Abstract
To clarify the K modified effects over activated carbon (AC) supported Mn–Ce oxide catalysts, several Mn–Ce/AC and xK–Mn–Ce/AC mixed oxide catalysts prepared via an impregnation method supported on AC were investigated for low-temperature selective catalytic reduction (SCR) of NO with NH3 in the simulated sintering flue gas. The Mn–Ce/AC catalyst with a K loading of 8% showed the highest catalytic activity, corresponding to 92.1% NO conversion and 92.5% N2 selectivity at 225 °C with a space velocity of 12,000 h–1. Furthermore, the 0.08K–Mn–Ce/AC catalyst exhibited better resistance to SO2 and H2O than Mn–Ce/AC, which could convert 72.3% and 74.1% of NO at the presence of 5% SO2 and H2O, respectively. After K modification, the relative ratios of Mn4+/Mnn+ as well as Ce3+/Cen+ and surface adsorbed oxygen increased. Additionally, the reduction performance of the catalyst was improved obviously, and both acid strength and quantity of acid sites increased significantly after the K species were introduced in Mn–Ce/AC. Especially, the NO adsorption capacity of the catalyst was enhanced, which remarkably promoted the denitration efficiency and SO2 resistance. The SCR of NO with NH3 on K–Mn–Ce/AC catalysts followed the L-H mechanism.
1. Introduction
Nitrogen oxide (NOx) is one of the main pollutants in industrial flue gas, which could bring about a series of serious environmental issues, such as acid rain and photochemical smog.1,2 Selective catalytic reduction with NH3 (NH3-SCR) is the most concerned technology for NOx removal. At present, V2O5/TiO2 and V2O5–WO3/TiO2 catalysts have high activity, which are widely used in industrial NOx removal at 300–400 °C.3,4 Nevertheless, the traditional catalysts are not suitable for industrial flue gas with the temperature below 250 °C, such as sintering flue gas produced from the iron and steel industry. There are more than 200 million tons per year of the emission loads of sintering flue gas in China, which has complex composition (including NOx, SO2, HCl, CO, dioxins, and dust). The iron and steel industry accounts for more than 10% of global emissions of NO, and more than 40% is from the iron ore sintering process,5 which is the major source of NO pollution in the steel industry. The temperature of sintering flue gas is between 120 and 180 °C and needs to be heated to the reaction window of high-temperature catalysts, which would consume a lot of energy. Therefore, it is necessary to develop a low-temperature NH3-SCR NOx removal catalyst.
The catalysts with transition metal oxides and rare earth oxides as active components have been demonstrated to exhibit superior catalytic activity at low temperature.6−12Zhu et al.9 prepared a CuO/AC catalyst, which could achieve 93% NO conversion at 150 °C. Yang et al.10 synthesized an Fe/AC catalyst, which obtained 95% NO conversion at 200 °C. Mn-based catalysts (e.g., Mn/TiO2, Mn/AC) has attracted increasing attention, and Mn/AC catalysts exhibited high NO conversion at 200 °C.11,12 In the presence of SO2, the CuO/AC catalyst is poisoned seriously; for Fe/AC catalysts, the NO conversion decreased rapidly from 90 to 40%. The Mn/AC catalyst is also easily deactivated by SO2 poisoning.13,14 Previous studies have found that loading other metal oxides on Mn-based catalysts could improve the SO2 resistance.15 Cao et al.16 found that Fe loading enhanced the surface acidity of the catalyst and improved the SO2 resistance of the Mn-based catalyst. Jin et al.17 supported Ce on Mn-based catalysts, and found that Ce modification could weaken the stability of the sulfate covering the surface of the catalyst and improve the SO2 resistance. However, the NO conversion of the Ce-supported catalyst in the presence of SO2 is still only 60%. The sulfur resistance of the Mn–Ce catalyst needs to be further strengthened.
In recent years, many researchers found that alkali metal K could be used as a promoter to modify catalysts.18 Pacultova et al.19 reported that K could promote the direct dissociation of NO on Co–Mn–Al oxides. Franken and Palkovits20 found that K has a positive effect on the decomposition of NO2 on the mixed spinel CuxCo3–xO4 catalysts. Xu et al.21 proposed that K modified catalysts could strengthen the NO removal path. The K/AC catalyst has a strong adsorption capacity for NO and good SO2 resistance. In addition, K could enhance the adsorption and storage of NOx on Pt/Al2O3 and Mg/Al2O3 catalysts.22,23 The promoting effect of K is related to alkalinity and electron donor properties, which facilitates the adsorption of oxygen-containing species.24 The nitrogen-containing functional groups on the AC surface also give alkaline and electron donor properties similar to K, which is the adsorption and activation site of NO.21,22 Therefore, K is a potential promoter to improve the catalytic activity and SO2 resistance of catalysts. On the other hand, researchers found that alkali metal can result in V2O5–WO3/TiO2 catalyst poisoning.25 However, the effects of K on the SCR activity over Mn–Ce/AC catalysts are not clear. Therefore, it is necessary to understand whether K modification can improve the NO removal activity and SO2 resistance of Mn–Ce/AC catalysts.
