Abstract
Chaigui granules were a traditional Chinese medicine (TCM) preparation with antidepressant effects derived from a famous antidepressant prescription. It was of great significance to clarify the antidepressant mechanism of Chaigui granules for the clinical application of this drug. In this study, a chronic unpredictable mild stress (CUMS) depression model was successfully established, and behavioral indicators were used to evaluate the antidepressant effect. Second, the CD4+, CD8+, and CD4+/CD8+ levels were detected in peripheral blood. Meanwhile, the amount of inflammatory cytokines was determined in serum. Correspondingly, LC/MS-based peripheral blood mononuclear cell (PBMC) metabolomics was used to investigate vital metabolic pathways participating in the antidepressive effects of Chaigui granules. Finally, bioinformatics technology was further employed to discover the potential antidepressant mechanism of Chaigui granules regulating the immune system. The results suggested that the administration of Chaigui granules significantly improved CUMS-induced depressive symptoms. Chaigui granules could improve immune function by regulating T lymphocyte subsets, increasing anti-inflammatory cytokine levels of IL-2 and IL-10, and reducing pro-inflammatory cytokine levels of TNF-α, IL-1β, and IL-6. In addition, metabolomics results of PBMCs showed that Chaigui granules improved 14 of the 25 potential biomarkers induced by CUMS. Metabolic pathway analyses indicated that purine metabolism was the critical metabolic pathway regulated by Chaigui granules. Furthermore, correlation analysis indicated that 13 key biomarkers were related to immune-related indicators. The metabolite–gene network of 13 key biomarkers was investigated by using bioinformatics. The investigation showed that 10 targets (5′-nucleotidase ecto; 5′-nucleotidase, cytosolic IB; 5′-nucleotidase, cytosolic II; etc.), mainly belong to the purine metabolism, might be potential targets for Chaigui granules to exert their antidepressant effects by improving immune function impairment. Together, our results suggested that Chaigui granules might exert antidepressant effects by improving immune function and regulating the purine metabolic pathway in PBMCs. This work used PBMCs metabolomics as an entry point to study the antidepressant mechanism of Chaigui granules, which provided a new way to elucidate the mechanism of a traditional Chinese medicine prescription.
1. Introduction
Depression is an affective mental illness caused by a variety of factors1,2 and has become one of the common mental health problems.3 At present, about 120 million people in the world are suffering from this disease.4,5 According to the World Health Organization forecast, depression will rise to the top of the global burden of illness in 2030.6,7 However, depressive mechanisms are not fully elucidated, and treatment remains a major challenge.8
Increasing evidence has indicated that inflammation and immune activation might be implicated the pathogenesis of depression.9−12 The inflammatory response theory of depression proposed by Maesetal in 1999 suggested that depression was related to the activation of the inflammatory response system.13−15 When inflammation occurs in the body, many cytokines that promote inflammation, such as interleukins, tumor apoptosis factors, etc., are produced.16 These pro-inflammatory cytokines enter the brain and affect the neurotransmitters or neural circuits related to mood regulation, thus promoting the development of depressive symptoms.17 Increased levels of pro-inflammatory cytokines are closely related to depression. The amounts of a variety of inflammatory products, including TNF-α, IL-1β, and IL-6 were increased in patients within the central nervous system and peripheral blood.18 In addition, a disturbed immune system is often reported in depressive patients and might be a crucial factor in the pathogenesis of depression.19,20 Inflammation or inflammatory responses were the result of immune system activation, often accompanied by the triggering of pro-inflammatory responses, including cytokines IL-1β, IL-6, and TNF-α, and adaptive T cell-mediated immune responses.21,22 Many types of immune cells maintain balance under normal conditions, but their dysregulation in action often leads to disease, with growing evidence that this occurs in mental illness, including depression.22 Peripheral blood mononuclear cells (PBMCs) contain a variety of immune cells such as T cells, B cells, natural killer cells, and dendritic cells, which play an essential role in the immune system and are now gradually being used in the study of psychiatric disorders such as depression.23,24 Several studies had suggested that the disturbed immunometabolism of PBMCs occurs in depressed rats.25 In particular, PBMCs and the brain have shown many parallel responses of the central nervous system.26,27 The disorder of lymphocyte function and metabolism was accompanied by changes in the neurotransmitter and hormone system.28 In summary, this provides evidence for possible crosstalk between the central nervous system and the peripheral immune system.29 Cerebrospinal fluid and brain biopsy samples were difficult to obtain, and it was impractical to collect these samples for routine clinical screening or diagnostic purposes.29,30 PBMCs have good practical advantages, including ease of access and low cost. They have great potential for use in studies as diagnostic biomarkers of depression and markers of antidepressant efficacy.31
At present, synthetic antidepressants play an important role in the treatment of depression, but serious adverse reactions have also begun to appear, such as headache, dizziness, anxiety, nervousness, insomnia, addiction, etc.32 Therefore, the development of a traditional Chinese medicine (TCM) with good antidepressant efficacy has gradually attracted more attention.33 Chaigui granules are derived from the classic prescription “Xiaoyao San”, which has numerous studies confirming a clear antidepressant effect.34−37 Currently, it has been approved by the Chinese National Medical Products Administration for clinical trials in depression.37 Chaigui granules consist of radix bupleuri (Bupleurum chinense (DC.)), radix paeoniae alba (Paeonia lactiflora (Pall.)), rhizoma atractylodis macrocephalae (Atractylodes macrocephala (Koidz.)), radix angelicae sinensis (Angelica sinensis (Oliv.) Diels), radix glycyrrhizae (Glycyrrhiza uralensis (Fisch.)), and herba menthae haplocalycis (Mentha haplocalyx (Briq.)). However, it was unclear whether Chaigui granules could exert antidepressant effects by regulating PBMC abnormalities. Therefore, it is vital to further clarify the potential antidepressant mechanism of Chaigui granules in PBMCs.
LC/MS metabolomics is a new omics approach that analyzes the low-molecular-weight metabolites of a certain organism in a specific physiological period.38,39 It is based on high-throughput mass spectrometry technology, cluster index analysis, and data processing to screen and identify the differential metabolites associated with the disease phenotype.38,40 Compared with other omics approaches, metabolomics is rather new and has already attracted substantial interest in the field of depression biomarkers.41
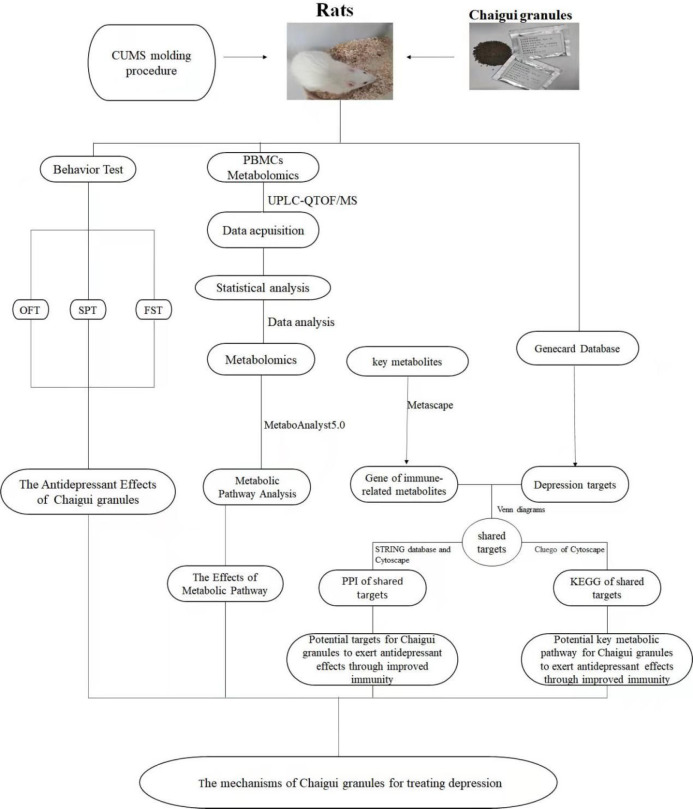
At present, the study of depression mechanisms is gradually becoming a research hotspot, but the antidepressant mechanism based on PBMCs is less studied. In this study, the chronic unpredictable mild stress (CUMS) rat model was used to evaluate the antidepressant effect of Chaigui granules. Next, peripheral blood T lymphocyte subpopulations were detected by flow cytometry, and serum inflammatory cytokine levels were measured. Subsequently, PBMC metabolomics was used to search the key metabolites and metabolic pathways regulated by Chaigui granules. Furthermore, a correlation analysis was used to explore the relationship between metabolites and immune indicators, focusing on key metabolites. Finally, bioinformatics technology was further employed to discover the potential antidepressant mechanism of Chaigui granules regulating the immune system (Figure 1). Notably, this study is the first to illustrate the underlying mechanism of the therapeutic effect of Chaigui granules on depressed rats from the perspective of PBMCs.
Figure 1.
Workflow for dissecting the mechanisms of Chaigui granules for treating depression.
2. Result
2.1. Evaluation of the Antidepressant Effect of Chaigui Granules
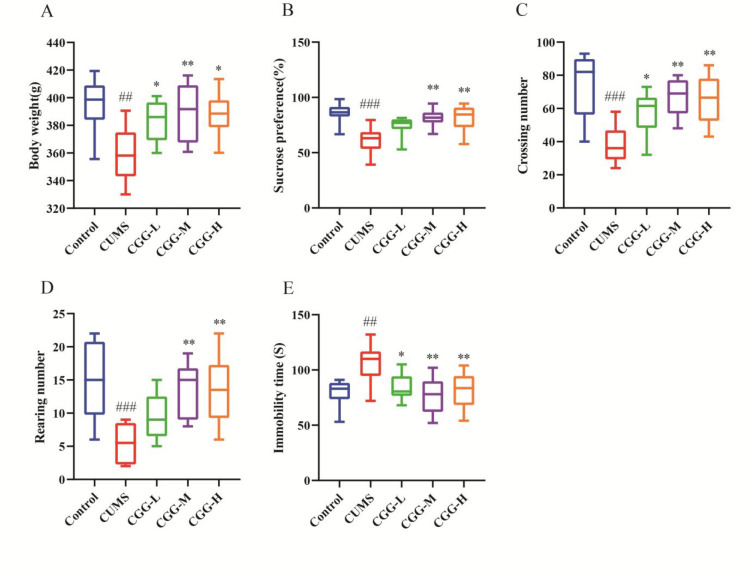
The depressive behaviors of CUMS rats and the antidepressant effects of Chaigui granules were estimated by the body weights, sucrose preference test (SPT), open field test (OFT), and forced swim test (FST) indicators. After four weeks of the CUMS procedure, the body weight (Figure 2A), sucrose preference rate (Figure 2B), crossing number (Figure 2C), and rearing number (Figure 2D) of OFT clearly decreased, and the immobile time of FST (Figure 2E) increased significantly in the model group of depression compared with that of the normal group. The rats in the CUMS group had significant depressive behavior, which was similar to clinical symptoms of depression. These results showed that the CUMS model of depression had been established successfully.
Figure 2.
