Abstract
Immune checkpoint inhibitors have greatly improved the prognoses of diverse advanced malignancies, including gastric and gastroesophageal junction (G/GEJ) cancer. However, the role of anti-programmed cell death protein-1 treatment in the neoadjuvant setting remains unclear. This phase 2 study aimed to evaluate sintilimab plus CapeOx as a neoadjuvant regimen in patients with advanced resectable G/GEJ adenocarcinoma. Eligible patients with resectable G/GEJ adenocarcinoma stage cT3-4NanyM0 were enrolled. Patients received neoadjuvant treatment with sintilimab (3 mg/kg for cases <60 kg or 200 mg for those ≥60 kg on day 1) plus CapeOx (oxaliplatin at 130 mg/m2 on D1 and capecitabine at 1000 mg/m2 two times per day on D1–D14) every 21 days, for three cycles before surgical resection, followed by adjuvant treatment with three cycles of CapeOx with the same dosages after surgical resection. The primary endpoint was pathological complete response (pCR) rate. Secondary endpoints included objective response rate, tumor regression grade per Becker criteria, survival and safety. As of July 30, 2020, 36 patients were enrolled. Totally 7 (19.4%) patients had GEJ cancer, and 34 (94.4%) patients were clinical stage III cases. A total of 35 (97.2%) patients completed three cycles of neoadjuvant treatment, and 1 patients received two cycles due to adverse events. All patients underwent surgery and the R0 resection rate was 97.2%. In this study, pCR and major pathological response were achieved in 7 (19.4%, 95% CI: 8.8% to 35.7%; 90% CI: 10.7% to 33.1%) and 17 (47.2%, 95% CI: 31.6% to 64.3%) patients, respectively. Thirty-one patients received adjuvant treatment. By December 20, 2021, three patients died after disease relapse, and two patients were alive with relapse. Median disease-free survival (DFS) and overall survival (OS) were not reached. The 1-year DFS and OS rates were 90.3% (95% CI: 80.4% to 100.0%) and 94.1% (95% CI: 86.5% to 100.0%), respectively. The most common (>1 patient) grade 3 treatment-related adverse events during neoadjuvant treatment were anemia and neutropenia (n=5 each, 13.9%). No serious adverse events (AEs) or grade 4–5 AEs were observed. Sintilimab plus oxaliplatin/capecitabine showed promising efficacy with encouraging pCR rate and good safety profile in the neoadjuvant setting. This combination regimen might present a new option for patients with locally advanced, resectable G/GEJ adenocarcinoma. Trial registration; NCT04065282.
Keywords: antibodies, neoplasm; clinical trials, phase II as topic; clinical trials as topic; gastrointestinal neoplasms; immunotherapy
Background
Gastric cancer (GC) is the fifth most commonly diagnosed malignancy, accounting for about 33% of cancer-related deaths worldwide, with the highest mortality and incidence rates reported in Eastern Asia.1 Surgical resection is the primary treatment option for early-stage and locally advanced tumors; nevertheless, most patients relapse after surgery, which is considered to be associated with the ‘residual tumor’2 or ‘tumor micrometastasis’.3
Neoadjuvant therapy is a well-established practice to improve efficacy beyond surgery alone in gastric (G) and gastroesophageal junction (GEJ) cancer with the advantages of reducing tumor burden and assessing tumor response before surgery, as well as improving overall survival (OS).4 Although studies have demonstrated the clinical benefit of neoadjuvant chemotherapy for G/GEJ cancer, pathological regression and long-term survival rate are still unsatisfactory.5–9
Immune checkpoint inhibitors have revolutionized the treatment of malignancies. Currently, anti-programmed cell death protein-1/programmed death-ligand 1 (PD-1/PD-L1) antibodies are approved mostly for unresectable or metastatic solid tumors, and its activity in the neoadjuvant setting is not well established. Liu et al recently demonstrated in preclinical mouse models the significantly greater therapeutic effect of neoadjuvant, compared with adjuvant, immunotherapy in eradicating metastases by systemically expanding tumor-specific CD8 +T cells in peripheral blood and organs.10 Based on these findings, neoadjuvant PD-1 blockade is likely to prime an effective systemic immunity, thus eradicating residual micrometastases after surgical resection of primary tumors. In addition, conventional chemotherapy has demonstrated properties of enhancing tumor antigenicity, interfering suppressive immune pathways, and increasing effector T-cell reactions.11 Therefore, neoadjuvant PD-1 blockade in combination with chemotherapy may potentially contribute to a stronger and broader tumor-specific T-cell response.
Sintilimab is a recombinant humanized lgG4 anti-PD-1 antibody, with greater affinity to human PD-1 than nivolumab and pembrolizumab.12 In a phase Ib trial in Chinese patients, sintilimab in combination with capecitabine and oxaliplatin (CapeOx) demonstrated a superior efficacy profile as first-line treatment regimen for advanced or metastatic G/GEJ adenocarcinoma, with acceptable safety outcomes.13
Here, we report the results of a phase 2 study evaluating the efficacy and safety of sintilimab in combination with CapeOx in the neoadjuvant setting in patients with resectable G/GEJ adenocarcinoma. To our best of knowledge, it is currently the first prospective study of neoadjuvant anti-PD-1 immunotherapy combined with chemotherapy in G/GEJ cancer.
