Abstract
Objective
To investigate whether antineutrophil cytoplasm antibody (ANCA)-negative and myeloperoxidase (MPO)-ANCA–positive granulomatosis with polyangiitis (GPA) differ from proteinase-3 (PR3)-ANCA–positive GPA.
Methods
Diagnostic characteristics and outcomes of newly diagnosed French Vasculitis Study Group Registry patients with ANCA-negative, MPO-ANCA–positive or PR3-ANCA–positive GPA satisfying American College of Rheumatology criteria and/or Chapel Hill Conference Consensus Nomenclature were compared.
Results
Among 727 GPA, 62 (8.5%) were ANCA-negative, 119 (16.4%) MPO-ANCA–positive and 546 (75.1%) PR3-ANCA–positive. ANCA-negative patients had significantly (p<0.05) more limited disease (17.7% vs 5.8%) and less kidney involvement (35.5% vs 58.9%) than those PR3-ANCA–positive or MPO-ANCA–positive, with comparable relapse-free (RFS) and overall survival (OS). MPO-ANCA–positive versus PR3-ANCA–positive and ANCA-negative patients were significantly more often female (52.9% vs 42.1%), older (59.8 vs 51.9 years), with more frequent kidney involvement (65.5% vs 55.2%) and less arthralgias (34.5% vs 55.1%), purpura (8.4% vs 17.1%) or eye involvement (18.5% vs 28.4%); RFS was similar but OS was lower before age adjustment. PR3-positive patients’ RFS was significantly lower than for ANCA-negative and MPO-positive groups combined, with OS higher before age adjustment. PR3-ANCA–positivity independently predicted relapse for all GPA forms combined but not when comparing only PR3-ANCA–positive versus MPO-ANCA–positive patients.
Conclusions
Based on this large cohort, ANCA-negative versus ANCA-positive patients more frequently had limited disease but similar RFS and OS. MPO-ANCA–positive patients had similar RFS but lower OS due to their older age. PR3-ANCA–positive GPA patients’ RFS was lower than those of the two other subsets combined but that difference did not persist when comparing only PR3 versus MPO-ANCA–positive patients.
Keywords: granulomatosis with polyangiitis, systemic vasculitis, autoimmune diseases, autoimmunity
Key messages.
What is already known about this subject?
Evidence suggests that antineutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) might be better classified as proteinase-3 (PR3)-positive or myeloperoxidase (MPO)-positive AAV than based on their phenotypic characteristics, but the data remain controversial for granulomatosis with polyangiitis (GPA).
What does this study add?
Patients with ANCA-negative GPA more frequently had limited disease than those ANCA–positive but relapse-free (RFS) and overall survival (OS) rates were similar.
RFS of MPO-ANCA–positive GPA patients was comparable to those of patients with PR3-ANCA–positive and ANCA-negative disease, but their OS rate was lower because of their older age.
PR3-ANCA–positive GPA patients’ RFS was lower than those of the two other subsets combined but that difference did not persist when comparing only PR3 versus MPO-ANCA–positive patients.
How might this impact on clinical practice or further developments?
These new findings reinforce that ANCA specificity must systematically be added (as a prefix) to the phenotype (GPA) but regardless of ANCA status, patients with GPA retain a high relapse rate.
Introduction
Antineutrophil cytoplasm antibody (ANCA)-associated vasculitides (AAVs) are necrotising vasculitides, predominantly affecting small vessels and associated with autoantibodies directed against neutrophil proteins: proteinase-3 (PR3) or myeloperoxidase (MPO). The Chapel Hill Conference Consensus (CHCC) defined granulomatosis with polyangiitis (GPA) according to clinical and histological features, including necrotising granulomatous inflammation, usually involving the upper and lower respiratory tract, and common necrotising glomerulonephritis and granulomatous and non-granulomatous extravascular inflammation.1 The European Medicines Agency algorithm was proposed for epidemiological studies to classify AAVs according to disease types: GPA, microscopic polyangiitis (MPA) or eosinophilic GPA.2 Those patients identified as having GPA according to the CHCC Nomenclature or that algorithm1 2 are more likely to relapse than those with MPA.3–7 Among Caucasian patients clinically diagnosed with GPA, 65%–75% are PR3-ANCA–positive, 20%–30% are MPO-ANCA–positive, with the remaining 5% having no detectable ANCA.6 However, Chinese and Japanese GPA patients predominantly express MPO-ANCA.8 9
Accumulating evidence suggests that ANCA specificity is a major determinant of AAV presentation and outcomes and that these clinical syndromes may be better classified as PR3-positive or MPO-positive AAVs.4–6 10–14 It has been recommended that ANCA specificity and the phenotype be used to categorise patients within the AAV spectrum.1 4 However, studies on only GPA patients yielded conflicting results, when evaluating whether ANCA-negative and MPO-ANCA–positive subsets differ from PR3-ANCA–positive patients.8 9 13 15–17 Based on a German cohort that included 177 GPA patients, those MPO-ANCA–positive more frequently had limited disease without severe organ involvement, especially less kidney involvement and subglottic stenosis; less frequently required cyclophosphamide or rituximab; and were less likely to relapse than PR3-ANCA–positive patients.13 In contrast, a pooled analysis of data from randomised controlled trials that included 321 GPA patients was unable to demonstrate major clinical differences between MPO-ANCA–positive and PR3-ANCA–positive patients.16 Furthermore, with mean follow-up at 18 months, the relapse risk was more closely associated with the AAV than ANCA specificity. Similar results were obtained in a retrospective analysis of a single-centre cohort of 150 AAVs, with 94 GPA, for whom relapse-free remission was better predicted by clinical GPA or MPA phenotypes than the ANCA subtype.17 The limited data available on ANCA-negative and MPO-ANCA–positive GPA, and discordant findings concerning the contributions of AAV and ANCA specificities to the initial phenotype and relapse risk for GPA patients prompted further investigations involving larger patient subsets.
The French Vasculitis Study Group (FVSG) Registry is a national database of AAV patients, whose clinical and laboratory information has been collected prospectively and longitudinally, thereby enabling description of clinical characteristics and long-term outcomes of a large cohort including GPA.18 The data from all GPA patients entered in this database were extracted and analysed to determine whether ANCA-negative and MPO-ANCA–positive GPA represent a clinically distinct subset—differing from proteinase-3 (PR3)-ANCA–positive GPA—with different outcomes within AAV, and whether ANCA status and specificity should be taken into account to predict relapse and adapt therapy.
Methods
Patients
This multicentre analysis was conducted on patients with newly diagnosed GPA entered prospectively into the FVSG Registry from 1983 to 2018. This database includes information from vasculitis patients referred to FVSG members, those who participated in our network’s trials19 and whose follow-up was pursued. Investigators from >60 French centres regularly contribute that information. For study enrolment, patients’ GPA diagnoses had to meet the 1990 American College of Rheumatology classification criteria and/or revised CHCC Nomenclature.1 20 All diagnoses of patients who fulfilled those criteria were reassessed independently by two of the authors (XP, MI) considering their entire clinical histories. All FVSG Registry patients gave their informed consent. Only patients entered in the Registry at diagnosis and followed for ≥6 months or who died within 6 months of entry were included. Patients with insufficient information were excluded.
