Abstract
Recent advances in quantifying nucleophilic reactivities in chemical reactions and intermolecular interactions of aromatic molecules are reviewed. This survey covers experimental (IR frequency shifts induced by hydrogen bonding) and theoretical (modeling of potential energy surfaces, atomic charges, molecular electrostatic potential) approaches in characterizing chemical reactivity. Recent advances in software developments assisting the evaluation of the reactive sites for electrophilic aromatic substitution are briefly discussed.
Introduction
Characterizing reactivities of molecular systems is a major theme of chemical research. Various methods have been employed in establishing relationships between chemical structure and reactivity. Possibilities to predict properties of complex molecules with the aid of theoretical or derived from experiment relationships are of critical importance in synthetic chemistry, the developments of new drugs for medical use, the synthesis of materials with specific functionalities, the synthesis of novel components for energy conversion systems, and many other applications. In organic chemistry, the seminal studies of Hammett1 were the most significant step forward in transforming the field into a quantitative science. The insights on the dependencies between specific structural characteristics and reactivity provided by the Hammett equation have enabled the establishment of major concepts in organic chemistry, such as inductive, mesomeric, and steric effects. The applications of the Hammett approach have also contributed to understanding the mechanisms of a number of organic reactions, especially when combined with kinetic measurements and spectroscopic observations. Besides numerous applications,2,3 more recent studies have focused on the theoretical concepts associated with the substituent constants, as well as the development of novel approaches for their evaluation.3−7
The impressive progress in the development of quantum mechanical methods and computational technologies during the past half-century has offered new opportunities for advances in the field of chemical reactivity. Even earlier, the frontier orbital methodology of Fukui8 provided key insights into the electronic factors governing chemical reactivity. The development of reactivity theory within the formalism of density functional theory9,10 has opened new opportunities for the quantitative description of the overall reactivity of chemical systems. Domingo et al.11 underlined the importance of the electron density distribution as a critical factor determining the ability of molecules to interact with other chemical systems. A significant breakthrough in the field was achieved by the application of the molecular electrostatic potential to the study of molecular structure and the energetics of intermolecular interactions and chemical reactions.12−14
Since the pioneering investigations of Hammett,1 aromatic systems have been the focus in the development of reactivity concepts, methodologies, and applications.1−8 The present Mini-Review covers some recent approaches in quantifying the reactivity of aromatic compounds using both experimental and theoretical approaches.
Results and Discussion
Modeling of Potential Energy Surfaces for Reactions of Aromatic Compounds
Numerous studies have investigated the potential energy surfaces for reactions involving aromatic compounds. In this section, we will only illustrate the potential of theoretical methods in characterizing aromatic reactivity by the recent studies of Brinck and co-workers15 and Liu16 on the potential energy surfaces for electrophilic aromatic substitution (EAS) reactions. Brinck and co-workers15 investigated the potential energy surfaces for 18 reactions of electrophilic halogenation of benzene derivatives with Cl2 in water medium. The strength of these studies is underlined by the excellent correspondence between the theoretically evaluated barrier heights and the experimental kinetic data. Table 1 presents the results of these authors. The experimental barrier heights are from literature sources (see ref (15) and references therein). The energies of critical structures along the reaction paths are evaluated using the M06-2X/6-311G(d,p) method. The effect of solvent is simulated with the IEF-PCM method. Both overall reactivity and positional selectivity are reliably predicted by these transition state theory computations. The results of Brinck et al.15 illustrate the power of contemporary theoretical methods in evaluating reaction mechanisms and characterizing reactivities of organic molecules
Table 1. Theoretically Evaluated Free Energy Differences (ΔG, in kcal·mol–1) for Critical Structures along the Reaction Paths–the First Transition State (TS1) and the σ-Complex Intermediate—for Chlorination of Monosubstituted Benzenes with Cl2 in Water Solvent with Comparison to Experimental Dataa.
| X | isomer | TS1 | σ-complex | exp.b |
|---|---|---|---|---|
| OCH3 | o- | 0.1 | 3.1 | 0.8 |
| m- | 9.1 | 19.8 | - | |
| p- | 0.0 | 0.0 | 0.0 | |
| CH3 | o- | 0.6 | 0.6 | 0.0 |
| m- | 3.2 | 4.8 | 2.8 | |
| p- | 0.0 | 0.0 | 0.2 | |
| F | o- | 1.2 | 2.7 | 1.3 |
| m- | 5.5 | 8.4 | - | |
| p- | 0.0 | 0.0 | 0.0 | |
| Cl | o- | 0.1 | 2.0 | 0.4 |
| m- | 3.7 | 5.7 | - | |
| p- | 0.0 | 0.0 | 0.0 | |
| CF3 | o- | 1.5 | 2.0 | 1.4 |
| m- | 0.0 | 0.0 | 0.0 | |
| p- | 2.3 | 2.4 | 1.8 | |
| CN | o- | 0.1 | 0.4 | 0.4 |
| m- | 0.0 | 0.0 | 0.0 | |
| p- | 1.4 | 1.6 | 1.0 |
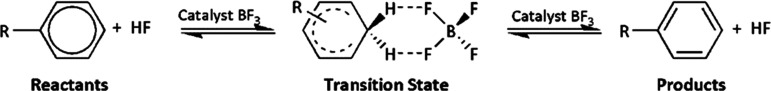
In a valuable theoretical work, Liu16 investigated the identity exchange reactions of a series of substituted benzenes with hydrogen fluoride, using boron trifluoride as catalyst in the gas phase (Scheme 1). The barrier heights for 37 reactions modeling substitutions at the meta and para positions of 18 monosubstituted benzenes derivatives as well as the unsubstituted benzene were theoretically evaluated with the M06-2X/aug-cc-pVDZ method by applying transition state theory computations (Table 2). In addition, Liu used theoretical Hirshfeld charges in rationalizing the reactivity of the benzene derivatives in the electrophilic substitutions investigated. Very good correlations between barrier heights and Hirshfeld charges at the ring carbons were obtained. The plot of ΔE vs qC(Hirsh) is shown in Figure 1.
Scheme 1. Identity EAS Exchange Reactions of Benzene Derivatives with Hydrogen Fluoride Studied in the Work of Liu.16 Adapted with Permission from Ref (16). Copyright 2015 American Chemical Society.