Methods
Patients
Eligible patients were previously untreated, histologically confirmed G/GEJ adenocarcinoma, clinical stage cT3–4NanyM0 according to eighth Edition Gastric Cancer Staging of American Joint Committee on Cancer (AJCC) as assessed by contrast-enhanced CT of the abdomen and pelvis. The tumor must be resectable before neoadjuvant therapy as evaluated by surgeons. Further inclusion criteria were 18–75 years of age, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1 and adequate hematopoietic, hepatic and renal functions. Patients were excluded with a history of gastrointestinal perforation or fistula within 6 months, high risk of gastrointestinal hemorrhage before enrollment, active infections, active or refractory autoimmune diseases, or uncontrolled systematic diseases. Full eligibility criteria are listed in the protocol in online supplemental materials.
jitc-2021-003635supp001.pdf (47.6KB, pdf)
Study design
This study was an investigator-initiated, single-arm, phase 2 study conducted at the First Affiliated Hospital of College of Medicine, Zhejiang University. All subjects provided written informed consent before enrollment. The study was prospectively registered at ClinicalTrials.gov.
Treatment
Neoadjuvant sintilimab and CapeOx was administered for three cycles before surgery. Each 3-week cycle consisted of sintilimab (3 mg/kg for cases <60 kg and 200 mg for those ≥60 kg) intravenously on day 1, oxaliplatin (130 mg/m2) intravenously on day 1 and capecitabine (1000 mg/m2 two times per day) orally at days 1–14. Dose modification or interruption was allowed and specified in the protocol (online supplemental materials). Surgery was scheduled within 1–4 weeks after completion of neoadjuvant treatment. Gastrectomy with D2 lymphadenectomy was required based on the study protocol. The scope of gastrectomy was determined by the location and extent of the primary tumor to ensure an adequate surgical resection margin. Additional three cycles of adjuvant CapeOx at the same dosages were scheduled within 4–6 weeks after surgery.
Assessments
Medical history, physical examination, weight, vital signs, ECOG PS, ECG, complete blood count, blood chemistry, urinary test, thyroid function test, gastroscopy with biopsy, and pathological evaluation were obtained or performed for each patient at baseline and before the start of each treatment cycle. Adverse events (AEs) during the neoadjuvant and adjuvant treatment periods were graded according to the National Cancer Institute’s Common Toxicity Criteria, V.5.0. Perioperative morbidity and mortality were recorded.
Tumors were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1 based on contrast-enhanced CT and PET Response Criteria in Solid Tumors (PERCIST) based on positron emission tomography and CT (PET/CT) at baseline and before surgery. Tumor staging was also performed at baseline (clinical TNM Classification of Malignant Tumors: cTNM) and after surgery (postneoadjuvant pathologic TNM Classification of Malignant Tumors: ypTNM) according to eighth Edition Gastric Cancer Staging of AJCC. Contrast-enhanced CT performed before the first dose of adjuvant treatment and every 3 months until disease relapse or death, for up to 2 years after surgery. Pathological response of the primary tumor after surgery was graded according to Becker criteria of Tumor Regression Grade (TRG)14: TRG1a (no residual tumor cells), which is equivalent to pathological complete response (pCR); TRG1b (<10% residual tumor cells); TRG2 (10%–50% residual tumor cells) and TRG3 (>50% residual tumor cells). Major pathological response (MPR) was defined as <10% residual tumor cells (TRG1a/b).
PD-L1 expression was assessed in formalin-fixed, paraffin-embedded tumor samples with the PD-L1 immunohistochemistry (IHC) 22C3 pharmDx assay (Dako, Glostrup, Denmark). Patients in this cohort were required to provide biopsy samples before treatment. PD-L1 evaluation was performed using the combined proportional score (CPS). PD-L1 positivity was defined as CPS ≥1, where CPS is the number of PD-L1-positive cells—tumor cells, lymphocytes, and macrophages— divided by the total number of tumor cells × 100. The mismatch repair (MMR) and Epstein-Barr virus (EBV) statuses of all patients were assessed by IHC.
Outcomes
The primary endpoint was the proportion of patients with pCR. Secondary endpoints included objective response rate (ORR, defined as the proportion of patients with the best overall response of complete response (CR) or partial response (PR)) before surgery, rate of each TRG after surgery, disease-free survival (DFS) defined as the time from enrollment to disease relapse or death from any cause, 1-year and 2-year OS rates and safety profile of the neoadjuvant regimen. Exploratory endpoints included the associations of pathological responses with survival outcomes and the associations of treatment efficacy with PD-L1 expression in the tumor tissue and biomarkers in peripheral blood. The primary analyses were performed in the intention-to-treat population. All AEs were analyzed in the safety population, defined as patients administered at least one dose of neoadjuvant or adjuvant treatment. Neoadjuvant or adjuvant treatment emergent AEs were reported separately due to the different regimens applied. Surgery-related morbidity and mortality were analyzed in the per-protocol population, defined as patients who were compliant with the study protocol and proceeded to surgery.
Statistical analysis
The sample size was calculated based on the assumption that the true pCR rate of neoadjuvant sintilimab in combination with CapeOx is 25%. Thus, 36 subjects would provide 70% power to ensure the lower boundary of 90% CI of a pCR rate higher than 10%. Descriptive statistics of baseline and clinicopathological characteristics were performed. The pCR, MPR, ORR and R0 resection rate were calculated, and the corresponding CIs were estimated by the Blaker’s binomial exact method. Kaplan-Meier estimates of DFS and OS probabilities were determined, with respective 95% CIs. Statistical analyses were performed with the R Statistical Software (V.3.5.3).
