Abstract
Background
Prostate cancer (PC) responds to androgen deprivation therapy (ADT) usually in a transient fashion, progressing from hormone-sensitive PC (HSPC) to castration-resistant PC (CRPC). We investigated a mouse model of PC as well as specimens from PC patients to unravel an unsuspected contribution of thymus-derived T lymphocytes and the intestinal microbiota in the efficacy of ADT.
Methods
Preclinical experiments were performed in PC-bearing mice, immunocompetent or immunodeficient. In parallel, we prospectively included 65 HSPC and CRPC patients (Oncobiotic trial) to analyze their feces and blood specimens.
Results
In PC-bearing mice, ADT increased thymic cellularity and output. PC implanted in T lymphocyte-depleted or athymic mice responded less efficiently to ADT than in immunocompetent mice. Moreover, depletion of the intestinal microbiota by oral antibiotics reduced the efficacy of ADT. PC reduced the relative abundance of Akkermansia muciniphila in the gut, and this effect was reversed by ADT. Moreover, cohousing of PC-bearing mice with tumor-free mice or oral gavage with Akkermansia improved the efficacy of ADT. This appears to be applicable to PC patients because long-term ADT resulted in an increase of thymic output, as demonstrated by an increase in circulating recent thymic emigrant cells (sjTRECs). Moreover, as compared with HSPC controls, CRPC patients demonstrated a shift in their intestinal microbiota that significantly correlated with sjTRECs. While feces from healthy volunteers restored ADT efficacy, feces from PC patients failed to do so.
Conclusions
These findings suggest the potential clinical utility of reversing intestinal dysbiosis and repairing acquired immune defects in PC patients.
Keywords: prostatic neoplasms, immunomodulation, adaptive immunity, translational medical research
Introduction
Prostate cancer (PC) is the most prevalent malignant disease in men, affecting the vast majority of octogenarians.1 Driven by a heterogeneous set of oncogenic drivers, PC usually develops in a multistep process from benign hypertrophy through adenoma to adenocarcinoma that first invades local tissues and then disseminates to distant sites.2 High-risk localized PC is initially treated by radical prostatectomy and/or local radiotherapy, often together with adjuvant androgen deprivation therapy (ADT) by surgical castration (bilateral orchiectomy), chemical castration (subcutaneously injected gonadotropin-releasing hormone receptor agonists) or antiandrogens (orally administered drugs such as abiraterone or enzalutamide that inhibit androgen synthesis or the nuclear translocation of the androgen receptor, respectively).3 ADT is also the therapy of choice for metastatic hormone-sensitive PC (HSPC). However, after a latency HSPC usually progresses to castration-resistant PC (CRPC), then requiring more toxic treatments including chemotherapy before the final switch to palliative care.4 Importantly, the prognosis of PC is not only dictated by cancer cell-autonomous alterations. Thus, infiltration of PC by CD8+ cytotoxic T lymphocytes indicates good prognosis, while infiltration by immunosuppressive CD4+ regulatory T cells (Tregs) has a negative prognostic impact.5–9
Although the efficacy of targeted therapies (including ADT) and chemotherapies has been usually considered to be mediated by cancer cell-autonomous effects,10 11 recent work suggests that highly successful molecules usually also affect the cancer-immune dialog, favoring antitumor immune responses.12 13 This has been well documented for widely used chemotherapeutic agents including single-agent anthracyclines,14 15 dactinomycin,16 oxaliplatin,17 taxanes,18 as well as specific combination therapies.19 20 Such agents induce immunogenic cell death, rendering malignant cells recognizable to the immune system.21 Moreover, multiple targeted agents including tyrosine or serine/threonine kinase inhibitors have been found to enhance immunosurveillance by a plethora of different mechanisms that help explain their long-term effects beyond treatment discontinuation.13 22 23 This applies as well to estrogen receptor antagonists, which are used for the treatment of hormone receptor-positive breast cancer.24 Both in preclinical models and in PC patients, ADT causes an increase of the immune infiltrate of the malignant tumor and the adjacent normal prostate25–29 suggesting that ADT triggers at least a transient immune response, likely against both normal and cancer-specific antigens.30 Of note, testosterone is one of the best known endogenous accelerators of thymic atrophy,31 32 suggesting that ADT might reverse thymic aging, which is one of the drivers of T cell aging and the senescence-associated decline in anticancer immunosurveillance.33 Hence, it is conceivable that ADT has local as well as systemic effects on antitumor immune responses.
It would be naïve to assume that the immune-cancer dialog would not be influenced by external factors. Indeed, recent work by multiple groups has revealed the cardinal importance of the gut microbiota in determining the general immune tonus,34–36 as well as cross-reactive cancer antigen-specific immune responses.37 38 Circumstantial evidence indicates that ADT modifies the gut microbiota,39–41 providing yet another possible mechanism through which ADT might affect PC immunosurveillance.
Driven by the aforementioned considerations, we decided to investigate the effects of ADT on thymic function and the microbiota while addressing the possibility that thymus-derived T cells as well as specific bacterial species might influence the efficacy of ADT against PC. Our preclinical data (in mice) as well as our clinical-translational results (in PC patients) suggest a complex relationship between ADT, gut microbiota, PC and thymic function that has a major impact on the therapeutic outcome of ADT.