Table 2. Barrier Heights (in kcal·mol–1) for the Identity Exchange EAS Reaction of Monosubstituted Benzenes with Hydrogen Fluoride and Hirshfeld Charges (in Electrons) Are Given for meta and para Carbons from M06-2X/aug-cc-pVDZ Computationsa.
| R | isomer | q(Hirsh) | barrier height | R | isomer | q(Hirsh) | barrier height |
|---|---|---|---|---|---|---|---|
| H | –0.0498 | 28.41 | tBu | m- | –0.0514 | 27.63 | |
| Cl | m- | –0.0414 | 31.57 | p- | –0.0554 | 25.34 | |
| p- | –0.0488 | 27.35 | CCl3 | m- | –0.0417 | 31.10 | |
| Et | m- | –0.0506 | 27.79 | p- | –0.0387 | 31.89 | |
| p- | –0.0554 | 25.23 | CF3 | m- | –0.0399 | 32.23 | |
| F | m- | –0.0419 | 31.64 | p- | –0.0368 | 33.16 | |
| p- | –0.0560 | 25.38 | CHO | m- | –0.0449 | 31.16 | |
| Me | m- | –0.0506 | 28.45 | p- | –0.0337 | 32.96 | |
| p- | –0.0558 | 24.59 | CN | m- | –0.0380 | 33.47 | |
| NH2 | m- | –0.0501 | 30.29 | p- | –0.0326 | 33.94 | |
| p- | –0.0717 | 11.09 | COF | m- | –0.0408 | 33.00 | |
| NMe2 | m- | –0.0525 | 29.83 | p- | –0.0292 | 34.90 | |
| p- | –0.0748 | 12.18 | NH3+ | m- | –0.0082 | 53.02 | |
| OH | m- | –0.0474 | 29.56 | p- | –0.0048 | 54.92 | |
| p- | –0.0658 | 16.69 | NO2 | m- | –0.0365 | 34.29 | |
| Pr | m- | –0.0508 | 28.49 | p- | –0.0296 | 35.37 | |
| p- | –0.0554 | 24.69 | NO | m- | –0.0420 | 31.85 | |
| p- | –0.0298 | 34.08 | |||||
| SO3H | m- | –0.0356 | 33.68 | ||||
| p- | –0.0296 | 35.33 |
Adapted from ref (16), with permission from the author.
Figure 1.
Strong correlation for 38 electrophilic aromatic substitution reactions between the computed barrier heights and the Hirshfeld charges on the regioselective carbon atoms in Scheme 1.
The theoretical research of Liu16 illustrates very well the effects of aromatic substituents on the reaction barriers for a considerable number of EAS reactions but also proposes a suitable theoretical quantity—Hirshfeld charge on the regiospecific carbon atom—as a nucleophilic reactivity descriptor. The investigation of Liu16 underlines the usefulness of theoretically evaluated quantities in describing the reactivity of aromatic molecules. The application of several differently defined atomic charges in quantifying aromatic reactivity is discussed later in the present survey.
Applications of O–H Stretching Frequency Shifts upon Hydrogen Bonding in Quantifying Aromatic Reactivity
The measurements of kinetic constants for a series of compounds provide a direct assessment of reactivity as well as insights concerning the structural factors determining the reaction rates. Kinetic studies involve, nonetheless, considerable experimental effort and the necessity of special equipment. For many systems, the investigator needs a more efficient approach in characterizing reactivity and positional selectivity. Quantum chemistry computations and various theoretical reactivity parameters provide appropriate solutions in this respect. In spite of the success of the theoretical procedures developed, it has always been important to employ suitable experimental quantities that would provide sufficiently reliable advance information on reactivity and positional selectivity. Basicity scales for molecules in the gas phase and in solution have found wide application in this respect. Laurence et al.17 defined the basicity of an extensive number of organic molecules belonging to different classes on the basis of measured infrared frequency shifts resulting from hydrogen bonding with proton donating species, containing an O–H group. In recent studies,18 we explored the possibility of applying the shifts of O–H stretching frequencies [Δν(OH)exp] for selected proton donor molecules upon hydrogen bonding as experimental measures of reactivity for series of aromatic molecules. We investigated the relationships between the Δν(OH)exp shifts induced by π-hydrogen bonding with the aromatic π-electron system and kinetic constants for electrophilic aromatic nitration reactions, as well as the quaternization of pyridine derivatives with methyl iodide.
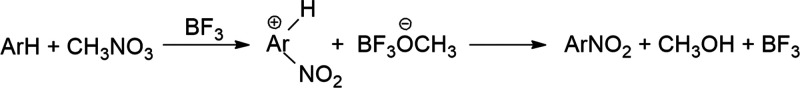
Nitration of Aromatic Derivatives by Methyl Nitrate
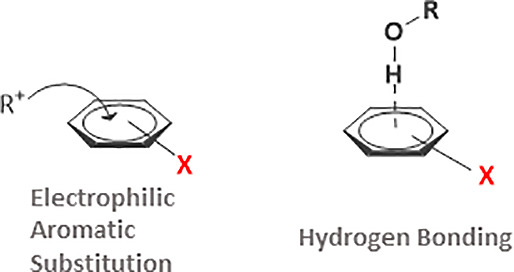
The mechanisms of electrophilic aromatic substitution (EAS) reactions involve an initial interaction of the attaching electrophile with the π-electrons of the aromatic species.2 Considering the nature of this initial step, we investigated the correlations between phenol Δν(OH)exp shifts induced by π-hydrogen bonding with aromatic substrates. The experimentally recorded phenol Δν(OH)exp shifts are juxtaposed to the relative rate constants (log krel) for the nitration of a series of aromatic derivatives (methylbenzenes, monoalkylbenzenes, halobenzenes, and anisole) by methyl nitrate in the presence of BF3 in nitromethane solvent (literature kinetic data, see footnotes to Table 1 in ref (18)). The reaction is illustrated in Scheme 2.
Scheme 2. Schematic Representation for CH3ONO2–BF3 Rrene Nitration.
The rate constants reported in Table 3 reflect the overall reactivity of the aromatic derivatives in the studied reactions, which may involve substitutions at different ring positions of the same compound. Therefore, no perfect correlation between the Δν(OH)exp shifts and log krel can be expected. It should be emphasized that measuring the shifts of the phenol O–H stretching frequency, as induced by π-hydrogen bonding with the aromatic ring, is a very simple experiment that only requires the recording of a single IR spectrum for the complex formed. Therefore, the established relationship (Figure 2) offers a straightforward experimental approach in characterizing the nucleophilic reactivity of aromatic molecules. Limitations to the proposed approach may arise from the necessity that the initial interaction between the aromatic substrate and the attacking electrophile controls the reaction rate. As is well-known, nonetheless, aromatic rings richer in electron density participate more readily in EAS reactions.2
Table 3. Relative Overall Rate Constants (log krel)a for the Nitration of Benzene, Alkylbenzenes, Halobenzenes, and Anisole. Also Reported Are Shifts of the Molecular Electrostatic Potential at 1.5 Å over the Centroid of the Aromatic Ring; and Shifts of Phenol ν(OH) Stretching Frequencies (in cm–1) upon π-Hydrogen Bonding in CCl4 Solventa.