Results
Patient characteristics
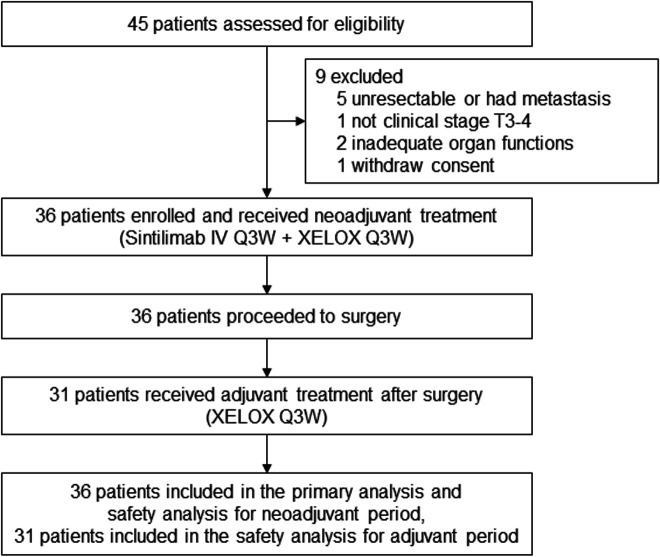
From August 1, 2019, to July 30, 2020, 45 patients were screened and 36 patients were eligible and included in this study. Nine patients were excluded for unresectable tumors or metastasis (n=5), not being clinical stage T3–T4 (n=1), inadequate organ functions (n=2), or consent withdrawal before treatment (n=1) (figure 1). The baseline characteristics of the 36 patients are shown in table 1. The median age was 65.5 years (range, 43–76). Totally 7 (19.4%) patients had GEJ cancer, and 34 (94.4%) cases were clinical stage III.
Figure 1.
Trial flowchart. IV, intravenous; Q3W, every 3 weeks.
Table 1.
Baseline demographic and patient characteristics
| Characteristic | All patients (N=36) |
| Age—year | |
| Median (range) | 65.5 (43–76) |
| Sex—no. (%) | |
| Male | 24 (66.7) |
| Female | 12 (33.3) |
| ECOG performance status—no. (%) | |
| 0 | 36 (100) |
| Primary tumor location—no. (%) | |
| Gastric | 29 (80.6) |
| Gastric-esophageal junction | 7 (19.4) |
| Histological type | |
| Adenocarcinoma | 35 (97.2) |
| Signet cell carcinoma | 1 (2.8) |
| Lauren’s classification—no. (%) | |
| Intestinal | 17 (47.2) |
| Diffuse | 13 (36.1) |
| Mixed | 5 (13.9) |
| Unknown or unclassifiable | 1 (2.8) |
| Clinical T stage—no. (%) | |
| cT3 | 11 (30.6) |
| cT4a | 25 (69.4) |
| Clinical N stage—no. (%) | |
| cN0 | 2 (5.6) |
| cN1 | 18 (50.0) |
| cN2 | 15 (41.7) |
| cN3 | 1 (2.8) |
| Clinical tumor, node, metastases stage—no. (%) | |
| IIB | 2 (5.6) |
| III | 34 (94.4) |
| PD-L1 status—no. (%) | |
| CPS ≥1 | 21 (58.3) |
| CPS <1 | 11 (30.6) |
| Unknown | 4 (11.1) |
CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed cell death ligand-1.
Treatments
By the cut-off date of December 20, 2021, all patients had discontinued the treatment. Thirty-five (97.2%) patients completed three cycles of neoadjuvant treatment, and one received two cycles due to grade 3 aspartate aminotransferase (AST) increase. Twenty-five patients completed three cycles, and six received one or two cycles of adjuvant treatment. Of the 11 patients who did not complete the planned adjuvant treatment, 8 discontinuations were requested by the patient and 3 were determined by investigators due to unsatisfied risk-benefit ratio.
Totally 13 (36.1%) and 19 (52.8%) patients experienced dose reductions of oxaliplatin and capecitabine, respectively. Median dose intensities for sintilimab, oxaliplatin and capecitabine were 100% (range, 66.7%–105.0%), 95.1% (32.1%–100.5%) and 97.2% (22.1%–120.0%), respectively.
All patients proceeded to surgery after neoadjuvant treatment. The median interval time between the last dose of sintilimab and surgery was 36 days (28–70). Totally 18 (50%) patients underwent total gastrectomy, and the other 18 received distal gastrectomy. D2 lymphadenectomy was performed in all patients. One patient underwent R1 resection with positive surgical margin. The R0 resection rate was 97.2% (95% CI: 85.7% to 99.9%).
The median time interval between surgery and the first dose of adjuvant treatment was 37 days (27–116).