Evaluation of the antidepressant effect of Chaigui granules. (A) Rat body weights on day 28. (B) Sucrose preference. (C) The crossing number in the open field test (OFT). (D) The rearing number in the OFT. (E) Immobility time in the forced swimming test (FST). All data are expressed as the median ± min to max (n = 8). #P < 0.05, ##P < 0.01, and ###P < 0.001 compared with control group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the CUMS group. CUMS is the the model group, CGG-L is the group that received a low dose of Chaigui granules, CGG-M is the group that received a medium dose of Chaigui granules, and CGG-H is the group that received the high dose of Chaigui granules.
After four weeks, the weights of rats in the low-dose group (CGG-L, 4.2 g/kg, P < 0.05), the middle-dose group (CGG-M, 8.3 g/kg, P < 0.01), and the high-dose group (CGG-H, 16.6 g/kg, P < 0.05) of the Chaigui granules were increased significantly after treatment compared with those of the CUMS group (Figure 2A). It showed that Chaigui granules could effectively reverse the weight loss caused by the CUMS model. In the SPT experiment (Figure 2B), the rate of sucrose preference was significantly higher in the CGG-M group (P < 0.01) and the CGG-H group (P < 0.01) compared with the CUMS model group, and there was a trend of increase in the CGG-L group. This indicated that the phenomenon of depression in CUMS rats had improved. Similarly, the OFT and the FST also showed improvements compared with the model group. On the 28th day, the Chaigui granules groups showed significant increases in the crossing number (Figure 2C) and the rearing number (Figure 2D) of the OFT compared with those of the CUMS group. The immobile time of the FST was remarkably reduced (Figure 2E) compared with that of the model group. All these findings suggested that Chaigui granules had a remarkable antidepressant effect. Furthermore, the antidepressant effect of Chaigui granules was better in the medium and high doses, so the medium and high doses were chosen for subsequent studies.
2.2. The Regulatory Effect of Chaigui Granules on the Immune Function of CUMS Depression in Rats
2.2.1. Changes of Peripheral Blood T Lymphocyte Subsets in CUMS-Depressed Rats and the Regulatory Effect of Chaigui Granules
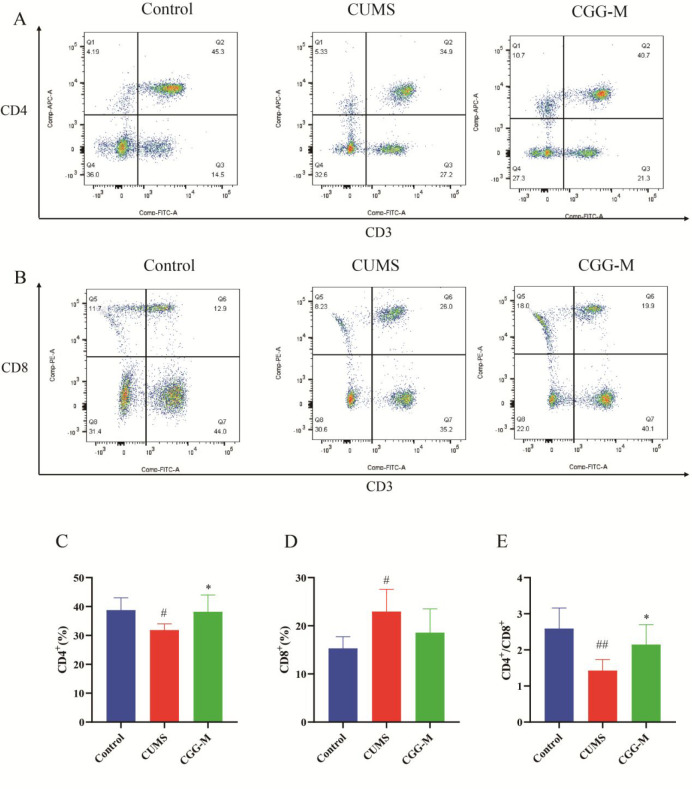
T lymphocyte subsets in peripheral blood play an essential role in regulating immune stability.42,43 The total numbers of CD4+ and CD8+ T lymphocytes and the ratio of CD4+/CD8+ T lymphocytes are important indices to reflect the immune status of an organism.44,45 Counts of main lymphocyte subpopulations (CD3, CD4, and CD8 T cells) were performed to test immune function using flow cytometry. As a result, representative flow cytometric dot plots of CD4+ and CD8+ in T lymphocytes are shown in Figure 3A and B, respectively. The level of CD8+ T cells in the CUMS group increased, while that of the CD4+ T cells and the ratio of CD4+/CD8+ T lymphocytes decreased compared with those of the control group (Figure 3C–E). Compared with the CUMS group, Chaigui granules could significantly increase the percentage of CD4+ T cells and the ratio of CD4+/CD8+ T lymphocytes in peripheral blood lymphocytes. The results showed that the T lymphocyte subsets of CUMS depressed rats had changed, and the immune function had been disordered. Chaigui granules had an antidepressant effect by regulating T lymphocyte subsets and improving immune function.
Figure 3.
Changes of peripheral blood T lymphocyte subsets in CUMS-depressed rats and the regulatory effect of Chaigui granules. Representative flow cytometric dot plots of (A) CD4+ and (B) CD8+ in T lymphocytes. The statistical analysis for (C) CD4+ and (D) CD8+T cells and (E) CD4+/CD8+. All data are expressed as the mean ± SD (n = 6). #P < 0.05 and ##P < 0.01 compared with the control group. *P < 0.05 and **P < 0.01, compared with the CUMS group. CUMS is the model group, and CGG-M is the group that received the medium dose of Chaigui granules.
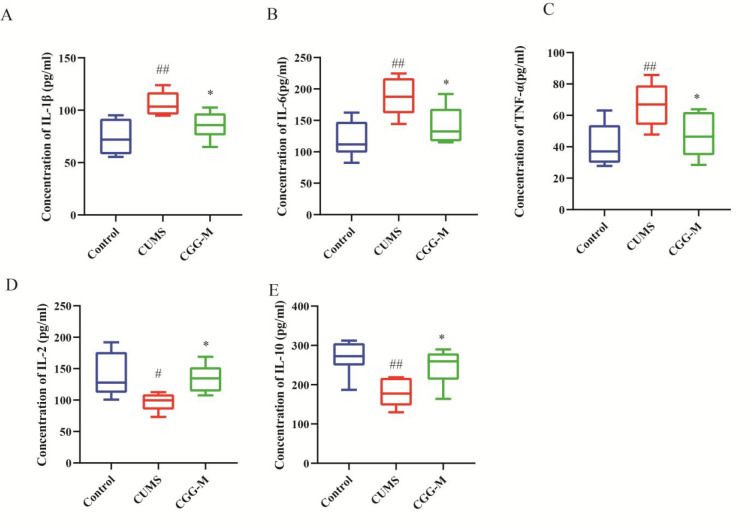
2.2.2. Chaigui Granules Improved CUMS-Induced Immune Inflammation
Cytokines are active signal molecules secreted by immune cells, including pro-inflammatory cytokines and anti-inflammatory cytokines, and play a crucial role in immune response; therefore, to a certain extent the changes in their expression levels could reflect the immune function of the body.46 To elucidate the effect of Chaigui granules on CUMS-induced inflammation, the levels of important pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 and anti-inflammatory cytokines IL-2 and IL-10 in serum were measured (Figure 4). In the CUMS group, the levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the serum were higher and the levels of anti-inflammatory cytokines (IL-2 and IL-10) in the serum were lower compared with those of the control group. After four weeks of administration of the Chaigui granules, the levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 were lower, and the secretion of anti-inflammatory cytokines IL-2 and IL-10 increased. The results indicated that Chaigui granules had obvious antidepressant effects by improving immune function and alleviating immune inflammation.
Figure 4.
Chaigui granules improve CUMS-induced immune inflammation. Effect of the Chaigui granules on the levels of (A) IL-1β, (B) IL-6, (C) TNF-α, (D) IL-2, and (E) IL-10 in the serum of rats. All data are expressed as the median ± min to max (n = 6). #P < 0.05 and ##P < 0.01 compared with the control group. *P < 0.05 and **P < 0.01 compared with the CUMS group. CUMS is the the model group, and CGG-M is the group that received the medium dose of Chaigui granules.
2.3. Results of the Metabolomics Analysis of PBMCs
2.3.1. Validation of the Metabolomics Method
The typical peak intensities based on chromatograms of PBMCs samples were obtained through UPLC-MS/MS positive and negative ion modes. Additionally, the principal component analysis (PCA) scores plot of the PBMCs (Figure S1) indicated that the quality control group (QC) was gathered. Meanwhile, 10 ions were taken from basic peak intensity chromatography of the QC samples for method validation (Tables S1). The RSDs for the retention times were 0.063–1.894%, and relative peak areas were 2.85–9.87%, which illustrated that the instrument was stable and the data process was reliable.47
2.3.2. Regulation of Chaigui Granules on Abnormal Metabolism of PBMCs Induced by CUMS
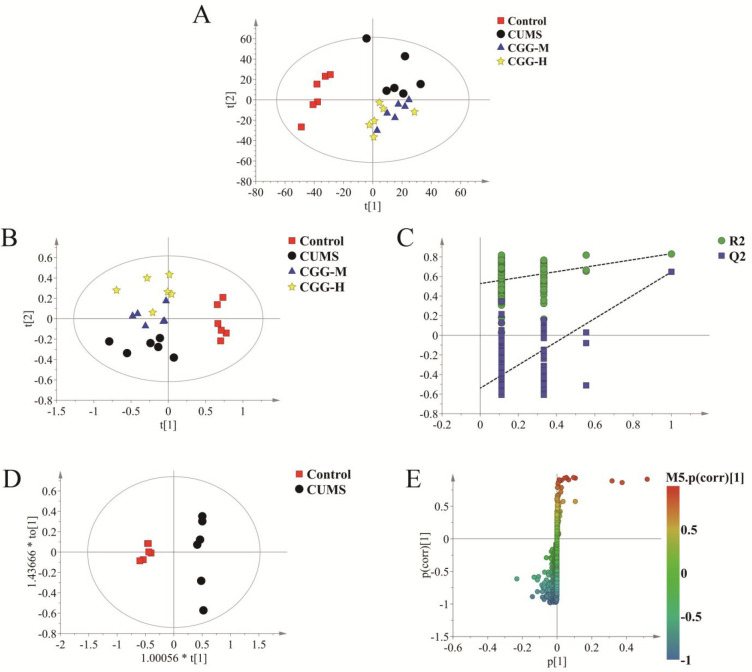
To obtain the metabolic differences among different groups, all metabolomics data were analyzed with Simca14.1 for multivariate data. As shown in Figure 5A, an obvious separation could be observed among the control, the CUMS model, and Chaigui granules treatment groups in the PCA score plot. This indicated that a biochemical perturbation occurred in the CUMS group and the Chaigui granules treatment groups. The PLS-DA model was further used to distinguish the different metabolites among the different groups, as shown in Figure 5B. The classification effect was significant, and the groups became obviously distinguished from each other.
Figure 5.
Metabolomics profiling analysis of PBMCs. (A) PCA score plot, (B) PLS-DA score plot, (C) PLS-DA validation plot, (D) OPLS-DA score plot, and (E) S-plot of OPLS-DA. CUMS is the model group, CGG-M is the group that received the medium dose of Chaigui granules, and CGG-H is the group that received the high dose of Chaigui granules.