Definitions
In accordance with EULAR recommendations, the following definitions were applied21: remission, the absence of signs of ‘new/worsening’ disease activity according to the Birmingham Vasculitis Activity Score (BVAS=0) version 322; and relapse, the reoccurrence or new appearance of disease activity attributable to active vasculitis. Limited GPA was defined according to the revised CHCC Nomenclature and referred particularly to disease confined to the upper or lower respiratory tract, or the eye.1 Severe GPA was defined as posing an immediate threat to either the patient’s life or vital organ function.23
Data collection and analysis
Demographic, clinical and biological information was collected on electronic case-report forms at diagnosis (baseline) and each follow-up visit. Information at diagnosis and outcomes were compared among ANCA-negative, MPO-ANCA–positive and PR3-ANCA–positive GPA patients.
Statistical analyses
Quantitative variables are reported as mean±SD or median (IQR) and qualitative variables as number (percentages). Continuous variables were compared with Student’s t-test or Mann-Whitney U test, and categorical variables with χ2 test or Fisher’s exact test, as appropriate. Kaplan-Meier estimations of relapse-free survival (RFS) and overall survival (OS) probabilities were compared using log-rank tests. RFS was calculated from GPA diagnosis to relapse, death or end of follow-up, whichever occurred first. OS was calculated from GPA diagnosis to death or the last follow-up visit. To identify independent predictors of RFS or OS, univariable and multivariable Cox regression analyses assessed potential associations with patient demographics, clinical, laboratory and therapeutic parameters. Results are expressed as HR and 95% CIs. A p value ≤0.05 defined significance. Statistical analyses were computed with R V.3.2.2 software (R Core Team, 2015, R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement statement
Patients and the public were not involved in the design, conduct, reporting or dissemination of this research.
Results
Overall description at diagnosis of FVSG Registry patients with GPA
The Registry database enrolled 795 patients with new-onset GPA.18 For the 727 with complete baseline clinical details, including ANCA-immunoassay results, median follow-up was 3.7 (1.8–6.6) years, 62 (8.5%) were ANCA-negative, 119 (16.4%) MPO-ANCA–positive and 546 (75.1%) PR3-ANCA–positive (table 1). Their RFS and OS probabilities (table 2) are shown in figure 1A, B according to ANCA specificity.
Table 1.
Main clinical characteristics and treatments at diagnosis of 727 French Vasculitis Study Group Registry GPA patients according to ANCA specificity
| Characteristic | Overall n=727 | P value (PR3-ANCA+ vs MPO-ANCA+ vs ANCA–) | No ANCA n=62 | P value (ANCA– vs PR3-ANCA+ and MPO-ANCA+) | MPO-ANCA+ n=119 | P value (MPO-ANCA+ vs PR3-ANCA+ and ANCA–) | PR3-ANCA+ n=546 | P value (PR3-ANCA+ vs MPO-ANCA+ and ANCA–) |
| Sex, female | 319 (43.9) | 0.082 | 28 (45.2) | 0.94 | 63 (52.9) | 0.04 | 228 (41.8) | 0.06 |
| Age at diagnosis, years, mean (SD) | 53 (16.3) | <0.001 | 52.5 (16.5) | 0.79 | 59.8 (17.2) | <0.001 | 51.6 (15.7) | <0.001 |
| Limited GPA | 50 (6.9) | 0.002 | 11 (17.7) | 0.001 | 8 (6.7) | 0.55 | 31 (5.7) | 0.04 |
| Severe GPA | 83 (11.4) | 0.08 | 2 (3.2) | 0.09 | 17 (14.3) | 0.71 | 64 (11.7) | 0.75 |
| General | 592 (81.4) | 0.83 | 51 (82.3) | 0.99 | 99 (83.2) | 0.70 | 442 (81) | 0.66 |
| Fever >38.5°C | 304 (41.8) | 0.05 | 23 (37.1) | 0.51 | 39 (32.8) | 0.04 | 242 (44.3) | 0.02 |
| Weight loss >3 kg within 3 months | 329 (45.3) | 0.16 | 21 (33.9) | 0.08 | 53 (44.5) | 0.94 | 255 (46.7) | 0.20 |
| Arthralgias | 376 (51.7) | <0.001 | 27 (43.5) | 0.23 | 41 (34.5) | <0.001 | 308 (56.4) | <0.001 |
| Myalgias | 197 (27.1) | 0.30 | 15 (24.2) | 0.70 | 39 (32.8) | 0.16 | 143 (26.2) | 0.39 |
| Ear, nose and throat | 582 (80.1) | 0.53 | 49 (79.0) | 0.96 | 91 (76.5) | 0.35 | 442 (81) | 0.35 |
| Sinusitis | 297 (40.9) | 0.44 | 28 (45.2) | 0.56 | 43 (36.1) | 0.30 | 226 (41.4) | 0.67 |
| Rhinitis | 390 (53.6) | 0.28 | 29 (46.8) | 0.32 | 59 (49.6) | 0.38 | 302 (55.3) | 0.14 |
| Nasal crusts | 276 (38) | 0.10 | 16 (25.8) | 0.05 | 44 (37) | 0.05 | 216 (39.6) | 0.15 |
| Epistaxis | 138(19) | 0.04 | 7 (11.3) | 0.15 | 16 (13.4) | 0.12 | 115 (21.1) | 0.02 |
| Saddle nose | 9 (1.2) | 0.06 | 0 (0) | 0.75 | 4 (3.4) | 0.07 | 5 (0.9) | 0.33 |
| Nasal obstruction | 183 (25.2) | 0.64 | 18 (29.0) | 0.56 | 27 (22.7) | 0.57 | 138 (25.3) | 0.99 |
| Otitis | 166 (22.8) | 0.32 | 11 (17.7) | 0.40 | 23 (19.3) | 0.38 | 132 (24.2) | 0.16 |
| Lung | 492 (67.7) | 0.35 | 37 (59.7) | 0.21 | 83 (69.7) | 0.67 | 372 (68.1) | 0.72 |
| Alveolar haemorrhage | 130 (17.9) | 0.21 | 7 (11.3) | 0.21 | 26 (21.8) | 0.27 | 97 (17.8) | 0.98 |
| Massive alveolar haemorrhage and/or Hb <90 g/L | 35 (4.8) | 0.16 | 1 (1.6) | 0.36 | 3 (2.5) | 0.30 | 31 (5.7) | 0.09 |