Material and methods
This section will be described in online supplemental file.
jitc-2021-004191supp001.pdf (99.3KB, pdf)
Results
T cell-dependent efficacy of ADT in mice with PC
The murine Myc-CaP cell line has an amplified androgen receptor gene and requires testosterone for optimal proliferation.42 Myc-CaP PCs implanted in syngeneic FVB/N mice respond to androgen depletion therapy (ADT resulting from subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix acetate)43 by growth arrest and partial shrinkage. This effect usually lasts 15 to 25 days until cancer growth resumes (figure 1A, B), indicating progression from HSPC to CRPC. Of note, depletion of T lymphocytes by intravenous injection of antibodies depleting CD4+and CD8+ cells attenuated tumor growth control by ADT (figure 1C) and accelerated time to progression (TTP) from HSPC to CRPC (figure 1D). A similar effect was observed when PCs were implanted into mice homozygous for the nude spontaneous mutation (Foxn1 nu) that are athymic and hence lack thymus-dependent T lymphocytes. As compared with PCs evolving in immunocompetent mice, PCs established in nu/nu mice responded less efficiently to ADT (figure 1E) and progressed more rapidly (figure 1F). Of note, intravenous transfer of thymocytes from FVB/N mice into nu/nu mice blunted the natural progression of PCs (figure 1G, H). However, similar to what has been found in PC patients 44 45 immune checkpoint blockade using antibodies targeting CTLA-4 and PD-1 failed to achieve a major improvement of ADT efficacy (online supplemental file 2).
Figure 1.
T cell-dependent efficacy of ADT in mice with prostate cancer (PC). (A) Experimental setup to investigate the effect of ADT in Myc-CaP prostate cancer model. (B) Tumor growth curves and representation of TTP defining hormone sensitive (HSPC) and castration resistance (CRPC) intervals. (C) Tumor growth kinetics of Myc-CaP following depletion of T cells using αCD4 and αCD8 monoclonal antibodies, 3 days before systemic therapy (ADT or sham control) and twice a week until sacrifice. (D) Kaplan-Meier curves illustrating TTP. (E, F) Tumor growth kinetics in Myc-CaP bearing nu/nu mice, treated with ADT or sham control (E) and TTP Kaplan-Meier curves (F). (G) experimental setup of thymocytes or vehicle transfer from FVB/N healthy mice to nu/nu by i.v tail injection before Myc-CaP inoculation the next day. (H) Tumor growth kinetics in the nu/nu groups compared with immunocompetent FVB/N mice. ADT, androgen deprivation therapy; TTP, time to progression.
jitc-2021-004191supp002.pdf (51KB, pdf)
Opposing thymic effects of PC and ADT in mice
Intrigued by the importance of thymus-derived T cells in PC immunosurveillance (see above), we investigated the impact of PC and ADT on the thymus. Histological analysis (figure 2A), followed by morphometric calculations (figure 2B–D) revealed that, as compared with tumor-free mice, mice bearing PCs and receiving sham treatment displayed a reduction of thymic area, in particular the medulla. ADT increased the thymus area, in particular the cortex, thus reversing the effect of PC on the cortical area (figure 2B–D). Immunophenotyping of thymocyte suspensions using immunofluorescence and cytometry unveiled a PC-induced increase in double-negative type-3 (DN3) cells with a CD4-CD8-CD44-CD25+ phenotype that was only partially reversed by ADT. PC also caused a relative increase of double-positive (DP) cells (phenotype: CD4+CD8+). The relative abundance of single-positive CD4+CD8- or CD4-CD8+ cells, as well as the percentage of TCRβ+ cells were reduced by PC irrespective of ADT (figure 2E). In the blood, ADT stimulated an increase in the percentage of DN T cells (phenotype: CD4-CD8-CD3+), reversed the increase of the CD4+/CD8+ T cell ratio and tended to attenuate the recirculation of DP cells (figure 2F). In contrast, the PC-induced reduction of TCRβ+ cells among CD3+ lymphocytes was not corrected by ADT or even worsened it (figure 2F).
Figure 2.
Thymic effect of prostate cancer (PC) and ADT in mice. (A) Micrograph pictures of thymi in naïve and PC tumor- bearing mice treated with ADT. From the left to the right: hematoxylin eosin-stained thymi sections from healthy controls (CO), sham-treated PC bearing mice (PC sham), and ADT -treated PC bearing mice (PC +ADT), sacrificed at D10 post-treatment (scale bars are indicated in the graphs). (B–D). Surface assessment of overall, cortex and medulla areas in the three groups described in (A). Each dot represents one thymus. (E) Flow cytometry determination of thymocyte phenotypes at D10 post -treatment. from left to right: proportion of DN3 thymocytes (CD44-CD25+ in dn), DP thymocytes (CD3+CD4+CD8+), sp CD4+ and CD8+, and TCRβ+in live cells (F). Flow cytometry determination of circulating lymphocytes at D10 post-treatment. From left to right: proportion of dn in CD3+ cells, DP thymocytes (CD3+CD4+CD8+), sp CD4+ and CD8+, and TCRβ+ in CD3+ cells. Means±SEM are depicted for 4–12 mice/group. A representative experiment is depicted for all graphs except (F) where a pool of two experiments is shown. ANOVA statistical analyses (Kruskal-Wallis test) were used for multiple comparisons. ADT, androgen deprivation therapy; ANOVA, analysis of variance.
Altogether, these results indicate subtle thymotropic effects of ADT that may favor the thymic efflux of DN T cells and reverse the PC-induced increase in the peripheral CD4+/CD8+ T cell ratio.
