Abstract

Novel KMnO4-modified loofah biochar loaded with nano-Fe2O3 (FMLB) was successfully synthesized for Cu(II) adsorption. Nitrogen adsorption method, scanning electron microscope, X-ray photoelectron spectroscopy, Fourier transform infrared spectrometer, and other characterization measurements were used to evaluate the physical and chemical properties of FMLB and nano-Fe2O3-loaded biochar (FLB). The results show that the adsorption behavior of Cu(II) can be best fitted by the Langmuir isotherm model and the pseudo-second-order (PSO) kinetic model, indicating that the surface of FMLB was composed of homogeneous adsorption, and chemical adsorption dominated the adsorption process under optimal reaction conditions. The adsorption capacity of FMLB is improved by 42.86% compared to FLB, and it remained over 75% after four cycles. The inner-sphere complexes with manganese oxide (MnOx) and oxygen-containing functional groups, as well as electrostatic interaction, physical adsorption, and ion exchange, play important roles in Cu(II) adsorption. The saturation magnetization of FMLB was 10.41 emu/g, ensuring that it can be easily separated from aqueous solutions. Therefore, magnetically recyclable biochar modified by KMnO4 is a feasible method for Cu(II) adsorption.
1. Introduction
A great deal of industrial effluents with heavy metals enter the natural water bodies and flow into rivers and lakes through surface runoff and seep into groundwater.1,2 Heavy metals are highly migratory, nondegradable, and toxic, which threaten human health through food chain aggregation.3−5 At present, treatments for heavy metals mainly include adsorption, coagulation, ion exchange, electrochemical technology, and other technical means, among which the adsorption method is simple and feasible for low-concentration heavy metals.6−8 Compared with traditional adsorbents, biochar has the characteristics of wide sources, low cost, large specific surface area, and abundant pore structures, making it widely used in water purification and other fields.9,10
Biochar is produced by pyrolysis of biomass at a temperature lower than 700 °C under oxygen-free conditions,11 the main sources of which are agricultural waste, industrial organic matter, and municipal sludge.12 However, with low specific surface area and a limited number of functional groups, unmodified biochar usually has a limited adsorption capacity for pollutants in aqueous solutions.13 Therefore, there is a need to modify biochar, and there are several mainstream modification methods, including chemical and physical modification, metal or metal oxide-supported, and clay mineral modification methods.14,15
Recently, potassium permanganate treatment was successfully used for biochar modification to enhance the absorption ability for heavy metal removal. Because potassium permanganate is not only a strong oxidant but also the precursor of manganese oxide (MnOx), and the oxidation reaction might promote the precursor to be added into the feedstock, it might lead to a new type of modified biochar after pyrolysis.16,17 For instance, in Li and Yang’s study,18 biochars were separately modified by potassium permanganate, ferric chloride hexahydrate, and alkaline for adsorption experiments for Cd(II) ions, and potassium permanganate-modified biochar showed the best adsorption capacity.
Copper, as one of the many enzyme components, participates in many physiological metabolic processes, but it is also a potentially toxic element, which is the main heavy metal polluting soil and groundwater.19 The addition of MnOx can enhance the surface properties significantly and increase the quantity of surface O-containing groups of biochar, providing abundant active adsorption sites for Cu(II).20 Besides, chemical complexation, coprecipitation, ion exchange, and Cu(II)−π interactions are the dominant mechanisms of Cu(II) adsorption.21,22 However, the adsorbed biochar is difficult to separate from the solution, and many studies have been performed for improving this,23,24 such as introducing magnetic particles on the biochar surface. There are two mainstream methods for magnetic modification: wet impregnation with iron oxides25 and pretreatment of biomass with iron ions.26 Also, a physical mixing method was reported to prepare magnetic biochar by comixing feedstock with Fe3O4 and evenly dispersing them in certain solvents.27
Nicolaou and Philippou28 prepared magnetic biochar from pine needles for adsorbing Cu(II) from wastewater, finding that Cu(II) cations are complexed by hydroxyl groups on the magnetite particles. In Xiao and Cheng’s study,29 uniform and consistent γ-Fe2O3 particles appeared on the surface of FeCl3-modified biochar, which could be easily separated from a solution with a saturation magnetization of 16.5 emu/g. Nevertheless, the common magnetic biochar usually has a low adsorption capacity for Cu(II) in aqueous solutions; therefore, developing a new type of biochar with both excellent heavy metal adsorption capacity and good magnetism is necessary.
To date, few studies have reported the use of potassium permanganate modification as well as increasing the magnetism of biochar at the same time for enhancing the adsorption capacity for Cu(II). Herein, by combining the advantages of magnetite, potassium permanganate, and suitable raw materials, in this study, KMnO4-modified magnetic loofah biochar (FMLB) was prepared by Fe(NO3)3·9H2O impregnation and modification of MnOx. In this study, for the raw material of biochar, loofah sponge was chosen since the annual output of loofah is nearly one million tons, and the output of its by-product is relatively large according to the Chinese agricultural website. The biochar prepared from loofah sponge possesses a stable structure, a large specific surface area, and abundant pore structures.30−32 The purposes of this study are as follows: (a) preparation of biochar and modified biochar; (b) characterizing and analyzing the physicochemical properties of the modified biochar; (c) studying the influence of solution conditions on the adsorption effect; (d) analyzing the adsorption thermodynamics and kinetics of Cu(II) by FMLB; and (e) exploring the adsorption mechanism of FMLB for Cu(II).
