Abstract

Anionic dyes are one of the most serious contaminants in water as these molecules are known to be toxic to many living organisms. Herein, we report the development of functionalized polyvinylidene fluoride membranes modified with chitosan-coated iron oxide nanomaterials (Fe-PVDF) for the efficient treatment of anionic dye-contaminated water. Aqueous solutions of anionic dyes could be captured rapidly by passing through the functionalized membrane under reduced pressure. Under neutral conditions, Fe-PVDF showed a maximum removal capacity of 74.6 mg/g for Evans blue (EB) through the adsorption process. In addition, the adsorption capacity was significantly enhanced up to 434.78 mg/g under acidic conditions. The adsorption process for EB matched well with the Langmuir model, indicating monolayer adsorption of the dye to the membrane surface. Moreover, Fe-PVDF can be reusable by a simple washing step in an alkaline solution, and thus, the composite membrane was applied several times without a significant decrease in its adsorption performance. The same composite membrane was further applied to the removal of five other different anionic dyes with high efficiencies. The adsorption mechanism can be explained by the electrostatic interaction between the positively charged chitosan and the negatively charged dye as well as the affinity of the sulfate groups in dye molecules for the surface of the iron oxide nanoparticles. The easy preparation and rapid decolorization procedures make this composite membrane suitable for efficient water treatment.
Introduction
As the discharge of pollutants increases due to rapid industrialization, the environment and public health are threatened by exposure to toxic contaminants.1,2 Therefore, it has become an urgent task to remove harmful substances from industrial wastewater. Among the pollutants generated from various processes such as the printing and textile industries, some anionic organic dyes are known to have several toxic effects on living organisms.3,4 Because most of the commercial anionic dyes are well dissolved and chemically stable in aqueous media, they can be persistent in water for long periods.5 Several studies previously reported conventional separation techniques for the treatment of these pollutants, including adsorption,6−11 extraction,12−14 coagulation,15−17 and degradation.18−20 These methods may be useful in certain cases; however, such physicochemical processes often suffer from several technical issues such as high preparation cost, low efficiency, and limited reusability.21−23 In the last decades, many researchers focused on the development of membrane-based techniques for water purification.24,25 Traditional membrane filtration is a technique whereby the contaminants are rejected based on their relative size to the membrane pore size. However, recent progress of nanotechnology enabled the obtention of additional functionalities to the bare polymeric membrane.26−31 Some nanoparticles including ZnO, Fe3O4, and TiO2, as well as various organic ligands incorporated into nanomaterials, have been employed for the preparation of functional membranes. These composites showed enhanced adsorption performance in the treatment of contaminants as the nanoadsorbents have a large area-to-volume ratio as well as high affinity to target pollutants. The purification capacity of composite membranes is determined by the amount of the substance incorporated and the type of chemical or physical interactions between the adsorbents and target pollutants.
We previously reported a gold nanoparticle-immobilized cellulose acetate membrane for the removal of radioactive iodine anions in water through the adsorptive process.32,33 These techniques exhibited improved performances in terms of rapidity, high removal efficiency, and high removal rate compared to conventional methods. Furthermore, the nanoadsorbents embedded in hybrid composites are designed to maintain their chemical stability during water treatment, allowing easy separation after treatment. Inspired by previous studies on nanoadsorbent-embedded membranes, we designed a new interfacial immobilizing strategy by employing a hydrophilic polyvinylidene fluoride (PVDF) membrane and chitosan-coated iron oxide nanoparticles for the removal of organic dyes through the adsorption process. PVDF has been widely applied to the water treatment as it possesses good mechanical properties, thermal resistance, and chemical stability. In addition, the cationic nature of chitosan polymer can be used for the removal of anionic dye contaminants by electrostatic interactions and also useful for the stable immobilization of the nanoadsorbent in the PVDF membrane. In the present work, the nanocomposite membrane is created by the vacuum filtration method. The adsorption and separation performance of the composite membrane using Evans blue (EB) dye is first demonstrated, and then, its reproducibility is evaluated. Moreover, the same functional membrane is further applied to the treatment of aqueous solutions containing distinct anionic dyes.
Experimental Section
Materials
EB, Congo red, Acid Yellow 25, Acid Green 25, and methyl blue were purchased from Sigma-Aldrich (Yongin, Korea). Chitosan-coated iron oxide nanomaterials (γ-Fe2O3, average hydrodynamic diameter: 50 nm) were purchased from Chemicell (Berlin, Germany). The hydrophilic PVDF flat sheet membrane (0.20 μm pore size, 47 mm diameter) was provided by Hyundai micro Co., LTD (Daejeon, Korea). Aqueous hydrochloric acid (aq. HCl, 37%), sodium hydroxide (NaOH), and acetic acid (CH3COOH, 99%) were purchased from the Duksan company (Daejeon, Korea). All reagents were of analytical grade and used without further purification.