| Lung nodules | 303 (41.7) | 0.61 | 25 (40.3) | 0.93 | 45 (37.8) | 0.41 | 233 (42.7) | 0.39 |
| Lung infiltrate | 134 (18.4) | 0.44 | 6 (9.7) | 0.18 | 18 (15.1) | 0.29 | 62 (11.4) | 0.58 |
| Subglottic stenosis | 11 (1.5) | 0.07 | 3 (4.8) | 0.09 | 2 (1.7) | 1.00 | 6 (1.1) | 0.22 |
| Kidney | 414 (56.9) | <0.001 | 22 (35.5) | 0.001 | 78 (65.5) | <0.05 | 314 (57.5) | 0.66 |
| Serum creatinine median (µmol/L) | 93 (73–177) | <0.001 | 77 (63–100) | <0.001 | 113 (78–199) | <0.001 | 92 (74–174.5) | <0.001 |
| Serum creatinine >150 µmol/L | 162/563 (28.8) | 0.06 | 7/42 (16.7) | 0.10 | 34/94 (36.2) | 0.11 | 121/427 (28.3) | 0.77 |
| Rise in creatinine level >30% | 178 (24.5) | <0.05 | 9 (14.5) | 0.08 | 37 (31.1) | 0.09 | 132 (24.2) | 0.81 |
| Proteinuria | 256 (35.2) | 0.004 | 10 (16.1) | 0.002 | 45 (37.8) | 0.59 | 201 (36.8) | 0.14 |
| Haematuria | 280 (38.5) | <0.001 | 9 (14.5) | 0.001 | 54 (45.4) | 0.11 | 217 (39.7) | 0.27 |
| Need for dialysis | 41 (5.6) | 0.10 | 0 (0) | 0.09 | 9 (7.6) | 0.48 | 32 (5.9) | 0.79 |
| Mucocutaneous | 232 (31.9) | 0.14 | 17 (27.4) | 0.52 | 30 (25.2) | 0.11 | 185 (33.9) | 0.06 |
| Purpura | 114 (15.7) | 0.01 | 6 (9.7) | 0.24 | 10 (8.4) | 0.02 | 98 (17.9) | 0.005 |
| Livedo reticularis | 26 (3.6) | 0.49 | 1 (1.6) | 0.61 | 6 (5.0) | 0.50 | 19 (3.5) | 0.99 |
| Gangrene | 16 (2.2) | 0.33 | 3 (4.8) | 0.30 | 2 (1.7) | 0.94 | 11 (2.0) | 0.76 |
| Eye | 195 (26.8) | 0.07 | 19 (30.6) | 0.58 | 22 (18.5) | 0.03 | 154 (28.2) | 0.17 |
| Exophthalmos | 24 (3.3) | 0.83 | 2 (3.2) | 1.00 | 5 (4.2) | 0.75 | 17 (3.1) | 0.80 |
| Episcleritis | 67 (9.2) | 0.13 | 4 (6.5) | 0.58 | 6 (5.0) | 0.12 | 57 (10.4) | 0.07 |
| Scleritis | 30 (4.1) | 0.16 | 1 (1.6) | 0.48 | 2 (1.7) | 0.22 | 27 (4.9) | 0.09 |
| Cardiovascular | 115 (15.8) | 0.53 | 7 (11.3) | 0.40 | 21 (17.6) | 0.65 | 87 (15.9) | 0.98 |
| Pericarditis | 31 (4.3) | 0.21 | 0 (0) | 0.16 | 5 (4.2) | 1.00 | 26 (4.8) | 0.35 |
| Myocarditis | 9 (1.2) | 0.34 | 1 (1.6) | 1.00 | 3 (2.5) | 0.35 | 5 (0.9) | 0.33 |
| Congestive heart failure | 10 (1.3) | 0.49 | 0 (0) | 0.77 | 1 (0.8) | 0.35 | 9 (1.6) | 0.47 |
| Gastrointestinal | 76 (10.5) | 0.55 | 5 (8.1) | 0.67 | 10 (8.4) | 0.53 | 61 (11.2) | 0.34 |
| Abdominal pain | 41 (5.6) | 0.47 | 2 (3.2) | 0.57 | 9 (7.6) | 0.44 | 30 (5.5) | 0.91 |
| Perforation | 2 (0.3) | 0.042 | 0 (0) | 1.00 | 1 (0.8) | 0.74 | 1 (0.2) | 1.00 |
| Neurological | 215 (29.6) | 0.79 | 16 (25.8) | 0.59 | 35 (29.4) | 1.00 | 164 (30.0) | 0.70 |
| Central nervous system | 21 (2.9) | 0.18 | 4 (6.5) | 0.18 | 2 (1.7) | 0.58 | 15 (2.7) | 0.89 |
| Peripheral neuropathy | 143 (19.7) | 0.06 | 5 (8.1) | 0.025 | 25 (21.0) | 0.78 | 113 (20.7) | 0.27 |
| BVAS at diagnosis, mean (SD) | 17.5 (8.8) | 0.001 | 13.3 (8.1) | <0.001 | 18.2 (8.6) | 0.36 | 17.8 (8.7) | 0.10 |
| Histology supporting GPA diagnosis | 324 (44.6) | 0.935 | 29 (46.8) | – | 53 (44.5) | – | 242 (44.3) | – |
| Intravenous cyclophosphamide | 561 (77.2) | <0.001 | 35 (56.5) | <0.001 | 86 (72.3) | 0.20 | 440 (80.6) | <0.001 |
| Oral cyclophosphamide | 41 (5.6) | 0.001 | 10 (16.1) | 0.001 | 6 (5.0) | 0.93 | 25 (4.6) | <0.05 |
| Rituximab | 50 (6.9) | 0.66 | 3 (4.8) | 0.69 | 10 (8.4) | 0.60 | 37 (6.8) | 0.99 |
| Methotrexate | 33 (4.5) | 0.13 | 6 (9.7) | 0.09 | 5 (4.2) | 1.00 | 22 (4.0) | 0.35 |
| Glucocorticoids | 709 (97.5) | 0.10 | 58 (93.5) | 0.09 | 116 (97.5) | 1.00 | 535 (98) | 0.27 |
| Glucocorticoid, mg/day mean (SD) | 60 (50–70) | 0.006 | 50.2 (29.7) | 0.05 | 52.0 (23.0) | 0.03 | 59.1 (21.4) | 0.002 |
| Methylprednisolone pulse | 208 (28.6) | 0.06 | 10 (16.1) | 0.03 | 39 (32.8) | 0.33 | 159 (29.1) | 0.66 |
| Plasma exchanges | 57 (7.8) | 0.21 | 2 (3.2) | 0.24 | 7 (5.9) | 0.50 | 48 (8.8) | 0.13 |
Values are expressed as number (percentage) unless stated otherwise.
ANCA, antineutrophil cytoplasm antibody; GPA, granulomatosis with polyangiitis; MPO, myeloperoxidase; PR3, proteinase-3.
Table 2.
Relapse-free survival and overall survival of 727 granulomatosis with polyangiitis patients according to ANCA specificity
| Overall n=727 | P value (PR3-ANCA+ vs MPO-ANCA+ vs ANCA–) | No ANCA n=62 | P value (ANCA– vs PR3-ANCA+ and MPO-ANCA+) | MPO-ANCA+ n=119 | P value (MPO-ANCA+ vs PR3-ANCA+ and ANCA–) | PR3-ANCA+ n=546 | P value (PR3-ANCA+ vs MPO-ANCA+ and ANCA–) | |
| Relapse-free survival | ||||||||
| 5-year (95% CI) | 38.6% (34.3 to 43.4) | 0.12 | 56.2% (43.8 to 72.1) | 0.18 | 47.2% (36.0 to 61.7) | 0.17 | 35.4% (30.7 to 40.9) | 0.04 |
| 10-year (95% CI) | 19.4% (14.7 to 25.5) | 37.0% (22.7 to 60.2) | 32.7% (20.5 to 52.0) | 14.8% (10.0 to 21.9) | ||||
| Overall survival | ||||||||
| 5-year (95% CI) | 92.6% (90.3 to 95.0) | 0.0058 | 93.0% (86.6 to 89.9) | 0.92 | 83.9% (75.2 to 93.5) | 0.001 | 94.2% (91.8 to 96.7) | <0.01 |
| 10-year (95% CI) | 86.8% (82.9 to 90.9) | 85.7% (75.0 to 98.0) | 81.5% (72.1 to 92.1) | 88.1% (83.5 to 92.9) | ||||
ANCA, antineutrophil cytoplasm antibody; MPO, myeloperoxidase; PR3, proteinase-3.
Figure 1.