2. Materials and Methods
2.1. Materials
The reagents used in this research, such as Cu(NO3)2·3H2O, KMnO4, Fe(NO3)3·9H2O, ethanol (99%), NaOH, HCl, and CH3COONa, were all of analytical grade. Loofah sponge was obtained from the local market in Guangzhou, Guangdong province in China.
2.2. Preparation of Modified Biochar
The loofah sponge or loofah cloth is a meshlike fiber inside the loofah of the Cucurbitaceae plant at maturity, and the part used for this test was the vascular bundle of the fruit after peeling and seeding. The loofah sponge was crushed with a pulverizer after washing with deionized water. The pulverized loofah sponge was placed in a porcelain boat, compacted, and heated for 2 h in a tube furnace at 600 °C while nitrogen was continuously passed through the whole process. After cooling down to room temperature, it was taken out and bagged for later use, marked as LB.
The loofah sponge and Fe(NO3)3·9H2O were mixed in an ethanol solution at the ratio of 1:1, stirred magnetically for 2 h, and evaporated to dryness at 50 °C after mixing evenly. The dried Fe(NO3)3·9H2O/loofah sponge mixture was carbonized at 600 °C for 1.5 h with the protection of a nitrogen atmosphere in a quartz tube furnace, and the heating rate was set at 10 °C/min. After carbonization, the magnetic modified biochar was rinsed several times with DI water, dried, and passed through a 120-mesh sieve and marked as FLB.
Magnetic biochar (FLB) was immersed in a potassium permanganate solution, and the mass ratio of FLB to KMnO4 was 2:1. Then, the mixture was vibrated ultrasonically, dried in an oven at 105 °C, and carbonized at 600 °C for 0.5 h in a nitrogen atmosphere. The obtained samples were marked as FMLB after rinsing several times with DI water and dried to constant weight in an oven.
2.3. Adsorbent Characterization
The BET surface area, pore size distribution, and total pore volume were characterized by performing N2 adsorption–desorption (IQ-2). Scanning electron microscopy (SEM) (S-4800) was used for surface morphology, and elemental composition analysis was measured by X-ray photoelectron spectroscopy (XPS) (ESCALAB 250Xi). Fourier infrared spectroscopy (Nicolet iN10) was used to characterize the types and contents of the functional groups of biochar. The concentration of Cu(II) was measured using an inductively coupled plasma emission spectrometer (Optima 8000).
2.4. Experimental Design of Cu(II) Adsorption
In the experiment of the influence of pH on adsorption performance, 10 mL of Cu(II) solution of 1000 mg/L was placed into a 15 mL test tube, 20 mg of adsorbent was added, and the pH was adjusted to 1.0–5.5; the final concentration was determined after filtration with a 0.22 μm syringe filter. In the experiment of the effect of adsorbent quality on the adsorption effect, the dosage of biochar ranged from 10 to 80 mg.
In the adsorption kinetics experiment, the reaction time interval was set from 0.5 to 48 h. However, in the experiment for adsorption isotherms, the initial concentration of Cu(II) was from 10 to 2000 mg/L, and other experimental conditions were the same as above.
The adsorption efficiency of Cu(II) by biochar is expressed by the adsorption capacity qe and the removal rate E, and the calculation formulas are shown in eqs 1 and 2, respectively.
| 1 |
| 2 |
In the formula, qe (mg/g) is the adsorption capacity at equilibrium of the biochar on Cu(II), c0 (mg/L) is the initial concentration of Cu(II), ce (mg/L) is the concentration of the Cu(II) solution at adsorption equilibrium, V (mL) is the volume of the Cu(II) solution, m (mg) is the quality of biochar added, and E (%) is the removal efficiency.
Eq 3 shows the pseudo-first-order (PFO) dynamic model, while the pseudo-second-order (PSO) dynamic model is shown in eq 4.
| 3 |
| 4 |
In the equation, k1 (min–1) and k2 (g/(mg·min)) represent the reaction rate constants of the first-order and second-order kinetic equations. T (min) is the reaction time and qt and qe (mg/g) are the adsorption capacities at time and the completion of adsorption equilibrium, respectively. The commonly used adsorption isotherm equations are the Freundlich and Langmuir equations. The Langmuir isotherm equation is shown in eq 5, and eq 6 shows the Freundlich isotherm model.
| 5 |
| 6 |
Here, ce (mg/L) is the concentration of the adsorbate at saturation, kF (mg/g) is the adsorption capacity, nF is a constant, qm (mg/g) is the maximum adsorption capacity of Langmuir, and kL (L/mg) is the Langmuir constant.
2.5. Desorption and Reuse Experiment
A total of 1 M CH3COONa was used in the desorption experiment of the adsorbing Cu(II) biochar, using a 200 mg/L Cu(II) solution, and the rest of the experimental setup was the same as the initial adsorption experiment.