Relationship between ADT and gut microbiota in mice
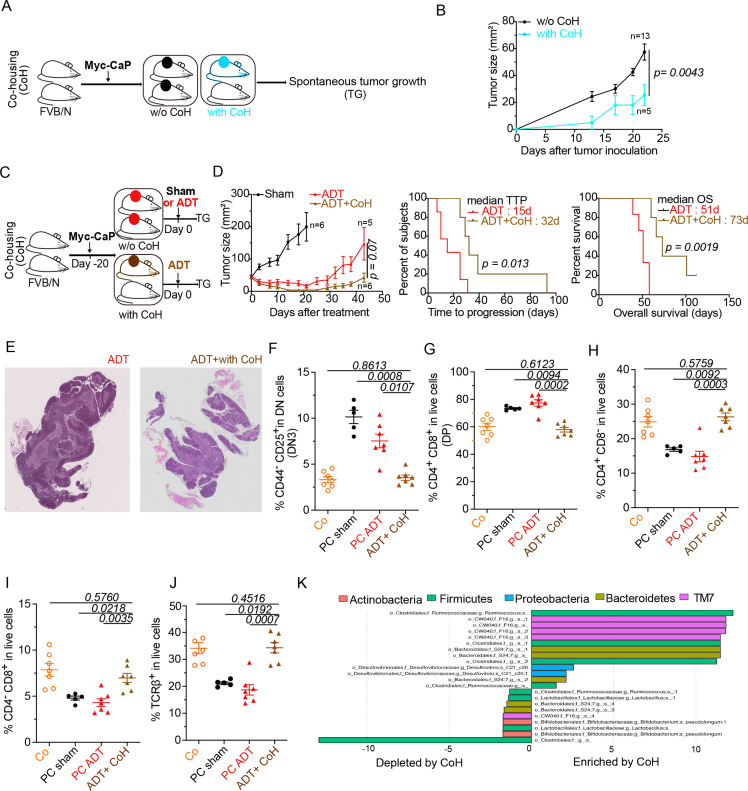
In the next step, we investigated the relationship between ADT and the intestinal microbiota. Oral supplementation of three broad-spectrum antibiotics (ampicillin, colistin, streptomycin), a combination that eliminates the majority of gut commensals46 (figure 3A), reduced the anticancer effects of ADT in two different laboratories located in Villejuif, France (figure 3B) and Wisconsin, USA (figure 3C). Hence, independently from its exact composition (which depends on the animal facility), the microflora can support the efficacy of ADT. Of note, comparative 16S ribosomal RNA sequencing of the fecal microbiota from tumor-free and PC-bearing mice revealed a reduction of alpha diversity of the microbiota determined by two different algorithms (figure 3D) with a tangible effect on the overall microbial composition detectable by principal component analyses (figure 3E). Linear discriminant analysis effect size revealed the PC-associated depletion of Verrucomicrobiaceae family members including the Akkermansia muciniphila species (figure 3F), Ruminococcaceae and Rikenellaceae (represented by Alistipes spp) known to be associated with response to PD1 blockade.47 48 The comparison of the microbiota of PC-bearing mice receiving ADT or sham treatments indicated that ADT restored richness that is, alpha diversity (figure 3G) and increased the relative abundance of A. muciniphila (figure 3H), echoing prior reports that oral antiandrogens, including bicalutamide, enzalutamide and abiraterone acetate increase the abundance of A. muciniphila in PC patients.39 40 Several Lachnospiraceae family members were also depleted by PC, but restored by ADT (figure 3F, H).
Figure 3.
Relationship between ADT and gut microbiota in mice. (A) Experimental setup of Myc-CaP tumor-bearing mice treated with ADT or sham control in the presence or absence of broad-spectrum antibiotics (ATB). ATB were delivered 3 days before ADT and then, 1 week on/1 week off. (B, C) Tumor growth kinetics and TTP in the French animal facility (B) duplicated in a second independent US animal facility (C). (D) Richness of the microbiota intestinal ecosystem estimated by two different methods monitoring the alpha diversity of stools in a longitudinal and paired mouse follow-up, in samples collected before and after tumor inoculation. (E) Beta-Diversity ordination plot based on principal coordinate analysis of normalized and standardized fecal microbiota composition in paired animals before (red dots) and after (black dots) Myc-CaP inoculation. Bray-Curtis distance and weighted UniFrac distance were used as beta diversity metrics and visualized through NMDS method. (F) Bar plots of fecal species that discriminate taxonomic composition between pretumor and post-tumor inoculation in mice by DESeq2 method. (G) Idem as in (D) before and 7 days after ADT. (H) Idem as in (F) pre-ADT and post-ADT in mice. Results were confirmed in two independent experiments; one representative experiment being shown. Tumor growth curves are depicted by means±SEM of tumor sizes over time and P values were calculated using two-way ANOVA for paired repeated measures. Kaplan-Meier curves were used for TTP. The Mann-Whitney U test and the Wilcoxon signed-rank test were used to determine significant differences among the different groups according to alpha-diversity. ADT, androgen deprivation therapy; ANOVA, analysis of variance; CSS, cumulative sum scaling; NMDS, non-metric multidimensional scaling; TTP, time to progression.
Collectively, these results indicate that both PC and ADT have an impact on the microbiota, which in turn influences the anticancer effects of ADT.
Thymic and microbial effect of ADT in PC patients
Next, we analyzed a cohort of PC patients (online supplemental figure S3 and table S1) with respect to thymus-relevant and microbial parameters. Comparisons of HSPC patients (before or after ADT) with individuals that have developed CRPC (and hence have been subjected to long-term ADT until therapeutic failure) revealed that CRPC was coupled to a reduction in circulating lymphocytes (figure 4A) but an increase in naïve CD4+ T cells (phenotype: CD45RA+CD127+CCR7+) (figure 4B) as well as signal-joint T cell receptor excision cycles (sjTREC) indicating enhanced thymic output under ADT (figure 4C). Metagenomic shotgun sequencing of the fecal microbiota detected significant differences in the intestinal ecosystems between HSPC and CRPC patients with an expansion of some anticancer immune response-associated species including Alistipes,49 Roseburia faecis (50) and Ruminococcus51 under ADT (figure 4D, E) that were lost in the PC mouse model. The overall variance of the microbiota was best explained by the patient status (HSPC vs CRPC) but also correlated with several immune parameters (in particular the frequency of naïve CD4+ T cells, sjTRECs, but also CD4+ T cells with a central memory (TCM) phenotype: CD45RA-CCR7+CD27+) and clinical parameters (patient age and ADT duration) (figure 4F). At the individual species level, the abundance of the health-associated Roseburia faecis was negatively associated with the frequency of circulating naïve CD4+ T cells (figure 4G).