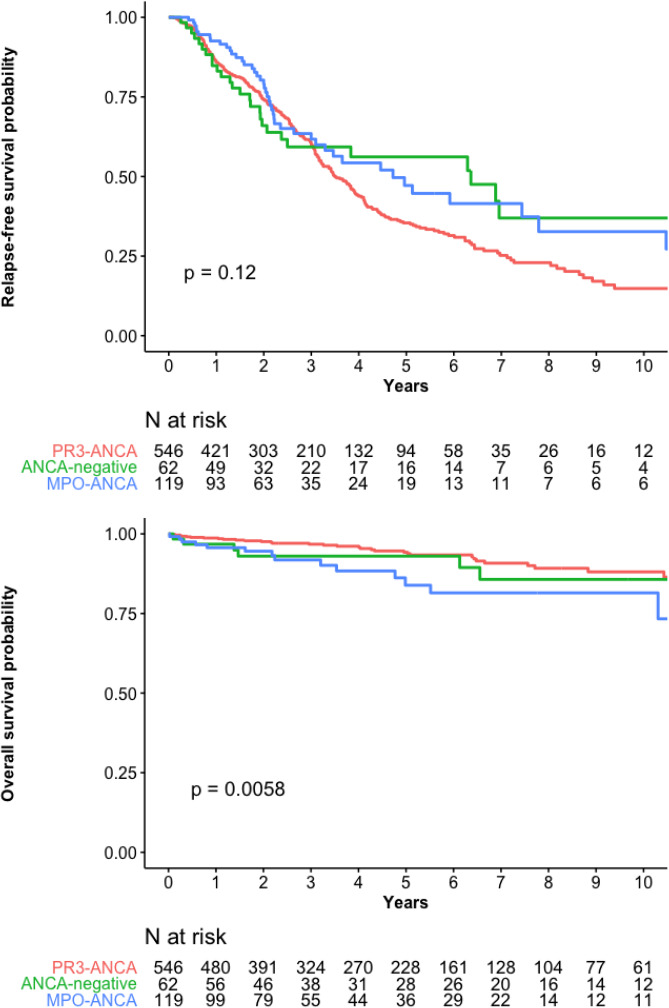
(A) Relapse-free survival and (B) overall survival probabilities for the 727 patients with granulomatosis with polyangiitis according to their ANCA status. ANCA, antineutrophil cytoplasm antibody; MPO, myeloperoxidase; PR3, proteinase-3.
Comparison of 62 ANCA-negative versus 665 PR3-ANCA–positive or MPO-ANCA–positive patients
Histological findings supported GPA diagnosis for 29 (47%) ANCA-negative patients. Among the 33 patients with no histological support of a GPA diagnosis, 21 had chronic sinusitis, otitis media or mastoiditis for >3 months, 12 had X-ray evidence of fixed pulmonary infiltrates, nodules or cavitations present for >1 month, seven had 2+ haematuria and 2+ proteinuria on urinalysis, and four had subglottic stenosis, bloody nasal discharge and crusting for >1 month, or nasal ulceration.2 At diagnosis, ANCA-negative patients had limited disease significantly more frequently than those PR3-ANCA–positive and MPO-ANCA–positive combined, less kidney involvement and peripheral neuropathy resulting in a lower baseline BVAS (table 1). They significantly less frequently received intravenous or oral cyclophosphamide induction therapy or methylprednisolone infusions, and had received a lower initial glucocorticoid dose. Their RFS and OS rates did not differ (table 2, figure 2A, B).
Figure 2.
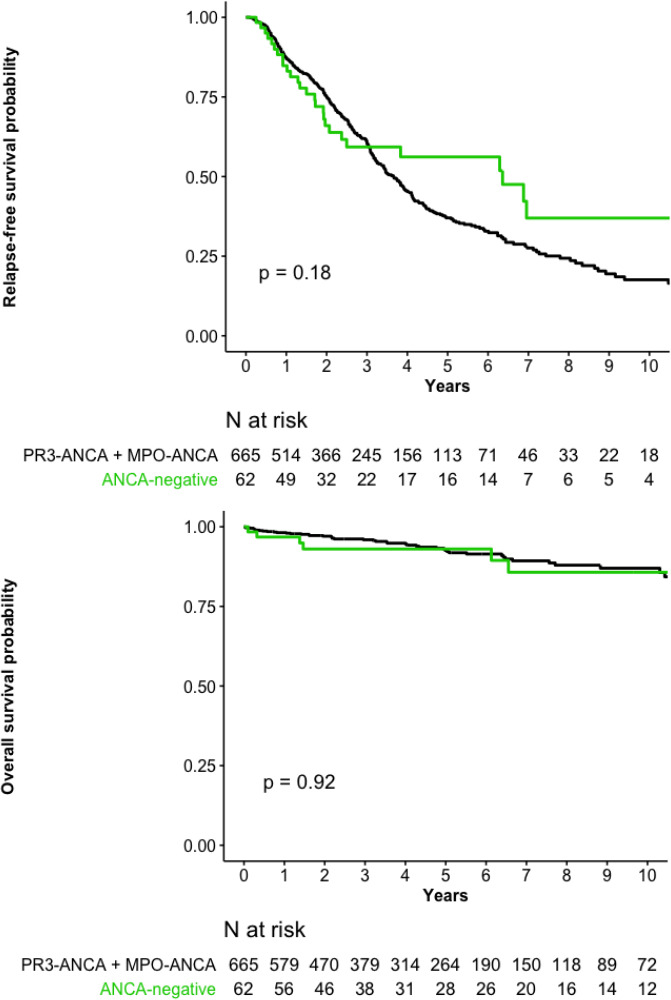
(A) Relapse-free survival and (B) overall survival probabilities for the 62 ANCA-negative granulomatosis with polyangiitis patients versus 665 PR3-ANCA–positive and MPO-ANCA–positive GPA patients. ANCA, antineutrophil cytoplasm antibody; MPO, myeloperoxidase; PR3, proteinase-3.
Comparison of 119 MPO-ANCA–positive versus 608 PR3-ANCA–positive or ANCA-negative patients
MPO-ANCA–positive patients versus PR3-ANCA–positive and ANCA-negative patients combined, were significantly more often female, older, with more frequent kidney involvement but less frequent arthralgias, purpura or eye involvement (table 1). Their mean baseline BVAS and induction therapies were comparable. Their RFS rate was similar but OS was lower (table 2, figure 3A, B).
Figure 3.
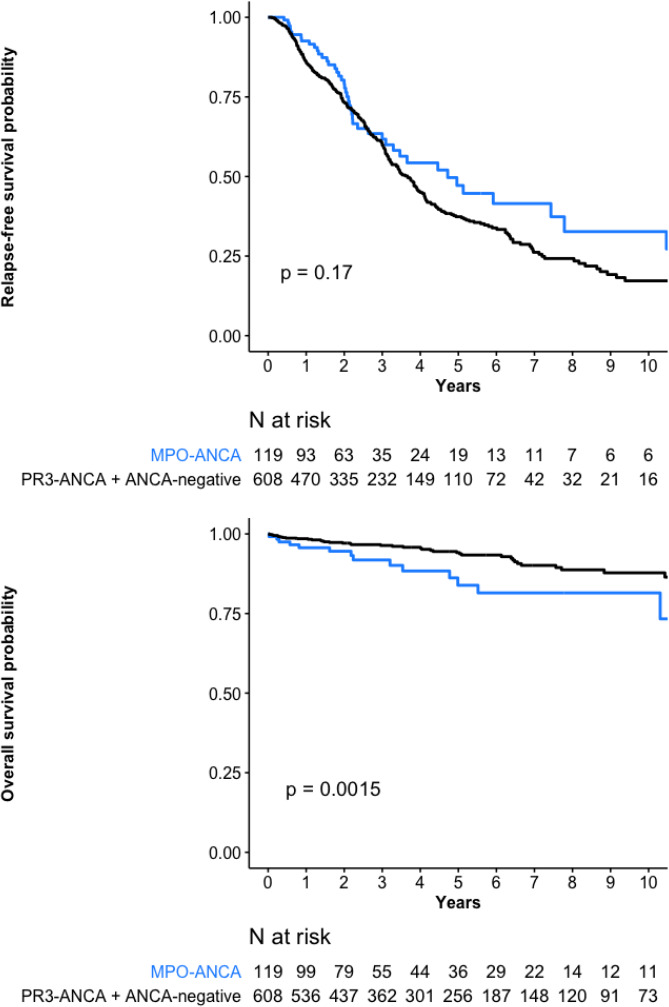
(A) Relapse-free survival and (B) overall survival probabilities for the 119 ANCA-MPO–positive granulomatosis with polyangiitis (GPA) patients versus 608 PR3-ANCA–positive and ANCA-negative GPA patients combined. ANCA, antineutrophil cytoplasm antibody; MPO, myeloperoxidase; PR3, proteinase-3.