Tumor response and survival outcomes
Based on pathological evaluation after surgery, pCR (TRG1a) was observed in seven patients (19.4%, 95% CI: 8.8% to 35.7%; 90% CI: 10.7% to 33.1%). TRG1b, TRG2 and TRG3 were observed in 10 (27.8%), 15 (41.7%) and 4 (11.1%) patients, respectively. The MPR (TRG1a/b) rate was 47.2% (95% CI: 31.6% to 64.3%), the pCR and MPR rates were 27.3% and 59.1%, respectively (table 2). Compared with clinical stage before treatment, 26 (72.2%) patients had T downstaging, 25 (69.4%) had N downstaging and 27 (75.0%) had overall tumor, node, metastases (TNM) downstaging (table 3). Among 36 patients whose pretreatment biopsy samples were screened for PD-L1 expression, 4 (11.1%) had no PD-L1 expression data due to poor sample quality, and 21 (58.3%) had PD-L1 positive expression (CPS ≥1). Here we analyzed the associations of pathological pCR and MPR with PD-L1 expression according to four thresholds (CPS <1, CPS ≥1, CPS ≥5 and CPS ≥10). In the PD-L1 CPS <1 subgroup (n=11), the pCR and MPR rates were 9.1% and 27.3%, respectively. In the 21 patients with PD-L1 CPS ≥1, the pCR and MPR rates were 28.6% and 57.1%, respectively. Among the 11 patients with PD-L1 CPS ≥5, the pCR and MPR rates were 27.3% and 54.5%, respectively. Among the six patients with PD-L1 CPS ≥10, the pCR and MPR rates were 33.3% and 50.0%, respectively.
Table 2.
Tumor responses
| Tumor responses | All patients |
| Radiological evaluation | |
| RECIST 1.1—no. (%) | N=36 |
| Patients with target disease | 6 (16.7) |
| Patients with non-target disease only | 30 (83.3) |
| Complete response (CR) | 0 |
| Partial response | 4 (66.7) |
| Stable disease | 2 (33.3) |
| Non-CR/non-PD | 30 (83.3) |
| Progressive disease (PD) | 0 |
| Objective response rate | 66.7 |
| Disease control rate | 100 |
| PERCIST—no. (%) | N=31 |
| Complete metabolic response | 0 |
| Partial metabolic response | 19 (61.3) |
| Stable metabolic response | 11 (35.5) |
| Progressive metabolic response | 1 (3.2) |
| Pathological evaluation | |
| Becker criteria—no. (%) | N=36 |
| pCR (TRG1a) | 7 (19.4) |
| TRG1b | 10 (27.8) |
| TRG2 | 15 (41.7) |
| TRG3 | 4 (11.1) |
| MPR (TRG1a/b) | 17 (47.2) |
MPR, major pathological response; pCR, pathological complete response; PERCIST, PET Response Criteria in Solid Tumors; RECIST, Response Evaluation Criteria in Solid Tumors; TRG, Tumor Regression Grade.
Table 3.
Tumor downstaging rates
| Clinical stage before treatment | Pathology stage after operation | No. (%) |
| T stage | ||
| cT3 | ypT0 | 3 (8.3) |
| ypT1 | 3 (8.3) | |
| ypT2 | 2 (5.6) | |
| ypT3 | 3 (8.3) | |
| cT4 | ypT0 | 4 (11.1) |
| ypT1 | 5 (13.9) | |
| ypT2 | 5 (13.9) | |
| ypT3 | 5 (13.9) | |
| ypT4a | 6 (16.7) | |
| N stage | ||
| cN0 | ypN0 | 2 (5.6) |
| cN1 | ypN0 | 14 (38.9) |
| ypN1 | 1 (2.8) | |
| ypN2 | 1 (2.8) | |
| ypN3 | 2 (5.6) | |
| cN2 | ypN0 | 6 (16.7) |
| ypN1 | 4 (11.1) | |
| ypN2 | 2 (5.6) | |
| ypN3 | 3 (8.3) | |
| cN3 | ypN0 | 1 (2.8) |
| Overall tumor, node, metastases stage | ||
| IIB | I | 2 (5.6) |
| III | 0 | 7 (19.4) |
| I | 12 (33.3) | |
| II | 6 (16.7) | |
| III | 9 (25.0) | |
In this cohort, only two (5.6%) patients were deficient MMR (dMMR). One of them, with a high PD-L1 expression (CPS=68), had pCR; the other patient whose PD-L1 expression is unknown had non-MPR (TRG2). Of two (5.6%) patients with EBV-positive status, CPS of 10 and 0 were found, respectively, and none of them achieved MPR. Furthermore, these two EBV-positive patients were proficient mismatch repair (pMMR).
Among the six patients with target lesions per RECIST V.1.1, four achieved PR and two had stable disease (SD). In the overall population, the disease control rate (percentage of patients who achieved PR, CR or SD) was 100%. No patients had progressive disease before surgery.
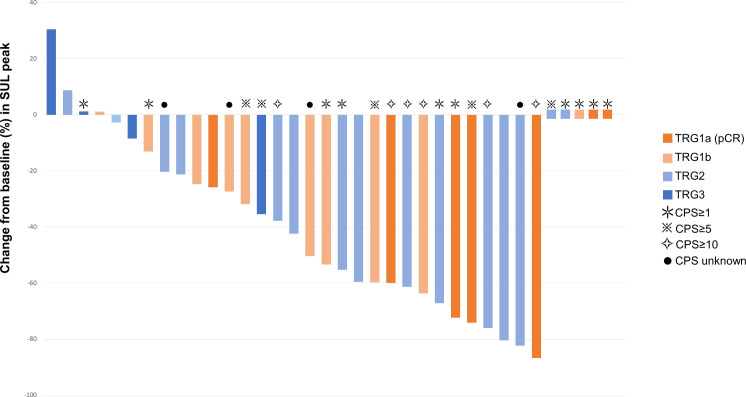
All patients underwent PET-CT both at baseline and before surgery. Among the 31 patients with measurable target lesions based on PERCIST, 19 (61.3%) achieved partial metabolic response (PMR), 11 (35.5%) had stable metabolic disease and 1 (3.2%) showed progressive metabolic disease (table 2). The changes of standardized uptake value(SUV) corrected for lean body mass (SUL) peak before surgery from baseline, as well as pathological response and PD-L1 CPS data are presented in a waterfall plot (figure 2). The PMR per PERCIST predicted pathological response in 4 (80.0%) of 5 patients with pCR and 9 (64.3%) of 14 patients with MPR.