The permutation test parameters presented the excellent predictive ability of the control and model groups, as shown in Figure 5C (intercepts for PBMCs R2 = 0.92 and Q2 = −0.438), indicating that the models reliably explained and did not overfit the data. Furthermore, the supervised OPLS-DA analysis was used to evaluate the different metabolites between the control and model groups, and significant separation was found between the control group and the model group in the PBMCs (Figure 5D). To further analyze the differences between the metabolites, the OPLS-DA S-plot (Figure 5E) was used to show the different metabolites between the control and the model group. In the S-plots, different points represent different in metabolites. The further away from the center of the variable, the more influence the variable had on the group separation. The above results demonstrated that the metabolic disorders induced by the CUMS model improved after treatment with Chaigui granules.
2.3.3. Selection of Differential Metabolites and the Regulatory Effects of Chaigui Granules
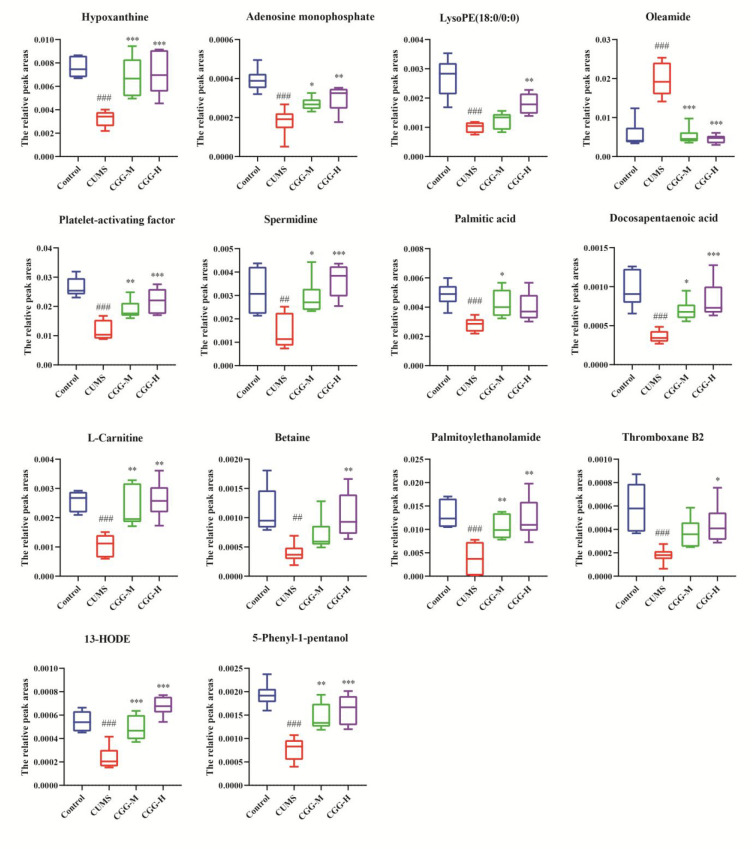
The differential metabolites were screened between the control and the CUMS groups based on VIP values greater than 1 and t-tests (p < 0.05). As a consequence, 25 differential metabolites were screened out (Table 1). Compared with the control group, the levels of five differential metabolites (palmitic amide, octadecanamide, linoleamide, etc.) were increased those of 20 differential metabolites (adenosine monophosphate, hypoxanthine, l-carnitine, etc.) were decreased in CUMS group. Among them, 14 metabolites were regulated after treatment with the Chaigui granules. Details of the metabolites significantly regulated by Chaigui granules in PBMCs are shown in Figure 6, indicating that both the CGG-M and CGG-H groups have regulatory effects on different metabolites. The results suggested that the metabolites could be obviously regulated, and the metabolic disturbances were improved in the model group of rats after treatment with Chaigui granules.
Table 1. Differential Metabolites Associated with Depression Were Detected by UPLC-MS/MS in PBMCsa.
| no. | metabolites | retention time (min) | m/z | formula | VIP | P | trend | scan mode |
|---|---|---|---|---|---|---|---|---|
| 1 | palmitic amide | 25.60 | 256.26 | C16H33NO | 19.38 | 0.00** | ↑ | + |
| 2 | octadecanamide | 28.60 | 284.29 | C18H37NO | 16.28 | 0.00** | ↑ | + |
| 3 | platelet-activating factor | 21.21 | 524.37 | C26H54NO7P | 6.89 | 0.00** | ↓ | + |
| 4 | oleamide | 25.83 | 265.25 | C18H35NO | 6.87 | 0.00** | ↑ | + |
| 5 | palmitoylethanolamide | 14.60 | 300.29 | C18H37NO2 | 5.90 | 0.02* | ↓ | + |
| 6 | hypoxanthine | 2.25 | 137.05 | C5H4 N4O | 3.77 | 0.00** | ↓ | + |
| 7 | linoleamide | 25.30 | 280.26 | C18H33NO | 4.77 | 0.00** | ↑ | + |
| 8 | palmitic acid | 10.66 | 274.27 | C16H32O2 | 2.95 | 0.00** | ↓ | + |
| 9 | spermidine | 1.15 | 146.17 | C7H19N3 | 2.44 | 0.02* | ↓ | + |
| 10 | l-carnitine | 1.41 | 162.11 | C7H15NO3 | 2.04 | 0.00** | ↓ | + |
| 11 | LysoPE (18:0/0:0) | 20.25 | 482.32 | C23H48NO7P | 2.24 | 0.04* | ↓ | + |
| 12 | 1-monopalmitoylglycerol | 25.93 | 331.28 | C19H38O4 | 2.18 | 0.00** | ↓ | + |
| 13 | docosatrienoic acid | 24.43 | 335.29 | C22H38O2 | 2.96 | 0.00** | ↓ | + |
| 14 | 5-phenyl-1-pentanol | 2.23 | 159.03 | C6H6O5 | 2.12 | 0.00** | ↓ | + |
| 15 | phytosphingosine | 10.84 | 318.30 | C18H39NO3 | 1.42 | 0.03* | ↓ | + |
| 16 | l-tyrosine | 2.73 | 182.08 | C9H11NO3 | 1.44 | 0.03* | ↓ | + |
| 17 | betaine | 1.48 | 118.09 | C5H11NO2 | 1.48 | 0.02* | ↓ | + |
| 18 | docosapentaenoic acid | 21.88 | 331.26 | C22H34O2 | 1.44 | 0.00** | ↓ | + |
| 19 | thromboxane B2 | 9.10 | 369.23 | C20H34O6 | 1.21 | 0.012* | ↓ | – |
| 20 | MG (18:0/0:0/0:0) | 28.80 | 359.32 | C21H42O4 | 1.26 | 0.00** | ↓ | + |
| 21 | 13-HODE | 18.17 | 295.23 | C18H32O3 | 1.10 | 0.00** | ↓ | – |
| 22 | hexadecenal | 25.93 | 239.24 | C16H30O | 1.23 | 0.00** | ↓ | + |
| 23 | docosadienoate (22:2n6) | 26.75 | 337.31 | C22H40O2 | 1.96 | 0.00** | ↓ | + |
| 24 | sphingosine 1-phosphate | 14.07 | 380.26 | C18H38NO5P | 1.06 | 0.04* | ↑ | + |
| 25 | adenosine 5′-monophosphate | 1.59 | 348.07 | C10H14N5O7P | 1.11 | 0.00** | ↓ | + |
“↓” or “↑” means the metabolite significantly decreased or increased in the CUMS group compared with the control group. *p < 0.05 and **p < 0.01 compared with the control group.
Figure 6.
Relative peak areas of the differential metabolites in PBMCs regulated by Chaigui granules. All data are expressed as the median ± min to max (n = 6). #P < 0.05, ##P < 0.01, and ###P < 0.001 for the CUMS group vs the control group. *P < 0.05, **P < 0.01, and ***P < 0.001 for the drug-treated groups vs the CUMS group. CUMS is the the model group, CGG-M is the group that received the medium dose of Chaigui granules, and CGG-H is the group that received the high dose of Chaigui granules.
2.4. Metabolic Pathway Analysis
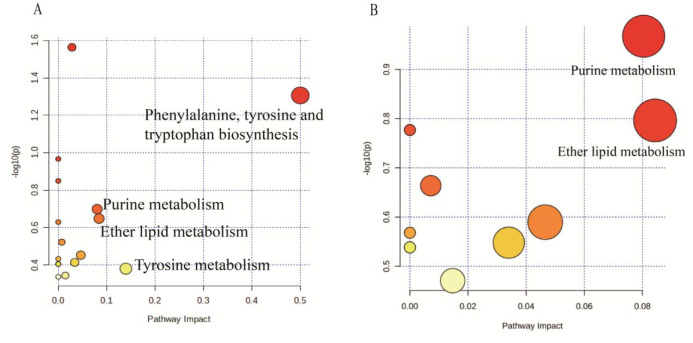
To identify the metabolic pathways most associated with depression, the MetaboAnalyst 5.0 program was applied to analyze the metabolic pathways to gain more information for potential biomarkers. In this study, pathways with impact values >0.05 were considered as the significant metabolism pathways. The results showed that four significant metabolic pathways were disordered, including phenylalanine, tyrosine, and tryptophan biosynthesis; tyrosine metabolism; ether lipid metabolism; and purine metabolism (Figure 7A). Among them, Chaigui granules were effective for regulating two metabolic pathways, namely purine metabolism and ether lipid metabolism, in PBMCs (Figure 7B). As a result, the metabolic pathway analysis showed that purine metabolism (impact of 0.08) was the vital metabolic pathway regulated by Chaigui granules.
Figure 7.
Metabolic Pathway Analysis. (A)The disorder metabolic pathways in the CUMS group. (B) The metabolic pathways regulated by Chaigui granules. The size and color of each circle indicate the significance of pathway ranked by the P-value and the pathway impact score, respectively. Red represents higher P-values, and yellow represents lower P-values. The larger the circle, the higher the impact score.
2.5. Correlation Analysis of Metabolites and Immune-Related Indicators
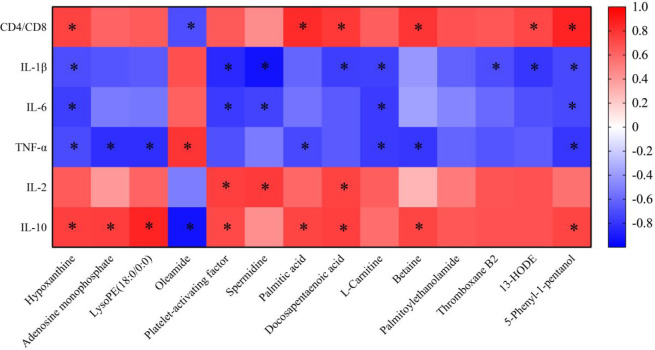
Metabolomic analysis revealed that 14 key metabolites were regulated after treatment with Chaigui granules, and these metabolites were closely related to the antidepressant effect of Chaigui granules. To investigate the potential link between metabolites and immune-related indicators, a Pearson correlation analysis was performed to screen key biomarkers for significant correlations with both depression and immune function. As a result, 13 different metabolites were related to more than one immune-related index, including oleamide, palmitic acid, docosapentaenoic acid, 13-hode, platelet-activating factor, hypoxanthine, thromboxane B2, spermidine, l-carnitine, lysope (18:0/0:0), 5-phenyl-1-pentanol, betaine, and adenosine monophosphate, among which hypoxanthine and 5-phenyl-1-pentanol had the strongest correlation with immune indicators and were closely related to the five indicators. Platelet-activating factor and docosapentaenoic acid were strong and were closely related to four indicators. Palmitoylethanolamide was weaker than the immune indicators, and it had no correlation with six immune indicators (Figure 8). Overall, the results indicated that 13 key biomarkers associated with the antidepressant effect of Chaigui granules were closely related to either immune function or inflammation.