3. Results and Discussion
3.1. Physicochemical Properties of Biochar
The SBET surface area and pore size structure of different adsorbents are shown in Table 1. The modification of KMnO4 under high temperature changed the surface morphology of the magnetic biochar, and the SBET of the biochar prepared with KMnO4 as the modifier increased greatly, which was due to the further reaction between KMnO4 and the organic matter in the loofah sponge biochar at high temperatures, promoting the formation of pore structures. Besides, the strong oxidizability of KMnO4 would destroy many micropores and form many mesopores and macropores at the same time, thus increasing the overall pore volume and the average pore diameter.22
Table 1. Analysis of Surface Area and Pore Size of Different Biochars.
| SBET (m2/g) | Vtot (mm3/g) | pore width (nm) | |
|---|---|---|---|
| LB | 96.70 | 50.16 | 2.08 |
| FLB | 113.24 | 64.89 | 2.29 |
| FMLB | 187.11 | 136.07 | 2.91 |
Table 2 shows the elemental composition analysis of each biochar, from which we can see that the main components of the unmodified loofah sponge biochar are C (79.82%) and O (9.74%), the high content of which might be due to the formation of abundant oxygen-containing functional groups and aromatic carbon in organic components after the pyrolysis. The iron content of FLB and FMLB was over 30%, and the manganese content of FMLB was over 20%, indicating that Fe and Mn have been successfully loaded on the surface and pores of the corresponding biochar, while potassium permanganate modification did not affect the magnetism of the biochar. After KMnO4 modification, the content of O increased; due to this, KMnO4 modification significantly enhanced its polarity and increased the oxygen-containing groups.
Table 2. Element Composition of Different Biochars.
| element
composition (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | C | H | O | Fe | Mn | K | P | Mg | Ca | |
| LB | 0.23 | 79.82 | 2.14 | 9.74 | 2.65 | 0.49 | 0.48 | 3.49 | ||
| FLB | 0.73 | 51.96 | 1.44 | 5.56 | 37.62 | 0.83 | 0.14 | 0.15 | 0.87 | |
| FMLB | 0.41 | 25.78 | 1.04 | 12.29 | 35.79 | 22.38 | 0.63 | 0.09 | 0.15 | 0.88 |
Transmission electron microscopy (TEM) spectrum and mapping analysis of FMLB are shown in Figure 1. It can be seen that the surface of FMLB mainly contains five elements: C, O, N, Mn, and Fe, which is consistent with the above elemental analysis results. Iron and manganese are uniformly distributed on the surface of the biochar, which indicates that potassium permanganate and magnetic modification have been successfully completed.
Figure 1.
TEM and mapping of FMLB.
The surface micromorphology of the adsorbent greatly affects its adsorption performance. Scanning electron microscopy can clearly and intuitively reflect the micromorphology of the biochar. The SEM images of the biochar before and after modification are shown in Figure 2. As can be seen from Figure 2a,b, the surface of the original biochar LB is relatively flat and has a large number of obvious pores. The pores of LB are related to the decomposition of lignocellulose materials at high temperatures. Pyrolysis at high temperatures will lead to the evaporation of volatile compounds and the formation of aromatic carbon structures, which provides a large number of sites for modifiers.33 After Fe(NO3)3·9H2O treatment, the surface of FLB became rougher and formed a layer of dense particles with a spherical morphology, which might be γ-Fe2O3. In Figure 2c,d, the particle size of γ-Fe2O3 is uniform and less than 100 nm, indicating that there is no large-scale agglomeration of nano iron particles. Compared with FLB, the surface of FMLB is rougher, with the stratified structure of micro-/nano-scale MnOx evenly dispersed on it, which proves that there is secondary cracking on the surface of the biochar under high-temperature activation of KMnO4.
Figure 2.
SEM images of (a) LB (5 μm), (b) LB (1 μm), (c) FLB (5 μm), (d) FLB (1 μm), (e) FMLB (5 μm), and (f) FMLB (1 μm).
To better understand the surface functional groups of the biochar before and after modification, XPS and Fourier transform infrared spectroscopy (FTIR) were used to characterize the changes before and after modification. According to the previous peak splitting method,34 O 1s in FMLB could be split into three peaks, which are located at 529.48, 531.36, and 532.88 eV. As can be seen from Figure 3b, the proportions of the metal oxide bond (M–O, lattice oxygen), metal-linked hydroxyl (M–OH), and carbon atom-linked hydroxyl (C–OH) are 38.64, 35.54, and 25.82%, respectively, indicating that most of the oxygen came from metal oxide and metal-linked hydroxyl. MnOx and Mn-OH provide rich active sites for adsorbing Cu(II). To find out the valence state of Mn in FMLB, the Mn 2p spectrum is shown in Figure 3c. The peak spacing between the Mn 2p1/2 peak and the Mn 2p3/2 peak is 11.8 eV. According to the peak splitting method of Tang,35 Mn (2p3/2) can be divided into three valence peaks, namely, Mn2+ (640.21 eV), Mn3+ (641.34 eV), and Mn4+ (644.71 eV), where the figure shows that 67.91% of Mn in FMLB presents the +3 valence state, and Mn2+ and Mn4+ account for about 20 and 10%, respectively.
Figure 3.
XPS spectra of (a) wide scan of FLB and FMLB, (b) O 1s of FMLB, (c) Mn 2p of FMLB, (d) Fe 2p of FLB, and (e) Fe 2p of FMLB.
The valence states of iron in FLB and FMLB are different. As shown in Figure 3d,e, the peaks of Fe 2p1/2 and Fe 2p3/2 are located at 724.43 and 709.75 eV. According to Lu and Fu’s peak separation method,36 octahedral Fe2+ is located at 709.75 eV and octahedral Fe3+ and tetrahedral Fe3+ are located at 710.98 and 714.36 eV, respectively. Before modification, the proportions of Fe2+ and Fe3+ in the magnetic biochar were 45.74 and 54.26%, respectively. After KMnO4 modification, the proportion of Fe2+ decreased to 18.08% and Fe3+ increased to 81.92%, which indicated that the iron-containing components in FLB were oxidized after KMnO4 modification, and the valence of Mn in FMLB also decreased accordingly.