In this work, blue coke-based activated carbon as raw material and K–Mn–Ce/AC catalysts with different K loading amounts were prepared by the wet impregnation method. The effects of K on the denitrification activity and SO2 and H2O resistances of Mn–Ce/AC catalysts were investigated with the reaction temperature range of 75–250 °C. Meanwhile, on the base of the specific surface area calculated by the BET method, XRD, XPS, NH3-TPD, H2-TPR, and FT-IR were used to reveal the influence mechanism of K modification.
2. Results and Discussion
2.1. SCR Performance
The NO conversion of catalysts with different K loadings between 75 and 250 °C is shown in Figure 1a. It can be seen that the NO conversion increased with the increase of reaction temperature for all catalysts. The NO conversion of the Mn–Ce/AC catalyst increased with increasing K loading up to the optimum value, and 0.08K–Mn–Ce/AC exhibited the highest activity at the temperature of 75–250 °C, corresponding to 92.1% NO conversion at 250 °C. However, with the further increase of the loading of K, the NO conversion of the Mn–Ce/AC catalyst decreased significantly. The excessive K could be harmful to the catalytic performance of Mn–Ce/AC catalysts, which may be due to a part of the active sites being covered with K.
Figure 1.
NO conversion (a) and N2 selectivity (b) for M/AC catalysts.
The values of N2 selectivity over the different catalyst samples are shown in Figure 1b. It was clear that the N2 selectivity decreased with increasing temperature for all catalysts, which could be attributed to the part of NH3 that was oxidized at high temperature. After K modification, the N2 selectivity of catalysts improved obviously. The 0.08K–Mn–Ce/AC exhibited the highest N2 selectivity, corresponding to 92.5% at 250 °C.
2.2. Effect of SO2 on Catalytic Activity
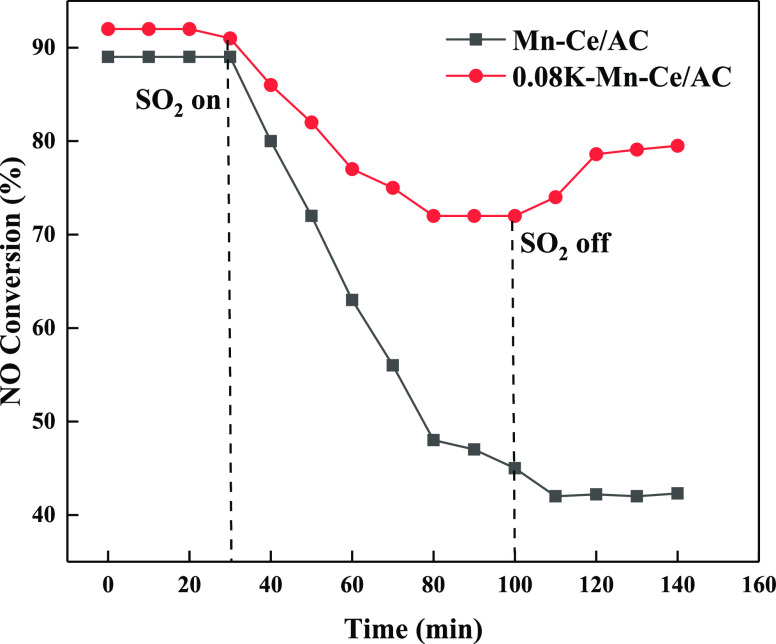
The NO conversion of the Mn–Ce/AC and 0.08KMn–Ce/AC catalysts measured at 120 ppm SO2 at 250 °C is shown in Figure 2. After the SO2 was added at 30 min, the NO conversion of 0.08K–Mn–Ce/AC decreased from 92.1% to 72.3% at 80 min and then remained stable, and that of Mn–Ce/AC rapidly decreased from 89.4% to 45.6% at 100 min. When SO2 was stopped, the NO conversion of 0.08K–Mn––Ce/AC recovered to 79.5%, while the NO conversion of the Mn–Ce/AC catalyst continued to decline and then remained 42.1% at 110 min. K modification could effectively improve the SO2 resistance of the Mn–Ce/AC catalyst.
Figure 2.
Effect of SO2 on the catalytic activity of the catalysts.
2.3. Effect of H2O on Catalytic Activity
The denitrification activity of Mn–Ce/AC and 0.08KMn–Ce/AC catalysts tested at 5% H2O is shown in Figure 3. After the H2O was added at 30 min, the NO conversion of Mn–Ce/AC decreased from 89.4% to 64.3%, and that of 0.08K–Mn–Ce/AC decreased from 92.0% to 74.1%. After cutting off H2O, the NO conversion rates of Mn–Ce/AC and 0.08KMn–Ce/AC recovered to 68.1% and 76.4%, respectively. Loading the proper amount of K could enhance the H2O resistance of the Mn–Ce/AC catalyst.
Figure 3.
Effect of H2O on the catalytic activity of the catalysts.