Figure 8.
Correlation analysis of metabolites and immune-related indicators. Correlation analysis between metabolites regulated by Chaigui granules and immune-related indicators according to the Pearson correlation coefficient. Red indicated that |r| was a positive value, and blue indicated that |r| was a negative value. The darker the color, the larger the |r| value. The asterisk (*) represents P < 0.05 and |r| > 0.7.
2.6. Biological Networks Analysis of Key Biomarkers
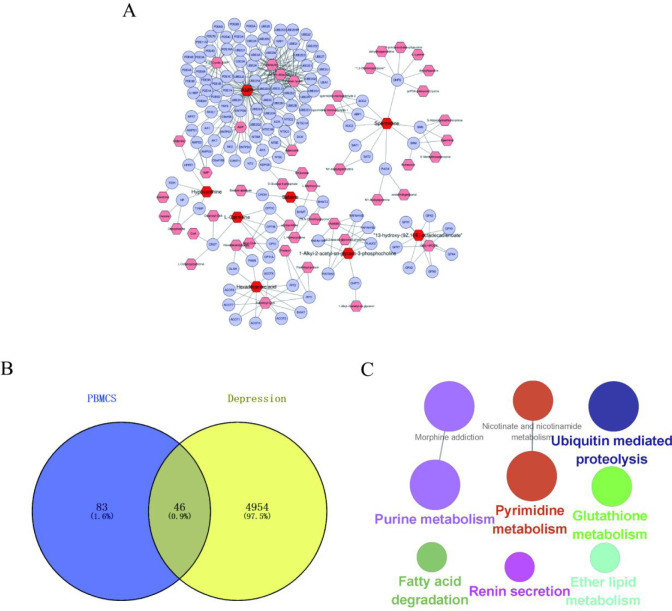
2.6.1. Construction and Analysis of 13 Key Biomarkers and Related Gene Networks
The correlation analysis indicated that 13 Chaigui granule-regulated metabolites were closely related to immune function or immune inflammation. Therefore, this study further investigated the potential key metabolic pathways related to immune function and inflammation that were regulated by Chaigui granules. The metabolites–gene networks were constructed using Metscape, and genes related to 13 key metabolites were obtained (Figure 9A). The genes related to key metabolites and genes associated with depression were intersected by Venn diagrams, and 46 shared targets were obtained (Figure 9B). KEGG pathway enrichment analysis was carried out using the ClueGo plug-in with a cutoff of p < 0.05 for the 46 shared targets to discover potential key metabolic pathways related to immune function and inflammation that were regulated by Chaigui granules. An enriched pathway network was constructed (Figure 9C), and a total of nine enriched pathways were obtained, specifically purine metabolism, pyrimidine metabolism, nicotinate and nicotinamide metabolism, ubiquitin-mediated proteolysis, morphine addiction, fatty acid degradation, glutathione metabolism, renin secretion, and ether lipid metabolism. These pathways are closely associated with the development of immunity and depression, which could help to further understand the antidepressant mechanism of Chaigui granules by improving immune function.
Figure 9.
Construction and analysis of 13 key biomarkers and related gene networks. (A) The network of potential biomarkers for Chaigui granules for its antidepressant effect in PBMCs. (B) The Venn diagram of the common genes, which was obtained by intersecting the metabolite-related genes with disease genes. (C) Network of the shared genes KEGG pathway from ClueGO.
2.6.2. Protein–Protein Interaction (PPI) Analysis of the Key Targets
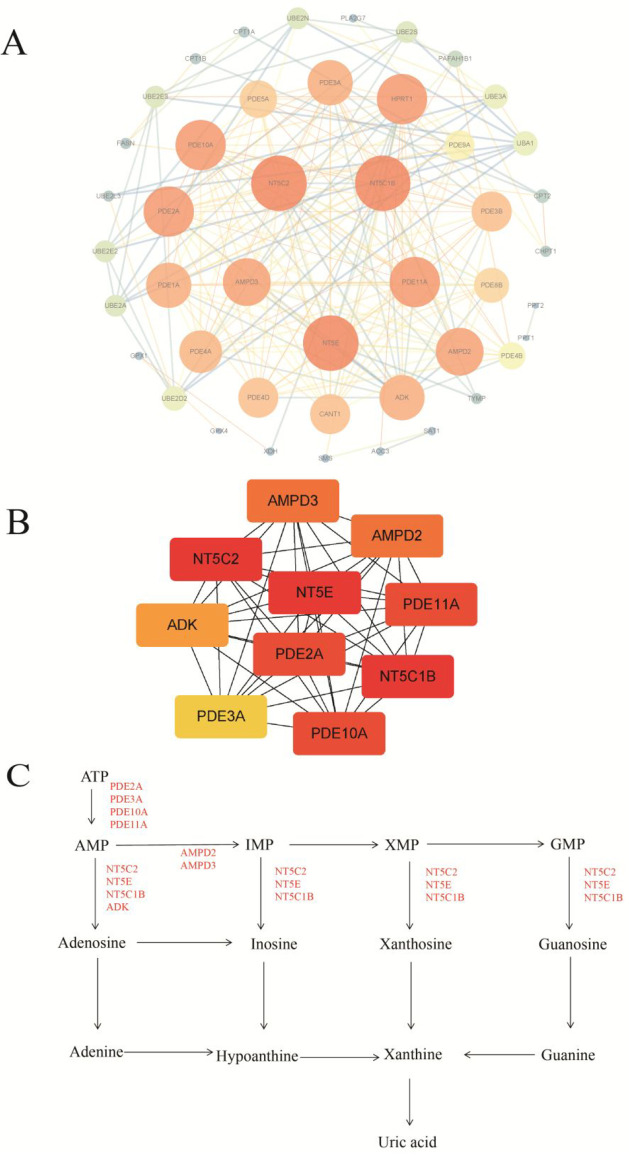
To obtain key targets for Chaigui granules to exert antidepressant effects through improved immunity, a string database was used to construct an interactive network of the 46 shared targets obtained by intersecting the key metabolite-related genes with disease genes. The constraint was “human species”. The network was constructed, and a “TSV” formated file of the gene interactions was downloaded and imported into Cytoscape software for modular and topological analysis. A protein network with 45 nodes and 208 edges was obtained (Figure 10A). The top ten targets with the highest maximal clique centrality (MCC) scores were ranked from yellow to red using the CytoHubba plugin, with redder colors indicating higher MCC scores and a preference for key targets (Table S2). The top ten targets were NT5E, NT5C1B, NT5C2, PDE11A, PDE10A, PDE2A, AMPD2, AMPD3, ADK, and PDE3A (Figure 10B), which played a major role in the network diagram (Figure 10C).
Figure 10.
Protein–protein interaction (PPI) analysis of the key targets. (A) PPI network of the shared targets and (B) the network of the protein targets with the top ten MCC values in the interaction network. (C) The position of the top ten targets in purine metabolism. For the interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.
3. Discussion
Although Chaigui granules have been demonstrated to treat depression, the elucidation of the mechanism still faced great challenges because of its multicomponent, multitarget, and multichannel efficacy. In this study, we first evaluated the antidepressant effect of Chaigui granules. After the administration of the Chaigui granules, the weight and levels of behavioral indicators were improved compared with those of the CUMS group. More importantly, we found that the immune function was disordered in the CUMS model rats, and Chaigui granules could improve immune function by regulating the ratio of T-lymphatic subpopulations, reducing the level of inflammatory cytokines and increasing the secretion of anti-inflammatory cytokines. Correspondingly, metabolomics of PBMCs was adopted to explore the antidepressant effects of Chaigui granules. Twenty-five depression-related metabolites were identified by LC/MS-based PBMCs metabolomics, and Chaigui granules groups could significantly retrieve 14 of them. Besides, the analysis of the metabolic pathways indicated that the purine metabolism was the most important metabolic pathway regulated by Chaigui granules. In addition, according to a Pearson correlation analysis, 13 key biomarkers related to depression and immune function damage were found. Finally, the metabolite–gene network of 13 key biomarkers was investigated using bioinformatics, which indicated that 10 targets (NT5E, NT5C1B, NT5C2, PDE11A, PDE10A, PDE2A, AMPD2, AMPD3, ADK, and PDE3A) mainly belong to the purine metabolism might be potential targets for Chaigui granules treating depression. To our knowledge, this was the first study to present a metabolomics analysis of PBMCs integrated with bioinformatics to further decipher the antidepressant pathogenesis of Chaigui granules.
3.1. Altered Immune Function and Inflammatory Response in Depressed Rats and the Regulatory Effects of Chaigui Granules
Studies demonstrated that chronic stress might cause changes in immune system functioning.48 In contrast to acute stress, chronic stressors are known to suppress immune function and raise susceptibility to inflammatory and mental illness.49,50 Studies have reported not only that there were higher levels of pro-inflammatory cytokines in the blood during chronic stress but also that the response and fighting ability of immune cells were lower.51 It was, for example, proved that chronic stress induced an inhibitory effect on innate and adaptive immune responses by altering the balance of type 1 and type 2 cytokines.52,53 On the other hand, in chronic stress the number of cytotoxic T cells and natural killer cells was reduced,54 lymphocyte proliferation in response to specific mitogens was decreased,55,56 and the percentages of CD4 helper T cells and CD8 cells were reduced,57 thus indicating a disorder of the immune system due to a chronic state of stress-related vigilance.58 Under normal conditions, T lymphocyte interactions maintain normal immune function. When the number and function of T lymphocytes change, however, it could lead to a disruption of cellular immune function.59,60 CD4+ T cells represent the key immune response cells, while CD8+ T cells represent the immune effector cells. A reduction of the CD4+/CD8+ ratio is an important feature of immune disorders.61 It has been shown that depression-like behavior in rats is related to a reduction in the amount of CD4+, an increase in CD8+ accumulation, and a reduction of the CD4+/CD8+ ratio.60 This study found that T lymphocyte subsets differed significantly among groups. Both the level of CD4+T cells and the CD4+/CD8+ ratio decreased, and the level of CD8+ T cells increased in rats in the model group after 28 days of CUMS modeling. Compared with the model group, Chaigui granules significantly improved the disruption of T-lymphocyte subsets. The results indicated that the T lymphocyte subsets of CUMS-depressed rats had changed and the immune function had been disordered. Chaigui granules could have an antidepressant effect by regulating the lymphocyte subsets and improving the immune function.