| species | log krela | Δν(OH)exp [cm–1] | ΔV (1.5) (a.u.) B3LYP/6-311++G(3df,2pd)b |
|---|---|---|---|
| benzene | 0.00 | 48 | 0.0000 |
| toluene | 1.41 | 58 | –0.0029 |
| ethylbenzene | 1.36 | 59 | –0.0033 |
| isopropylbenzene | 1.32 | 55 | –0.0030 |
| tert-butylbenzene | 1.29 | 60 | –0.0033 |
| 1,2-dimethylbenzene | 2.28 | 68 | –0.0048 |
| 1,3-dimethylbenzene | 2.46 | 69 | –0.0051 |
| 1,4-dimethylbenzene | 2.47 | 71 | –0.0050 |
| 1,2,3-trimethylbenzene | 2.96 | 75 | –0.0073 |
| 1,2,4-trimethylbenzene | 3.03 | 78 | –0.0072 |
| 1,3,5-trimethylbenzene | 2.98 | 77 | –0.0075 |
| 1,2,3,4-tetramethylbenzene | 3.33 | 87 | –0.0095 |
| 1,2,3,5-tetramethylbenzene | 3.27 | 88 | –0.0098 |
| 1,2,4,5-tetramethylbenzene | 3.34 | 85 | –0.0091 |
| pentamethylbenzene | 3.41 | 93 | –0.0117 |
| fluorobenzene | –0.92 | 38 | 0.0114 |
| chlorobenzene | –1.52 | 33 | 0.0134 |
| bromobenzene | –1.52 | 37 | 0.0140 |
| iodobenzene | –1.00 | 38 | |
| anisole | 2.27 | 60 | –0.0020 |
| rc | 0.991 | 0.969 |
Relative rate with respect to benzene (kbenzene = 1). See ref (18) and references therein for the sources of experimental kinetic data and Δν(OH)exp shifts.
Theoretical computations for modeled nitromethane solvent.
The correlation coefficients (absolute values are shown) refer to the linear regressions between the experimental log krel values and the predicted values according the 2nd order polynomial expressions.
Figure 2.
Plot (2nd order polynomial) of relative rate constants (log krel) for the nitration of benzene, alkylbenzenes, halobenzenes, and anisole vs shifts of phenol ν(OH) stretching frequency shifts upon π-hydrogen bonding.
An alternative theoretical approach in rationalizing reactivities was also tested for the nitration reaction considered. It was established that the change of the molecular electrostatic potential (MESP) over the centroid of the aromatic ring (at 1.5 Å over the ring) may also be employed in quantifying reactivity. The MESP is defined by the relation:12
| 1 |
In this expression, ZA and RA are the charge and position vector of nucleus A, ρ(r) is the electron density function, and r′ is an integration variable. The last column in Table 3 lists the shifts of MESP values [ΔV(1.5)], relative to benzene] for the compounds of the series investigated. The B3LYP/6-311++G(3df,2pd) and MP2/6-311++G(3df,2pd) computations were applied in estimating ΔV(1.5).18 The integral equation formalism of the polarizable continuum model (IEF-PCM) method was applied in modeling the nitromethane solvent. Table 3 shows the results from B3LYP computations. A quite satisfactory correlation (r = 0.969) between log krel and ΔV(1.5) was found. As discussed, for a number of compounds from the series, monosubstitutions at different positions in the ring are possible. Thus, the reported rate constants reflect the overall reactivity of the aromatic species. If the analysis is restricted to aromatic substrates with identical positions for monosubstitution (Table 4), an excellent correlation between log krel and ΔV(1.5) is obtained (r = 0.998).
Table 4. Relative Rate Constants (log krel)a for the Nitration of Alkylbenzenes with Identical Position for Substitution, Computed Values of Molecular Electrostatic Potentials at 1.5 Å over the Ring Centroid (in a.u., Relative to Benzene) from MP2/6-311++G(3df,2pd) Computations.
| species | log krela | ΔV(1.5) |
|---|---|---|
| benzene | 0.00 | 0.0000 |
| 1,4-dimethylbenzene | 2.47 | –0.0050 |
| 1,3,5-trimethylbenzene | 2.98 | –0.0076 |
| 1,2,3,4-tetramethylbenzene | 3.33 | –0.0094 |
| 1,2,3,5-tetramethylbenzene | 3.27 | –0.0098 |
| 1,2,4,5-tetramethylbenzene | 3.34 | –0.0090 |
| pentamethylbenzene | 3.41 | –0.0116 |
| r | 0.998 |
From ref (18) and references therein.
Quaternization of Substituted Pyridines with Ethyl Iodide
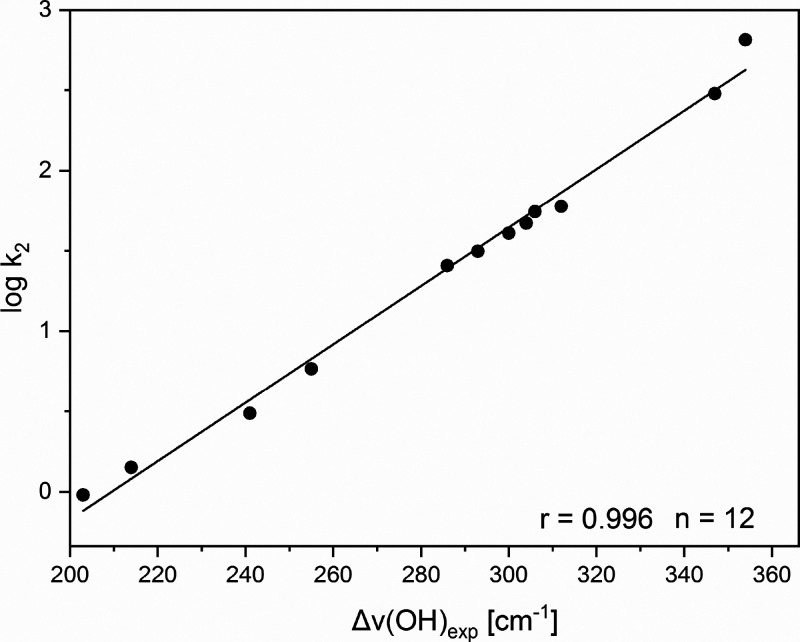
In a recent study,19 the relationships between electronic structure parameters and shifts of O–H stretching frequency upon hydrogen bonding for series of substituted pyridines were analyzed. Johnson et al.20 reported the rates of quaternization of 3- and 4-substituted pyridines with ethyl iodide in different solvents. As already discussed, Laurence et al.17 have characterized the basicity of organic molecules belonging to different classes by studying the IR frequency shifts resulting from hydrogen bonding with O–H group containing proton donors. These investigations included a series of six-membered aromatic N-heterocycles.21Table 5 illustrates the dependence between the measured rate constants for the quaternization reaction and the shifts of O–H stretching frequencies of 4-fluorophenole (in CCl4 solvent) induced by hydrogen bonding with substituted pyridines. A very high correlation coefficient (r = 0.996) characterizes the relationship between these two quantities. Table 5 includes derivatives, for which both rate constants and Δν(OH)exp shifts are available. The plot between log k2 and Δν(OH)exp is shown in Figure 3.