Fourier transform infrared spectroscopy can test the chemical structure of compounds by detecting the molecular vibration and rotation, including various surface functional groups, such as amino, carbonyl, carboxyl, and so on. As Figure 4 shows, the peaks around 3400 cm–1 indicated the vibrations of −OH, and those of 1610 cm–1 were relevant to C=C as well as C=O.37,38 Moreover, the peak around 1100 cm–1 corresponded to the Si–O–Si absorption.39 It should be noted that the content of oxygen-containing groups in LB increased by modification with potassium permanganate.
Figure 4.
FTIR spectra of biochar prepared with different modification processes.
The wettability of the magnetic biochar and the modified magnetic biochar will also affect the adsorption performance,40 so the contact angle between the surfaces of FLB and FMLB and deionized water was measured in this study (Figure 5). Two kinds of biochar materials were obtained by carbonization at medium and low temperatures, and they are hydrophilic materials because they have more functional groups.41 After KMnO4 modification, FMLB showed better hydrophilic performance than FLB, and the enhancement of hydrophilic performance was mainly due to the production of more O-containing functional groups by surface oxidation, such as −OH. The enhancement of hydrophilicity can make FMLB accelerate the transport of pollutants and substances on the biochar surface in the process of removing heavy metals and improve the adsorption rate of the adsorbent.42
Figure 5.
Contact angles of (a) FLB and (b) FMLB in deionized water.
3.2. Cu(II) Adsorption Kinetics and Isotherm Study
3.2.1. Adsorption Isotherm
The adsorption isotherm describes how the adsorbate interacts with the adsorbent, which is very important for optimizing the use of the adsorbent.43Figure 6 presents the adsorption isotherms of FLB and FMLB adsorbing Cu(II), and Table 3 lists their fitting parameters. As the Cu(II) concentration in the solution increased, the adsorption capacity of the biochar increased. In the initial stage, the adsorption capacity increased rapidly, which indicated that there are many active sites on the biochar surface. The more the active sites, the more favorable it is to adsorb Cu(II). However, when the concentrations reached about 35 and 50 mg/L, the adsorption capacity did not increase greatly, which was limited by the active sites.44 The R2 values (0.9704 and 0.9474) of the Langmuir model of FLB and FMLB are greater than those of the Freundlich model (0.8619 and 0.9106), indicating that the adsorption is homogeneous adsorption, which assumes monolayer adsorption, and maximum adsorption occurs when adsorbed molecules on the surface of the adsorbent form a saturated layer.45
Figure 6.
Adsorption isotherms of FLB and FMLB.
Table 3. Regression Parameters of the Cu(II) Adsorption Isotherm.
| Freundlich |
Langmuir |
|||||
|---|---|---|---|---|---|---|
| KF | n | R2 | Qmax | KL | R2 | |
| FLB | 5.8541 | 0.2699 | 0.8619 | 40.9836 | 0.0090 | 0.9704 |
| FMLB | 15.7484 | 0.1611 | 0.9106 | 47.6417 | 0.0291 | 0.9474 |
Table 4 lists the comparison of the maximum adsorption capacity of different adsorbents for Cu(II) in previous studies. The compiled data suggested that the FMLB showed a comparable adsorption capacity to biomass-derived adsorbent materials reported in previous studies for Cu(II) removal.
Table 4. Comparison of Adsorption Capacities of Cu(II) by Different Biochar Adsorbents.
3.2.2. Adsorption Kinetics
Pseudo-first-order (PFO) and pseudo-second-order (PSO) adsorption kinetic models represent the mononuclear and binuclear adsorption processes in a liquid–solid system, respectively.51Figure 7 shows that the adsorption kinetics for heavy metals fit well with the PSO model, and Table 5 shows that their correlation coefficients (R2) are better than PFO. During the first few minutes of the reaction, the adsorption rate was faster due to more adsorption sites. With the passage of time, the active sites were gradually occupied by Cu(II) ions, making the adsorption rate slow down and finally reach equilibrium. The PSO model is account of the assumption that the rate-limiting step of adsorption is chemical adsorption.52 Therefore, the adsorption of Cu(II) by FMLB belongs to chemical adsorption, and the adsorption process of FLB and FMLB for heavy metals reaches equilibrium within about 10 h, indicating that KMnO4 modification can enhance the adsorption capacity, while the adsorbing rate was not accelerated.
Figure 7.
Adsorption kinetic curves of FLB and FMLB.
Table 5. Regression Parameters on Cu(II) Adsorption Kinetics.
| PFO |
PSO |
|||||
|---|---|---|---|---|---|---|
| Qe | k1 | R2 | Qe | k2 | R2 | |
| FLB | 23.5704 | 0.4029 | 0.9507 | 25.5704 | 0.0195 | 0.9605 |
| FMLB | 36.7547 | 0.3776 | 0.9425 | 40.6143 | 0.0115 | 0.9648 |
3.3. Influence of Solution Parameters on the Adsorption Capacities of Cu(II)
3.3.1. Influence of Adsorbent Dosage
The amount of adsorbent is an important consideration affecting the adsorption process. Too little adsorbent may lead to a poor effect, while too much adsorbent may lead to low efficiency. Figure 8 shows the adsorption effect with different doses of biochar. With the increase of the amount of adsorbent, the amount of pollutants adsorbed by the unit adsorbent decreases. With the increase of adsorbent dosage, the removal rate increases, while the amount of pollutants adsorbed by the unit adsorbent decreases. When the amount of adsorbent rises from 10 to 20 mg, the adsorption capacity slightly reduces, while the removal ratio increases sharply. Then, with the continuous increase of adsorbent dosage, the adsorption capacity per unit decreased sharply, and the removal ratio of the adsorbent rose slowly. As a result, 20 mg was the optimal dosage for Cu(II) removal in this experiment.