In recent years, research had found that pro-inflammatory cytokines are especially closely associated with the development of depression.62,63 The cytokine hypothesis suggests that depression is related to abnormalities of the immune system, namely an inflammatory disorder caused by disturbances in neuroimmune regulation.64 Cytokines are a class of small-molecule proteins secreted by immune cells, which regulate a wide range of cellular physiological functions.65 Cytokines are divided into pro-inflammatory cytokines and anti-inflammatory cytokines, which play different roles in inflammation.66 For example, IL-1β, IL-6, and TNF-α are important pro-inflammatory cytokines, while IL-4 and IL-10 are anti-inflammatory cytokines.67 Clinical studies have indicated that depressed patients often had different levels of inflammatory activation, indicating that the occurrence of depression might be closely linked to cytokines.64,68 Several studies suggested that levels of a variety of inflammatory cytokines such as IL-1β, IL-6 and TNF-α were significantly elevated and those of anti-inflammatory cytokines such as IL-4 and IL-10 were reduced in the serum or plasma of depressed patients.69,70 In addition, meta-analyses indicated that levels of inflammatory cytokines in depressed patients, including IL-1, IL-6, and TNF-α, were clearly above those of healthy individuals and were positively related to the severity of depressive symptoms.71−73 Such an abnormal change in inflammatory factor levels suggested that depression might activate the inflammatory process.74 The results of this experiment showed that the serum levels of IL-1β, IL-6, and TNF-α were higher in CUMS model rats than in normal rats, while treatment with Chaigui granules reduced the levels of inflammatory cytokines.
3.2. Depression-Induced Dysfunction of the PBMC Pathway and the Modulatory Effect of Chaigui Granules
In our work, metabolomics identified purine metabolism as the main pathway regulated by Chaigui granules in depressed rats. Hypoxanthine and AMP levels were reduced in the model group. Hypoxanthine is a reaction intermediate in the metabolism of adenosine and the formation of nucleic acids via the nucleotide remediation pathway.75 Some researchers have also found that the disorder of purine metabolism might play an important role in the development of depression.76 The levels of 7-methylhypoxanthine, hypoxanthine, uric acid, and methylguanine in the urine of depressed rats with olfactory bulb resection are reduced.77 Similarly, a meta-analysis of metabolic profile data from a large number of depression patients showed that the level of hypoxanthine in the peripheral blood of patients with MDD also changed.78 This was consistent with the results of our study. AMP consists of a phosphate group, pentose ribose, and the nucleobase adenine and is produced by the enzyme adenylate kinase during the synthesis of ATP.79 The onset of depression could lead to altered levels of AMP. Studies had shown that the preventive use of ketamine can increase AMP levels in the hippocampus and plasma of stress-exposed mice.80 In recent years, studies have described that purinergic system dysfunction is associated with the pathology of mental diseases, such as anxiety, schizophrenia, and major depression.76,81 Purine metabolism and depression-related mechanisms of action were mainly focused on purine catabolism. Clinical studies of metabolomics have shown that the levels of purine-related metabolites in the plasma of children and adolescents with depression are variable.76 A recent study found that purine metabolism in the hypothalamus of mice with lipopolysaccharide-induced depression was disturbed and proved that the abnormal function of purine metabolism is one of the main features of inflammation-mediated depression.82 At the same time, changes in the purinergic system can lead to an increase in the number of purine P2X7 receptors, which in turn promotes immune inflammation and aggravates depression.83 PBMCs are composed of immune cells, which are an important part of the body’s peripheral immune system. Excessive stress could cause disturbances in the purine metabolism of PBMCs and affect the peripheral immune system. The latest research also showed that long-term stress could cause the destruction of the mitochondria of CD4+T cells, which are important immune cells, and produce a large amount of purines.84 Enhancing the immune function of PBMCs by regulating purine metabolism might be an important way for Chaigui granules to exert their antidepressant effect, but the deeper mechanism needs to be studied further.
3.3. Bioinformatics Analysis of Important Metabolites
PBMCs are key immune cells capable of secreting cytokines, which are closely related to immune-related indicators. KEGG pathway enrichment analysis results indicated that nine metabolic pathways, specifically purine metabolism, pyrimidine metabolism, nicotinate and nicotinamide metabolism, ubiquitin-mediated proteolysis, morphine addiction, fatty acid degradation, glutathione metabolism, renin secretion and ether lipid metabolism, might be important ways for Chaigui granules to exert their antidepressant effect by improving the body’s immunity. The results of the PPI analysis indicated that ten targets, such as NT5E, NT5C1B, NT5C2, PDE11A, PDE10A, PDE2A, AMPD2, AMPD3, ADK, and PDE3A, might be potential key targets for Chaigui granules to exert an antidepressant effect. Joint pathway analysis of the ten key targets indicated that they were mainly in purine metabolism, which further suggested that purine metabolism was an important way for Chaigui granules to exert an antidepressant effect. Recent studies have demonstrated that stress causes changes in the metabolites of peripheral blood purine metabolism and that the regulation of peripheral blood purine metabolism might be one way to alleviate depression.84−86 CD4+ T cells are an important component of PBMCs, and excessive stress could also contribute to disturbances in purine metabolism in peripheral blood CD4+ T lymphocytes. This could lead to the accumulation of interferon-1 (IRF-1), levels of enzymes associated with xanthine synthesis, resulting in significantly increased levels of xanthine.84 The results of this study were consistent with those reported in the literature and indicated that Chaigui granules could exert antidepressant effects by modulating the purine metabolic pathway of PBMC and improving the immune function of the body. However, deeper mechanisms need to be investigated further.
4. Conclusions
In summary, Chaigui granules could obviously improve the depressive symptoms induced by CUMS. The results suggested that Chaigui granules could exert antidepressant effects by improving immune function and regulating the purine metabolic pathway in PBMCs. This work was the first to use the metabolomics of PBMCs as an entry point to study the antidepressant mechanism of Chaigui granules, which provided a new approach to elucidate the underlying mechanism of a Chinese medicine prescription.
5. Materials and Methods
5.1. Chemicals and Reagents
The isolation kit for rat PBMCs and the rat peripheral blood lymphocyte separation solution were purchased from Tianjin Haoyang Biological Products Technology Co., Ltd. (Tianjin, China). IL-1β, IL-6, TNF-α, IL-2, and IL-10 kits were purchased from Shanghai Xitang Biotechnology Co., Ltd. (Shanghai, China). Chromatography-grade acetonitrile, formic acid, and methanol were provided from Thermo Fisher Scientific. The following antibodies were used: FITC antirat CD3 (lot B279666, Biolegend, San Diego, CA), APC antirat CD4 (lot B334589; Biolegend, San Diego, CA), and PE antirat CD8 (lot B331642; Biolegend, San Diego, CA). BD LSRFortessa X-20 was purchased from BD Biosciences. A Scientz-IID Ultrasonic Cell Shredder was purchased from Ningbo Xinzhi Biological Technology Co., Ltd. (Ningbo, China). A high-speed refrigerated centrifuge was purchased from Shanghai Lishen Scientific Instrument Co., Ltd. (Shanghai, China). A multifunctional microplate reader (TECAN Infinite M200 Pro) was purchased from TECAN.
5.2. Preparation of Chaigui Granules
Chaigui granules (batch no. 20181009) was prepared by the Center of Shanxi Academy of Traditional Chinese Medicine. The ratio of radix bupleurum, radix angelicae sinensis, radix paeoniae alba, atractylodes macrocephala, semen mint, and glycyrrhiza uralensis is 3:3:3:3:2:1. Our research team had conducted a systematic study on the chemical composition of Chaigui granules, which mainly contain albiflorin, paeoniflorin, saikosaponin A, ferulic acid, licoricin, and atractylenolide III.37
5.3. Experimental Animals
All procedures involving animals were approved by the Animal Ethics Committee of Shanxi University (approval no. SXULL2020028) to ensure ethical use and humane treatment of the animals. All animal experiments were conducted under the National Guidelines for Experimental Animal Welfare (MOST, China, 2006) and the NIH Guide for the Care and Use of Laboratory Animals. A total of 40 male Sprague–Dawley (SD) rats, weighing 200 ± 20g, were acquired from Beijing Vital Laboratory Animal Co. Ltd. (Beijing, China). All animals were kept at standard laboratory conditions (25 ± 2 °C temperatures and 56 ± 5% relative humidity) with a 12 h light–dark cycle and food and water. All experimental procedures attempted to minimize the suffering of the experimental animals.
5.4. Administration of Chaigui Granules and Experimental Design
After 1 week of acclimation, rats were randomized into four groups: the control group (control), the CUMS model group (CUMS), and Chaigui granules treatment groups at three doses (the low-dose group, CGG-L; the medium-dose group, CGG-M; and the high-dose group, CGG-H;). In the three Chaigui granules treatment groups, rats were given Chaigui granules at doses of 4.2, 8.3, and 16.6 g/kg, respectively. Rats in the control and CUMS groups were offered water (10 mL/kg). The best possible care was provided to minimize the suffering of the experimental animals.
5.5. Establishment of the CUMS Model
The CUMS program was performed as previously described, with some modifications.87,88 With the exception of the control group, rats in the other groups were exposed to one of the following chronic unpredictable mild stressors in random order daily for four weeks: swimming in cold water (4 °C) (5 min), banning water (24 h), 50 °C exposure (10 min), tail clamping for 2 min, fasting (24 h), ultrasound stimulation (60 MHz) (3 h), restraint (3h), foot shock every 10s (2 min), and breaking circadian rhythms.89 At the same time, rats were kept separately under the same conditions except for the control rats, which were kept undisturbed in their cages.
5.6. Body Weight and Behavioral Testing
The open field test (OFT), forced swim test (FST), and sucrose preference test (SPT) were performed during the experimental period, and body weight was measured prior to each behavioral test as previously described.90,91 These factors were used to evaluate the antidepressant effect of Chaigui granules as well as to assess whether the CUMS model of depression was successfully replicated. The detailed procedures for the SPT, the OFT, and the FST can be found in the Supporting Information.
5.7. Detection of Lymphocyte Subtypes in Rats
Flow cytometry was used to detect T lymphocyte subpopulations in Sprague–Dawley rat peripheral blood. Briefly, lymphocytes were obtained by orbital blood sampling and isolated using the peripheral blood lymphocyte isolation solution. After centrifugation, the single-cell suspension density was adjusted to 1 × 106 cells/mL. Second, CD3+ T cells, CD8+ T cells, and CD4+ T cells were labeled with antibody staining. Furthermore, the percentages of different immune cell subpopulations were determined by flow cytometry (BD LSRFortessa X-20).
5.8. ELISA for Inflammatory Cytokines
The levels of inflammatory cytokines, including IL-1β, IL-6, TNF-α, IL-2, and IL-10, in the serum were detected using ELISA kits according to the manufacturer’s instructions (Shanghai Xitang Biotechnology Co., Ltd., Shanghai, China). A microplate reader was used to measure the optical density (OD) at 450 nm.
5.9. Collection of PBMC Samples
When the rats were anesthetized by an intraperitoneal injection of chloral hydrate, blood was collected from the abdominal aorta using a sodium heparin vacuum tube. After sacrifice, blood samples were collected to prepare the PBMC samples. The PBMC sample preparation was carried out in accordance with the instructions of the rat PBMC isolation kit. The PBMCs were carefully aspirated into a 15 mL centrifuge tube and washed three times with the appropriate amount of washing solution, and the cells were counted and stored in a refrigerator at −80 °C for storage.