Comparison of 546 PR3-positive versus 181 ANCA-negative or MPO-positive patients
Patients with PR3-ANCA–positive GPA, compared with ANCA-negative and MPO-positive patients combined, were significantly younger at diagnosis, had significantly less frequent limited GPA, more frequent arthralgias and purpura. They had significantly received intravenous or oral cyclophosphamide more frequently and higher initial glucocorticoid dose. Compared with ANCA-negative and MPO-positive groups, PR3-positive patients’ RFS rate was lower but their OS rate higher (table 2, figure 4A, B).
Figure 4.
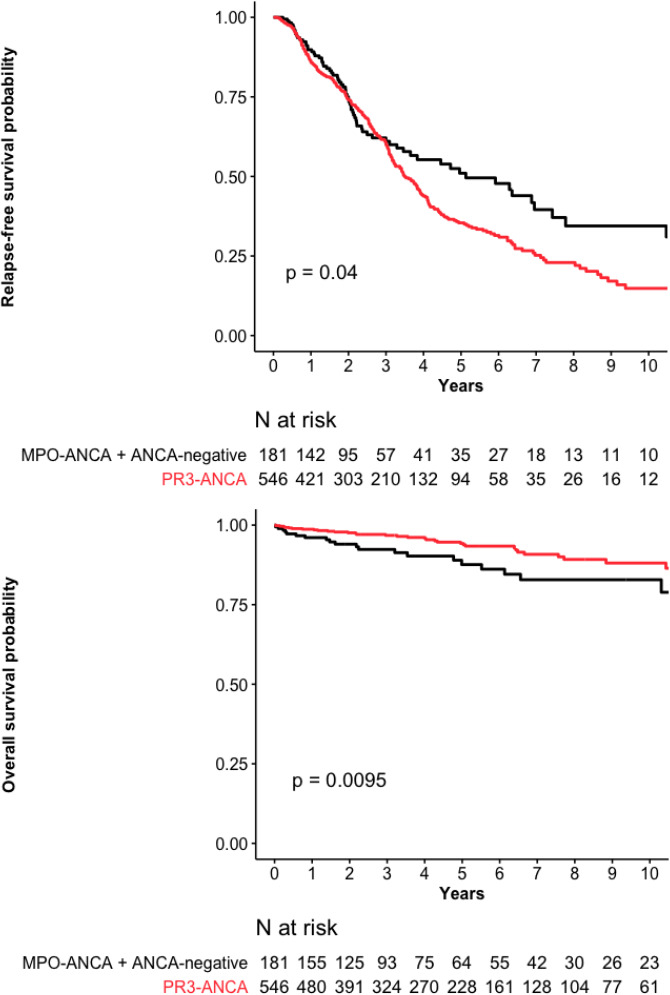
(A) Relapse-free survival and (B) overall survival probabilities for the 546 PR3-ANCA–positive granulomatosis with polyangiitis (GPA) patients versus 181 MPO-ANCA–positive and ANCA-negative GPA patients combined. ANCA, antineutrophil cytoplasm antibody; MPO, myeloperoxidase; PR3, proteinase-3.
Univariable and multivariable predictors of death or relapse for the entire GPA cohort
Univariable analyses selected only the PR3-ANCA–positivity as being associated with a higher relapse-risk probability, which persisted even after adjustment (table 3). Multivariable analysis retained age and congestive heart failure as significant and independent predictors of death and not MPO-ANCA–positivity (table 3).
Table 3.
Univariable and multivariable analyses of factors associated with death and relapse for the entire Registry cohort of 727 granulomatosis with polyangiitis patients
| Factor | Death | Relapse | ||||||
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.07 (1.04 to 1.09) | <0.001 | 1.07 (1.04 to 1.09) | <0.001 | 0.99 (0.99 to 1.00) | 0.520 | ||
| Female | 0.64 (0.37 to 1.08) | 0.09 | 1.03 (0.78 to 1.19) | 0.773 | ||||
| Lung | 0.46 (0.88 to 2.90) | 0.123 | 0.98 (0.78 to 1.22) | 0.864 | ||||
| Alveolar haemorrhage | 2.14 (1.23 to 3.72) | 0.006 | 1.08 (0.83 to 1.42) | 0.547 | ||||
| Renal | 3.19 (1.77 to 5.79) | 0.0001 | 1.02 (0.83 to 1.26) | 0.827 | ||||
| Serum creatinine >150 µmol/L | 2.16 (1.24 to 3.76) | 0.006 | 0.92 (0.70 to 1.21) | 0.572 | ||||
| Skin | 1.42 (0.84 to 2.40) | 0.180 | 1.04 (0.83 to 1.29) | 0.734 | ||||
| Ear, nose and throat | 0.77 (0.42 to 1.41) | 0.409 | 1.12 (0.86 to 1.47) | 0.396 | ||||
| Eye | 0.75 (0.42 to 1.34) | 0.347 | 1.02 (0.81 to 1.28) | 0.826 | ||||
| Cardiovascular | 2.70 (1.56 to 4.70) | 0.0004 | 1.06 (0.80 to 1.42) | 0.653 | ||||
| Congestive heart failure | 10.73 (4.56 to 25.22) | <0.0001 | 3.20 (1.12 to 9.16) | 0.029 | 0.69 (0.22 to 2.14) | 0.521 | ||
| Gastrointestinal | 1.27 (0.54 to 2.97) | 0.571 | 0.97 (0.67 to 1.41) | 0.905 | ||||
| Neurological | 1.61 (0.96 to 2.69) | 0.067 | 0.98 (0.78 to 1.24) | 0.926 | ||||
| BVAS | 1.07 (1.04 to 1.10) | <0.001 | 0.99 (0.98 to 1.00) | 0.283 | ||||
| Anti-myeloperoxidase | 2.25 (1.28 to 3.94) | 0.004 | 0.82 (0.60 to 1.12) | 0.219 | ||||
| Anti-proteinase-3 | 0.51 (0.30 to 0.85) | 0.01 | 0.43 (0.18 to 1.03) | 0.060 | 1.31 (1.01 to 1.69) | 0.040 | 1.29 (1.00 to 1.68) | 0.047 |
| ANCA negative | 1.04 (0.47 to 2.29) | 0.921 | 0.76 (0.51 to 1.13) | 0.185 | ||||
| Intravenous CYC | – | – | 0.85 (0.66 to 1.10) | 0.232 | ||||
| Oral CYC | – | – | 0.63 (0.37 to 1.07) | 0.091 | ||||
| Rituximab | – | – | 0.77 (0.43 to 1.38) | 0.385 | ||||
| Methotrexate | – | – | 1.02 (0.63 to 1.65) | 0.912 | ||||
ANCA, antineutrophil cytoplasm antibody; BVAS, Birmingham Vasculitis Activity Score.