Preparation of the Composite Membrane (Fe-PVDF)
A glass vacuum filter assembly was used to prepare the chitosan-coated iron oxide-immobilized PVDF membrane (Fe-PVDF). The vacuum filter unit consisted of a 300 mL graduated funnel, a filter holder fritted-glass support, a recover flask, and a vacuum pump. A hydrophilic PVDF (diameter: 47 mm and pore size: 0.22 μm) was placed between the filter holder fritted-glass support and the graduated funnel (Figure S1). A suspension of chitosan-coated iron oxide (25 mg/mL) was diluted with an aqueous acetic acid (2 wt %) solution. This suspension (17.6 mg/L, 200 mL) was poured into the graduated funnel, and then, vacuum was applied until all suspension passed through the membrane to provide Fe-PVDF. After washing the membrane with deionized water several times, the prepared Fe-PVDF was maintained under ambient conditions until it was used in the adsorption experiment.
Analysis and Characterization of the Composite Membrane (Fe-PVDF)
The anionic dye concentrations in aqueous solutions were measured using a UV–vis spectrophotometer (Shimadzu, UV-1800, Kyoto, Japan). The surface of Fe-PVDF was observed using an FEI Verios 460L field-emission scanning electron microscope under high-performance conditions with accelerating voltages of up to 15 kV. The elemental composition of the composite nanomaterials was determined by scanning electron microscopy (SEM) energy-dispersive X-ray (EDX) (AMITEC) analysis with accelerating voltages of up to 20 kV. EDX spectra were recorded in an area scan mode by focusing the electron beam onto a region of the sample surface. Fourier-transform infrared (FT-IR) spectra of the composite membrane (Fe-PVDF) in the range of 4000–400 cm–1 were recorded using an FT-IR/NIR spectrophotometer (PerkinElmer Inc, Seoul, Korea). The composite membrane decomposition was performed by thermal gravimetric analysis (TGA, TA Instruments, Discovery SDT 650) at a temperature range of 25–800 °C. The heating rate of the TGA was 10 °C/min at an airflow rate of 100 mL/min. The surface charge of the membrane was analyzed using the streaming potential method in an electro-kinetic analyzer (Anton Paar GmbH, Surpass 3, Seoul, Korea).
Stability Test
Fe-PVDF was immersed into a 0.1 N HCl, 0.1 N NaOH, and 1.0 M NaCl solution for 12 h. To determine the amount of iron oxide nanomaterials detached from PVDF, the concentration of iron ions in the supernatant was measured using inductively coupled plasma–mass spectrometry (ICP–MS, PerkinElmer Inc, NexION 2000, Seoul, Korea).
Adsorption of Dyes Using Fe-PVDF under Continuous In-Flow Conditions
The pH of dye solutions was adjusted by adding aqueous HCl or NaOH. To measure the adsorption efficiency of Fe-PVDF, an aqueous solution (50 mL) of varying concentrations (1–100 μmol/L) of anionic dye was poured into Fe-PVDF at a rate of approximately 140 mL/min with an operating pressure of 0.5 bar. The dye concentration in the eluate was determined using UV–vis absorbance spectroscopy. All experiments were performed in triplicate, and the mean values are presented. The adsorption capacity (Qe, mg/g), which is the amount of dye absorbed per unit mass of the absorbent, was calculated using eq 1.
| 1 |
where V is the volume of solution (L), C0 and Cf are the initial and final concentration of the dye in the eluate (mg/L), respectively, and m is the mass of the adsorbent (g).
The removal efficiency (%) defined by eq 2 was used to evaluate the adsorption capability of Fe-PVDF.
| 2 |
To determine the adsorption isotherm, adsorption experiments were conducted with different initial concentrations of EB (1–100 μmol/L). The dye solutions (50 mL) were passed through Fe-PVDF under a reduced pressure (ca. 0.5 bar). The residual EB concentration in the eluate was measured, and Qe at varied initial dye concentration was determined using eq 1. These adsorption data were plotted and fitted using the Langmuir (eq 3) and Freundlich isotherm (eq 4) as follows.
| 3 |
| 4 |
where C0, Ce, and Qe are the same parameters described in eq 1 and Qm (mg/g) is the maximum adsorption capacity of the adsorbent. KL (L/mg) and KF (L–1/n mg1–1/n/g) are the Langmuir and Freundlich constants, respectively.
Regeneration Study
After the adsorption experiment was accomplished, Fe-PVDF was added to an aqueous 0.1 M NaOH solution and agitated for 30 min to accomplish the anionic dye desorption. Subsequently, it was washed with deionized water for 30 min. The same procedure was repeated twice, and then, the composite membrane was reused for the treatment of the same dye solution.