Figure 4.
Thymic and microbial effect of ADT in prostate cancer patients. (A) Routine blood monitoring of lymphocyte counts. Absolute blood lymphocyte counts in patients with HSPC at baseline (pre-ADT) and 4–6 months after ADT (post-ADT) as well as in patients with CRPC. (B, C) Flow cytometric determination of blood cell populations. Naïve CD4 +cell proportion (B) and circulating sj TREC cells (C) in patients with (HSPC) pre-ADT and 4–6 months post-ADT and in CRPC patients compared with age-matched and sex-matched healthy controls. (A–C). Each dot represents one patient. The graph depicts means±SEM of lymphocyte counts. ANOVA statistical analyses (Kruskal-Wallis test) were used for multiple comparison. (D–F) Patient fecal microbiota composition and flow cytometry-based blood analysis. Principal coordinate analysis (PcoA) using Bray-Curtis distances calculated using species level relative abundances in CRPC (dark gray dots) and HSPC patients (red dots) (D) prevalence and relative abundances of differentially abundant species between CRPC and HSPC patients (E). Associations between the overall microbial community composition and flow cytometry-based blood analyses (F). Spearman’s correlations between immune cell profiles and species’ abundances controlling for time-point, age and patient (G). ADT, androgen deprivation therapy; ANOVA, analysis of variance; CRPC, castration-resistant prostate cancer; HSPC, hormone-sensitive PC.
jitc-2021-004191supp004.pdf (46.5KB, pdf)
jitc-2021-004191supp005.pdf (38.9KB, pdf)
Altogether, these results validate the preclinical results at the patient level. PC and ADT cause interlinked alterations in the microbiota and the immune system, and ADT induces the export of thymocytes resulting in an increase in naïve CD4+ T cells in peripheral blood.
Microbial improvement of ADT effects in mice
In the final step of this study, we investigated whether the microbial shifts induced by PC and ADT favor the progression from HSPC to CRPC. In an initial round of experiments, all mice in the same cage were inoculated with PC cells or, alternatively, only half of the mice received PC cells, meaning that these PC-bearing mice were cohoused with tumor-free mice (figure 5A). Cohousing reduced initial PC growth (figure 5B), suggesting that environmental factors including the microbiota (which is transferred between mice due to their coprophagic behavior)52 have a major impact on PC progression. To evaluate the influence of cohousing on the efficacy of ADT, we subjected mice with established PC to cohousing with cancer-free mice and later ADT (figure 5C). In this context, cohousing improved tumor growth control, enhanced TTP and increased overall survival of PC-bearing mice (figure 5D). Moreover, cohousing plus ADT improved the histological appearance of thymic architecture, divided into a morphologically distinct cortex and medulla, with a clear separation at the corticomedullary junction (figure 5E) and fully corrected the PC-induced increase in DN3 and DP thymocytes (figure 5F, G), as well as the depletion of SP and TCRβ+ thymocytes (figure 5H–J). Finally, cohousing induced major microbial shifts, including a relative enrichment in Ruminococcus spp (figure 5K), which is reputed for its stimulatory effects on tumor immunosurveillance47 48 53 and has been found to be increased in PC patients under ADT.41 In sum, transfer of the microbiota from tumor-free to PC-bearing mice had a major positive impact on PC-induced perturbations of the thymus and ameliorated the therapeutic efficacy of ADT.
Figure 5.
Co-housing (CoH) improved ADT-mediated taxonomic composition of the intestinal ecosystem and anticancer effects. (A–C) Experimental setup of cohousing experiments where tumor-bearing mice were housed with naive littermates prior to (A) or after (C) treatment with ADT. (B–D) Spontaneous and ADT-mediated tumor growth kinetics and TTP with and without cohousing and ADT (D). (E) Idem as in figure 2A. Representative animal for each micrograph picture: left and right panel (without and with cohousing) at D10 post-ADT. Bar scale: 200 mm. (F–J) Flow cytometric determination of thymocytes. proportion of DN3 thymocytes (CD44-CD25+ in dn) (F) DP thymocytes (CD3+CD4+CD8+) (G) sp CD4+ thymocytes (H) sp CD8+ thymocytes (I) and TCRβ+in live cells (J). (K) Idem as in figure 3H where metagenomic species discriminating between ADT-treated cohoused and non-cohoused mice. Tumor growth curves are depicted showing means±SEM of tumor sizes over time and p values were calculated using two-way ANOVA for paired repeated measures. Kaplan Meier curves were used for TTP and OS. A typical experiment comprizing at least 5 males/group is depicted, out of 2–3 experiments conducted and yielding similar conclusions. ADT, androgen deprivation therapy; ANOVA, analysis of variance; OS, overall survival; TTP, time to progression.
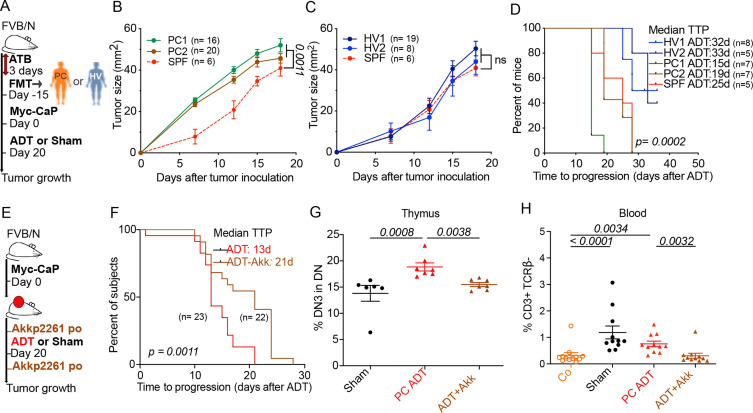
Of note, the transfer of feces from two PC-bearing patients (one with HSPC and one with CRPC, PC1 and PC2, respectively) accelerated the growth of PC in mice. In contrast, feces from two healthy volunteers (HV1, HV2) failed to affect PC growth as compared with normal mice housed in specific pathogen-free conditions (figure 6A–C) Fecal microbial transplantation of HV1 and HV2 (but not that of PC1 and PC2) also increased the efficacy of ADT (figure 6D).