Table 5. Rate Constants for the Quaternization of 3- and 4-Substituted Pyridines with Ethyl Iodidea Compared to Δν(OH)exp Shifts of 4-Fluorophenol upon Hydrogen Bonding.
Figure 3.
Rates of quaternization of substituted pyridines by ethyl iodide vs Δν(OH)exp shifts of 4-fluorophenole induced by hydrogen bonding with the pyridine derivatives. Reprinted with permission from ref (19). Copyright 2022 Elsevier.
Hydrogen Bonding Probes Electron Density Variations at the Basic Center in Series of Aromatic Molecules
In this section, we cover recent research that focuses on the possibilities of gaining insights into the trend of electron density variations at a reaction center within a series of structurally related aromatic molecules. The shifts of O–H stretching frequency of a selected proton-donating molecule upon hydrogen bonding is employed as an experimental probe. In the general case, the frequency shifts induced by hydrogen bonding have been shown to characterize the overall basicity of molecules.17 In addition to the electron density at the proton-accepting basic center, other factors may also influence the reactivity of molecules in hydrogen bonding within a series of related molecules. These factors include steric hindrance, effects of molecular strain, as well as the presence strongly polarizing substituents in close proximity of the basic center. These additional factors may obscure the potential good correspondence between electron density variations and experimental spectroscopic shifts. It is, therefore, of interest to explore the correlations between Δν(OH)exp shifts induced by hydrogen bonding and theoretical electronic structure parameters for structurally related aromatic molecules. Two series of aromatic derivatives were investigated: substituted alkyl benzoates and substituted pyridines.
Substituted Alkyl Benzoates
The relationships between the shifts of methanol O–H stretching frequencies upon hydrogen bonding with the carbonyl basic center for a series of substituted methyl and ethyl benzoates and theoretical quantities characterizing the electron densities at the proton accepting atom were examined in recent research.22 Four different types of atomic charges for the monomeric aromatic molecules were evaluated from ωB97X-D/6-311++G(3df,2pd) and MP2/6-311++G(3df,2pd) computations. The conductor-like polarized continuum model (CPCM) was employed in simulating the CCl4 solvent used in recording the experimental IR spectra. Table 6 presents the results from the ωB97X-D method. Strong correlations were found between the Δν(OH)exp shifts and atomic charges (Hirshfeld, CM5, NPA) for the carbonyl oxygen (qO) in the monomeric benzoates. Because of the different assumptions in evaluating atomic charges, relationships involving these theoretical quantities are not expected to be characterized by very high correlation coefficients. For the series of meta- and para-substituted alkyl benzoates, however, excellent correlations between qO and Δν(OH)exp shifts were established. As discussed, the Δν(OH)exp shifts characterize the overall basicity of molecules.17 This investigation22 goes a step further by considering the relationship between electron densities and spectroscopic shifts. The strong correlations established between the Δν(OH)exp and qO for the series of molecules considered shows that the approach adopted provides an experimental approach to assessing in a quantitative way the trend of variation of the electron density at the proton-accepting atom. Contributions from atom-centered basis function to the molecular electron density are employed in defining the NPA charges. The Hirshfeld and CM5 charges are obtained from procedures involving partitioning of the electron density into atomic domains. The absolute magnitude of charges is quite different for the three methods tested. Nonetheless, the correlations of with the Δν(OH)exp shifts are quite coherent. The results obtained suggest that the variation of charges provides physical insights, while their magnitude as obtained from the different methods is of lesser significance. The magnitude of charge densities, associated with a given atom in a molecule, is certainly outside the scope of the above spectroscopic experiments.
Table 6. Experimental Hydrogen Bond Induced Δν(OH)exp shifts (cm–1) vs Atomic Charges at the Carbonyl Oxygen of the Monomer Esters (in Electrons)a,b for a Series of Substituted Alkyl Benzoates.
| molecule | qO(Hirsh) | qO(CM5) | qNPAOqO(NPA) | Δν(OH)exp |
|---|---|---|---|---|
| ethyl benzoate | –0.2919 | –0.3420 | –0.6368 | 81 |
| ethyl 4-methylbenzoate | –0.2949 | –0.3450 | –0.6402 | 85 |
| ethyl 3-methylbenzoate | –0.2928 | –0.3431 | –0.6384 | 83 |
| ethyl 4-methoxybenzoate | –0.2982 | –0.3482 | –0.6446 | 88 |
| ethyl 4-(dimethylamino)benzoate | –0.3065 | –0.3562 | –0.6546 | 106 |
| ethyl 4-fluorobenzoate | –0.2920 | –0.3422 | –0.6374 | 76 |
| ethyl 3-fluorobenzoate | –0.2876 | –0.3377 | –0.6310 | 74 |
| ethyl 4-(trifluoromethyl)benzoate | –0.2851 | –0.3353 | –0.6285 | 67 |
| ethyl 4-bromobenzoate | –0.2890 | –0.3392 | –0.6333 | 72 |
| ethyl 4-cyanobenzoate | –0.2827 | –0.3329 | –0.6254 | 60 |
| diethyl terephthalate | –0.2872 | –0.3374 | –0.6306 | 69 |
| ethyl 4-nitrobenzoate | –0.2809 | –0.3312 | –0.6232 | 57 |
| methyl benzoate | –0.2901 | –0.3402 | –0.6351 | 76 |
| methyl 4-methylbenzoate | –0.2933 | –0.3432 | –0.6386 | 80 |
| methyl 4-methoxybenzoate | –0.2976 | –0.3475 | –0.6444 | 90 |
| methyl 4-(dimethylamino)benzoate | –0.3051 | –0.3548 | –0.6532 | 107 |
| methyl 4-fluorobenzoate | –0.2903 | –0.3403 | –0.6358 | 78 |
| methyl 4-chlorobenzoate | –0.2878 | –0.3378 | –0.6323 | 74 |
| methyl 4-bromobenzoate | –0.2873 | –0.3373 | –0.6316 | 74 |
| methyl 4-cyanobenzoate | –0.2808 | –0.3309 | –0.6235 | 60 |
| methyl 4-nitrobenzoate | –0.2792 | –0.3294 | –0.6214 | 58 |
| correlation coefficient, rb | 0.988 | 0.987 | 0.989 |
Atomic charges evaluated using the Hirshfeld, CM5 and NPA methods from ωB97X-D/6-311++G(3df,2pd) density functional theory computations (for references to the theoretical methods employed and the experimental Δν(OH)exp shifts in CCl4 solvent see ref (22)).
Absolute values of correlation coefficients.