Water Flux Measurement
The pure water flux of Fe-PVDF in each adsorption cycle with pure PVDF was measured using a glass vacuum filter assembly. For 1 min, the volume of pure water filtered using a functional membrane filtration area of 11.34 cm2 was measured under 0.5 bar, which was reduced using a vacuum source. The pure water flux was calculated using eq 5.
| 5 |
where V is the volume of permeated water (L), A is the functional membrane area (m2), Δt is the sampling time (h), and J is the pure water flux (L/m2/h). Deionized water was passed through the membrane under the same conditions used for the measurement of pure water flux before each EB adsorption cycle.
Results and Discussion
Fabrication and Characterization of the Chitosan-Iron Oxide-Incorporated Membrane
The experimental procedure for the adsorption of anionic dyes using the composite membrane is shown in Figure 1. As the first step, chitosan-coated iron oxide nanoparticles were immobilized to the PVDF membrane (Fe-PVDF). Next, the dye solution was passed through Fe-PVDF using a vacuum filtration system. To construct nanoadsorbents, the chitosan-functionalized iron oxide suspension was filtered through a commercially available hydrophilic PVDF, which was placed between the fritted-glass support (Figure S1). This method quickly allowed the preparation of organic–inorganic composite membranes. SEM analysis of the surface of nonmodified PVDF and Fe-PVDF (Figure 2a,b) showed that the nanoparticles were incorporated stably on the fibrils of PVDF. Elemental analysis of the composite membrane using EDX spectroscopy exhibited a set of peaks representing iron, along with fluoride and carbon elements, which were observed from PVDF (Figure 2c,d and Table S1). The obtained Fe-PVDF was also characterized by FT-IR. Figure 3 shows the peaks at approximately 3300 and 600 cm–1 attributed to −OH groups and Fe–O bonds, respectively. Additionally, the bending and stretching vibrations of N–H bond peaks corresponding to the introduction of chitosan-coated nanomaterials to PVDF are observed at approximately 1650 and 3350 cm–1. Moreover, the characteristic adsorption peaks at 1150–1050 cm–1 indicate the aliphatic ether bonds of chitosan. The C–F stretching bands are also observed in the 1400–1000 cm–1 region. These results indicate the incorporation of chitosan-coated nanomaterials on the PVDF membrane.
Figure 1.
Strategy for the removal of anionic dye using the composite membrane (Fe-PVDF).
Figure 2.
SEM images and EDX spectra of pure PVDF (a,c) and Fe-PVDF (b,d), respectively.
Figure 3.

FTIR spectra of pure PVDF (black), Fe-PVDF (blue), and chitosan-coated iron oxide nanoparticles (red).
To investigate the stability of the composite membrane, Fe-PVDF was immersed into aqueous solutions (0.1 M HCl, 0.1 M NaOH, and 1.0 M NaCl) for 12 h. The amount of liberated nanoparticles was determined by measuring iron ions in the solution using ICP–MS. As shown in Figure 4a, less than 0.3% of iron oxide was released from the solid support under low and high pH for 12 h, and most of the nanoparticles remained stable in the PVDF membrane. Considering that the adsorption of dye molecules can be accomplished in a short time compared to the above stability test, the observed stability is good enough to use Fe-PVDF in the adsorption experiment. Furthermore, the PVDF-supported iron oxide nanoparticles were bench-stable under dry conditions and thus could be stored for several weeks without losing stability. The stability may be attributed to the strong electrostatic interactions between the positively charged chitosan-coated nanoparticles (zeta potential = +21.2 mV at neutral pH) and negatively charged membrane (zeta potential = −18.9 mV at neutral pH). In addition, dipole interactions between electron-poor methylene (−CH2−) groups in the PVDF chain and oxo- or hydroxy groups on the surface of iron oxide resulted in the stable immobilization of nanoadsorbents in the polymeric membrane. Subsequently, TGA analysis was performed to investigate the amount of inorganic components incorporated in the composite membrane (Figure 4b). The weight of both nonmodified PVDF and Fe-PVDF dropped sharply at approximately 400 °C due to the defluoridation and carbonization of the polymeric backbone (red and blue lines, respectively). Moreover, the weights of the membranes remained constant above 550 °C because of the completely decomposed organic molecules. The results showed that the weight difference between bare PVDF and Fe-PVDF was 3.52% above 600 °C. Considering that the average weight of Fe-PVDF was 63 mg, the amount of inorganic iron oxide in the composite membrane was 2.22 mg. A weight loss of 17.1% was observed when chitosan-coated iron oxide nanoparticles were exposed to high temperatures (>600 °C), resulting in the decomposition of organic ligands containing chitosan. Therefore, approximately 2.68 mg of chitosan-functionalized nanoparticles could be immobilized on the membrane using the immobilization procedure.
Figure 4.
(a) Quantification of detached iron oxide nanoparticles under acidic, basic, and high-salt conditions analyzed by ICP–MS. (b) TGA curves of chitosan-coated iron oxides, pure PVDF, and Fe-PVDF.