Figure 6.
Improving ADT antitumor effects using FMT or Akkermansiap2261 (Akkp2261) (A) Experimental setup of FMT of stool samples from hormone sensitive patient (PC1) and CRPC patient (PC2) and 2 healthy volunteers (HV1 and HV2). (B) ADT-induced tumor growth after FMT from hormone sensitive patient (PC1) and CRPC patient (PC2) compared with mice without FMT under SPF conditions. (C) Idem as in (B) using FMT from healthy volunteers (HV1 and HV2). (D) TTP Kaplan-Meier curves post-ADT in different FMT arms (PC1, PC2, HV1, HV2, SPF). (E) Experimental setup using oral administration of Akkp2261 or PBS vehicle just before and next after ADT. (F) TTP Kaplan-Meier curves post-ADT with or without Akkp2261. (G–H) Flow cytometric determination of thymic (G) and blood lymphocytes (H) at day 10 post-ADT. Proportion of DN3 thymocytes (CD44-CD25+ in DN) and blood CD3+ TCRβ- δ- in three different groups: sham control, ADT without and with A. muciniphila. Results from one representative experiment out of two or a pool of two experiments are shown. At least five mice per experimental condition. Each dot represents one animal. P values were calculated using two-way ANOVA for paired repeated measures. Kaplan-Meier curves were used for TTP and OS. ADT, androgen deprivation therapy; ANOVA, analysis of variance; CRPC castration-resistant prostate cancer; FMT, fecal microbiota transplantation; OS, overall survival; SPF, specific pathogen-free; PBS, phosphate-buffered saline; TTP, time to progression.
Importantly, the gavage of mice with Akkermansia (Akkp2261) enhanced the efficacy of ADT against PC (figure 6E, F) and normalized the frequency of DN3 cells in the thymus (figure 6G) as well as the number of circulating immature T cells (phenotype: CD3+TCRβ-) (figure 6H). Also, changes have been noticed in thymus weight and cellularity under microbiota manipulation conditions (cohousing or oral administration of Akkermansia (online supplemental file 3).
jitc-2021-004191supp003.pdf (41KB, pdf)
Taken together, these results suggest that the microbiota from healthy mice or humans, as well as specific health-associated bacteria such as A. muciniphila, impact the immune system and ameliorate the efficacy of ADT in preclinical models.
Discussion
This study unravels the existence of a network of relationships between PC, ADT, thymus-dependent T lymphocytes and the intestinal microbiota. In several cases these relationships cannot be interpreted to represent a hierarchical order but instead are bilateral or even multilateral. For instance, the relationship between PC and the cellular immune system is non-hierarchical. On one hand, PC is under immunosurveillance, meaning that PC evolves and relapses following ADT more quickly in athymic nu/nu mice or after antibody-mediated depletion of T lymphocytes. However, in line with a previous study on PC,54 PC appears to induce thymic atrophy, and ADT reverses selective features of this PC-induced effect. Thus, PC induced a β-TCR deficiency probably related to a TCR rearrangement defect, consistent with the maturation block at the DN3 stage. Indeed, in mice with targeted mutations in the recombinase-activating gene or the TCRβ gene, DN thymocytes pass the CD44+CD25± stage (DN1 and DN2), and development is blocked at the CD44-CD25+ stage (DN3).55 Thus, our results suggest that PC compromises TCRβ chain rearrangement and consequently β-selection leading to a maturation blockage. Of note, ADT ultimately favored a surge of DN T cells in the blood from mice, as well as an increase in naïve CD4+ T cells in patients, that might reflect an increase in thymic output. In accord with this speculation, CRPC patients that had undergone prolonged ADT manifested an increase in recent thymic emigrants positive for sjTREC. Altogether, it appears that PC and its treatment by ADT affect the immune system in opposite manners and that thymus-dependent T cells are involved in the control of PC progression from HSPC to CRPC.
Yet another example of a non-hierarchical, bilateral relationship concerns the mutual influence between PC and the gut microflora. On one hand, the presence of PC apparently alters the microbiota (with the depletion of beneficial bacteria including A. muciniphila and Lachnospiraceae), and ADT reverses these effects, as found in our preclinical studies, echoing prior clinical reports that ADT enriches A. muciniphila in the gut from PC patients.39 40 On the other hand, normalization of the PC-driven microbial shifts can be achieved by three different methods: (1) cohousing with cancer-free mice, (2) fecal microbiota transplantation using the natural product of human volunteers (but not PC patients), and (3) oral administration of Akkermansia. These three manipulations reduce PC growth and/or improve the efficacy of ADT against PC, retarding the progression from HSPC to CRPC.56 We used oral administration of live Akkermansia to improve the outcome of ADT. It remains to be determined whether these effects involve adjuvant effects (perhaps mediated by the stimulation of Toll-like receptor two by the protein Amuc_1100),57 metabolic effects (that may be mediated by a bacterial protein resembling glucagon-like peptide-1 or alternatively by an increase in PPARα agonistic mono-palmitoyl-glycerol and immunostimulatory polyamines)58–60 or a yet-to-be-discovered cross-reactivity between bacterial and tumor antigens. Indeed, a recent study reports that intravenous injection of A. muciniphila-derived extracellular vesicles can induce CD8+ cytotoxic T cells responses against mouse PC established in immunocompetent C57BL/6 mice that slow down tumor growth in the absence of ADT.61 At this point, the mechanisms explaining how PC and its hormonal treatment affect the gut microflora or vice versa are mostly elusive. Recent data reported that the intestinal commensal microbiota in mice and patients with CRPC were enriched for species having the ability to convert precursors into active androgens.56
There is some evidence suggesting that the relationship between the gut microbiota and the immune system may be reciprocal as well. The immune system controls the homeostasis of the microbiota as well as the inflammatory status of the gut wall, while the microflora controls local and systemic immune responses62–64 including those involved in cancer immunosurveillance.34–38 Accordingly, when PC-bearing mice receiving ADT were cohoused with cancer-free mice, the shift to a more normal intestinal microflora was accompanied by an improvement of thymic architecture, as well as restoration of normal thymopoiesis. Moreover, in a heterogeneous cohort of PC patients comprizing both HSPC and CRPC, the variations in the gut microbiota correlated with the abundance of sjTREC, suggesting a functional link between the intestinal ecosystem and thymic output. The mechanistic underpinnings of such a hypothetical link remain to be elucidated.