Substituted Pyridines
Comparing experimental Δν(OH)exp shifts with theoretically evaluated charges in the series of pyridine derivatives offers another excellent platform for testing the application of spectroscopic quantities in following electron density variations. This possibility was explored recently19 by analyzing the relationships between theoretical charge density parameters and Δν(OH)exp shifts for the series of meta- and para-substituted pyridines. Atomic charges from the Hirshfeld population analysis and electrostatic potential at nuclei for the pyridine nitrogen (VN) were evaluated using ωB97X-D/6-311++G(3df,2pd) and MP2/6-311++G(3df,2pd) computations. The CPCM method was applied in modeling the tetrachloromethane solvent. This solvent was applied in the experimental measurement of the Δν(OH)exp shifts. As already discussed, the Hirschfeld charges describe quite well the reactivity of substituted benzenes (Table 2, Figure 1).16 An alternative theoretical quantity was also tested. The electrostatic potential at a nucleus (EPN) is a rigorously defined quantum mechanical quantity. It has been shown previously23 that the electrostatic potential at nuclei provides an accurate description of the ability of molecules to participate in hydrogen bonding. The electrostatic potential at atom Y is defined according to the equation:12
| 2 |
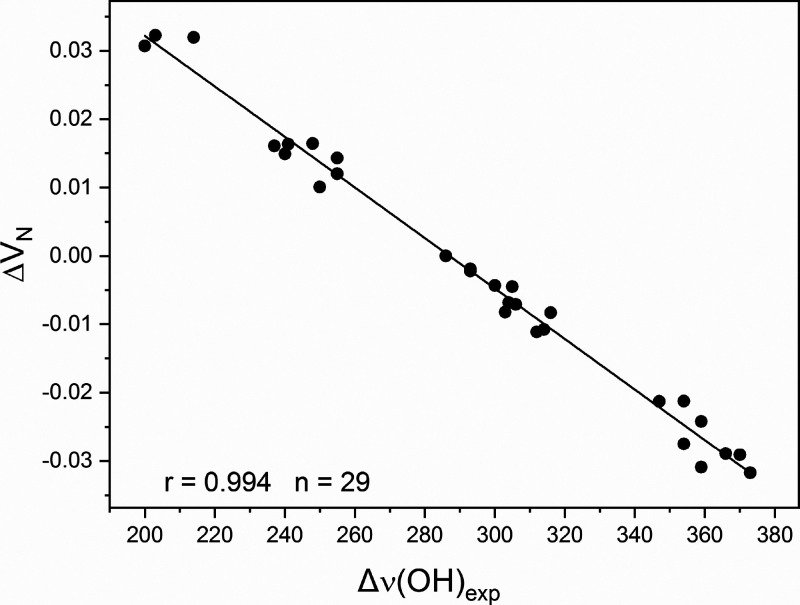
In eq 2, ZA is the charge on nucleus A positioned at RA, and ρ(r) is the electron density function. The singular term for RA = RY is excluded. VY is determined by the electrostatic potential induced at point RY by the positive charges of all nuclei, except for atom Y, and the entire electron density of the molecules. Notably, because of the inverse relationship to RA and r, in series of molecules with substituents positioned at a distance from the atom Y, the electron density in close proximity of the atomic center Y is expected to be a strong factor in determining VY. This is excellently illustrated by the established strong correlation (r = 0.994) between Δν(OH)exp and ΔVN (Table 7, Figure 4). The ΔVN values are the shifts of VN with respect to the unsubstituted pyridine. Politzer, Murrey, and Clark13 argued that electrostatic forces dominate the formation of hydrogen bonds.
Table 7. Experimental Hydrogen Bond Induced Δν(OH)exp shiftsa (cm–1), Hirshfeld, CM5 and NPA Atomic Charges (in electrons),b and Shifts of the Electrostatic Potential at the Nitrogen Atom (ΔVN, a.u.)b,c of Monomer Pyridine Derivatives.
| qN(Hirsh) | qN(CM5) | qN(NPA) | ΔVN | Δν(OH)exp | |
|---|---|---|---|---|---|
| pyridine | –0.1825 | –0.3776 | –0.4790 | 0.0000 | 286 |
| 3-fluoropyridine | –0.1700 | –0.3653 | –0.4563 | –0.0149 | 240 |
| 3-chloropyridine | –0.1691 | –0.3648 | –0.4588 | –0.0161 | 237 |
| 3-bromopyridine | –0.1687 | –0.3645 | –0.4587 | –0.0163 | 241 |
| 3-benzoylpyridine | –0.1709 | –0.3666 | –0.4697 | –0.0164 | 248 |
| 3-cyanopyridine | –0.1635 | –0.3595 | –0.4632 | –0.0323 | 203 |
| 3-ethylpyridine | –0.1845 | –0.3799 | –0.4743 | 0.0045 | 305 |
| 3-methylpyridine | –0.1842 | –0.3797 | –0.4742 | 0.0043 | 300 |
| 4-methylpyridine | –0.1904 | –0.3854 | –0.4892 | 0.0069 | 304 |
| 4-ethylpyridine | –0.1902 | –0.3852 | –0.4883 | 0.0071 | 306 |
| 4-methoxypyridine | –0.2014 | –0.3960 | –0.5098 | 0.0112 | 312 |
| 4-vinylpyridine | –0.1850 | –0.3799 | –0.4806 | 0.0019 | 293 |
| 4-acetylpyridine | –0.1697 | –0.3651 | –0.4573 | –0.0143 | 255 |
| 4-cyanopyridine | –0.1607 | –0.3563 | –0.4506 | –0.0320 | 214 |
| 4-N,N-dimethylaminopyridine | –0.2184 | –0.4121 | –0.5331 | 0.0289 | 366 |
| 4-phenylpyridine | –0.1854 | –0.3804 | –0.4812 | 0.0022 | 293 |
| 4-piperidinopyridine | –0.2200 | –0.4137 | –0.5347 | 0.0309 | 359 |
| 4-aminopyridine | –0.2122 | –0.4060 | –0.5260 | 0.0213 | 347 |
| 4-chloropyridine | –0.1786 | –0.3740 | –0.4770 | –0.0120 | 255 |
| 4-methylaminopyridine | –0.2182 | –0.4117 | –0.5344 | 0.0275 | 354 |
| 4-pyrrolidinopyridine | –0.2209 | –0.4144 | –0.5373 | 0.0317 | 373 |
| 4-tert-butylpyridine | –0.1906 | –0.3859 | –0.4864 | 0.0083 | 303 |
| 3,4-dimethylpyridine | –0.1918 | –0.3872 | –0.4839 | 0.0108 | 314 |
| 3,5-dimethylpyridine | –0.1859 | –0.3816 | –0.4698 | 0.0083 | 316 |
| 3,5-dichloropyridine | –0.1566 | –0.3529 | –0.4396 | –0.0307 | 200 |
| N-methyl-N-pyridin-4-ylhydrazine | –0.2138 | –0.4077 | –0.5271 | 0.0212 | 354 |
| 4-(4-methylpiperidino)pyridine | –0.2118 | –0.4060 | –0.5216 | 0.0242 | 359 |
| 4-N,N-diethylaminopyridine | –0.2199 | –0.4137 | –0.5344 | 0.0302 | 370 |
| methyl nicotinate | –0.1740 | –0.3693 | –0.4751 | –0.0101 | 250 |
| r (cor. coeff.)d | 0.973 | 0.974 | 0.921 | 0.994 |
In cm–1, from ref (21).