Adsorption of Anionic Dyes Using the Composite Membrane
To investigate the removal efficiency of an anionic dye under continuous in-flow conditions, aqueous solutions in the range of 1–100 μmol/L of EB, which contains four sulfonate groups (net charge = −4), were prepared. In the purification step, the EB solution (pH = 7) was passed through Fe-PVDF at a rate of ca. 140 mL/min under reduced pressure. After each adsorption experiment, the amount of residual dye in the eluate was determined using UV–vis absorbance spectroscopy. As shown in Figure 5, the observed adsorption capacity of the composite membrane under neutral conditions was 74.6 mg/g. The IR spectra of the dye-adsorbed composite membrane exhibited characteristic peaks of EB and Fe-PVDF (Figure S2). Conversely, the removal ability of the nonmodified bare membrane was insignificant.
Figure 5.
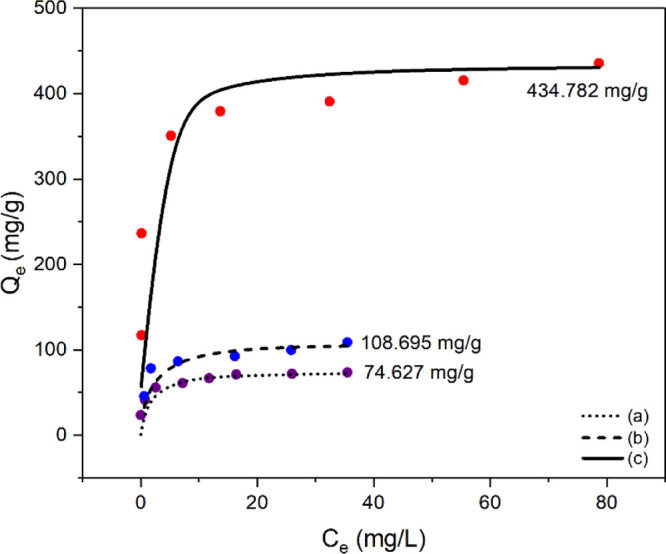
Adsorption capacity (Qe) of Fe-PVDF after filtration of the EB solution (a) under neutral conditions, (b) after pretreatment of the membrane with aqueous HCl, and (c) under pH = 1 solution.
The solution pH is an important factor to enhance adsorption capacity. As shown in Figure S3a, the surface charge of Fe-PVDF depends on the pH value. The incorporation of nanoadsorbents slightly increased the membrane potential, and the isoelectric point of Fe-PVDF was shifted to a higher pH. Therefore, more efficient removal of the anionic dye by Fe-PVDF is expected to occur under low pH than in neutral or alkaline media. As expected, the adsorption capacity (Qe) of Fe-PVDF increased significantly (434.782 mg/g) under pH = 1 (Figure 5). In addition, Figure S3b demonstrated that the Qe value largely depended on the solution pH. These results indicated that the protonation of the chitosan layer (i.e., amino groups in the polymer) in low pH resulted in the enhanced adsorption of anionic dyes by electrostatic interaction. Conversely, at a higher pH, more hydroxyl ions (−OH) in the aqueous media would compete with the negatively charged dye, leading to less overall removal efficiency. Besides, it is known that the sulfate group has a sufficient affinity to be used as an anchoring group on the iron oxide nanoparticles.34,35 Therefore, the sulfate anion(s) of EB can interact with the surface of the iron oxide nanoparticles. Also, the interaction between the functional group of EB (i.e., amino and hydroxy groups) and the functional group of chitosan occurs by hydrogen bonding, which can be another factor contributing to the adsorption of the dye molecule on the composite membrane.
To improve the removal performance, the protonation step was performed before the membrane was subjected to the adsorption procedure. For this, Fe-PVDF was immersed into 0.1 M HCl for several hours to enhance the positive surface charge of the membrane and washed multiple times with pure water. Thereafter, an aqueous solution of EB was passed through the acid-treated Fe-PVDF using the same procedure performed before. As shown in Figure 5, the pretreated membrane provided an enhanced performance (Qe = 108.7 mg/g) compared to the nontreated Fe-PVDF (Qe = 74.6 mg/g). Although this procedure was not as good as the previous experiment, which was conducted with a low pH solution, it does not require adjusting the pH of the dye solution. When a large volume of aqueous contaminants is treated with the composite membrane, the pretreatment approach can be a method of choice for efficient water treatment.
To investigate the adsorption isotherm of EB, the adsorption data were analyzed using the Langmuir and Freundlich equations, respectively. The linear fittings of the graphs with eqs 3 and 4 are shown in Figure S4, and the corresponding adsorption parameters for these models are summarized in Table S2. According to the calculated values, the Langmuir model matches more closely than the other as it provides a higher correlation coefficient value (R2 = 0.9985). Therefore, the adsorption of EB by the Fe-PVDF is monolayer adsorption.