Univariable and multivariable predictors of death or relapse for PR3-ANCA or MPO-ANCA–positive GPA patients
Excluding ANCA-negative GPA patients from the analysis, predictors of death remain unchanged but PR3-ANCA–positivity was no longer associated with an increased relapse-risk probability (table 4).
Table 4.
Univariable and multivariable analyses of factors associated with death and relapse for the 665 granulomatosis with polyangiitis antineutrophil cytoplasm antibody-positive patients
| Factor | Death | Relapse | ||||
| Univariable analysis | Multivariable analysis | Univariable analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.07 (1.04 to 1.09) | <0.001 | 1.07 (1.04 to 1.09) | <0.001 | 1.00 (0.80 to 1.24) | 0.999 |
| Female | 0.58 (0.33 to 1.02) | 0.061 | 0.99 (099 to 1.00) | 0.561 | ||
| Lung | 1.62 (0.85 to 3.07) | 0.141 | 0.98 (0.78 to 1.24) | 0.921 | ||
| Alveolar haemorrhage | 2.10 (1.17 to 3.78) | 0.012 | 1.06 (0.80 to 1.40) | 0.665 | ||
| Renal | 3.32 (1.71 to 6.45) | 0.0001 | 1.03 (0.77 to 1.20) | 0.744 | ||
| Serum creatinine >150 µmol/L | 1.87 (1.03 to 3.38) | 0.038 | 0.86 (0.65 to 1.13) | 0.292 | ||
| Haemodialysis | 3.37 (1.51 to 7.53) | 0.003 | 1.31 (0.83 to 2.06) | 0.240 | ||
| Skin | 1.42 (0.82 to 2.47) | 0.206 | 1.01 (0.78 to 1.24) | 0.912 | ||
| Ear, nose and throat | 0.77 (0.42 to 1.41) | 0.409 | 1.11 (0.84 to 1.45) | 0.445 | ||
| Eye | 0.74 (0.39 to 1.38) | 0.349 | 1.08 (0.85 to 1.36) | 0.513 | ||
| Cardiovascular | 2.52 (1.40 to 4.53) | 0.001 | 1.03 (0.76 to 1.38) | 0.838 | ||
| Congestive heart failure | 9.57 (3.77 to 24.3) | <0.0001 | 4.58 (1.79 to 11.70) | 0.001 | 0.70 (0.22 to 2.19) | 0.547 |
| Gastrointestinal | 0.98 (0.35 to 2.74) | 0.976 | 0.87 (0.59 to 1.30) | 0.521 | ||
| Neurological | 1.43 (0.82 to 1.48) | 0.205 | 1.00 (0.79 to 1.27) | 0.949 | ||
| Birmingham Vasculitis Activity Score | 1.07 (1.03 to 1.10) | <0.001 | 0.99 (0.97 to 1.00) | 0.169 | ||
| Anti-myeloperoxidase | 2.33 (1.31 to 4.14) | 0.003 | 0.80 (0.60 to 1.12) | 0.159 | ||
| Anti-proteinase-3 | 0.39 (0.22 to 0.70) | 0.001 | 0.43 (0.18 to 1.03) | 0.057 | 1.28 (0.93 to 1.75) | 0.119 |
| Intravenous cyclophosphamide | – | – | 0.85 (0.64 to 1.11) | 0.244 | ||
| Oral cyclophosphamide | – | – | 0.72 (0.40 to 1.28) | 0.267 | ||
| Rituximab | – | – | 0.73 (0.40 to 1.34) | 0.319 | ||
| Methotrexate | – | – | 1.1 (0.65 to 1.84) | 0.719 | ||
Discussion
Based on data from one of the largest cohorts of GPA patients, ANCA status was associated with patients’ phenotypes, and ANCA-negativity and MPO-ANCA–positivity distinguished distinct subsets. ANCA-negative GPA patients, compared with those ANCA-positive, more frequently had limited disease but similar RFS and OS rates. Relapse probability for MPO-ANCA–positive GPA patients was similar to those of ANCA-negative and PR3-ANCA–positive patients but their OS was lower because of their older age. PR3-ANCA–positivity was independently associated with a higher relapse probability when all GPA patients were considered but was not retained when comparing only PR3-ANCA–positive versus MPO-ANCA–positive subgroups. Thus, there was no appreciable difference in relapse rate between ANCA-positive GPA patients based on ANCA type and regardless of ANCA status, patients with GPA retain a high relapse rate.
Several classification criteria have been proposed to define syndromes within the AAV spectrum and then categorise patients accordingly.1 20 They all relied on combinations of clinical, radiographic and histological findings. In practice, they poorly predicted outcomes and prognoses.4 12 Several lines of evidence suggest that classifying patients by ANCA specificity produces more clearly defined groups than their classification by clinical diagnosis (GPA vs MPA).4 12 However, although demonstrated when GPA and MPA patients were considered together, data from observational studies and pooled trials yielded discordant outcome results for GPA patients according to ANCA specificity.13 16 24 We hypothesised that those discrepancies could result from too few GPA patients studied and aimed to clarify those findings by analysing the data from one of the largest GPA subsets.
Herein, ANCA-negative GPA patients more frequently had limited GPA and less kidney involvement, thereby justifying their less aggressive treatments. Very few authors have investigated this patient subset, which represents 8.5% of our GPA cohort and approximately 5% of GPA patients described in the literature.6 Our observed sex ratio of ~1 was unable to confirm the significant female predominance reported, perhaps attributable to chance in that smaller study.16 However, our results are in line with lower BVAS, primarily due to the lower likelihood of kidney involvement in ANCA-negative GPA patients. At the same time, these findings are consistent with previous findings suggesting that non-severe or limited GPA is more frequently ANCA-negative.25 In agreement with pooled-trial data showing that the ANCA-negative GPA patients’ relapse rate was similar to those PR3-positive,16 we found that the ANCA-negative GPA patients’ RFS and OS rates were similar to those of PR3-ANCA–positive or MPO-ANCA–positive GPA subsets. Also, it was recently reported that the risk of limited-GPA relapse was similar to that of systemic GPA.26 Our high relapse and mortality rates for patients with ANCA-negative GPA highlight that it is not always a benign disease and should be managed accordingly, with regular follow-up.