Figure 2.
Tumor responses per PERCIST and Becker criteria. CPS, combined proportional score; pCR, pathological complete response; TRG, Tumor Regression Grade; SUL, standardized uptake value (SUV) corrected for lean body mass; PRECIST: Response Evaluation Criteria in Solid Tumors.
By the cut-off date, the median follow-up time was 19.1 months, and 30.6% patients were follow-up for over 2 years. Three patients died after disease relapse, and two were alive with relapse. Median DFS and OS were not reached. In these cases, 1-year DFS and OS rates were 90.3% (95% CI: 80.4% to 100.0%) and 94.1% (95% CI: 86.5% to 100.0%), respectively (figure 3).
Figure 3.
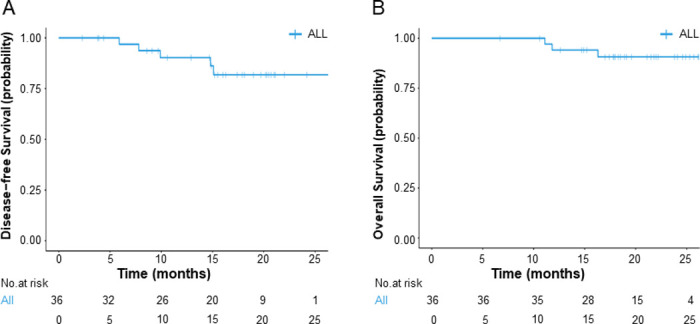
Survival outcomes. (A) Disease-free survival of all patients; (B) overall survival of all patients.
Safety
During the neoadjuvant treatment period, 35 (97.2%), 33 (91.7%) and 4 (11.1%) patients experienced treatment emergent adverse events (TEAEs), treatment-related adverse events to any drug (TRAEs) and sintilimab-related AEs, respectively. The most common (>10%) TRAEs were anemia (n=26, 72.2%), leukopenia (n=18, 50.0%), neutropenia (n=15, 41.7%), vomiting (n=10, 27.8%), alanine transaminase (ALT) and AST increases (n=7 each, 19.4%), hypokalemia (n=6, 16.7%), thrombocytopenia (n=6, 16.7%) and γ-glutamyl transferase (GGT) increase (n=4, 11.1%). Most of the TRAEs were grade 1 or 2. Ten (27.8%) patients experienced grade 3 TRAEs. The most common (>1 patient) grade 3 TRAEs were anemia and neutropenia (n=5 each, 13.9%); other grade 3 TRAEs included leukopenia, AST increase, thrombocytopenia and γ-GGT increase (n=1 each, 2.8%). No serious AEs and grade 4 or grade 5 AEs were observed. Three (8.3%) patients experienced AEs with potential immunologic etiology, including two grade 1 hyperthyroidism and one grade 1 hypothyroidism cases (table 4).
Table 4.
Neoadjuvant and adjuvant treatment-related adverse events
| Neoadjuvant treatment (N=36) | Adjuvant treatment (N=31) | |||
| Any grade | Grade 3 | Any grade | Grade 3 | |
| Treatment-related AEs, n (%) | 33 (91.7) | 10 (27.8) | 28 (90.3) | 2 (6.5) |
| Anemia | 26 (72.2) | 5 (13.9) | 5 (16.1) | 0 |
| White blood cell count decrease | 18 (50.0) | 1 (2.8) | 20 (64.5) | 0 |
| Neutrophil count decrease | 15 (41.7) | 5 (13.9) | 20 (64.5) | 2 (5.6) |
| Vomiting | 10 (27.8) | 0 | 9 (29.0) | 1 (2.8) |
| Alanine aminotransferase increase | 7 (19.4) | 0 | 3 (9.7) | 0 |
| Aspartate aminotransferase increase | 7 (19.4) | 1 (2.8) | 4 (12.9) | 0 |
| Hypokalemia | 6 (16.7) | 0 | 3 (9.7) | 0 |
| Platelet count decrease | 6 (16.7) | 1 (2.8) | 6 (19.4) | 0 |
| Gamma-glutamyl transferase increase | 4 (11.1) | 1 (2.8) | 5 (16.1) | 0 |
| Constipation | 3 (8.3) | 0 | 0 | 0 |
| Fever | 3 (8.3) | 0 | 0 | 0 |
| Rash | 3 (8.3) | 0 | 0 | 0 |
| Hypophagia | 2 (5.6) | 0 | 2 (6.5) | 0 |
| Hyperthyroidism | 2 (5.6) | 0 | 0 | 0 |
| Urinary tract infection | 1 (2.8) | 0 | 0 | 0 |
| Nausea | 1 (2.8) | 0 | 1 (3.2) | 0 |
| Lymphocyte count decrease | 1 (2.8) | 0 | 0 | 0 |
| Hypothyroidism | 1 (2.8) | 0 | 3 (9.7) | 0 |
| Enteritis | 1 (2.8) | 0 | 0 | 0 |
| Dermatitis allergic | 1 (2.8) | 0 | 0 | 0 |
| Lower gastrointestinal hemorrhage | 0 | 0 | 1 (3.2) | 0 |
| Palmar–plantar erythrodysesthesia syndrome | 0 | 0 | 1 (3.2) | 0 |
| Diarrhea | 0 | 0 | 1 (3.2) | 0 |
| Blood alkaline phosphatase increase | 0 | 0 | 1 (3.2) | 0 |
| Immune-related AEs, n (%) | 1 (2.8) | 0 | 0 | 0 |
| Hyperthyroidism | 2 (5.6) | 0 | 0 | 0 |
| Hypothyroidism | 1 (2.8) | 0 | 0 | 0 |
AEs, adverse events.