Figure 8.
Influence of adsorbent dosage on Cu(II) removal by (a) FLB and (b) FMLB.
3.3.2. Influence of Solution pH
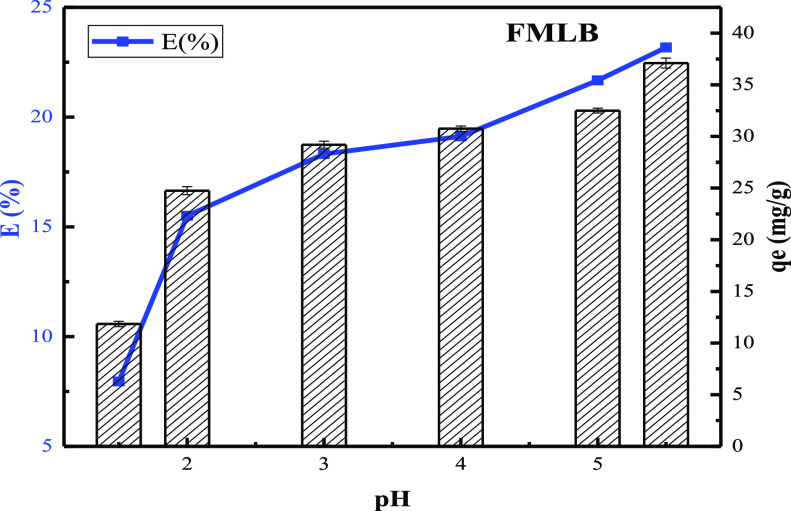
The pH degree of aqueous solutions is one of the most significant control parameters in the process of heavy metal adsorption. It influences the surface charge of the adsorbent and the ionization degree and the existing form of heavy metal ions in solution.53Figure 9 exhibits the influence of solution pH value on removing Cu(II). With the increase of pH value from 1 to 5, the removal ratio of Cu(II) rose and attained the maximum at a pH value of 5.5. This can be explained as follows: at a lower pH, the solution has a high concentration of H+ ions, and its high mobility will compete with metal ions for the active sites on the adsorbent surface. With the increase of pH, the H+ ion concentration decreases, resulting in the increase of copper ion adsorption, which leads to the increase of the adsorption capacity of Cu(II). The zero charge point of FMLB is 3.09, which implies that the surface of FMLB is negatively charged at the optimum pH (5.5). This suggests that electrostatic interactions may be the predominant mechanism for the adsorption of Cu(II) under this condition.
Figure 9.
Influence of solution pH on Cu(II) removal by FMLB.
3.3.3. Cycle Performance of the Adsorbent
The reusability of the adsorbent is also very important for adsorption performance. Figure 10 shows the adsorption characteristics of FMLB for Cu(II) under four cycles, and Cu(II) desorption was carried out by 1 M CH3COONa in the cycle gap. The figure shows that the removal efficiency of FMLB for Cu(II) reaches 92.5% after the first cycle and gradually decreases to 75.7% after three cycles, which is about 37 mg/g. The decrease of adsorption capacity may be caused by structural damage and loss of surface-active mineral components.54
Figure 10.
Desorption–adsorption cycle of Cu(II) by FMLB.
3.4. Adsorption Mechanism of Cu(II)
For the purpose of analyzing the adsorption behaviors of Cu(II) on the surface of FMLB, Figure 11 shows the FTIR spectrum of FMLB before and after adsorption. The absorption peak at 3600–3200 cm–1 after adsorption of Cu(II) is passivated, which is weaker than that before adsorption. It may be that Cu(II) reacts with −OH or −NH on the surface of FMLB by forming monodentate inner-sphere complexes. The peak intensity of the carboxyl group (C=C and C=O) at 540–1620 cm–1 decreased, indicating that the carboxyl group was consumed during adsorption.
Figure 11.
FTIR spectrum of FMLB before and after Cu(II) adsorption.
XPS spectra were used for characterization before and after adsorption. According to the peak separation results of the FMLB high-resolution O 1s spectrum of the adsorbent in Figure 12, after adsorption of heavy metal Cu(II), the area of the M–OH peak increased from 35.54 to 72.79%, while the area of the M–O peak at 530.1 eV decreased from 38.64 to 21.89%, and the area of the C–OH peak at 532.4 eV decreased from 25.5% to 5.31%. This indicated that the sites participating in the reaction in the adsorption process are mainly hydroxyl linked by carbon −OH and metal oxide bonds M–O.
Figure 12.
O 1s spectra of FMLB (a) before and (b) after adsorbing Cu(II).
In general, the MnOx particles loaded on the surface of magnetic biochar after KMnO4 modification were the main reason for the increase of adsorption capacity, while the increase of oxygen-containing functional groups on the surface of magnetic biochar due to the oxidation of KMnO4 also increased the adsorption capacity.