FVSG Registry patients with MPO-ANCA–positive GPA were more often older women, as usually reported.8 9 13 15 16 Our results also agree with the classical 10-year age discrepancy between patients with MPO-ANCA versus PR3-ANCA.4 8 27–29 Herein, this subset had significantly less frequent arthralgias, purpura or eye involvement, consistent with the data from the only other study that also included >100 MPO-ANCA–positive GPA patients.9 No such significant disease-manifestation differences were observed in studies with smaller enrolments of MPO-ANCA–positive GPA patients.16 17 30 Our MPO-ANCA–positive GPA patients also had significantly more frequent kidney involvement (65.5%), unlike this subset in German and Chinese cohorts.9 13 Further studies are still required to address these discrepancies. Our MPO-positive patients’ RFS rate was similar to those of ANCA-negative and PR3-positive subgroups, in accordance with the similar relapse rates observed across ANCA specificities among GPA patients in several cohorts.9 16 17 In addition, in several studies, the relapse risk was associated more closely with AAV type than ANCA specificity.16 17 We also found that there was no detectable difference in relapse rate between ANCA-positive GPA patients based on ANCA type. Taken together, those findings illustrated that GPA patients are at high risk of relapse, regardless of their ANCA status/specificity at diagnosis and confirmed that GPA phenotype remains a major factor associated with the probability of relapse.4 6 In addition, our observed lower OS rate of these older GPA patients did not remain statistically significant after adjustment for age at diagnosis, as observed in most studies.12 27 31
Our 546 PR3-ANCA–positive GPA patients represent one of the largest groups yet constituted, with median follow-up exceeding 3 years, enabling identification of long-term outcomes with a sufficient level of evidence.14 32 Compared with the other subsets, PR3-positive patients’ RFS rate was lower. That finding agrees with accumulating knowledge that PR3-positivity is independently associated with a higher relapse risk for AAV patients10 12 and allows us to extend the relapse risk associated with PR3-postivity to the subgroup of GPA.4 14 24 Our results demonstrate that GPA classification and diagnostic criteria incorporating ANCA-specificity are more useful than the clinical diagnosis alone to predict relapse and that PR3-ANCA–positivity was the sole factor significantly associated with a higher probability of relapse among all GPA. GPA patients’ high relapse risk should lead to appropriate patient education and vigilant monitoring to assure more favourable clinical outcomes. With the advent of rituximab as maintenance therapy, the treatment paradigm has changed from managing frequent relapses on maintenance methotrexate or azathioprine to adjusting rituximab duration to further limit relapses.33 However, the higher reported risk of severe SARS-CoV-2 infection in rituximab-treated patients may have changed the risk/benefit of maintenance rituximab approach. Recommendations for the management of COVID-19 vaccination and prophylaxis in patients receiving rituximab for systemic vasculitis have been proposed.34 Since most of those relapses were minor and rapidly treated,18 the higher relapse rate of our PR3-ANCA–positive GPA patients was not associated with shorter long-term survival, as previously reported.10 30 In contrast, better OS of PR3-ANCA–positive patients could be explained by the fact that MPO-ANCA–positive patients were older and had poorer renal outcomes, as in most cohorts.4 12 28–30
Our results add important clarifying information to the ongoing discussion of the relative contributions of ANCA specificity, as opposed to AAV clinical diagnosis, to GPA phenotype and outcomes. They reconcile the seemingly contradictory literature data and open the way for more tailored treatment, based not only on the AAV type but also on ANCA specificity. Current trials already take this dichotomy into account by stratifying enrolment according to ANCA specificity35 36 or even by including exclusively patients with one ANCA specificity (ClinicalTrials.gov Identifier: NCT00405860 or NCT03920722 for MPO-ANCA–positive and NCT03967925 for PR3-ANCA–positive patients). Our data suggest, even among patients receiving a clinically based GPA diagnosis, that those PR3-ANCA–positive are more prone to relapse than those of the two other subsets combined. Patients with critical organ damage that could become life-limiting if further injury occurs (eg, lungs and kidneys) or those at high risk of relapse, such as those who have previously relapsed or whose PR3-ANCA test is positive, should receive longer rituximab maintenance therapy, as already proposed,33 37 and new therapeutic strategies should be evaluated (ClinicalTrials.gov Identifier: NCT03967925).
Our study has major strengths. Our Registry collected the clinical characteristics and long-term outcomes of patients, originating from several medical specialties, enabling analysis according to immunoassay results of a truly representative distribution of different GPA presentations and evolutions. ANCA-negative GPA patients were also included and we enrolled more than twice the number of patients reported in each of the previous studies,4 9 13 15 16 thereby characterising one of the largest GPA-patient populations ever analysed. All diagnoses were reassessed independently by two of the authors, who considered patients’ entire follow-up.
Some limitations also need to be addressed. This study enrolled patients over a long period, during which general clinical practices evolved. Very few Asian patients were enrolled and it is not clear whether our results can be extrapolated to them. Even though patients had to meet the 1990 American College of Rheumatology GPA classification criteria and/or revised CHCC Nomenclature to be enrolled and were reassessed considering their entire clinical histories, some patients without documented evidence of vasculitis in the ANCA-negative subset may have been misclassified. The analysis was conducted retrospectively, engendering all the biases inherent to such a design, including that all relapses occurring during follow-up may not have been captured. However, all patient information was entered prospectively into the FVSG Registry database. Although ANCA-PR3–positivity was associated with a higher relapse risk in the multivariable analysis of our entire GPA population, that finding was mitigated in the subgroup analysis restricted to ANCA-positive patients, perhaps because clinicians may have already prescribed those patients longer maintenance therapies and the detection of a potential small difference may require an even larger sample size.
In conclusion, our results finally reconcile the seemingly contradictory data reported in the GPA literature and support the concept that ANCA specificity enables identification of distinct GPA subsets with clinical and prognostic relevance. ANCA-negative GPA patients more frequently had limited disease but RFS and OS rates comparable to the other subgroups. MPO-ANCA–positive GPA patients and the other subsets had similar RFS rates, with their lower OS rate being attributable to their older age. PR3-ANCA–positive GPA patients’ RFS was lower than those of the two other subsets combined but that difference did not persist when comparing only PR3 versus MPO-ANCA–positive patients and it did not lead to a lower OS rate. These new findings support adding ANCA specificity to the phenotype and taking it into account, along with a history of relapse or damage, when discussing therapeutic strategies for those patients diagnosed with GPA.
rmdopen-2021-002160supp001.pdf (177.9KB, pdf)
Acknowledgments
The authors thank Janet Jacobson for editorial assistance.
Footnotes
Collaborators: Other investigators are listed in the online supplemental appendix.
Contributors: XP, MI and LG designed the study and were involved in acquisition, analysis and interpretation of data. CP was involved in acquisition and interpretation of data. AK, PC, FM, TQ, FL, MH were involved in acquisition of data. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. XP accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: Individual sources of potential conflict are as follows. XP: speaking fees and honoraria: Boehringer Ingelheim, Sanofi; Congress inscription/travel/accommodations: Sanofi. CP: consultancies, speaking fees and honoraria: Hoffman-La Roche, Sanofi, Chemocentryx, InflaRx GmbH, GSK, AstraZeneca. AK: consultancies, speaking fees and congress inscription/travel/accommodations: Roche, AstraZeneca, Vifor. TQ: congress inscription/travel/accommodations: MSD, Sanofi-Genzyme, LFB. BT: consultancies, speaking fees and honoraria: Roche, Grifols, LFB, AstraZeneca. LM: consultancies, speaking fees and honoraria: Roche. All authors have been coinvestigators in academic studies for which rituximab was provided by Roche Pharma.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: We propose to list and designate all the site investigators as 'collaborators' as per Medline designation. This means their names are searchable on Medline. This is an important method to appropriately recognise the work of the many coinvestigators of this study and is consistent with approaches taken by major journals for such work. A full list of collaborators is provided in the online supplemental appendix 1. Several authors of this publication are members of the European Reference Network for Rare Immunodeficiency, Autoinflammatory and Autoimmune Diseases, Project ID No. 739543.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Additional data may be obtained upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Cochin University Hospital Ethics Committee (ID: AAA-2020-08033). Participants gave informed consent to participate in the study before taking part.