It should be noted that our work diverges from that recently published by Pernigoni et al56 in several points. Indeed, the work by Pernigoni et al56 postulated that antibiotic-mediated elimination of the gut microbiota may improve the outcome of ADT against PC by eliminating bacteria that produce androgens, thus annihilating the effects of ADT. However, there are several important methodological differences between our study and that by Pernigoni et al.56 First, Pernigoni et al56 used an antibiotic association of neomycin, ampicillin, vancomycin and metronidazole. In our study, we used a different antibiotic cocktail including ampicillin, streptomycin, and colistin without vancomycin. We have accumulated data to show that vancomycin depletes immunosuppressive bacteria such as Clostridia spp65, suggesting that this point is indeed crucial. Second, Pernigoni et al56 performed surgical castration (which is rarely used in the treatment of human PC), while we used chemical castration with an LHRH antagonist (Degarelix). Third, Pernigoni et al56 used the murine TRAMP C1 model, syngeneic from C57BL/6 mice while we focused on the Myc-CaP model growing in FVB/N mice. Altogether, these multiple differences (at (1) the levels of antibiotic cocktails, (2) treatments to induced ADT and (3) preclinical models) preclude a direct comparison of our study with that by Pernigoni et al.56
In conclusion, within the uncertainties of experimental and translational oncology, our work reveals that both the immune system and the intestinal microflora determine the efficacy of ADT against PC. Although immunotherapy with immune checkpoint inhibitors targeting CTLA-4 and PD-1 fails to improve ADT, the restoration of a healthy gut microbiota may constitute a valid strategy for prolonging tumor control by ADT.
Acknowledgments
We thank Dr. Charles G. Drake (Department of Hematology/Oncology, Columbia University Medical Center, New York, USA) for providing Myc-CaP cell lines and are grateful for his advices. We thank all members of Gustave Roussy Animal facility.
Footnotes
Twitter: @andrew_m_thomas, @ClaveEmmanuel
KF, GK and LZ contributed equally.
Contributors: LZ, GK, ST: designed, performed, involved in experiment analysis, writing the manuscript, access to the data and controlled the decision to publish. KF: designed, included patients, provided patient clinical data. AGG: involved in the design and in the analysis of mice and human experiments. KU: involved in the performance and analysis of mice experiments. LD: involved in the design and mice experiments. AMT, NS, FA, FP, FA: performed the metagenomics and bio-informatic analysis of human specimens. VQ, HP: performed the bio-informatic analysis of murine experiments. CR, BR: involved in bio-informatic and stastical analysis. GD, AS, FG: Flow cytometry in human specimens. CT, GF, PL, MGH, DGM, CR, CACS, JEF, MM: involved in mice experiments. MF: involved in statisctics and figures design. RD, EP: involved in bacterial manipulations and culture. EC and AT: performed human sj trecs experiment. SY, PO: Pathological analysis. SC, PB: Patients and clinical data.
Funding: ST was supported by a Poste d’ accueil INSERM fellowship (Plan Cancer). LZ laboratory was supported by the Germano-French ANR Ileobiome - 19-CE15-0029-01 and H2020 ONCOBIOME No 825410, RHU Torino Lumière ANR-16-RHUS-0008; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE). GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence Nationale de la Recherche (ANR)—Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association 'Ruban Rose'; Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Program on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; the and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. AMT and EC are supported by the French Government’s Investissement d’Avenir Program, Laboratoire d’Excellence “Milieu Intérieur” Grant ANR-10-LABX-69-01. INSERM U.1160 is a member of OPALE Carnot Institute, The Organization for Partnerships in Leukemia. MG-H and DGM are supported by the grant funding NIH/NCI P01 CA250927.
Competing interests: LZ and GK are scientific cofounders of everImmune, a company that develops bacteria for the treatment of cancer. GK is a scientific cofounder of Samsara Therapeutics and Therafast Bio. Acknowledgments: LZ laboratory was supported by the Germano-French ANR Ileobiome—19-CE15-0029-01 and H2020 ONCOBIOME N°825410, RHU Torino Lumière ANR-16-RHUS-0008; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE). GK is supported by Agence Nationale de la Recherche (ANR)—Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association 'Ruban Rose'; Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; the and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001. AMT and EC are supported by the French Government’s Investissement d’Avenir Program, Laboratoire d’Excellence 'Milieu Intérieur' Grant ANR-10-LABX-69-01. INSERM U.1160 is a member of OPALE Carnot Institute, The Organization for Partnerships in Leukemia. MG-H and DGM are supported by the grant funding NIH/NCI P01 CA250927.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The metagenomes have been deposited in NCBI under the bioproject PRJNA791751. Once public, they will be accessible using this link: https://www.ncbi.nlm.nih.gov/bioproject/791751.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by Gustave Roussy Cancer Campus, France in 'Oncobiotics' clinical study, B2M ethics protocol number PP: 15-013. Written informed consent in accordance with the Declaration of Helsinki was obtained from all patients. Participants gave informed consent to participate in the study before taking part.