The composite membrane reusability was investigated to evaluate the possibility of practical application. The reusability was determined based on repetitive EB removal experiments. After each adsorption experiment, Fe-PVDF was washed with 0.1 M NaOH solution for the rapid desorption of anionic dye. The composite membrane reusability was investigated for four repeated experiments under the same conditions at an EB concentration of 2.5 μM. As can be seen in Figure 6, the removal efficiencies were retained more than 93% during three cycles of experiments, and then, it was reduced to 81% in the fourth filtration. Repetitive treatment of Fe-PVDF under a strong basic condition for regeneration may lead to a decrease of the membrane capacity. After the fourth cycle, a slightly decreased pure water flux was observed (10,750 L/m2/h) as compared with that obtained in the first experiment (11,800 L/m2/h). These results suggested that Fe-PVDF developed in this study could perform repeated dye removal up to four times while maintaining more than 80% of removal efficiency.
Figure 6.

Effect of adsorption–desorption cycles on anionic dye removal.
Finally, the Fe-PVDF membrane was also applied to remove other anionic molecules, including Acid Yellow 25, Congo red, Acid Green 25, and methyl blue. Table S3 shows the structures and physical characters of the dyes, including charges and maximum UV–vis absorption wavelengths. To investigate the removal efficiency, dye solutions with different initial concentrations were passed through Fe-PVDF. The removal efficiency (%) obtained for the dyes (Figure 7a) was calculated using eq 2; the images of Fe-PVDF after the treatment of anionic dyes are shown in Figure 7b. The removal efficiency gradually decreased as the initial dye concentration increased. This is because the occupied sites of the adsorbent under high concentrations hinder the adsorption of additional anionic dye molecules. Furthermore, under acidic conditions, the decolorization of anionic dyes was accomplished more evidently compared to those obtained under neutral conditions (Figure S5). This phenomenon further proves the electrostatic interaction mechanism for the removal of anionic dyes.
Figure 7.
(a) Comparison of the removal efficiency of five different anionic dyes for Fe-PVDF and (b) images of the membranes before (Fe-PVDF) and after the treatment of anionic dyes.
To date, several functionalized nanomaterials have been employed in batch processing for the removal of organic dyes. In particular, the magnetic property of iron oxide nanomaterials allows the facile separation of the dye-containing adsorbents from water using an external magnet after the purification procedure was accomplished.36−46 The nanoadsorbent-immobilized adsorptive membrane developed in this study does not require the recovery of adsorbents from water, and thus, this approach provides a more convenient decolorization method than the batch process. It should be noted that with a single filtration process using Fe-PVDF, anionic dyes can be quickly removed, and the observed removal efficiency is favorably compared with those obtained in previous studies (Table S4). Moreover, Fe-PVDF exhibited superior performances compared with the similar approach using the functionalized iron oxide-embedded affinity column, which exhibited quite a slow rate of decolorization (ca. 2 mL/min) as well as intrinsic low stability under acidic conditions (pH < 3).47
Conclusions
In the present study, a new adsorptive membrane was developed by loading chitosan-coated iron oxide nanoparticles on the support layer of hydrophilic PVDF. The embedded nanoadsorbents were maintained stably in the polymeric membrane under varied pH and high salt conditions. The immobilization of chitosan-coated iron oxide on the PVDF membrane provided high adsorption capability for the continuous in-flow removal of anionic dyes. Under acidic conditions, the dye removal capacity of Fe-PVDF significantly increased as the positively charged surface of the adsorbent interacted more efficiently with the target pollutant. By using the same approach, we successfully demonstrate the rapid decolorization of five different anionic dyes. Moreover, this membrane shows a favorable regeneration ability up to four cycles without significant reduction in removal efficiency and water flux. These results indicate that Fe-PVDF possesses great potential for removing anionic dyes from water. Further optimization and validation of the adsorption process will be necessary to apply Fe-PVDF to the large-scale treatment of dye-containing wastewater.
Acknowledgments
This research was funded by the National Research Foundation of Korea (grant number: 2019R1F1A106159) and the Basic Study and Interdisciplinary R&D Foundation Fund of the University of Seoul (2020).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06991.