One patient experienced grade 3 AST increase after two cycles of neoadjuvant treatment and skipped the third cycle. Four (11.1%) patients experienced surgery delay (>28 days after the end of the last neoadjuvant cycle) due to AEs or deterioration of performance status after neoadjuvant treatment.
Seven (19.4%) patients experienced grade 3 surgery-related complications, including anemia (n=5, 13.9%), AST increase (n=2, 5.6%), ALT increase (n=1, 2.8%), γ-GGT increase (n=1, 2.8%) and thrombocytopenia (n=1, 2.8%). No emergency reoperation or intensive care was required. No postoperative mortality was observed.
During the adjuvant treatment period, TEAEs occurred in 28 (90.3%) patients, and all were treatment related. Most of these TRAEs were grade 1 or grade 2. Two (6.5%) patients experienced grade 3 TRAEs that included neutrophil count decrease (n=2, 6.5%) and vomiting (n=1, 3.2%) (table 4).
Discussion
In this phase 2 study, we evaluated the efficacy and safety of neoadjuvant sintilimab in combination with CapeOx followed by gastrectomy with D2 lymphadenectomy in patients with locally advanced, resectable G/GEJ adenocarcinoma. The study met the pre-specified primary endpoint with a pCR rate of 19.4% (95% CI: 8.8% to 35.7%; 90% CI: 10.7% to 33.1%). To our knowledge, this is the first report evaluating the efficacy and safety of neoadjuvant anti-PD-1 immunotherapy combined with chemotherapy in G/GEJ cancer.
Perioperative chemotherapy has been the standard of care for locally advanced GC since the MAGIC study.6 Pathological response is commonly recommended as a surrogate short-term endpoint, while pCR is believed to predict the long-term benefit in neoadjuvant trials.15 16 CapeOx is one of regimens widely adopted in the perioperative treatment of GC. The pCR rate of neoadjuvant CapeOx ranged from 4% to 9% in previous studies.17–19 Chemotherapy with the FLOT regimen achieved the highest pCR (16%) and MPR (37%) rates in the neoadjuvant setting in GC, but also showed greater toxicity, with 27% patients experiencing treatment-related serious AEs in the FLOT4 study.20 In this study, adding sintilimab to CapeOx achieved higher pCR (19.4%) and MPR (47.2%) rates with better safety profile in a small cohort with cT3–4 and a large proportion of cN +GC cases, compared with the FLOT4 study. Camrelizumab, another PD-1 inhibitor, combined with FOLFOX as neoadjuvant therapy for GC, achieved a pCR rate of 8%.21 We attribute our favorable results to the combination of different drugs, more patients with good performance status, more accurate clinical staging with less occult metastasis during surgery, and a higher rate of PD-L1 positive patients (CPS ≥1, 58.3%). In addition to the promising pCR and MPR results, a remarkable downstaging effect was also observed in this study, even though it may be potentially confounded by preoperative overstaging. Compared with previously reported proportions, from 43.3% to 55.2%,22 23 downstaging of overall TNM stage was achieved in 75% patients, which was also translated into a high R0 resection rate (97.2%) in this study. Notably, 21 (58.3%) patients achieved nodal downstage to ypN0 after neoadjuvant treatment. This is reportedly an important hallmark demonstrating the effectiveness of preoperative therapy.24 The high pCR and downstage rates also led to the promising survival rate observed in this study, although continued follow-up is required to demonstrate the long-term benefit of this treatment.
PD-L1 expression is a potential biomarker for anti-PD-1/PD-L1 treatment. However, its predictive value in GC remains unclear. CPS assesses PD-L1 positive cells, including tumor cells, lymphocytes and macrophages, and has been used as a stratification factor or a companion diagnostic in unresectable and metastatic GC studies.25–27 In these studies, patients with metastatic GC with a higher CPS showed a longer survival and better response to anti-PD-1 combination therapies.26 28 29 In the KEYNOTE-062 study, pembrolizumab in combination with chemotherapy had a greater ORR benefit versus chemotherapy in patients with CPS ≥10 (52.5% vs 37.8%), compared with those with CPS ≥1 (48.6% vs 37.2%) in unresectable or metastatic GC.28 29 In recently completed CheckMate-649 study, nivolumab plus chemotherapy prolonged the median survival time over chemotherapy in similar patients with GC, by 2.2, 2.7 and 3.3 months in the overall, CPS ≥1, and CPS ≥5 populations, respectively. A similar predictive value of CPS was observed with PD-1 inhibitor plus chemotherapy in locally advanced, patients with resectable GC in this study. Higher pCR and MPR rates were observed in 6 (28.6%) and 12 (57.1%) among the 21 patients with CPS ≥1, compared with overall population (19.4% and 47.2%, respectively). The predictive value with CPS ≥1 was consistent with these previous studies, but the response rate did not further rise when the cut-off was escalated to CPS ≥5 or CPS ≥10. This may due to the small sample size of this study. These results support CPS as a predictive biomarker for selecting patients who may benefit more from neoadjuvant anti-PD-1 treatment; however, this prediction warrants further investigation.