References
- 1.Beyer K, Moris L, Lardas M, et al. Diagnostic and prognostic factors in patients with prostate cancer: a systematic review protocol. BMJ Open 2021;11:e040531. 10.1136/bmjopen-2020-040531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong D. Unravelling the molecular mechanisms of prostate cancer evolution from genotype to phenotype. Crit Rev Oncol Hematol 2021;163:103370. 10.1016/j.critrevonc.2021.103370 [DOI] [PubMed] [Google Scholar]
- 3.Lokeshwar SD, Klaassen Z, Saad F. Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol 2021;18:433–42. 10.1038/s41585-021-00470-4 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt KT, Huitema ADR, Chau CH, et al. Resistance to second-generation androgen receptor antagonists in prostate cancer. Nat Rev Urol 2021;18:209–26. 10.1038/s41585-021-00438-4 [DOI] [PubMed] [Google Scholar]
- 5.Flammiger A, Bayer F, Cirugeda-Kühnert A, et al. Intratumoral T but not B lymphocytes are related to clinical outcome in prostate cancer. APMIS 2012;120:901–8. 10.1111/j.1600-0463.2012.02924.x [DOI] [PubMed] [Google Scholar]
- 6.Davidsson S, Ohlson A-L, Andersson S-O, et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod Pathol 2013;26:448–55. 10.1038/modpathol.2012.164 [DOI] [PubMed] [Google Scholar]
- 7.Ness N, Andersen S, Valkov A, et al. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 2014;74:1452–61. 10.1002/pros.22862 [DOI] [PubMed] [Google Scholar]
- 8.Nardone V, Botta C, Caraglia M, et al. Tumor infiltrating T lymphocytes expressing FOXP3, CCR7 or PD-1 predict the outcome of prostate cancer patients subjected to salvage radiotherapy after biochemical relapse. Cancer Biol Ther 2016;17:1213–20. 10.1080/15384047.2016.1235666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Attwood K, Bshara W, et al. High intratumoral CD8+ T-cell infiltration is associated with improved survival in prostate cancer patients undergoing radical prostatectomy. Prostate 2021;81:20–8. 10.1002/pros.24068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70. 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 12.Galluzzi L, Humeau J, Buqué A, et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020;17:725–41. 10.1038/s41571-020-0413-z [DOI] [PubMed] [Google Scholar]
- 13.Petroni G, Buqué A, Zitvogel L, et al. Immunomodulation by targeted anticancer agents. Cancer Cell 2021;39:310–45. 10.1016/j.ccell.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 14.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005;202:1691–701. 10.1084/jem.20050915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007;14:1848–50. 10.1038/sj.cdd.4402201 [DOI] [PubMed] [Google Scholar]
- 16.Humeau J, Sauvat A, Cerrato G, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med 2020;12:e11622. 10.15252/emmm.201911622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Naour J, Liu P, Zhao L, et al. A TLR3 ligand Reestablishes chemotherapeutic responses in the context of FPR1 deficiency. Cancer Discov 2021;11:408–23. 10.1158/2159-8290.CD-20-0465 [DOI] [PubMed] [Google Scholar]
- 18.Senovilla L, Galluzzi L, Marino G, et al. Immunosurveillance against cancer-associated hyperploidy. Oncotarget 2012;3:1270–1. 10.18632/oncotarget.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezu L, Gomes-de-Silva LC, Dewitte H, et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol 2015;6:187. 10.3389/fimmu.2015.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfirschke C, Engblom C, Rickelt S, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 2016;44:343–54. 10.1016/j.immuni.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72. 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 22.Zitvogel L, Rusakiewicz S, Routy B, et al. Immunological off-target effects of imatinib. Nat Rev Clin Oncol 2016;13:431–46. 10.1038/nrclinonc.2016.41 [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Zhao L, Pol J, et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat Commun 2019;10:1486. 10.1038/s41467-019-09415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buqué A, Bloy N, Perez-Lanzón M, et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun 2020;11:3819. 10.1038/s41467-020-17644-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake CG, Doody ADH, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 2005;7:239–49. 10.1016/j.ccr.2005.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page ST, Plymate SR, Bremner WJ, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab 2006;290:E856–63. 10.1152/ajpendo.00484.2005 [DOI] [PubMed] [Google Scholar]
- 27.Mercader M, Bodner BK, Moser MT, Kwon PS, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 2001;98:14565–70. 10.1073/pnas.251140998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gamat M, McNeel DG. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr Relat Cancer 2017;24:T297–310. 10.1530/ERC-17-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorrentino C, Musiani P, Pompa P, et al. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res 2011;17:1571–81. 10.1158/1078-0432.CCR-10-2804 [DOI] [PubMed] [Google Scholar]
- 30.Zitvogel L, Perreault C, Finn OJ, et al. Beneficial autoimmunity improves cancer prognosis. Nat Rev Clin Oncol 2021;18:591–602. 10.1038/s41571-021-00508-x [DOI] [PubMed] [Google Scholar]
- 31.Pearce P, Khalid BA, Funder JW. Androgens and the thymus. Endocrinology 1981;109:1073–7. 10.1210/endo-109-4-1073 [DOI] [PubMed] [Google Scholar]
- 32.Aspinall R, Andrew D. Gender-Related differences in the rates of age associated thymic atrophy. Dev Immunol 2001;8:95–106. 10.1155/2001/17064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol 2021;22:687–98. 10.1038/s41590-021-00927-z [DOI] [PubMed] [Google Scholar]
- 34.Zitvogel L, Ma Y, Raoult D, et al. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 2018;359:1366–70. 10.1126/science.aar6918 [DOI] [PubMed] [Google Scholar]