Preparation of Fe-PVDF using a vacuum filtration method, FT-IR spectra of EB and Fe-PVDF-adsorbed EB, zeta potential measurements of PVDF and Fe-PVDF at different pH values, effect of the solution pH on the removal efficiency of EB, adsorption isotherm of EB on Fe-PVDF, adsorption isotherm parameters of EB onto Fe-PVDF, molecular structures and characteristics of the anionic dyes, and comparison of removal capacities for organic dye using iron oxide-based nanoadsorbents (PDF)
Author Contributions
⊥ J.-H.S. and J.E.Y. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Schwarzenbach R. P.; Egli T.; Hofstetter T. B.; von Gunten U.; Wehrli B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. 10.1146/annurev-environ-100809-125342. [DOI] [Google Scholar]
- Tkaczyk A.; Mitrowska K.; Posyniak A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. 10.1016/j.scitotenv.2020.137222. [DOI] [PubMed] [Google Scholar]
- Salleh M. A. M.; Mahmoud D. K.; Karim W. A. W. A.; Idris A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. 10.1016/j.desal.2011.07.019. [DOI] [Google Scholar]
- Shanker U.; Rani M.; Jassal V. Degradation of Hazardous Organic Dyes in Water by Nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. 10.1007/s10311-017-0650-2. [DOI] [Google Scholar]
- Tang Y.; Li M.; Mu C.; Zhou J.; Shi B. Ultrafast and Efficient Removal of Anionic Dyes from Wastewater by Polyethyleneimine-Modified Silica Nanoparticles. Chemosphere 2019, 229, 570–579. 10.1016/j.chemosphere.2019.05.062. [DOI] [PubMed] [Google Scholar]
- Singh N. B.; Nagpal G.; Agrawal S.; Rachna Water Purification by Using Adsorbents: A Review. Environ. Technol. Innovat. 2018, 11, 187–240. 10.1016/j.eti.2018.05.006. [DOI] [Google Scholar]
- Tehrani M. S.; Zare-Dorabei R. Competitive removal of hazardous dyes from aqueous solution by MIL-68(Al): Derivative spectrophotometric method and response surface methodology approach. Spectrochim. Acta, Part A 2016, 160, 8–18. 10.1016/j.saa.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Saghanejhad Tehrani M.; Zare-Dorabei R. Highly efficient simultaneous ultrasonic-assisted adsorption of methylene blue and rhodamine B onto metal organic framework MIL-68(Al): central composite design optimization. RSC Adv. 2016, 6, 27416–27425. 10.1039/c5ra28052d. [DOI] [Google Scholar]
- Ramezani F.; Zare-Dorabei R. Simultaneous ultrasonic-assisted removal of malachite green and methylene blue from aqueous solution by Zr-SBA-15. Polyhedron 2019, 166, 153–161. 10.1016/j.poly.2019.03.033. [DOI] [Google Scholar]
- Yao Y.; Miao S.; Liu S.; Ma L. P.; Sun H.; Wang S. Synthesis, Characterization, and Adsorption Properties of Magnetic Fe3O4@graphene Nanocomposite. Chem. Eng. J. 2012, 184, 326–332. 10.1016/j.cej.2011.12.017. [DOI] [Google Scholar]
- Kiani A.; Haratipour P.; Ahmadi M.; Zare-Dorabei R.; Mahmoodi A. Efficient removal of some anionic dyes from aqueous solution using a polymer-coated magnetic nano-adsorbent. J. Water Supply: Res. Technol. 2017, 66, 239–248. 10.2166/aqua.2017.029. [DOI] [Google Scholar]
- Jin W.; Zhang Y. Sustainable Electrochemical Extraction of Metal Resources from Waste Streams: From Removal to Recovery. ACS Sustainable Chem. Eng. 2020, 8, 4693–4707. 10.1021/acssuschemeng.9b07007. [DOI] [Google Scholar]
- Khanpour R.; Sheikhi-Kouhsar M. R.; Esmaeilzadeh F.; Mowla D. Removal of Contaminants from Polluted Drilling Mud Using Supercritical Carbon Dioxide Extraction. J. Supercrit. Fluids 2014, 88, 1–7. 10.1016/j.supflu.2014.01.004. [DOI] [Google Scholar]
- Guedes-Alonso R.; Sosa-Ferrera Z.; Santana-Rodríguez J. J. An On-Line Solid Phase Extraction Method Coupled with UHPLC-MS/MS for the Determination of Steroid Hormone Compounds in Treated Water Samples from Waste Water Treatment Plants. Anal. Methods 2015, 7, 5996–6005. 10.1039/c5ay00807g. [DOI] [Google Scholar]