3.5. Magnetic Recovery Characteristics of Modified Biochar
Magnetic properties of FMLB were studied by a hysteresis loop. The hysteresis loop in Figure 13 is an S-shaped curve, and the FMLB sample possesses magnetism. The saturation magnetization reached 10.41 emu/g, ensuring that the permanent magnet can be separated from the aqueous solution. The magnetism of FMLB can be used to separate the adsorbent from the solution, thus saving cost and avoiding secondary pollution. In addition, the magnetic loss of FMLB after adsorption is less than that of FLB, which indicates that after KMnO4 treatment, the magnetic properties of the biochar are more stable, which is beneficial to the long-term recycling of adsorbents.
Figure 13.
Hysteresis loops of FLB and FMLB before and after adsorption.
4. Conclusions
FMLB, the KMnO4-modified magnetic loofah biochar, was successfully synthesized for Cu(II) adsorption. The adsorption process of Cu(II) was well fitted by the pseudo-second-order (PSO) kinetic model and the Langmuir isotherm model. The adsorption process on the surface of FMLB is a homogeneous adsorption, and chemical adsorption dominated the adsorption process. The optimal adsorbent dosage for Cu(II) removal was 20 mg, and the ideal pH value was 5.5. The adsorption performance of FMLB can be maintained above 75% after four cycles. The complexes with MnOx and oxygen-containing functional groups enhance the adsorption process, and the analysis of the adsorption mechanism shows that electrostatic interaction, physical adsorption, and ion exchange also play an important part in removing Cu(II). The saturation magnetization of FMLB was about 10.41 emu/g, ensuring that it could be a stable adsorbent for long-term recycling.
Acknowledgments
This research was financially supported by Key-Area Research and Development Program of GuangDong Province (2019B110209003); the National Natural Science Funds of China (51906045); and the Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), (GML2019ZD0101); Guangdong Basic and Applied Basic Research Foundation (2021B1515020068).
Author Contributions
# F.Z. and R.S. authors contributed equally to this work.
The authors declare no competing financial interest.
References
- Bolisetty S.; Peydayesh M.; Mezzenga R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. 10.1039/C8CS00493E. [DOI] [PubMed] [Google Scholar]
- Zheng B. Y.; Huang G. H.; Liu L. R.; Zhai M. Y.; Li Y. Inter-regional cluster analysis of heavy-metal emissions. J. Cleaner Prod. 2021, 282, 124439 10.1016/j.jclepro.2020.124439. [DOI] [Google Scholar]
- Nagajyoti P. C.; Lee K. D.; Sreekanth T. V. M. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 2010, 8, 199–216. 10.1007/s10311-010-0297-8. [DOI] [Google Scholar]
- Li C. M.; Wang H. C.; Liao X. L.; Xiao R.; Liu K. H.; Bai J. H.; Li B.; He Q. Heavy metal pollution in coastal wetlands: A systematic review of studies globally over the past three decades. J. Hazard. Mater. 2022, 424, 127312 10.1016/j.jhazmat.2021.127312. [DOI] [PubMed] [Google Scholar]
- Antonovics J.; Bradshaw A. D.; Turner R. G.. Heavy Metal Tolerance in Plants. In Advances in Ecological Research; Cragg J. B., Ed.; Academic Press, 1971; pp 1–85. [Google Scholar]
- Fu F. L.; Wang Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage. 2011, 92, 407–418. 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Bailey S. E.; Olin T. J.; Bricka R. M.; Adrian D. D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. 10.1016/S0043-1354(98)00475-8. [DOI] [Google Scholar]
- Afrooz M. R.; Moghadas B. K.; Tamjidi S. Performance of functionalized bacterial as bio-adsorbent for intensifying heavy metal uptake from wastewater: A review study. J. Alloys Compd. 2022, 893, 162321 10.1016/j.jallcom.2021.162321. [DOI] [Google Scholar]
- Qiu B. B.; Tao X. D.; Wang H.; Li W. K.; Ding X.; Chu H. Q. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081 10.1016/j.jaap.2021.105081. [DOI] [Google Scholar]
- Lu Z.; Zhang H.; Shahab A.; Zhang K.; Zeng H. T.; Bacha A. U. R.; Nabi I.; Ullah H. Comparative study on characterization and adsorption properties of phosphoric acid activated biochar and nitrogen-containing modified biochar employing Eucalyptus as a precursor. J. Cleaner Prod. 2021, 303, 127046 10.1016/j.jclepro.2021.127046. [DOI] [Google Scholar]
- Beesley L.; Moreno-Jimenez E.; Gomez-Eyles J. L.; Harris E.; Robinson B.; Sizmur T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. 10.1016/j.envpol.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Tan X. F.; Liu Y. G.; Zeng G. M.; Wang X.; Hu X. J.; Gu Y. L.; Yang Z. Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. 10.1016/j.chemosphere.2014.12.058. [DOI] [PubMed] [Google Scholar]