From ωB97X-D/6-311++G(3df,2pd) computations.
Shifts with respect the VN for unsubstituted pyridine.
Absolute values of correlation coefficients.
Figure 4.
Plot of ΔVN vs Δν(OH)exp shifts in substituted pyridines. Reprinted with permission from ref (19). Copyright 2022 Elsevier.
The Hirshfeld charges at the carbonyl oxygen are shown in the second column of Table 6. The relationship between Δν(OH)exp shifts and qN(Hirsh) is characterized by a correlation coefficient r = 0.973. The results obtained show that, as in the case of alkyl benzoates (Table 6), the Δν(OH)exp shifts characterize quite satisfactorily the trend of variations of the electron density at the basic center within the series investigated. As already discussed, the Δν(OH)exp shifts may be taken to represent a measure of the overall basicity of molecules.17 The results obtained reveal that the O–H stretching frequency shifts upon hydrogen bonding may be employed also as a probe of the variations of electron density at a particular basic center in series of structurally related molecules.
The approach outlined is only applicable for cases where the proton accepting site is at a distance from the structural variations, as well as in the absence of significant steric effects. These results underline the importance of hydrogen bonding as a sensitive probe of molecular properties.
Atomic Charges and Aromatic Reactivity
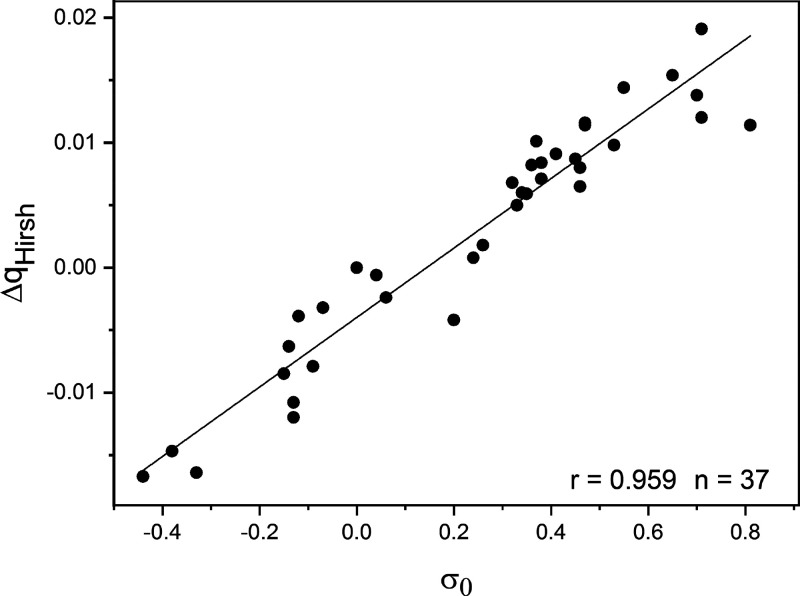
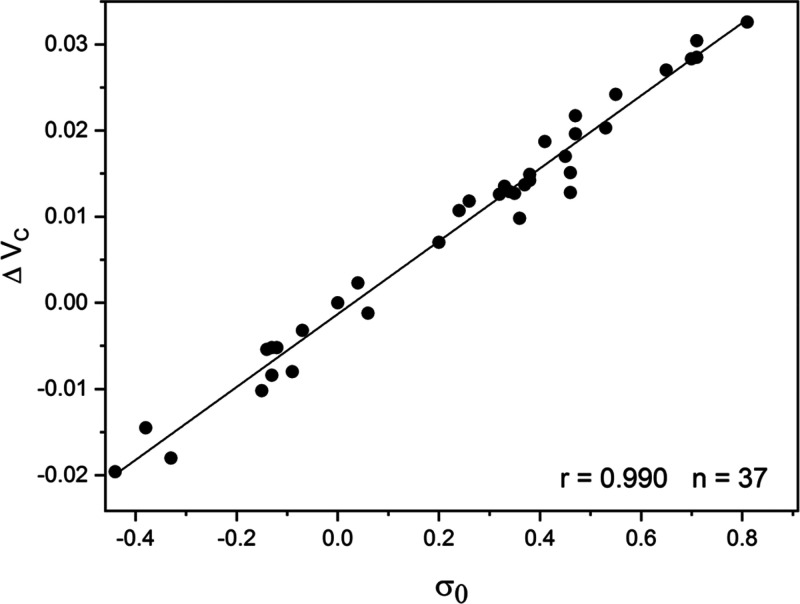
In a recent research study,24 the applicability of several frequently employed methods for atomic charges evaluation in characterizing properties of aromatic molecules was examined. Atomic charges for the ring carbon atoms of monosubstituted benzenes, derived using Mulliken, Hirshfeld, natural population analysis and QTAIM (for references to these theoretical methods, see ref (24)), were correlated with substituent constants for meta and para positions with respect the substituents. The B3LYP and ωB97X-D density functional and MP2 methods combined with the 6-311++G(3df,2pd) basis set were applied in deriving the theoretical quantities. The set of σ0 constants for monosubstituted benzenes, evaluated from experimental kinetic data, were correlated with the theoretical atomic charges (qC). Since the kinetic data are obtained for reactions in different solvents, the theoretical computations refer to molecules in isolation (gas phase). Among the methods tested, the Hirshfeld charges for the meta and para ring carbons provided the most significant correlation with the σ0 constants. The best results are obtained from MP2/6-311++G(3df,2pd) method, with a correlation coefficient r = 0.959. This plot between ΔqHirsh and σ0 constants is illustrated in Figure 5 (for table data, see ref (24)). ΔqHirsh is the difference in carbon charge between the substituted derivative and benzene. The electrostatic potential at the respective carbon atoms (ΔVC) provides superior correlations with the σ0 constants: r = 0.990 for the two DFT methods applied and r = 0.980 for the MP2 results. The correlation between VC and σ0 is shown in Figure 6 (for table data, see ref (24)).
Figure 5.
Correlation between shifts of Hirshfeld charges (ΔqHirsh) for carbon atoms at meta and para positions in monosubstituted benzenes [calculated with the MP2/6-311++G(3df,2pd) method] and σm0 and σp0 substituent constants.
Figure 6.
Plot between σ0 constants and ΔVC (in a.u.) from ωB97X-D/6-311++G(3df,2pd) computations. ΔVC is the difference in VC values for the monosubstituted derivative and benzene.
As already discussed, in an earlier study, Liu16 showed that Hirshfeld charges for the ring carbons are reliable descriptors of reactivity and regioselectivity for the identity exchange reactions of substituted benzenes with hydrogen fluoride.
