment of steal syndrome.
| Technique | Description |
|---|---|
| Flow reduction procedures | |
| Simple plication | Bare sutures narrow a section of the vein |
| Banding | Synthetic cuff narrows the vein |
| MILLER | Angiography with balloon angioplasty-guided banding |
| Loop inter-position | Inter-positioned synthetic loop graft elongates the vein |
| Flow restoration procedures | |
| DRIL | Inter-positioned arterial bypass graft (inflow proximal to and outflow distal to the AV fistula) restores flow into the native artery which is ligated just distal to the fistula |
| Extension | Distal creation of fistula with either one of the two arteries (radial or ulnar) to preserve half the blood supply to the hand |
| PAI | Conversion of fistula inflow to a proximal artery with a graft |
DRIL: distal revascularization with interval ligation; MILLER: minimally invasive limited ligation endoluminal-assisted revision; PAI: proximalization of the arterial inflow; AV: arteriovenous.
Flow reduction procedures for high-flow steal syndrome
Ligation alleviates the pain associated with SS but results in permanent loss of the AV access. Banding and plication are the techniques used to narrow the venous limb and increase resistance, and thus restore and improve distal arterial perfusion. 8 Banding involves circumferential placement of a synthetic cuff to narrow the vein. Advantages of banding include dissection at and exposure of the anastomosis only, reversibility, and immediate use of the fistula; 9 its disadvantages include risk of stenosis and thrombosis secondary to focal narrowing. 6 Newer interventions such as the minimally invasive limited ligation endoluminal-assisted revision (MILLER) technique use angiography with balloon angioplasty to determine the optimal degree of narrowing and as a guide for banding.10,11 Simple plication, a technique that may be more effective in autologous fistulas over synthetic grafts, entails narrowing the vein proximal to the anastomosis with uninterrupted polypropylene suture. 5 As with banding, this technique increases resistance within the vein and improves flow through the artery. An alternative procedure for flow reduction that involves subcutaneously tunneled inter-positioning of a loop polytetrafluoroethylene (PTFE) AV graft employs Poiseuille’s law, whereby lengthening the lumen reduces rate of blood flow through the AV fistula. 6
Arterial flow restoration procedures for low-flow steal syndrome
The DRIL procedure does not change the hemodynamics of the fistula but simply bypasses it to restore distal arterial flow. It involves creation of a bypass, using inter-positioned autologous reversed vein or PTFE graft (grafted end-to-side of the artery), between the proximal and distal arterial limb across the AV fistula. 7 The native artery is ligated proximal to the new anastomosis. DRIL restores function but multiple anastomoses on the same artery risk arterial stenosis(es). The “extension technique,” performed either prophylactically or therapeutically to ensure distal flow, entails creation of an arterio-cephalic fistula distal to the brachial bifurcation. 12 The distal anastomosis, with either the radial or ulnar artery, aims to preserve half the blood supply of the hand; however, it does not eliminate the risk for steal. Another method used to restore arterial flow is proximalization of the arterial inflow (PAI). This method involves using a graft to convert the arterial supply of the fistula to a more proximal artery. 13 This method eliminates the ligation used in the DRIL procedure, maintaining arterial continuity.
Pledgeted plication procedure
Our described technique is innovative with anticipated benefits. As the pledgeted plication technique is most suited for high-flow situations, most comparisons will be drawn to the flow reduction techniques as opposed to the arterial flow restoration techniques that are used for low-flow SSs.
Our technique described above involves only one dissection site, as opposed to the PAI technique. Plication with suture to ensure gradual narrowing of the vein may disrupt flow patterns, however expectedly less than the abrupt banding seen in the banding technique, which may potentiate turbulence, stenosis, and consequent thrombosis. Thus, modification with PTFE felt pledgets used as buttresses under fine polypropylene suture protects the dilated, thin-walled vein from the narrow area of pressure that would be exerted by bare suture, causing tension and/or tear. Furthermore, longitudinally buttressed pledgets with interrupted, horizontal mattress, fine polypropylene sutures appose the vein’s “roof sides” in a gradual, flush fashion unlike the “ribbed” texture from uninterrupted suture in the unmodified plication technique which may cause or propagate thrombosis.
Of note, contrary to other operative techniques where the goal is to reduce the flow rate to within a 600–1500 mL/min range, we aimed for a 25% reduction in the flow rate to account for the anticipated narrowing effect secondary to inflammatory scarring from surgery. Using this metric as our goal, we avoid the complexity and risks of angiography associated with the MILLER technique.