- Li R. H.; Wang J. J.; Zhou B. Y.; Zhang Z. Q.; Liu S.; Lei S.; Xiao R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Cleaner Prod. 2017, 147, 96–107. 10.1016/j.jclepro.2017.01.069. [DOI] [Google Scholar]
- Cheng N.; Wang B.; Wu P.; Lee X.; Xing Y.; Chen M.; Gao B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448 10.1016/j.envpol.2021.116448. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Singh E.; Mishra R.; Kumar S. Biochar as environmental armour and its diverse role towards protecting soil, water and air. Sci. Total. Environ. 2022, 806, 150444 10.1016/j.scitotenv.2021.150444. [DOI] [PubMed] [Google Scholar]
- Zou W. H.; Han R. P.; Chen Z. Z.; Zhang J. H.; Shi J. Kinetic study of adsorption of Cu(II) and Pb(II) from aqueous solutions using manganese oxide coated zeolite in batch mode. Colloids Surf., A 2006, 279, 238–246. 10.1016/j.colsurfa.2006.01.008. [DOI] [Google Scholar]
- Kamran U.; Park S. J. MnO2-decorated biochar composites of coconut shell and rice husk: An efficient lithium ions adsorption-desorption performance in aqueous media. Chemosphere 2020, 260, 127500 10.1016/j.chemosphere.2020.127500. [DOI] [PubMed] [Google Scholar]
- Li B.; Yang L.; Wang C. Q.; Zhang Q. P.; Liu Q. C.; Li Y. D.; Xiao R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. 10.1016/j.chemosphere.2017.02.061. [DOI] [PubMed] [Google Scholar]
- Giachino A.; Focarelli F.; Marles-Wright J.; Waldron K. J. Synthetic biology approaches to copper remediation: bioleaching, accumulation and recycling. FEMS Microbiol. Ecol. 2021, 97, fiaa249 10.1093/femsec/fiaa249. [DOI] [PubMed] [Google Scholar]
- Wang H. Y.; Gao B.; Wang S. S.; Fang J.; Xue Y. W.; Yang K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. 10.1016/j.biortech.2015.08.132. [DOI] [PubMed] [Google Scholar]
- Xiao J.; Hu R.; Chen G. C.; Xing B. S. Facile synthesis of multifunctional bone biochar composites decorated with Fe/Mn oxide micro-nanoparticles: Physicochemical properties, heavy metals sorption behavior and mechanism. J. Hazard. Mater. 2020, 399, 123067 10.1016/j.jhazmat.2020.123067. [DOI] [PubMed] [Google Scholar]
- Song Z.; Lian F.; Yu Z.; Zhu L.; Xing B.; Qiu W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. 10.1016/j.cej.2013.12.061. [DOI] [Google Scholar]
- Li X.; Wang C.; Zhang J.; Liu J.; Liu B.; Chen G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847 10.1016/j.scitotenv.2019.134847. [DOI] [PubMed] [Google Scholar]
- Yi Y. Q.; Huang Z. X.; Lu B. Z.; Xian J. Y.; Tsang E. P.; Cheng W.; Fang J. Z.; Fang Z. Q. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468 10.1016/j.biortech.2019.122468. [DOI] [PubMed] [Google Scholar]
- Han Z. T.; Sani B.; Mrozik W.; Obst M.; Beckingham B.; Karapanagioti H. K.; Werner D. Magnetite impregnation effects on the sorbent properties of activated carbons and biochars. Water Res. 2015, 70, 394–403. 10.1016/j.watres.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Gao B.; Varnoosfaderani S.; Hebard A.; Yao Y.; Inyang M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. 10.1016/j.biortech.2012.11.132. [DOI] [PubMed] [Google Scholar]
- Li Y. F.; Zhang X. Y.; Zhang P. Z.; Liu X.; Han L. J. Facile fabrication of magnetic bio-derived chars by co-mixing with Fe3O4 nanoparticles for effective Pb2+ adsorption: Properties and mechanism. J. Cleaner Prod. 2020, 262, 121350 10.1016/j.jclepro.2020.121350. [DOI] [Google Scholar]
- Nicolaou E.; Philippou K.; Anastopoulos I.; Pashalidis I. Copper Adsorption by Magnetized Pine-Needle Biochar. Processes 2019, 7, 903 10.3390/pr7120903. [DOI] [Google Scholar]
- Xiao F. F.; Cheng J. H.; Cao W.; Yang C.; Chen J. F.; Luo Z. F. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. 10.1016/j.jcis.2019.01.068. [DOI] [PubMed] [Google Scholar]
- Ren L. T.; Yi X. L.; Yang Z. S.; Wang D. F.; Liu L. Q.; Ye J. H. Designing Carbonized Loofah Sponge Architectures with Plasmonic Cu Nanoparticles Encapsulated in Graphitic Layers for Highly Efficient Solar Vapor Generation. Nano Lett. 2021, 21, 1709–1715. 10.1021/acs.nanolett.0c04511. [DOI] [PubMed] [Google Scholar]
- Gupta V. K.; Agarwal S.; Singh P.; Pathania D. Acrylic acid grafted cellulosic Luffa cylindrical fiber for the removal of dye and metal ions. Carbohydr. Polym. 2013, 98, 1214–1221. 10.1016/j.carbpol.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Zeng L. L.; Liu Q.; Lu M.; Liang E. X.; Wang G. X.; Xu W. Y. Modified natural loofah sponge as an effective heavy metal ion adsorbent: Amidoxime functionalized poly(acrylonitrile-g-loofah). Chem. Eng. Res. Des. 2019, 150, 26–32. 10.1016/j.cherd.2019.07.021. [DOI] [Google Scholar]