5.10. LC-MS Metabolomics
5.10.1. Sample Preparation for Metabolomics of PBMCs
To 2 mL of water cooled with 80% methanol every 10–7 pieces of water were added PBMCs at 4 °C. The sample was vortexed for 2 min, sonicated on ice for 2 min, and centrifuged at 4 °C and 12000 rpm for 20 min. The supernatant was aspirated and then freeze-dried. Then, the sample was reconstituted with 100 uL of 80% methanol–water and centrifuged at 4 °C and 12000 rpm for 20 min. The supernatant was taken for LC-MS analysis. From each sample was extracted a 10 μL aliquot, and the aliquot was mixed as a quality control (QC) sample. A QC sample was inserted for every 5 samples, and the stability and performance of the instrument were checked.90,91
5.10.2. UPLC-QTOF/MS Analysis
A Thermo-Fisher Dionex UltiMate 3000 UHPLC-Q Exactive Orbitrap-MS, a Thermo Compound Discoverer 3.1 system, and an Xcalibur workstation were used to get LC-MS raw data. The chromatographic separation of the PBMC sample was performed on an Acquity UPLC HSS T3 column. The experimental metabolomics procedure is based on the previous article.90,91 The details of the mobile phase system and gradient elution are listed in the Supporting Information.
5.11. Data Analysis
Matched peak data were obtained from the raw LC-MS data using Compound Finder 3.1 software, and the peak area data were normalized. Then, principal component analysis (PCA), partial least-squares discriminant analysis (PLS-DA), and orthogonal partial least-squares discriminant analysis (OPLS-DA) were carried out using SIMCA-P14.1 software (Umetrics, Sweden).92 Differential metabolites among the control and model groups were selected based on VIP values greater than 1 and t-tests (P < 0.05) and were identified according to the following online databases: mzCloud (https://www.mzcloud.org/), ChemSpider (http://www.chemspider.com), and KEGG (http://www.kegg.jp). MetaboAnalyst 5.0 software was used for the pathway analysis.
5.12. Bioinformatics Analysis of Key Metabolites
5.12.1. Construction and Analysis of Metabolites and Related Gene Networks
The metabolites in PBMCs modulated by the administration of Chaigui granules were introduced into Metscape to establish a metabolic–gene network to obtain metabolite-related genes to better explain the antidepressant effects of Chaigui granules.93,94 The GeneCards database (https://genecards.weizmann.ac.il/v3/) was used to find targets associated with depression.95 Use “depression” as a keyword to search and filter for depression-related targets. Meanwhile, depression-related genes in the pathogenesis of depression were obtained. The Venn diagram of the common genes was obtained by intersecting metabolite-related genes with the depression-related genes.
5.12.2. KEGG Pathway Enrichment Analysis
To further understand the relevant functions of the target genes and illustrate the pathogenesis of depression, we performed KEGG pathway enrichment analyses of the target genes. ClueGo, a plug-in of Cytoscape software, integrates Gene Ontology, KEGG, WikiPathways, and Reactome to map groups of genes to specific functions.96 We performed a KEGG analysis of the targets based on the Cluego plug-in. We ran the ClueGO plugin and chose a cutoff threshold of P < 0.05 to display the paths.97
5.12.3. PPI Network and Hub Gene Analysis
The protein–protein interaction network (PPI network) was obtained by importing the common targets from the Wayne diagram into the STRING (https://string-db.org/) online database for analysis.98 Moreover, Cytoscape software was used to create PPI networks and identify hub proteins with the Cytohubba plugin.99 The top ten proteins were ranked as potential therapeutic targets using their CytoHubba plug-in with maximal clique centrality (MCC).100 The joint-pathways analyst tool of MetaboAnalyst 5.0 was used to explore the crucial metabolic pathway of the top ten targets.
5.13. Statistical Analysis
Statistical analysis was performed using Graphpad Prism 8.0 software and SPSS26.0. The statistical results were obtained using the Newman–Keuls multiple comparison test in ANOVA, the value of comparing the control and treatment groups. P < 0.05 was regarded as statistically significant, and P < 0.01 was regarded as a very significant difference.
Acknowledgments
This study was funded by the National Nature Science Foundation of China (nos. 82074323 and 81673572) and the major science and technology project for “Significant New Drugs Creation” (no. 2017ZX09301047). This research project is supported by Shanxi Scholarship Council of China (no. 2020019) and the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (no. 201991).
Glossary
Abbreviations
- PBMCs
peripheral blood mononuclear cells
- TCM
traditional Chinese medicine
- CUMS
chronic unpredictable mild stress
- OFT
open field test
- FST
forced swim test
- SPT
sucrose preference test
- PCA
principal component analysis
- PLS-DA
partial least-squares discriminant analysis
- OPLS-DA
orthogonal partial least-squares discriminant analysis
- PPI
protein–protein interaction
- NT5E
5′-nucleotidase ecto
- NT5C1B
5′-nucleotidase, cytosolic IB
- NT5C2
5′-nucleotidase, cytosolic II
- PDE11A
phosphodiesterase 11A
- PDE10A
phosphodiesterase 10A
- PDE2A
phosphodiesterase 2A
- AMPD2
adenosine monophosphate deaminase 2
- AMPD3
adenosine monophosphate deaminase 3
- ADK
adenosine kinase
- PDE3A
phosphodiesterase 3A
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06046.
PCA score plot of QC samples, stability of the UHPLC-MS method, top 10 targets ranked by the MCC method, and detailed experimental procedures (PDF)
Author Contributions
Y.Z., X.Q., Y.W., and G.D. conceived of and designed the experiments; D.H. and L.W. performed the experiments; D.H. and Y.Z. drafted the manuscript; and Y.Z. and X.Q. reviewed the paper. All authors have read, revised, and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Michels S.; Ganjam G. K.; Martins H.; Schratt G. M.; Wohr M.; Schwarting R.; Culmsee C. Downregulation of the psychiatric susceptibility gene Cacna1c promotes mitochondrial resilience to oxidative stress in neuronal cells. Cell Death Discov 2018, 4, 54. 10.1038/s41420-018-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K.; Li X. Risk Factors for Depression in Patients with Chronic Obstructive Pulmonary Disease. Med. Sci. Monit 2018, 24, 1417–1423. 10.12659/MSM.904969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang X.; Zhang Y.; Li J.; Li Z.; Zhang Y.; Ye X.; Tang Q.; Sun W. Comparative Efficacy and Acceptability of Anti-inflammatory Agents on Major Depressive Disorder: A Network Meta-Analysis. Front Pharmacol 2021, 12, 691200. 10.3389/fphar.2021.691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D.; Sardesai U.; Razdan R. C-reactive protein level in late-onset depression: A case-control study. Indian J. Psychiatry 2018, 60, 467–471. 10.4103/psychiatry.IndianJPsychiatry_127_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzakhani L.; Poursafa P. The Association between Depression and Climatic Conditions in the Iran Way to Preventive of Depression. Int. J. Prev Med. 2014, 5, 947–951. [PMC free article] [PubMed] [Google Scholar]
- Lee K. W.; Shin D. Association of Night Eating with Depression and Depressive Symptoms in Korean Women. Int. J. Environ. Res. Public Health 2019, 16, 4831. 10.3390/ijerph16234831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annette S.; Stephan G.; Mueser K. T.; Martin H.; Elisabeth R.; Ulrich G.; Marketa C.; Rolf E.; Hans-Jurgen M.; Peter F. A 2-year longitudinal study of neuropsychological functioning, psychosocial adjustment and rehospitalisation in schizophrenia and major depression. Eur. Arch Psychiatry Clin Neurosci 2020, 270, 699–708. 10.1007/s00406-020-01118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J.; Preskorn S.; Teague T. K.; Drevets D.; Yates W.; Drevets W. Minocycline and aspirin in the treatment of bipolar depression: A protocol for a proof-of-concept, randomised, double-blind, placebo-controlled, 2 × 2 clinical trial. BMJ. Open 2012, 2, e000643 10.1136/bmjopen-2011-000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G. M.; Stochl J.; Zammit S.; Goodyer I.; Lewis G.; Jones P. B. Childhood inflammatory markers and intelligence as predictors of subsequent persistent depressive symptoms: a longitudinal cohort study. Psychol. Med. 2018, 48, 1514–1522. 10.1017/S0033291717003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R.; O’Connor J. C.; Freund G. G.; Johnson R. W.; Kelley K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 2008, 9, 46–56. 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.; Fei G. Q.; Liu W. J.; Ding J.; Wang Y.; Wang H.; Ji J. L.; Wang X. Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-kappaB signaling pathways. Acta Pharmacol. Sin 2020, 41, 612–619. 10.1038/s41401-019-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. H.; Raison C. L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 2016, 16, 22–34. 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 1999, 461, 25–46. 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhang H.; Xie J.; Wang X. Identification of Gene Co-Expression Modules and Core Genes Related to Immune Disorders in Major Depression Disorder. Int. J. Gen Med. 2021, 14, 7983–7993. 10.2147/IJGM.S336686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadrina M.; Bondarenko E. A.; Slominsky P. A. Genetics Factors in Major Depression Disease. Front Psychiatry 2018, 9, 334. 10.3389/fpsyt.2018.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than A.; Liu C.; Chang H.; Duong P. K.; Cheung C.; Xu C.; Wang X.; Chen P. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat. Commun. 2018, 9, 4433. 10.1038/s41467-018-06981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Henriquez G.; Simon M. S.; Burger B.; Weidinger E.; Wijkhuijs A.; Arolt V.; Birkenhager T. K.; Musil R.; Muller N.; Drexhage H. A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front Psychiatry 2019, 10, 458. 10.3389/fpsyt.2019.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Bakky M. S.; Amin E.; Faris T. M.; Abdellatif A. Mental depression: Relation to different disease status, newer treatments and its association with COVID-19 pandemic (Review). Mol. Med. Rep 2021, 24, 839. 10.3892/mmr.2021.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.; Coope A.; Falkenberg C.; Dunlop B. W.; Czamara D.; Provencal N.; Craighead W. E.; Mayberg H. S.; Nemeroff C. B.; Binder E. B.; et al. Investigation of MORC1 DNA methylation as biomarker of early life stress and depressive symptoms. J. Psychiatr. Res. 2020, 120, 154–162. 10.1016/j.jpsychires.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M.; Williams L. J.; Jacka F. N.; O’Neil A.; Pasco J. A.; Moylan S.; Allen N. B.; Stuart A. L.; Hayley A. C.; Byrne M. L.; et al. So depression is an inflammatory disease, but where does the inflammation come from?. BMC Med. 2013, 11, 200. 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. H.; Maletic V.; Raison C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E.; Toups M.; Nemeroff C. B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi M. A.; Memon M. A.; Jamil T.; Naqvi S. Z.; Aimulajiang K.; Gadahi J. A.; Xu L.; Song X.; Li X.; Yan R. Galectin Domain Containing Protein from Haemonchus contortus Modulates the Immune Functions of Goat PBMCs and Regulates CD4+ T-Helper Cells In Vitro. Biomolecules 2020, 10, 116. 10.3390/biom10010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncarevic S.; Lossner C.; Kuhn K.; Prinz T.; Pike I.; Zucht H. D. In-depth profiling of the peripheral blood mononuclear cells proteome for clinical blood proteomics. Int. J. Proteomics 2014, 2014, 129259. 10.1155/2014/129259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Tang G.; Cheng K.; Yang D.; Chen G.; Liu Z.; Zhang R.; Zhou J.; Fang L.; Fang Z.; et al. Peripheral blood mononuclear cell-based metabolomic profiling of a chronic unpredictable mild stress rat model of depression. Mol. Biosyst 2014, 10, 2994–3001. 10.1039/C4MB00388H. [DOI] [PubMed] [Google Scholar]