As with any surgical technique, ours is not without risks. The risk for stenosis and thrombosis remains but as noted above, we hypothesize it to be less than other techniques. In addition, there is some risk for reaction or infection with the introduction of any foreign material. We believe the routine use of pledgets in cardiovascular surgery makes it a safe option. Also, promising outcomes in our patients warrant further use and study of this novel technique.
Conclusion
SS is a potential complication of an AV fistula that can result in significant morbidity for the patient. While multiple operative techniques have been used to treat the condition, our modification of the plication procedure has the potential benefits of protection of the vein from bare suture, less turbulent flow, and thus lower risk of thrombosis. We propose to continue following the patients in this study and to perform the procedure on other suitable candidates to further study the long-term outcomes and potential complications, including recurrence of SS. The pledgeted plication technique detailed in this article has produced a successful outcome in the applied patients and warrants further study.
Acknowledgments
The authors appreciate the support of the Avera Transplant Institute in the creation of this manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval to report this case was obtained from The Avera Institutional Review Board (2018.039-100559).
Informed consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article. Figure 1 was created by Dr. Sujit Vijay Sakpal and full permission is granted for its use in this article.
ORCID iDs: Thomas Vierhout  https://orcid.org/0000-0001-5504-720X
https://orcid.org/0000-0001-5504-720X
Sujit Vijay Sakpal  https://orcid.org/0000-0002-1752-4105
https://orcid.org/0000-0002-1752-4105
References
- 1. Holman E. The physiology of an arteriovenous fistula. Am J Surg 1955; 89: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 2. Mwipatayi BP, Bowles T, Balakrishnan S, et al. Ischemic steal syndrome: a case series and review of current management. Curr Surg 2006; 63(2): 130–135. [DOI] [PubMed] [Google Scholar]
- 3. Knox RC, Berman SS, Hughes JD, et al. Distal revascularization-interval ligation: a durable and effective treatment for ischemic steal syndrome after hemodialysis access. J Vasc Surg 2002; 36(2): 250–255; discussion256. [DOI] [PubMed] [Google Scholar]
- 4. Hansrani V, Muhammad K, Charlswood N, et al. The efficacy of the secondary extension technique in the management of arterio-venous fistula-associated steal syndrome. J Vasc Access 2019; 20(6): 592–596. [DOI] [PubMed] [Google Scholar]
- 5. Yaghoubian A, de Virgilio C. Plication as primary treatment of steal syndrome in arteriovenous fistulas. Ann Vasc Surg 2009; 23(1): 103–107. [DOI] [PubMed] [Google Scholar]
- 6. Henriksson AE, Bergqvist D. Steal syndrome after brachiocephalic fistula for vascular access: correction with a new simple surgical technique. J Vasc Access 2004; 5(1): 13–15. [DOI] [PubMed] [Google Scholar]
- 7. Korzets A, Kantarovsky A, Lehmann J, et al. The “DRIL” procedure—a neglected way to treat the “steal” syndrome of the hemodialysed patient. Isr Med Assoc J 2003; 5(11): 782–785. [PubMed] [Google Scholar]
- 8. Wixon CL, Hughes JD, Mills JL. Understanding strategies for the treatment of ischemic steal syndrome after hemodialysis access. J Am Coll Surg 2000; 191(3): 301–310. [DOI] [PubMed] [Google Scholar]
- 9. Stary D, Thaow C-P. Banding of the venous limb of surgically created arteriovenous fistula in the treatment of ischaemic steal syndrome. ANZ J Surg 2002; 72(5): 367–368. [DOI] [PubMed] [Google Scholar]
- 10. Miller GA, Goel N, Friedman A, et al. The MILLER banding procedure is an effective method for treating dialysis-associated steal syndrome. Kidney Int 2010; 77(4): 359–366. [DOI] [PubMed] [Google Scholar]
- 11. Sheaffer WW, Hangge PT, Chau AH, et al. Minimally invasive limited ligation endoluminal-assisted revision (MILLER): a review of the available literature and brief overview of alternate therapies in dialysis associated steal syndrome. J Clin Med Res 2018; 7: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ehsan O, Bhattacharya D, Darwish A, et al. “Extension technique”: a modified technique for brachio-cephalic fistula to prevent dialysis access-associated steal syndrome. Eur J Vasc Endovasc Surg 2005; 29(3): 324–327. [DOI] [PubMed] [Google Scholar]
- 13. Zanow J, Kruger U, Scholz H. Proximalization of the arterial inflow: a new technique to treat access-related ischemia. J Vasc Surg 2006; 43: 1216–1221; discussion1221. [DOI] [PubMed] [Google Scholar]