- Yu J.; Jiang C.; Guan Q.; Ning P.; Gu J.; Chen Q.; Zhang J.; Miao R. Enhanced removal of Cr(VI) from aqueous solution by supported ZnO nanoparticles on biochar derived from waste water hyacinth. Chemosphere 2018, 195, 632–640. 10.1016/j.chemosphere.2017.12.128. [DOI] [PubMed] [Google Scholar]
- Liu J. T.; Ge X.; Ye X. X.; Wang G. Z.; Zhang H. M.; Zhou H. J.; Zhang Y. X.; Zhao H. J. 3D graphene/delta-MnO2 aerogels for highly efficient and reversible removal of heavy metal ions. J. Mater. Chem. A 2016, 4, 1970–1979. 10.1039/C5TA08106H. [DOI] [Google Scholar]
- Tang W. X.; Wu X. F.; Li D. Y.; Wang Z.; Liu G.; Liu H. D.; Chen Y. F. Oxalate route for promoting activity of manganese oxide catalysts in total VOCs’ oxidation: effect of calcination temperature and preparation method. J. Mater. Chem. A 2014, 2, 2544–2554. 10.1039/C3TA13847J. [DOI] [Google Scholar]
- Lu J. W.; Fu F. L.; Ding Z. C.; Li N.; Tang B. Removal mechanism of selenite by Fe3O4-precipitated mesoporous magnetic carbon microspheres. J. Hazard. Mater. 2017, 330, 93–104. 10.1016/j.jhazmat.2017.01.056. [DOI] [PubMed] [Google Scholar]
- Liu J. H.; Huang Z. J.; Chen Z. Y.; Sun J.; Gao Y. H.; Wu E. Y. Resource utilization of swine sludge to prepare modified biochar adsorbent for the efficient removal of Pb(II) from water. J. Cleaner Prod. 2020, 257, 120322 10.1016/j.jclepro.2020.120322. [DOI] [Google Scholar]
- Biniak S.; Szymański G.; Siedlewski J.; Świątkowski A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. 10.1016/S0008-6223(97)00096-1. [DOI] [Google Scholar]
- Dehkhoda A. M.; Ellis N.; Gyenge E. Effect of activated biochar porous structure on the capacitive deionization of NaCl and ZnCl2 solutions. Microporous Mesoporous Mater. 2016, 224, 217–228. 10.1016/j.micromeso.2015.11.041. [DOI] [Google Scholar]
- Cederlund H.; Borjesson E.; Lundberg D.; Stenstrom J. Adsorption of Pesticides with Different Chemical Properties to a Wood Biochar Treated with Heat and Iron. Water, Air, Soil Pollut. 2016, 227, 203 10.1007/s11270-016-2894-z. [DOI] [Google Scholar]
- Suliman W.; Harsh J. B.; Abu-Lail N. I.; Fortuna A. M.; Dallmeyer I.; Garcia-Perez M. The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci. Total Environ. 2017, 574, 139–147. 10.1016/j.scitotenv.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Jayalakshmi A.; Rajesh S.; Senthilkumar S.; Mohan D. Epoxy functionalized poly(ether-sulfone) incorporated cellulose acetate ultrafiltration membrane for the removal of chromium ions. Sep. Purif. Technol. 2012, 90, 120–132. 10.1016/j.seppur.2012.02.010. [DOI] [Google Scholar]
- Foo K. Y.; Hameed B. H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. 10.1016/j.cej.2009.09.013. [DOI] [Google Scholar]
- Dong X. L.; Ma L. N. Q.; Li Y. C. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J. Hazard. Mater. 2011, 190, 909–915. 10.1016/j.jhazmat.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Cao X. D.; Ma L. N.; Gao B.; Harris W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. 10.1021/es803092k. [DOI] [PubMed] [Google Scholar]
- Chen X.; Chen G.; Chen L.; Chen Y.; Lehmann J.; McBride M. B.; Hay A. G. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour. Technol. 2011, 102, 8877–8884. 10.1016/j.biortech.2011.06.078. [DOI] [PubMed] [Google Scholar]
- Kołodyńska D.; Wnętrzak R.; Leahy J. J.; Hayes M. H. B.; Kwapiński W.; Hubicki Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. 10.1016/j.cej.2012.05.025. [DOI] [Google Scholar]
- Yang G.-X.; Jiang H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. 10.1016/j.watres.2013.09.050. [DOI] [PubMed] [Google Scholar]
- Wang H.; Gao B.; Wang S.; Fang J.; Xue Y.; Yang K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. 10.1016/j.biortech.2015.08.132. [DOI] [PubMed] [Google Scholar]
- Zuo X.; Liu Z.; Chen M. Effect of H2O2 concentrations on copper removal using the modified hydrothermal biochar. Bioresour. Technol. 2016, 207, 262–267. 10.1016/j.biortech.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Ho Y. S.; McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. 10.1016/S0032-9592(98)00112-5. [DOI] [Google Scholar]
- Vadivelan V.; Kumar K. V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. 10.1016/j.jcis.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Dai M. G. Mechanism of adsorption for dyes on activated carbon. J. Colloid Interface Sci. 1998, 198, 6–10. 10.1006/jcis.1997.5254. [DOI] [Google Scholar]
- Shan R.; Shi Y. Y.; Gu J.; Bi J. W.; Yuan H. R.; Luo B.; Chen Y. Aqueous Cr(VI) removal by biochar derived from waste mangosteen shells: Role of pyrolysis and modification on its absorption process. J. Environ. Chem. Eng. 2020, 8, 103885 10.1016/j.jece.2020.103885. [DOI] [Google Scholar]