- Szyguła A.; Guibal E.; Ariño Palacín M.; Ruiz M.; Sastre A. M. Removal of an Anionic Dye (Acid Blue 92) by Coagulation–Flocculation Using Chitosan. J. Environ. Manag. 2009, 90, 2979–2986. 10.1016/j.jenvman.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Matilainen A.; Vepsäläinen M.; Sillanpää M. Natural Organic Matter Removal by Coagulation During Drinking Water Treatment: A Review. Adv. Colloid Interface Sci. 2010, 159, 189–197. 10.1016/j.cis.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Cui H.; Huang X.; Yu Z.; Chen P.; Cao X. Application Progress of Enhanced Coagulation in Water Treatment. RSC Adv. 2020, 10, 20231–20244. 10.1039/d0ra02979c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashefi S.; Borghei S. M.; Mahmoodi N. M. Covalently Immobilized Laccase onto Graphene Oxide Nanosheets: Preparation, Characterization, and Biodegradation of Azo Dyes in Colored Wastewater. J. Mol. Liq. 2019, 276, 153–162. 10.1016/j.molliq.2018.11.156. [DOI] [Google Scholar]
- Sekar S.; Surianarayanan M.; Ranganathan V.; MacFarlane D. R.; Mandal A. B. Choline-Based Ionic Liquids-Enhanced Biodegradation of Azo Dyes. Environ. Sci. Technol. 2012, 46, 4902–4908. 10.1021/es204489h. [DOI] [PubMed] [Google Scholar]
- Panthi G.; Park M.; Kim H.-Y.; Lee S.-Y.; Park S.-J. Electrospun ZnO Hybrid Nanofibers for Photodegradation of Wastewater Containing Organic Dyes: A Review. J. Ind. Eng. Chem. 2015, 21, 26–35. 10.1016/j.jiec.2014.03.044. [DOI] [Google Scholar]
- Rajkumar D.; Song B. J.; Kim J. G. Electrochemical Degradation of Reactive Blue 19 in Chloride Medium for the Treatment of Textile Dyeing Wastewater with Identification of Intermediate Compounds. Dyes Pigm. 2007, 72, 1–7. 10.1016/j.dyepig.2005.07.015. [DOI] [Google Scholar]
- Crini G. Non-Conventional Low-Cost Adsorbents for Dye Removal: A Review. Bioresour. Technol. 2006, 97, 1061–1085. 10.1016/j.biortech.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Sala M.; Gutierrez-Bouźan M. C. Electrochemical Techniques in Textile Processes and Wastewater Treatment. Int. J. Photoenergy 2012, 2012, 629103. 10.1155/2012/629103. [DOI] [Google Scholar]
- Pendergast M. M.; Hoek E. M. V. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. 10.1039/c0ee00541j. [DOI] [Google Scholar]
- Abdullah N.; Yusof N.; Lau W. J.; Jaafar J.; Ismail A. F. Recent Trends of Heavy Metal Removal from Water/Wastewater by Membrane Technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. 10.1016/j.jiec.2019.03.029. [DOI] [Google Scholar]
- Yin J.; Deng B. Polymer-Matrix Nanocomposite Membranes for Water Treatment. J. Membr. Sci. 2015, 479, 256–275. 10.1016/j.memsci.2014.11.019. [DOI] [Google Scholar]
- Ng L. Y.; Mohammad A. W.; Leo C. P.; Hilal N. Polymeric Membranes Incorporated with Metal/Metal Oxide Nanoparticles: A Comprehensive Review. Desalination 2013, 308, 15–33. 10.1016/j.desal.2010.11.033. [DOI] [Google Scholar]
- Zheng Y.-M.; Zou S.; Nanayakkara K. G. N.; Matsuura T.; Chen J. P. Adsorptive Removal of Arsenic from Aqueous Solution by a PVDF/Zirconia Blend Flat Sheet Membrane. J. Membr. Sci. 2011, 374, 1–11. 10.1016/j.memsci.2011.02.034. [DOI] [Google Scholar]
- Werber J. R.; Osuji C. O.; Elimelech M. Materials for Next-Generation Desalination and Water Purification Membranes. Nat. Rev. Mater. 2016, 1, 16018. 10.1038/natrevmats.2016.18. [DOI] [Google Scholar]
- Zhang X.; Fang X.; Li J.; Pan S.; Sun X.; Shen J.; Han W.; Wang L.; Zhao S. Developing New Adsorptive Membrane by Modification of Support Layer with Iron Oxide Microspheres for Arsenic Removal. J. Colloid Interface Sci. 2018, 514, 760–768. 10.1016/j.jcis.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Liu C.; Cheng L.; Zhao Y.; Zhu L. Interfacially Crosslinked Composite Porous Membranes for Ultrafast Removal of Anionic Dyes from Water Through Permeating Adsorption. J. Hazard. Mater. 2017, 337, 217–225. 10.1016/j.jhazmat.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Park J. E.; Shim H. E.; Mushtaq S.; Choi Y. J.; Jeon J. A Functionalized Nanocomposite Adsorbent for the Sequential Removal of Radioactive Iodine and Cobalt Ions in Aqueous Media. Korean J. Chem. Eng. 2020, 37, 2209–2215. 10.1007/s11814-020-0668-1. [DOI] [Google Scholar]