- 35.Finlay BB, Goldszmid R, Honda K, et al. Can we harness the microbiota to enhance the efficacy of cancer immunotherapy? Nat Rev Immunol 2020;20:522–8. 10.1038/s41577-020-0374-6 [DOI] [PubMed] [Google Scholar]
- 36.Baruch EN, Wang J, Wargo JA. Gut microbiota and antitumor immunity: potential mechanisms for clinical effect. Cancer Immunol Res 2021;9:365–70. 10.1158/2326-6066.CIR-20-0877 [DOI] [PubMed] [Google Scholar]
- 37.Fluckiger A, Daillère R, Sassi M, et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 2020;369:936–42. 10.1126/science.aax0701 [DOI] [PubMed] [Google Scholar]
- 38.Sepich-Poore GD, Zitvogel L, Straussman R, et al. The microbiome and human cancer. Science 2021;371. 10.1126/science.abc4552. [Epub ahead of print: 26 03 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sfanos KS, Markowski MC, Peiffer LB, et al. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis 2018;21:539–48. 10.1038/s41391-018-0061-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daisley BA, Chanyi RM, Abdur-Rashid K, et al. Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat Commun 2020;11:4822. 10.1038/s41467-020-18649-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang LL, Wong CYP. A cross-sectional study on gut microbiota in prostate cancer patients with prostatectomy or androgen deprivation therapy. Prostate Cancer Prostatic Dis 2021. 10.1038/s41391-021-00360-1 [DOI] [PubMed] [Google Scholar]
- 42.Watson PA, Ellwood-Yen K, King JC, et al. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res 2005;65:11565–71. 10.1158/0008-5472.CAN-05-3441 [DOI] [PubMed] [Google Scholar]
- 43.Marangoni E, Vincent-Salomon A, Auger N, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 2007;13:3989–98. 10.1158/1078-0432.CCR-07-0078 [DOI] [PubMed] [Google Scholar]
- 44.Venkatachalam S, McFarland TR, Agarwal N, et al. Immune checkpoint inhibitors in prostate cancer. Cancers 2021;13. 10.3390/cancers13092187. [Epub ahead of print: 02 05 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma P, Pachynski RK, Narayan V, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell 2020;38:489–99. 10.1016/j.ccell.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 46.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Routy B, Gopalakrishnan V, Daillère R, et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol 2018;15:382–96. 10.1038/s41571-018-0006-2 [DOI] [PubMed] [Google Scholar]
- 48.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. 10.1126/science.1240527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamanai-Shacoori Z, Smida I, Bousarghin L, et al. Roseburia spp.: a marker of health? Future Microbiol 2017;12:157–70. 10.2217/fmb-2016-0130 [DOI] [PubMed] [Google Scholar]
- 51.Daillère R, Vétizou M, Waldschmitt N, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016;45:931–43. 10.1016/j.immuni.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 52.Shabat Y, Lichtenstein Y, Ilan Y. Short-Term Cohousing of sick with healthy or treated mice alleviates the inflammatory response and liver damage. Inflammation 2021;44:518–25. 10.1007/s10753-020-01348-0 [DOI] [PubMed] [Google Scholar]
- 53.Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504–14. 10.1038/s41591-020-01224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borodin YI, Lomshakov AA, Astashov VV, et al. Thymus in experimental carcinogenesis of the prostate gland. Bull Exp Biol Med 2014;157:724–7. 10.1007/s10517-014-2652-4 [DOI] [PubMed] [Google Scholar]
- 55.Mombaerts P, Clarke AR, Rudnicki MA, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature 1992;360:225–31. 10.1038/360225a0 [DOI] [PubMed] [Google Scholar]
- 56.Pernigoni N, Zagato E, Calcinotto A, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 2021;374:216–24. 10.1126/science.abf8403 [DOI] [PubMed] [Google Scholar]
- 57.Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 2017;23:107–13. 10.1038/nm.4236 [DOI] [PubMed] [Google Scholar]
- 58.Depommier C, Vitale RM, Iannotti FA, et al. Beneficial effects of Akkermansia muciniphila are not associated with major changes in the circulating Endocannabinoidome but linked to higher Mono-Palmitoyl-Glycerol levels as new PPARα agonists. Cells 2021;10:185. 10.3390/cells10010185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grajeda-Iglesias C, Durand S, Daillère R, et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging 2021;13:6375–405. 10.18632/aging.202739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoon HS, Cho CH, Yun MS, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol 2021;6:563–73. 10.1038/s41564-021-00880-5 [DOI] [PubMed] [Google Scholar]
- 61.Wu Q, Tian A-L, Li B, et al. IGF1 receptor inhibition amplifies the effects of cancer drugs by autophagy and immune-dependent mechanisms. J Immunother Cancer 2021;9:e002722. 10.1136/jitc-2021-002722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011;145:745–57. 10.1016/j.cell.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009;139:485–98. 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.López-Otín C, Kroemer G. Hallmarks of health. Cell 2021;184:33–63. 10.1016/j.cell.2020.11.034 [DOI] [PubMed] [Google Scholar]
- 65.Yonekura S, Terrisse S, Alves Costa Silva C, et al. Cancer induces a stress ileopathy depending on B-adrenergic receptors and promoting dysbiosis that contribute to carcinogenesis. Cancer Discov 2021. 10.1158/2159-8290.CD-21-0999. [Epub ahead of print: 20 Dec 2021]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-004191supp001.pdf (99.3KB, pdf)
jitc-2021-004191supp002.pdf (51KB, pdf)
jitc-2021-004191supp004.pdf (46.5KB, pdf)
jitc-2021-004191supp005.pdf (38.9KB, pdf)
jitc-2021-004191supp003.pdf (41KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. The metagenomes have been deposited in NCBI under the bioproject PRJNA791751. Once public, they will be accessible using this link: https://www.ncbi.nlm.nih.gov/bioproject/791751.