As previously reported, microsatellite instability-high/dMMR occurs in a sizeable share of GC cases (8%–22%),30 31 and EBV-positivity is present in about 9% of GC cases.31 Patients with GC with dMMR or EBV positivity are likely to respond to immunotherapy,31 32 and both are mutually exclusive. In this study, two patients with dMMR and two with EBV were identified, which was consistent with the prevalence reported previously. Although one patient with dMMR achieved pCR, no conclusion can be drawn due to the limited sample size of this study.
RECIST V.1.1 is a standard radiological evaluation criterion for various solid tumors, but not always considered optimal in neoadjuvant treatment of GC. In this study, only six patients had measurable lesions, which made it inadequate to use RECIST V.1.1 for tumor assessment. In order to monitor tumor response during the neoadjuvant period, we also performed PET-CT in all patients and adopted PERCIST criteria to quantify tumor metabolic changes. A few studies have proposed that 18F-fluoro-2-deoxy-D-glucose (18FDG) changes in terms of PERCIST criteria are highly predictive of treatment efficacy in patients with non-small cell lung cancer undergoing immune checkpoint inhibitor therapies, although not immune-chemo-combinations therapy.33–35 However, 18FDG changes were not well correlated with pathological responses in this study examining patients with GC. A longer follow-up is required to evaluate whether 18FDG changes predict survival benefit in patients with GC. In addition, new 18FDG PET-based response assessment criteria, for example, PECRIT36 and PET Response Evaluation Criteria for Immunotherapy (PERCIMT),37 have also been developed to better fit PET imaging into immunotherapy.38 High-quality prospective trials are still warranted to determine whether patients with GC could benefit from PET-CT in terms of prognosis of immunotherapy.
This study was designed to employ sintilimab only in the preoperative treatment phase, rather than for perioperative treatment, with several considerations. At the time of study design, both efficacy and long-term safety of sintilimab in patients with resectable GC remained unknown. As an investigator-initiated study with the primary endpoint of pCR, only preoperative sintilimab treatment was approved by the institutional ethics committee. This design was proposed to meet the study endpoint and to mitigate the safety risk of exposure to extra treatment. However, the pathological response and safety data obtained in this study support further studies to fully evaluate sintilimab in combination with chemotherapy in the perioperative setting.
The toxicity of sintilimab in combination with CapeOx was manageable with the most frequent TRAEs being anemia, leukopenia and neutropenia, which is consistent with previous findings.13 Most TRAEs were grade 1 or grade 2. There was only one patient who skipped the last cycle of preoperative treatment due to grade 3 AST increase and grade 2 ALT increase, which were manageable and attenuated to grade 1 within 9 days after treatment interruption and without corticosteroid given.
There were several limitations in this study. First, this was a phase 2 study with a small sample size, the results may not be generalized beyond the study population. Second, survival data were not obtained due to a short follow-up time. All patients are still on follow-up. The survival results will be reported when data are available. Finally, the clinical response observed in this study should be further accompanied and explained with biomarker and translational studies. These analyses are still ongoing.
In conclusion, neoadjuvant sintilimab, oxaliplatin and capecitabine showed encouraging clinical benefits and acceptable safety, providing a promising treatment option for locally advanced, resectable G/GEJ adenocarcinoma. Several well-designed phase 3 randomized controlled trials have been initiated to confirm the role of neoadjuvant anti-PD-1/PD-L1 antibodies in combination with chemotherapy for G/GEJ adenocarcinoma (NCT03221426, NCT04592913, NCT04139135).
Footnotes
HJ, XY, NL and MK contributed equally.
Contributors: LT is responsible for the overall content as guarantor. HJ contributed to conception, design, acquisition of data, interpretation of data, drafted the manuscript, and critically revised the manuscript; XY, NL, MK contributed to design, acquisition of data, interpretation of data, and drafted the manuscript; ZM, DZ, WW, HaoWang, HaiWang, KH, ZL, YL, JZ, KZ, YZ, NX contributed to acquisition of data; ZL, YL, YanWang, YisWang contributed to design, analysis of data, interpretation of data; LT contributed to conception, design, and critically revised the manuscript; All authors have read and approved the final manuscript.
Funding: The study was supported by the National Health and Family Planning Commission Research Fund & Zhejiang Provincial Medical and Health Major Science and Technology Plan Project (Grant No. KWJ-ZJ-2108); the Public Welfare Technology Application Research Project of Zhejiang Province (Grant No. LGF20E030004) and Innovent Biologics, Inc.