- Mushtaq S.; Yun S.-J.; Yang J. E.; Jeong S.-W.; Shim H. E.; Choi M. H.; Park S. H.; Choi Y. J.; Jeon J. Efficient and Selective Removal of Radioactive Iodine Anions Using Engineered Nanocomposite Membranes. Environ. Sci.: Nano 2017, 4, 2157–2163. 10.1039/c7en00759k. [DOI] [Google Scholar]
- Walter A.; Garofalo A.; Parat A.; Martinez H.; Felder-Flesch D.; Begin-Colin S. Functionalization Strategies and Dendronization of Iron Oxide Nanoparticles. Nanotechnol. Rev. 2015, 4, 581–593. 10.1515/ntrev-2015-0014. [DOI] [Google Scholar]
- Yee C.; Kataby G.; Ulman A.; Prozorov T.; White H.; King A.; Rafailovich M.; Sokolov J.; Gedanken A. Self-Assembled Monolayers of Alkanesulfonic and -phosphonic Acids on Amorphous Iron Oxide Nanoparticles. Langmuir 1999, 15, 7111–7115. 10.1021/la990663y. [DOI] [Google Scholar]
- Chatterjee S.; Guha N.; Krishnan S.; Singh A. K.; Mathur P.; Rai D. K. Selective and Recyclable Congo Red Dye Adsorption by Spherical Fe3O4 Nanoparticles Functionalized with 1,2,4,5-Benzenetetracarboxylic Acid. Sci. Rep. 2020, 10, 111. 10.1038/s41598-019-57017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.; Zhao H.; Chen S.; Long F.; Huang B.; Yang B.; Pan X. A Magnetically Recyclable Chitosan Composite Adsorbent Functionalized with EDTA for Simultaneous Capture of Anionic Dye and Heavy Metals in Complex Wastewater. Chem. Eng. J. 2019, 356, 69–80. 10.1016/j.cej.2018.08.222. [DOI] [Google Scholar]
- Khosravi M.; Azizian S. Adsorption of Anionic Dyes from Aqueous Solution by Iron Oxide Nanospheres. J. Ind. Eng. Chem. 2014, 20, 2561–2567. 10.1016/j.jiec.2013.10.040. [DOI] [Google Scholar]
- Li X.; He Y.; Sui H.; He L. One-Step Fabrication of Dual Responsive Lignin Coated Fe3O4 Nanoparticles for Efficient Removal of Cationic and Anionic Dyes. Nanomaterials 2018, 8, 162. 10.3390/nano8030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergis B. R.; Kottam N.; Hari Krishna R.; Nagabhushana B. M. Removal of Evans Blue Dye from Aqueous Solution Using Magnetic Spinel ZnFe2O4 Nanomaterial: Adsorption Isotherms and Kinetics. Nano-Struct. Nano-Objects 2019, 18, 100290. 10.1016/j.nanoso.2019.100290. [DOI] [Google Scholar]
- Essandoh M.; Garcia R. A.; Gayle M. R.; Nieman C. M. Performance and Mechanism of Polypeptidylated Hemoglobin (Hb)/Iron Oxide Magnetic Composites for Enhanced Dye Removal. Chemosphere 2020, 247, 125897. 10.1016/j.chemosphere.2020.125897. [DOI] [PubMed] [Google Scholar]
- Yao S.; Liu Z.; Shi Z. Arsenic Removal from Aqueous Solutions by Adsorption onto Iron Oxide/Activated Carbon Magnetic Composite. J. Environ. Health Sci. Eng. 2014, 12, 58. 10.1186/2052-336x-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.-C.; Tran H. N.; Chen X.-H.; Juang R.-S. Preparation of Polyaminated Fe3O4@chitosan Core-Shell Magnetic Nanoparticles for Efficient Adsorption of Phosphate in Aqueous Solutions. J. Ind. Eng. Chem. 2020, 83, 235–246. 10.1016/j.jiec.2019.11.033. [DOI] [Google Scholar]
- Nithya R.; Thirunavukkarasu A.; Sathya A. B.; Sivashankar R. Magnetic materials and magnetic separation of dyes from aqueous solutions: a review. Environ. Chem. Lett. 2021, 19, 1275–1294. 10.1007/s10311-020-01149-9. [DOI] [Google Scholar]
- Mudhoo A.; Sillanpää M. Magnetic nanoadsorbents for micropollutant removal in real water treatment: a review. Environ. Chem. Lett. 2021, 19, 4393–4413. 10.1007/s10311-021-01289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.; Zhang Q.; Lu Y.; Wu S.; Wang W. High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study. Sep. Purif. Technol. 2020, 238, 116400. 10.1016/j.seppur.2019.116400. [DOI] [Google Scholar]
- Lee S. Y.; Shim H. E.; Yang J. E.; Choi Y. J.; Jeon J. Continuous Flow Removal of Anionic Dyes in Water by Chitosan-Functionalized Iron Oxide Nanoparticles Incorporated in a Dextran Gel Column. Nanomaterials 2019, 9, 1164. 10.3390/nano9081164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