Molecular Electrostatic Potential and Aromatic Reactivity
Novel studies of chemical reactivity often employ theoretically estimated molecular electrostatic potential values. An excellent review on the subject was recently published by Gadre et al.14 The authors provide a historical overview of theoretical concepts associated with the molecular electrostatic potential as well as recent advances in its applications in characterizing molecular properties and reactivity. In an early investigation Politzer et al.25 employed minima of the surface electrostatic potential (Vmin) in interpreting the effects of amino and nitro substituents on properties of the aromatic ring. Later Gadre and Suresh4 applied Vmin estimates in analyzing the positional reactivity of substituted benzenes.
As already discussed in the present survey, MESP values at selected points in the molecular space may be employed in rationalizing and predicting chemical reactivity trends for reactions of aromatic compounds. It was also shown in earlier studies that the electrostatic potential at nuclei (eq 2) may be employed as a reactivity descriptor of the reactivity of molecules in hydrogen bonding.23 The theoretically evaluated VC values for the meta and para carbon atoms of an extended series of monosubstituted benzenes characterize quantitatively both overall reactivity and positional selectivity.7 On the basis of these results, an efficient computational approach for evaluation of aromatic substituent constants was proposed.7 The power of EPN in rationalizing and predicting the experimental O–H frequency shifts induced by hydrogen bonding for the N-heteroaromatic pyridine derivatives was already discussed in the present survey (as illustrated in Figure 4).
Software Developments and Machine Learning
The search for fast and efficient approaches to molecular design has led to the development of specialized software and machine learning methods in achieving these goals. Aromatic and heteroaromatic molecules are basic structures employed in the developments of novel drugs and various derivatives, finding application in material science as well as energy conversion and conservation devices. These developments are based on established correlations between structural characteristics and regiospecific reactivities.
Kromann et al.26 designed software for prediction of the regiospecific reactivity of electrophilic aromatic substitution reactions. The RegioSQM software, developed by these authors, is based on the well-known dependence between site-specific reactivities and proton affinities at the ring carbons for aromatic molecules. Semiempirical methods (PM6-DH+, PM6, PM7, PM3, or AM1) are applied for the efficient evaluation of proton affinities. The method was tested for 525 literature examples of EAS reactions and is established to predict correctly the site of substitution in over 96% of cases. The input is simplified and requires the SMILES string of the molecule analyzed.
With the aim of facilitating the search for molecules with suitable pharmacokinetic properties, Tomberg et al.27 proposed a machine learning model for predicting the most reactive site for electrophilic aromatic substitution. Six quantum mechanical descriptors are employed in defining the structure–reactivity relationship. These include partial charges, C–H bond orders, sum of bond orders around a carbon atom, condensed Fukui coefficients for electrophilic attack, atomic solvent accessible surface, and reduced-RegioSQM energies, employing the RegioSQM software.26 Based on a considerable number of literature experimental data for EAS reactions, mostly halogenations, the procedure provided 93% accuracy of predictions.
The present Mini-Review covers selected developments in describing the reactivity of aromatic compounds from the past few years. A comprehensive survey of the literature on the topic published prior to 2016 is provided in the review of Brinck and Liljenberg.28
Conclusions
Recent studies reveal novel approaches in describing the reactivity of aromatic systems in chemical reactions and intermolecular interactions. A simple experimental methodology utilizing the shifts of O–H stretching frequency in the IR spectra of selected proton donor molecules upon hydrogen bonding predicts with good accuracy the nucleophilic reactivity for several series of aromatic and heteroaromatic molecules. Theoretical quantities obtained from quantum mechanical computations for the aromatic reactants in the ground state also reveal an excellent potential in rationalizing and predicting the regiospecific aromatic reactivities. Strong correlations of molecular electrostatic potential values at selected points of the molecular space (above the centroid of the aromatic ring, at the positions of the ring carbons, and at the minima of the surface electrostatic potential) for the aromatic reactants and experimental kinetic data are obtained. Atomic charges from the Hirshfeld population analysis offer a good basis for interpretation and prediction of both overall reactivity and positional selectivity for reactions of aromatic compounds. Theoretically evaluated potential energy surfaces for electrophilic aromatic substitution reactions via applications of transition state theory were shown to provide comprehensive information on both reaction mechanisms and reactivity trends. Specialized software products, based on quantum reactivity descriptors, have been designed aiming at automated determination of the most reactive sites for electrophilic aromatic substitutions.
Acknowledgments
Financial support from the Bulgarian National Science Fund (BNSF), Grant KP-06-H39/11/2019 (Project SOFIa), and the U.S. National Science Foundation, Grant CHE No. 2134792, is gratefully acknowledged.
Biographies
Boris Galabov – Prof. Boris Galabov received his Ph.D. in 1975 from the University of Sofia. His current research interests include studies on the application of computational and spectroscopic methods in studies of organic reaction mechanisms, including electrophilic aromatic substitutions, ester aminolysis, amide hydrolysis, carbamate aminolysis, as well as in hydrogen bonding and describing chemical reactivity.
Henry F. Schaefer III – Before moving to Georgia, Henry F. Schaefer III was for 18 years a Professor of Chemistry at the University of California, Berkeley. At the University of Georgia, Schaefer is Graham Perdue Professor of Chemistry and Director of the Center for Computational Quantum Chemistry.
Sonia Ilieva – Prof. DSc Sonia Ilieva, is head of the Department of Pharmaceutical and Applied Organic Chemistry, Faculty of Chemistry and Pharmacy, Sofia University. Her scientific interests are in the fields of computational chemistry and biochemistry, solving organic reaction mechanisms with quantum chemistry methods and molecular spectroscopy, and in QSAR studies.
Diana Cheshmedzhieva – Associate professor Diana Cheshmedzhieva received her Ph.D. in 2009 from the University of Sofia. Her research interests are focused on modeling the mechanisms of organic reactions and quantitative description of the reactivity of organic molecules.
Valya Nikolova – Associate professor Valya Nikolova received her Ph.D. degree in 2008 from the University of Sofia. Her research interests include studies of hydrogen bonding, characterizing chemical reactivity of molecules and host–guest interactions of biomolecules.
Vassil A. Popov – A student with strong interests in the application of computational methods in research.