- Liu M. L.; Zhang X. T.; Du X. Y.; Fang Z.; Liu Z.; Xu Y.; Zheng P.; Xu X. J.; Cheng P. F.; Huang T.; et al. Severe disturbance of glucose metabolism in peripheral blood mononuclear cells of schizophrenia patients: A targeted metabolomic study. J. Transl. Med. 2015, 13, 226. 10.1186/s12967-015-0540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruso-Julve F.; Pombero A.; Pilar-Cuellar F.; Garcia-Diaz N.; Garcia-Lopez R.; Juncal-Ruiz M.; Castro E.; Diaz A.; Vazquez-Bourgon J.; Garcia-Blanco A.; et al. Dopaminergic control of ADAMTS2 expression through cAMP/CREB and ERK: Molecular effects of antipsychotics. Transl Psychiatry 2019, 9, 306. 10.1038/s41398-019-0647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.; Kwon Y. A.; Lee Y.; Kim H.; Yun J. H.; Kim S.; Kim D. K. G1/S cell cycle checkpoint defect in lymphocytes from patients with Alzheimer’s disease. Psychiatry Investig 2012, 9, 413–417. 10.4306/pi.2012.9.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Zhang S. X.; Wang W.; Cheng K.; Guo H.; Rao C. L.; Yang D. Y.; He Y.; Zou D. Z.; Han Y.; et al. Potential antidepressant and resilience mechanism revealed by metabolomic study on peripheral blood mononuclear cells of stress resilient rats. Behav. Brain Res. 2017, 320, 12–20. 10.1016/j.bbr.2016.11.035. [DOI] [PubMed] [Google Scholar]
- Das Gupta S.; Lipponen A.; Paldanius K.; Puhakka N.; Pitkanen A. Dynamics of clusterin protein expression in the brain and plasma following experimental traumatic brain injury. Sci. Rep 2019, 9, 20208. 10.1038/s41598-019-56683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. Y.; Xia Q. H.; Xia Q. R.; Zhang X. L.; Liang J. MicroRNA-Based Biomarkers in the Diagnosis and Monitoring of Therapeutic Response in Patients with Depression. Neuropsychiatr Dis Treat 2019, 15, 3583–3597. 10.2147/NDT.S237116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee Z.; Zandiyeh Z.; Moeini M.; Gholami A. The Effect of Spiritual Intervention on Postmenopausal Depression in Women Referred to Urban Healthcare Centers in Isfahan: A Double-Blind Clinical Trial. Nurs Midwifery Stud 2016, 5, e32990 10.17795/nmsjournal32990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Wang P.; Liu M.; Tang L.; Fang J.; Zhao Y.; Zhang Y.; Li D.; Xu H.; Yang H. A 1H-NMR-Based Metabonomic Study on the Anti-Depressive Effect of the Total Alkaloid of Corydalis Rhizoma. Molecules 2015, 20, 10047–10064. 10.3390/molecules200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Zheng X. F.; Gao X. X.; Zhou Y. Z.; Guo X. Q.; Tian J. S.; Qin X. M. Anti-depressant effect and mechanism of supercritical CO2 extract from Compound Chaigui Fang. Zhongguo Zhong Yao Za Zhi 2014, 39, 2744–2750. [PubMed] [Google Scholar]
- Chen J. L.; Gao Y.; Qin X. M.; Tian J. S. Anti-depression mechanism of supercritical CO (2) extract from Compound Chaigui Fang based on network pharmacology. Yao Xue Xue Bao 2016, 51, 388–395. [PubMed] [Google Scholar]
- Chen L.; Liu H.; Chen J. L.; Gao X. X.; Zhou Y. Z.; Tian J. S.; Qin X. M. Anti-depressive mechanism of Fufang Chaigui prescription based on neuroendocrine hormone and metabolomic correlation analysis. Zhongguo Zhong Yao Za Zhi 2015, 40, 4080–4087. [PubMed] [Google Scholar]
- Gao X.; Li Y.; Meng M.; Wang P.; Feng Y.; Jia J.; Qin X. Exploration of chemical composition and absorption characteristics of Chaigui granules based on UHPLC-Q-orbitrap-MS/MS. J. Pharm. Biomed Anal 2020, 187, 113293. 10.1016/j.jpba.2020.113293. [DOI] [PubMed] [Google Scholar]
- Zhang S. Q.; Pan S. M.; Liang S. X.; Han Y. S.; Chen H. B.; Li J. C. Research status and prospects of biomarkers for nasopharyngeal carcinoma in the era of highthroughput omics (Review). Int. J. Oncol. 2021, 58, 9. 10.3892/ijo.2021.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y.; Shuai P.; Wang H.; Zhang S.; Li J.; Du M.; Huang P.; Qu C.; Huang L. Untargeted metabolomics for uncovering plasma biological markers of wet age-related macular degeneration. Aging (Albany NY) 2021, 13, 13968–14000. 10.18632/aging.203006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijas C.; Montenegro-Burke J. R.; Warth B.; Spilker M. E.; Siuzdak G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. 10.1038/nbt.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C. I.; Lin G.; Chiang M. H.; Chiu C. Y. Metabolomics-based discrimination of patients with remitted depression from healthy controls using 1H-NMR spectroscopy. Sci. Rep 2021, 11, 15608. 10.1038/s41598-021-95221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao C.; Tian M.; Gao H.; Gao Y.; Ruan J.; Wu L.; Gao G.; Yi L.; Liu J. Effects of Radon From Hot Springs on Lymphocyte Subsets in Peripheral Blood. Dose Response 2020, 18, 155932582090233. 10.1177/1559325820902338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J.; Ling X.; Peng B.; Ding G. miR-142–5p regulates CD4+ T cells in human non-small cell lung cancer through PD-L1 expression via the PTEN pathway. Oncol. Rep. 2018, 40, 272–282. 10.3892/or.2018.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M.; Huang T.; Hou B.; Guo Y. Role of Demyelination Efficiency within Acellular Nerve Scaffolds during Nerve Regeneration across Peripheral Defects. Biomed Res. Int. 2017, 2017, 4606387. 10.1155/2017/4606387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Li X.; Hou W. J.; Dong L. X.; Cao J. Endothelial function and T-lymphocyte subsets in patients with overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Chin Med. J. (Engl) 2019, 132, 1654–1659. 10.1097/CM9.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Wei X.; Li Y.; Feng T.; Jiang L.; Zhu H.; Yu X.; Tang J.; Chen G.; Zhang J.; et al. PM2.5 in poultry houses synergizes with Pseudomonas aeruginosa to aggravate lung inflammation in mice through the NF-kappaB pathway. J. Vet. Sci. 2020, 21, e46 10.4142/jvs.2020.21.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K.; Gao X. X.; Feng Y.; Li J.; Wang H.; Lv S. L.; Wang P. Y.; Zhang B.; Qin X. M. Integrated adrenal and testicular metabolomics revealed the protective effects of Guilingji on the Kidney-Yang deficiency syndrome rats. J. Ethnopharmacol 2020, 255, 112734. 10.1016/j.jep.2020.112734. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B.; Pavlov Y. G.; Kleber B. Music in Research and Rehabilitation of Disorders of Consciousness: Psychological and Neurophysiological Foundations. Front Psychol 2015, 6, 1763. 10.3389/fpsyg.2015.01763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J.; Lee H.; Lee G.; Oh S. J.; Shin M. K.; Shim I.; Bae H. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One 2012, 7, e42054 10.1371/journal.pone.0042054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D.; Peterlik D.; Reber S. O.; Lechner A.; Mannel D. N. Induction of Suppressor Cells and Increased Tumor Growth following Chronic Psychosocial Stress in Male Mice. PLoS One 2016, 11, e0159059 10.1371/journal.pone.0159059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo J. T.; Berntson G. G.; Malarkey W. B.; Kiecolt-Glaser J. K.; Sheridan J. F.; Poehlmann K. M.; Burleson M. H.; Ernst J. M.; Hawkley L. C.; Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann. N.Y. Acad. Sci. 1998, 840, 664–673. 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Qin A.; Zhong T.; Zou H.; Wan X.; Yao B.; Zheng X.; Yin D. Critical role of Tim-3 mediated autophagy in chronic stress induced immunosuppression. Cell Biosci 2019, 9, 13. 10.1186/s13578-019-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F. S. Enhancing versus Suppressive Effects of Stress on Immune Function: Implications for Immunoprotection versus Immunopathology. Allergy Asthma Clin Immunol 2008, 4, 2. 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Echevarria C. B.; Lamboy-Caraballo R.; Aquino-Acevedo A. N.; Armaiz-Pena G. N. Neuroendocrine Regulation of Tumor-Associated Immune Cells. Front Oncol 2019, 9, 1077. 10.3389/fonc.2019.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen R.; Vassend O. Effects of examination stress on some cellular immunity functions. J. Psychosom. Res. 1987, 31, 693–701. 10.1016/0022-3999(87)90018-3. [DOI] [PubMed] [Google Scholar]
- Dobbin J. P.; Harth M.; McCain G. A.; Martin R. A.; Cousin K. Cytokine production and lymphocyte transformation during stress. Brain Behav. Immun 1991, 5, 339–348. 10.1016/0889-1591(91)90029-A. [DOI] [PubMed] [Google Scholar]
- Glacer R.; Kiecolt-Glacer J. K.; Stout J. C.; Tarr K. L.; Speicher C. E.; Holliday J. E. Stress-related impairments in cellular immunity. Psychiatry Res. 1985, 16, 233–239. 10.1016/0165-1781(85)90111-8. [DOI] [PubMed] [Google Scholar]