Competing interests: HJ received advisory fees from Innovent Biologics. ZL, YL and Yan Wang are employees of Innovent Biologics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the institutional Ethics Committee of the First Affiliated Hospital of College of Medicine, Zhejiang University (approval number: 2019-1083). Participants gave informed consent to participate in the study before taking part.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Figueiredo PC, Pimentel-Nunes P, Libânio D, et al. A systematic review and meta-analysis on outcomes after RX or R1 endoscopic resection of superficial gastric cancer. Eur J Gastroenterol Hepatol 2015;27:1249–58. 10.1097/MEG.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z-yu, Ge H-yan,. Micrometastasis in gastric cancer. Cancer Lett 2013;336:34–45. 10.1016/j.canlet.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 4.Shitara K, Al-Batran S-E, Bang Y-J, et al. 198TiP spotlight: phase III study of zolbetuximab + mFOLFOX6 versus placebo + mFOLFOX6 in first-line Claudin18.2⁺/HER2⁻ advanced or metastatic gastric or gastroesophageal junction adenocarcinoma (G/GEJ). Ann Oncol 2020;31:S1317. 10.1016/j.annonc.2020.10.462 [DOI] [Google Scholar]
- 5.Ychou M, Boige V, Pignon J-P, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–21. 10.1200/JCO.2010.33.0597 [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 7.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European organisation for research and treatment of cancer randomized trial 40954. J Clin Oncol 2010;28:5210–8. 10.1200/JCO.2009.26.6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Batran S-E, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697–708. 10.1016/S1470-2045(16)30531-9 [DOI] [PubMed] [Google Scholar]
- 9.Blackham AU, Greenleaf E, Yamamoto M, et al. Tumor regression grade in gastric cancer: predictors and impact on outcome. J Surg Oncol 2016;114:434–9. 10.1002/jso.24307 [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382–99. 10.1158/2159-8290.CD-16-0577 [DOI] [PubMed] [Google Scholar]
- 11.Yan Y, Kumar AB, Finnes H, et al. Combining immune checkpoint inhibitors with conventional cancer therapy. Front Immunol 2018;9:9. 10.3389/fimmu.2018.01739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Fei K, Jing H, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs 2019;11:1443–51. 10.1080/19420862.2019.1654303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang H, Zheng Y, Qian J, et al. Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase Ib clinical trial. BMC Cancer 2020;20:760. 10.1186/s12885-020-07251-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521–30. 10.1002/cncr.11660 [DOI] [PubMed] [Google Scholar]
- 15.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–72. 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 16.Hellmann MD, Chaft JE, William WN, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–50. 10.1016/S1470-2045(13)70334-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue K, Ying X, Bu Z, et al. Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II-III randomized trial. Chin J Cancer Res 2018;30:516–25. 10.21147/j.issn.1000-9604.2018.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y, Fang Y, Shen Z, et al. Oxaliplatin plus capecitabine in the perioperative treatment of locally advanced gastric adenocarcinoma in combination with D2 gastrectomy: NEO-CLASSIC study. Oncologist 2019;24:1311–989. 10.1634/theoncologist.2019-0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Cheng X, Cui Y-H, et al. Efficacy after preoperative capecitabine and oxaliplatin (XELOX) versus docetaxel, oxaliplatin and S1 (DOS) in patients with locally advanced gastric adenocarcinoma: a propensity score matching analysis. BMC Cancer 2018;18:702. 10.1186/s12885-018-4615-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–57. 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Han G, Li H. Camrelizumab combined with FOLFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma. American Society of Clinical Oncology 2020. [Google Scholar]
- 22.D'Ugo D, Persiani R, Rausei S, et al. Response to neoadjuvant chemotherapy and effects of tumor regression in gastric cancer. Eur J Surg Oncol 2006;32:1105–9. 10.1016/j.ejso.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Yang X, Yan C, et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: a single-arm, open-label, phase II trial. Eur J Cancer 2020;130:12–19. 10.1016/j.ejca.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 24.Ikoma N, Estrella J, Hofstetter WL. Nodal downstaging in gastric cancer in relation to survival when ypN0 is achieved. American Society of Clinical Oncology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shitara K, Van Cutsem E, Bang Y-J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 2020;6:1571–80. 10.1001/jamaoncol.2020.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wainberg ZA, Fuchs CS, Tabernero J. Efficacy of pembrolizumab monotherapy for advanced Gastric/Gastroesophageal junction cancer with programmed death ligand 1 combined positive score ≥10. Clin Cancer Res 2021. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013. 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabernero J, Van Cutsem E, Bang Y-J. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. American Society of Clinical Oncology 2019. [Google Scholar]
- 29.Moehler M, Shitara K, Garrido M, et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol 2020;31:S1191. 10.1016/j.annonc.2020.08.2296 [DOI] [Google Scholar]
- 30.Miceli R F, Raimondi A, et al. Patient data meta-analysis of the value of microsatellite instability as a biomarker in gastric cancer. J Clin 2019;37:3392–400. [DOI] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449–58. 10.1038/s41591-018-0101-z [DOI] [PubMed] [Google Scholar]
- 33.Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging 2018;45:56–66. 10.1007/s00259-017-3806-1 [DOI] [PubMed] [Google Scholar]
- 34.Spigel DR, Chaft JE, Gettinger S, et al. Fir: efficacy, safety, and biomarker analysis of a phase II open-label study of Atezolizumab in PD-L1-Selected patients with NSCLC. J Thorac Oncol 2018;13:1733–42. 10.1016/j.jtho.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humbert O, Cadour N, Paquet M, et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging 2020;47:1158–67. 10.1007/s00259-019-04573-4 [DOI] [PubMed] [Google Scholar]
- 36.Cho SY, Lipson EJ, Im H-J, et al. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J Nucl Med 2017;58:1421–8. 10.2967/jnumed.116.188839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anwar H, Sachpekidis C, Winkler J, et al. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 2018;45:376–83. 10.1007/s00259-017-3870-6 [DOI] [PubMed] [Google Scholar]
- 38.Unterrainer M, Ruzicka M, Fabritius MP, et al. Pet/Ct imaging for tumour response assessment to immunotherapy: current status and future directions. Eur Radiol Exp 2020;4:63. 10.1186/s41747-020-00190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-003635supp001.pdf (47.6KB, pdf)