Boriana Hadjieva – Associate professor Boriana Hadjieva received her Ph.D. in 1990 from the University of Sofia. Her research interests are in synthetic organic chemistry, spectroscopic studies of organic reactions, including electrophilic aromatic substitutions, amide hydrolysis, ester aminolysis, as well as spectroscopic characterization of the conformation of amides and thioamides.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Hammett L. P. Some relations between reaction rates and equilibrium constants. Chem. Rev. 1935, 17, 125–136. 10.1021/cr60056a010. [DOI] [Google Scholar]
- Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds; Mortier J., Ed.; Wiley: Hoboken, NJ, 2016. [Google Scholar]
- Krygowski T. M.; Stepien B. T. Sigma- and pi-electron delocalization: focus on substituent effects. Chem. Rev. 2005, 105, 3482–3512. 10.1021/cr030081s. [DOI] [PubMed] [Google Scholar]
- Gadre S. R.; Suresh C. H. Electronic Perturbations of the Aromatic Nucleus: Hammett Constants and Electrostatic Potential Topography. J. Org. Chem. 1997, 62, 2625–2627. 10.1021/jo961679l. [DOI] [PubMed] [Google Scholar]
- Seybold P. G. Quantum Chemical-QSPR Estimation of the Acidities and Basicities of Organic Compounds. Adv. Quantum Chem. 2012, 64, 83–104. 10.1016/B978-0-12-396498-4.00015-6. [DOI] [Google Scholar]
- Galabov B.; Ilieva S.; Schaefer H. F. An Efficient Computational Approach for the Evaluation of Substituent Constants. J. Org. Chem. 2006, 71, 6382–6387. 10.1021/jo0605288. [DOI] [PubMed] [Google Scholar]
- Szatylowicz H.; Siodla T.; Stasyuk O. A.; Krygowski T. M. Towards Physical Interpretation of Substituent Effects: The Case of meta- and para- substituted Anilines. Phys. Chem. Chem. Phys. 2016, 18, 11711–11721. 10.1039/C5CP06702B. [DOI] [PubMed] [Google Scholar]
- Fukui K.; Yonezawa T.; Shingu H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. 10.1063/1.1700523. [DOI] [Google Scholar]
- Parr R. G.; Yang W.. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, 1989. [Google Scholar]
- Geerlings P.; De Proft F.; Langenaeker W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1873. 10.1021/cr990029p. [DOI] [PubMed] [Google Scholar]
- Domingo L. R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. 10.3390/molecules21101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politzer P.; Truhlar D. G.. Chemical Applications of Atomic and Molecular Electrostatic Potentials; Plenum Press: New York, 1981. [Google Scholar]
- Politzer P.; Murray J. S.; Clark T. Mathematical Modeling and Physical Reality in Noncovalent Interactions. J. Mol. Mod. 2015, 21, Article No. 52. 10.1007/s00894-015-2585-5. [DOI] [PubMed] [Google Scholar]
- Gadre S. R.; Suresh C. H.; Mohan N. Electrostatic Potential Topology for Probing Molecular Structure, Bonding and Reactivity. Molecules 2021, 26, 3289. 10.3390/molecules26113289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenberg M.; Stenlid H. J.; Brinck T. Theoretical Investigation into Rate-Determining Factors in Electrophilic Aromatic Halogenation. J. Phys. Chem. A 2018, 122, 3270–3279. and references therein 10.1021/acs.jpca.7b10781. [DOI] [PubMed] [Google Scholar]
- Liu S. Quantifying Reactivity for Electrophilic Aromatic Substitution Reactions with Hirshfeld Charge. J. Phys. Chem. A 2015, 119, 3107–3111. 10.1021/acs.jpca.5b00443. [DOI] [PubMed] [Google Scholar]
- Laurence C.; Graton J.; Berthelot M.; Besseau F.; Le Questel J.-Y.; Lucon M.; Ouvrard C.; Planchat A.; Renault E. An Enthalpic Scale of Hydrogen-Bond Basicity. 4. Carbon π Bases, Oxygen Bases, and Miscellaneous Second-Row, Third-Row, and Fourth-Row Bases and a Survey of the 4-Fluorophenol Affinity Scale. J. Org. Chem. 2010, 75, 4105–4123. and references therein 10.1021/jo100461z. [DOI] [PubMed] [Google Scholar]
- Galabov B.; Koleva G.; Hadjieva B.; Schaefer H. F. III π-Hydrogen Bonding Probes the Reactivity of Aromatic Compounds: Nitration of Substituted Benzenes. J. Phys. Chem. A 2019, 123, 1069–1076. 10.1021/acs.jpca.8b12508. [DOI] [PubMed] [Google Scholar]
- Galabov B.; Popov V. A.; Cheshmedzhieva D.; Ilieva S.; Schaefer H. F. Hydrogen Bonding as a Probe of Electron Density Variations: Substituted Pyridines. Chem. Phys. Lett. 2022, 791, 139378. 10.1016/j.cplett.2022.139378. [DOI] [Google Scholar]
- Johnson C. D.; Roberts I.; Taylor P. G. Pyridine Nitrogen Reactivity. J. Chem. Soc., Perkin Trans. 2 1981, 409–413. 10.1039/p29810000409. [DOI] [Google Scholar]
- Berthelot M.; Laurence C.; Safar M.; Besseau F. Hydrogen-bond Basicity pKHB Scale of Six-Membered Aromatic N-Heterocycles. J. Chem. Soc., Perkin Trans. 2 1998, 283–290. 10.1039/a706696a. [DOI] [Google Scholar]
- Cheshmedzhieva D.; Ilieva S.; Hadjieva B.; Galabov B. Hydrogen Bonding Probes Electron Density Variations at the Basic Center in Substituted Alkyl Benzoates: Theory and Experiment. J. Phys. Org. Chem. 2021, 34, e4258 10.1002/poc.4258. [DOI] [Google Scholar]
- Galabov B.; Bobadova-Parvanova P. Molecular Electrostatic Potential as Reactivity Index in Hydrogen Bonding: An Initio Molecular Orbital Study of Complexes of Nitrile and Carbonyl Compounds with Hydrogen Fluoride. J. Phys. Chem. A 1999, 103, 6793–6799. 10.1021/jp984313g. [DOI] [Google Scholar]
- Nikolova V.; Cheshmedzhieva D.; Ilieva S.; Galabov B. Atomic Charges in Describing Properties of Aromatic Molecules. J. Org. Chem. 2019, 84, 1908–1915. 10.1021/acs.joc.8b02908. [DOI] [PubMed] [Google Scholar]
- Politzer P.; Abrahmsen L.; Sjoberg P. Effects of Amino and Nitro Substituents Upon the Electrostatic Potential of an Aromatic Ring. J. Am. Chem. Soc. 1984, 106, 855–860. 10.1021/ja00316a005. [DOI] [Google Scholar]
- Kromann J. M.; Jensen J. H.; Kruszyk M.; Jessing M.; Jorgensen M. Fast and Accurate Prediction of the Regioselectrivity of Electrophilic Aromatic Substitution Reactions. Chem. Science 2018, 9, 660–665. 10.1039/C7SC04156J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomberg A.; Johansson M. J.; Norrby P.-O. A Predictive Tool for Electrophilic Aromatic Substitutions Using Machine Learning. J. Org. Chem. 2019, 84, 4695–4703. 10.1021/acs.joc.8b02270. [DOI] [PubMed] [Google Scholar]
- Brinck T.; Liljenberg M. In Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds; Mortier J., Ed., Wiley: Hoboken, NJ, 2016; Chapter 4. [Google Scholar]