- Peifer C.; Hagemann V.; Claus M.; Larra M. F.; Aust F.; Kuhn M.; Owczarek M.; Brode P.; Pacharra M.; Steffens H.; et al. Low self-reported stress despite immune-physiological changes in paramedics during rescue operations. Excli J. 2021, 20, 792–811. 10.17179/excli2021-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X.; Sun X.; Kong H.; Peng S.; Yu Z.; Wen J.; Yuan B. Chinese Herbal Medicine for the Treatment of Children and Adolescents With Refractory Mycoplasma Pneumoniae Pneumonia: A Systematic Review and a Meta-Analysis. Front Pharmacol 2021, 12, 678631. 10.3389/fphar.2021.678631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M.; Tang Q.; Li X.; Zhao R.; Li J.; Xu H.; Gao Y.; Mao Y. Shen-Qi-Jie-Yu-Fang has antidepressant effects in a rodent model of postpartum depression by regulating the immune organs and subsets of T lymphocytes. Neuropsychiatr Dis Treat 2015, 11, 1523–1540. 10.2147/NDT.S83964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S. Z.; Liu J. W.; Fang E. F.; Ng T. B.; Lian Y. L.; Ge H. Chronic unpredictable mild stress impairs erythrocyte immune function and changes T-lymphocyte subsets in a rat model of stress-induced depression. Environ. Toxicol Pharmacol 2014, 37, 414–422. 10.1016/j.etap.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Meng L.; Li J.; Cheng Y.; Wei T.; Du Y.; Peng S. Dysmenorrhea increased the risk of postpartum depression in Chinese Han parturients. Sci. Rep 2019, 9, 16579. 10.1038/s41598-019-53059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchesin K. D.; Becker-Krail D.; McClung C. A. Mood-related central and peripheral clocks. Eur. J. Neurosci 2020, 51, 326–345. 10.1111/ejn.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.; Sun L. H.; Yang W.; Cui R. J.; Xu S. B. The Role of BDNF in the Neuroimmune Axis Regulation of Mood Disorders. Front Neurol 2019, 10, 515. 10.3389/fneur.2019.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wang T.; Tian Y.; Zhang C.; Ge K.; Zhang J.; Chang J.; Wang H. Gold nanorods-mediated efficient synergistic immunotherapy for detection and inhibition of postoperative tumor recurrence. Acta Pharm. Sin B 2021, 11, 1978–1992. 10.1016/j.apsb.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto Rodrigues Bicalho P.; Magna Ribeiro F.; Henrique Ferreira Marcal P.; Gomes de Alvarenga D.; Silva F. d. S. Does Helium Pneumoperitoneum Reduce the Hyperinflammatory Response in Septic Animals during Laparoscopy?. Surg Res. Pract 2020, 2020, 5738236. 10.1155/2020/5738236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Wu L.; Yan G.; Chen Y.; Zhou M.; Wu Y.; Li Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target Ther 2021, 6, 263. 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich F. E. Inflammatory cytokine-associated depression. Brain Res. 2015, 1617, 113–125. 10.1016/j.brainres.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus G. Z.; Jansen K.; Titus S.; Carvalho A. F.; Gabbay V.; Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J. Psychiatr. Res. 2015, 68, 316–328. 10.1016/j.jpsychires.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang A.; Hao H.; Zheng X.; Liang Y.; Xie Y.; Xie T.; Dai C.; Zhao Q.; Wu X.; Xie L.; et al. Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. J. Neuroinflammation 2011, 8, 100. 10.1186/1742-2094-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos I. C.; Vasconcelos-Moreno M. P.; Costa L. G.; Kunz M.; Brietzke E.; Quevedo J.; Salum G.; Magalhaes P. V.; Kapczinski F.; Kauer-Sant’Anna M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- Jiang M.; Qin P.; Yang X. Comorbidity between depression and asthma via immune-inflammatory pathways: a meta-analysis. J. Affect Disord 2014, 166, 22–29. 10.1016/j.jad.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Dowlati Y.; Herrmann N.; Swardfager W.; Liu H.; Sham L.; Reim E. K.; Lanctot K. L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Zou W.; Feng R.; Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naive patients with major depression. PLoS One 2018, 13, e0197267 10.1371/journal.pone.0197267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K.; Katakami N.; Yamamoto Y.; Ninomiya H.; Takahara M.; Matsuoka T. A.; Bamba T.; Fukusaki E.; Shimomura I. Identification of Metabolites Associated with Onset of CAD in Diabetic Patients Using CE-MS Analysis: A Pilot Study. J. Atheroscler. Thromb 2019, 26, 233–245. 10.5551/jat.42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Liu L.; Lan X.; Cohen D.; Zhang Y.; Ravindran A. V.; Yuan S.; Zheng P.; Coghill D.; Yang L.; et al. Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents. Mol. Psychiatry 2019, 24, 1478–1488. 10.1038/s41380-018-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. F.; Feng L.; Liu X. M.; Tao X.; Wang L. S.; Zhang M. D.; Wang Z.; Chen S. G.; Chang Q. Urinary metabolic disturbance in the olfactory bulbectomized rats and the modulatory effects of fluoxetine. Life Sci. 2019, 234, 116751. 10.1016/j.lfs.2019.116751. [DOI] [PubMed] [Google Scholar]
- Pu J.; Liu Y.; Zhang H.; Tian L.; Gui S.; Yu Y.; Chen X.; Chen Y.; Yang L.; Ran Y.; et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol. Psychiatry 2021, 26, 4265–4276. 10.1038/s41380-020-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barido F. H.; Lee S. K. Changes in proteolytic enzyme activities, tenderness-related traits, and quality properties of spent hen meat affected by adenosine 5′-monophosphate during cold storage. Poult Sci. 2021, 100, 101056. 10.1016/j.psj.2021.101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. C.; Hill C.; Mastrodonato A.; LaGamma C. T.; Kitayev A.; Brachman R. A.; Narain N. R.; Kiebish M. A.; Denny C. A. Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacol 2018, 43, 1813–1821. 10.1038/s41386-018-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S.; Akundi R. S. Mitochondria: A Connecting Link in the Major Depressive Disorder Jigsaw. Curr. Neuropharmacol 2019, 17, 550–562. 10.2174/1570159X16666180302120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Li Y.; Jia Y.; Wei C.; Xu H.; Guo R.; Li Y.; Jia J.; Qi X.; Gao X. Imbalance in amino acid and purine metabolisms at the hypothalamus in inflammation-associated depression by GC-MS. Mol. Biosyst 2017, 13, 2715–2728. 10.1039/C7MB00494J. [DOI] [PubMed] [Google Scholar]
- Irrera N.; Pizzino G.; Calo M.; Pallio G.; Mannino F.; Fama F.; Arcoraci V.; Fodale V.; David A.; Francesca C.; et al. Lack of the Nlrp3 Inflammasome Improves Mice Recovery Following Traumatic Brain Injury. Front Pharmacol 2017, 8, 459. 10.3389/fphar.2017.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K. Q.; Li Y. Y.; Wang H. L.; Mao X. T.; Guo J. X.; Wang F.; Huang L. J.; Li Y. N.; Ma X. Y.; Gao Z. J.; et al. Stress-Induced Metabolic Disorder in Peripheral CD4(+) T Cells Leads to Anxiety-like Behavior. Cell 2019, 179, 864–879. 10.1016/j.cell.2019.10.001. [DOI] [PubMed] [Google Scholar]
- Hamilton P. J.; Chen E. Y.; Tolstikov V.; Pena C. J.; Picone J. A.; Shah P.; Panagopoulos K.; Strat A. N.; Walker D. M.; Lorsch Z. S.; et al. Chronic stress and antidepressant treatment alter purine metabolism and beta oxidation within mouse brain and serum. Sci. Rep 2020, 10, 18134. 10.1038/s41598-020-75114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y.; Wang Y.; Wu Z.; Lan T.; Tian Y.; Chen X.; Li Y.; Dang R.; Bai M.; Cheng K.; et al. Metabolomic abnormalities of purine and lipids implicated olfactory bulb dysfunction of CUMS depressive rats. Metab. Brain Dis 2020, 35, 649–659. 10.1007/s11011-020-00557-8. [DOI] [PubMed] [Google Scholar]
- Wang G.; Lei C.; Tian Y.; Wang Y.; Zhang L.; Zhang R. Rb1, the Primary Active Ingredient in Panax ginseng C.A. Meyer, Exerts Antidepressant-Like Effects via the BDNF-Trkb-CREB Pathway. Front Pharmacol 2019, 10, 1034. 10.3389/fphar.2019.01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Yin Q.; Tian J.; Gao X.; Qin X.; Du G.; Zhou Y. Studies on the Changes of Pharmacokinetics Behaviors of Phytochemicals and the Influence on Endogenous Metabolites After the Combination of Radix Bupleuri and Radix Paeoniae Alba Based on Multi-Component Pharmacokinetics and Metabolomics. Front Pharmacol 2021, 12, 630970. 10.3389/fphar.2021.630970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Wei F.; Liu H.; Zhao S.; Du G.; Qin X. Integrating hippocampal metabolomics and network pharmacology deciphers the antidepressant mechanisms of Xiaoyaosan. J. Ethnopharmacol 2021, 268, 113549. 10.1016/j.jep.2020.113549. [DOI] [PubMed] [Google Scholar]
- Gong W.; Zhu S.; Chen C.; Yin Q.; Li X.; Du G.; Zhou Y.; Qin X. The Anti-depression Effect of Angelicae Sinensis Radix Is Related to the Pharmacological Activity of Modulating the Hematological Anomalies. Front Pharmacol 2019, 10, 192. 10.3389/fphar.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Yin Q.; Tian J.; Gao X.; Qin X.; Du G.; Zhou Y. Studies on the potential link between antidepressant effect of Xiaoyao San and its pharmacological activity of hepatoprotection based on multi-platform metabolomics. J. Ethnopharmacol 2020, 249, 112432. 10.1016/j.jep.2019.112432. [DOI] [PubMed] [Google Scholar]
- Liu X.; Wang X.; Sun H.; Guo Z.; Liu X.; Yuan T.; Fu Y.; Tang X.; Li J.; Sun W.; et al. Urinary metabolic variation analysis during pregnancy and application in Gestational Diabetes Mellitus and spontaneous abortion biomarker discovery. Sci. Rep 2019, 9, 2605. 10.1038/s41598-019-39259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W.; Wei Z.; Dong J.; Duan F.; Chen K.; Chen C.; Liu J.; Yang X.; Chen L.; Xiao H.; et al. Global Metabolomics Reveals the Metabolic Dysfunction in Ox-LDL Induced Macrophage-Derived Foam Cells. Front Pharmacol 2017, 8, 586. 10.3389/fphar.2017.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.; Ma J.; Kang A. N.; Kang S. Y.; Jung H. W.; Park Y. K. A Novel Approach Based on Metabolomics Coupled With Intestinal Flora Analysis and Network Pharmacology to Explain the Mechanisms of Action of Bekhogainsam Decoction in the Improvement of Symptoms of Streptozotocin-Induced Diabetic Nephropathy in Mice. Front Pharmacol 2020, 11, 633. 10.3389/fphar.2020.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Hao L.; Fang K.; Han X. X.; Yu H.; Zhang J. J.; Cai L. J.; Fan T.; Zhang W. D.; Pang K.; et al. A network pharmacology perspective for deciphering potential mechanisms of action of Solanum nigrum L. in bladder cancer. BMC Complement Med. Ther 2021, 21, 45. 10.1186/s12906-021-03215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X.; Chen J.; Wu L. Identification of candidate biomarkers associated with apoptosis in melanosis coli: GNG5, LPAR3, MAPK8, and PSMC6. Biosci Rep 2019, 39, BSR20181369. 10.1042/BSR20181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Liu L.; Zhuang J.; Liu C.; Zhou C.; Yang J.; Gao C.; Liu G.; Sun C. Identification of key candidate targets and pathways for the targeted treatment of leukemia stem cells of chronic myelogenous leukemia using bioinformatics analysis. Mol. Genet Genomic Med. 2019, 7, e851 10.1002/mgg3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J.; Meng H.; Lv B.; Wang J.; Lu Y.; Su L.; Zhao S.; Li W. High expression levels of TLR9 and PD-L1 indicates a poor prognosis in patients with angioimmunoblastic T-cell lymphoma: a retrospective study of 88 cases in a single center. J. Cancer 2020, 11, 57–68. 10.7150/jca.37033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemira M.; Collin F.; Szalkowska A.; Bielska A.; Chwialkowska K.; Reszec J.; Niklinski J.; Kwasniewski M.; Kretowski A. Molecular Signature of Subtypes of Non-Small-Cell Lung Cancer by Large-Scale Transcriptional Profiling: Identification of Key Modules and Genes by Weighted Gene Co-Expression Network Analysis (WGCNA). Cancers (Basel) 2020, 12, 37. 10.3390/cancers12010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng P.; Wang X.-M.; Su H.-F.; Zhang T.; Ning L.-N.; Shi Y.; Yang S.-S.; Lin L.; Tian Q. Protective effects of Da-cheng-qi decoction in rats with intracerebral hemorrhage. Phytomedicine 2021, 90, 153630. 10.1016/j.phymed.2021.153630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.