References
- 1. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international chapel Hill consensus conference Nomenclature of vasculitides. Arthritis Rheum 2013;65:1–11. 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 2. Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7. 10.1136/ard.2006.054593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003;349:36–44. 10.1056/NEJMoa020286 [DOI] [PubMed] [Google Scholar]
- 4. Lionaki S, Blyth ER, Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum 2012;64:3452–62. 10.1002/art.34562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis 2013;72:1003–10. 10.1136/annrheumdis-2012-201750 [DOI] [PubMed] [Google Scholar]
- 6. Kitching AR, Anders H-J, Basu N, et al. Anca-Associated vasculitis. Nat Rev Dis Primers 2020;6:71. 10.1038/s41572-020-0204-y [DOI] [PubMed] [Google Scholar]
- 7. Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003;41:776–84. 10.1016/S0272-6386(03)00025-8 [DOI] [PubMed] [Google Scholar]
- 8. Ono N, Niiro H, Ueda A, et al. Characteristics of MPO-ANCA-positive granulomatosis with polyangiitis: a retrospective multi-center study in Japan. Rheumatol Int 2015;35:555–9. 10.1007/s00296-014-3106-z [DOI] [PubMed] [Google Scholar]
- 9. Chang D-Y, Li Z-Y, Chen M, et al. Myeloperoxidase-ANCA-positive granulomatosis with polyangiitis is a distinct subset of ANCA-associated vasculitis: a retrospective analysis of 455 patients from a single center in China. Semin Arthritis Rheum 2019;48:701–6. 10.1016/j.semarthrit.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 10. Hogan SL, Falk RJ, Chin H, et al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 2005;143:621–31. 10.7326/0003-4819-143-9-200511010-00005 [DOI] [PubMed] [Google Scholar]
- 11. Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 2012;367:214–23. 10.1056/NEJMoa1108735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cornec D, Cornec-Le Gall E, Fervenza FC, et al. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol 2016;12:570–9. 10.1038/nrrheum.2016.123 [DOI] [PubMed] [Google Scholar]
- 13. Schirmer JH, Wright MN, Herrmann K, et al. Myeloperoxidase-Antineutrophil Cytoplasmic Antibody (ANCA)-Positive Granulomatosis With Polyangiitis (Wegener's) Is a Clinically Distinct Subset of ANCA-Associated Vasculitis: A Retrospective Analysis of 315 Patients From a German Vasculitis Referral Center. Arthritis Rheumatol 2016;68:2953–63. 10.1002/art.39786 [DOI] [PubMed] [Google Scholar]
- 14. Puéchal X, Pagnoux C, Perrodeau Élodie, et al. Long-Term outcomes among participants in the WEGENT trial of Remission-Maintenance therapy for granulomatosis with polyangiitis (Wegener's) or microscopic polyangiitis. Arthritis Rheumatol 2016;68:690–701. 10.1002/art.39450 [DOI] [PubMed] [Google Scholar]
- 15. Chen M, Yu F, Zhang Y, et al. Characteristics of Chinese patients with Wegener's granulomatosis with anti-myeloperoxidase autoantibodies. Kidney Int 2005;68:2225–9. 10.1111/j.1523-1755.2005.00679.x [DOI] [PubMed] [Google Scholar]
- 16. Miloslavsky EM, Lu N, Unizony S, et al. Myeloperoxidase-Antineutrophil Cytoplasmic Antibody (ANCA)-Positive and ANCA-Negative Patients With Granulomatosis With Polyangiitis (Wegener's): Distinct Patient Subsets. Arthritis Rheumatol 2016;68:2945–52. 10.1002/art.39812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deshayes S, Martin Silva N, Khoy K, et al. Clinical impact of subgrouping ANCA-associated vasculitis according to antibody specificity beyond the clinicopathological classification. Rheumatology 2019;58:1731–9. 10.1093/rheumatology/kez016 [DOI] [PubMed] [Google Scholar]
- 18. Iudici M, Pagnoux C, Courvoisier DS, et al. Granulomatosis with polyangiitis: study of 795 patients from the French vasculitis Study Group registry. Semin Arthritis Rheum 2021;51:339–46. 10.1016/j.semarthrit.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 19. Guillevin L. Treatment of systemic necrotizing vasculitides: the 40-year experience of the French vasculitis Study Group. Presse Med 2020;49:104034. 10.1016/j.lpm.2020.104034 [DOI] [PubMed] [Google Scholar]
- 20. Leavitt RY, Fauci AS, Bloch DA, et al. The American College of rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum 1990;33:1101–7. 10.1002/art.1780330807 [DOI] [PubMed] [Google Scholar]
- 21. Hellmich B, Flossmann O, Gross WL, et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis 2007;66:605–17. 10.1136/ard.2006.062711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis 2009;68:1827–32. 10.1136/ard.2008.101279 [DOI] [PubMed] [Google Scholar]
- 23. Wegener's Granulomatosis Etanercept Trial (WGET) Research Group . Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med 2005;352:351–61. 10.1056/NEJMoa041884 [DOI] [PubMed] [Google Scholar]
- 24. Pagnoux C, Springer J. Editorial: Classifying Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitides According to ANCA Type or Phenotypic Diagnosis: Salt or Pepper? Arthritis Rheumatol 2016;68:2837–40. 10.1002/art.39860 [DOI] [PubMed] [Google Scholar]
- 25. Nölle B, Specks U, Lüdemann J, et al. Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener granulomatosis. Ann Intern Med 1989;111:28–40. 10.7326/0003-4819-111-1-28 [DOI] [PubMed] [Google Scholar]
- 26. Iudici M, Pagnoux C, Courvoisier DS, et al. Localised versus systemic granulomatosis with polyangiitis: data from the French vasculitis Study Group registry. Rheumatology 2021:keab719. 10.1093/rheumatology/keab719 [DOI] [PubMed] [Google Scholar]
- 27. Flossmann O, Berden A, de Groot K, et al. Long-Term patient survival in ANCA-associated vasculitis. Ann Rheum Dis 2011;70:488–94. 10.1136/ard.2010.137778 [DOI] [PubMed] [Google Scholar]
- 28. de Joode AAE, Sanders JSF, Stegeman CA. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol 2013;8:1709–17. 10.2215/CJN.01020113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohammad AJ, Segelmark M. A population-based study showing better renal prognosis for proteinase 3 antineutrophil cytoplasmic antibody (ANCA)-associated nephritis versus myeloperoxidase ANCA-associated nephritis. J Rheumatol 2014;41:1366–73. 10.3899/jrheum.131038 [DOI] [PubMed] [Google Scholar]
- 30. Monti S, Felicetti M, Delvino P, et al. Anti-Neutrophil cytoplasmic antibody specificity determines a different clinical subset in granulomatosis with polyangiitis. Clin Exp Rheumatol 2021;39 Suppl 129:107–13. [DOI] [PubMed] [Google Scholar]
- 31. Wallace ZS, Fu X, Harkness T, et al. All-Cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology 2020;59:2308–15. 10.1093/rheumatology/kez589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puéchal X, Iudici M, Pagnoux C, et al. Sustained remission of granulomatosis with polyangiitis after discontinuation of glucocorticoids and immunosuppressant therapy: data from the French vasculitis Study Group registry. Arthritis Rheumatol 2021;73:641–50. 10.1002/art.41551 [DOI] [PubMed] [Google Scholar]
- 33. Charles P, Perrodeau Élodie, Samson M, et al. Long-Term rituximab use to maintain remission of antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2020;173:179–87. 10.7326/M19-3827 [DOI] [PubMed] [Google Scholar]
- 34. Puéchal X, Cottin V, Faguer S, et al. French vasculitis Study Group recommendations for the management of COVID-19 vaccination and prophylaxis in patients with systemic vasculitis. Presse Med 2022;51:104107. 10.1016/j.lpm.2021.104107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walsh M, Merkel PA, Peh C-A, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 2020;382:622–31. 10.1056/NEJMoa1803537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jayne DRW, Merkel PA, Schall TJ, et al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 2021;384:599–609. 10.1056/NEJMoa2023386 [DOI] [PubMed] [Google Scholar]
- 37. Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016;75:1583–94. 10.1136/annrheumdis-2016-209133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002160supp001.pdf (177.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Additional data may be obtained upon reasonable request.