2.4. Analysis of N2 Adsorption–Desorption Isotherm
The nitrogen absorption–desorption isotherms for all the samples are shown in Figure 4. All samples presented high adsorption capacities in high relative pressure (P/P0 > 0.8), indicating the existence of abundant mesopores and macropores in catalysts. The Mn–Ce/AC and K–Mn–Ce/AC have similar nitrogen absorption–desorption isotherms. The K-modification had no obvious effect on the adsorption capacity of the Mn–Ce/AC catalyst. The specific surface area, pore volume, and pore size of the catalysts are shown in Table 1. After modification, the specific surface area and pore diameter of all catalysts decreased, and the pore volume increased slightly. 0.08K–Mn–Ce/AC exhibited the highest NO conversion, but both the specific surface area and pore diameter of 0.08K–Mn–Ce/AC were smaller than those of Mn–Ce/AC. Therefore, the change of the pore structure was not the main reason to improve the catalytic activity.
Figure 4.
N2 adsorption–desorption isotherms for AC, Mn–Ce/AC, 0.06K–Mn–Ce/AC, 0.08K–Mn–Ce/AC, 0.10K–Mn–Ce/AC, and 0.12K–Mn–Ce/AC.
Table 1. The Pore Structure Data of the Catalysts Measured by Nitrogen Adsorption.
| catalyst | surface area (m2·g–1) | pore volume (cm3·g–1) | pore diameter (nm) |
|---|---|---|---|
| AC | 221 | 0.088 | 4.03 |
| Mn–Ce/AC | 231 | 0.043 | 4.12 |
| 0.06K–Mn–Ce/AC | 222 | 0.049 | 4.05 |
| 0.08K–Mn–Ce/AC | 212 | 0.051 | 3.99 |
| 0.10K–Mn–Ce/AC | 210 | 0.053 | 4.03 |
| 0.12K–Mn–Ce/AC | 207 | 0.048 | 4.07 |
2.5. XRD Analysis
The crystal phase of the catalyst was determined by XRD, and the results are shown in Figure 5. The diffraction peak representing SiO2 (PDF #85-0794) appeared at 26.6° in all samples. For the Mn–Ce/AC catalyst, the diffraction peaks at 2θ values of 31.3, 35.2, and 68.3° were attributed to Mn3O4 (JCPDS no. 75-1560), and the diffraction peaks at 47.5° and 59.9° were assigned to CeO2 (JCPDS 34-0394). The diffraction peaks of metal oxides were weak, which indicated the low loading amount of metal oxides or that the metal oxides had extremely poor crystallinity and/or highly dispersed on the catalyst surface. After K modification, the diffraction peaks representing K2O could be observed at 29.4, 36.6, and 39.4°. With the increase of K content, the diffraction peaks of K2O were almost unchanged. K adhered to the surface of the catalyst and did not agglomerate after increasing K loading. The diffraction peaks of Mn3O4 and CeO2 for the K-modified catalysts were similar to the normal catalysts. Therefore, K had no obvious effect on the crystallinity and the particle dispersion of active components on the catalysts.
Figure 5.
XRD patterns of AC and M/AC catalysts.
2.6. XPS Analysis
The XPS technique was applied to identify the state of the elements on the surface of catalysts and surface element concentrations of catalysts. The XPS spectra of Mn 2p, Ce 3d, O 1s, and N 1s in Mn–Ce/AC and 0.08K–Mn–Ce/AC catalysts are shown in Figure 6. The Mn–Ce/AC and 0.08K–Mn–Ce/AC catalysts after reaction in the presence of SO2 were marked as Mn–Ce–D/AC and 0.08K–Mn–Ce–D/AC.
Figure 6.
XPS spectra for the catalyst surface: (a) Mn 2p, (b) Ce 3d, (c) O 1s, (d) N 1s, and (e) K 2p.
It could be found from Figure 6a that the Mn 2p spectra were composed of Mn 2P3/2 and Mn 2p1/2. The peaks at 639.3, 640.2, and 641.2 eV were attributed to Mn2+, Mn3+, and Mn4+ in the Mn–Ce/AC catalyst, respectively. There were only two characteristic peaks of Mn4+ and Mn3+ in the 0.08K–Mn–Ce/AC catalyst. The characteristic peak of Mn2+ disappeared on the 0.08K–Mn–Ce/AC catalyst. After K modification, the average valence state of Mn ions increased. The relative atoms of Mn4+/Mnn+ and Mn3+/Mnn+ on the surface of the catalyst were calculated according to the relative area of the characteristic peak, as shown in Table 2. The concentration of high valence state Mn4+ on the surface of Mn–Ce/AC was 22.2%, and on 0.08K–Mn–Ce/AC was 26.5%. K modification could increase the concentration of Mn4+ on the surface of the Mn–Ce/AC catalyst. Although both Mn3+ and Mn4+ can adsorb NO and NH3, it was considered that one of them played a major role in the activation on the same catalyst. The change of Mn3+/Mnn+ and Mn4+/Mnn+ ratios before and after the reaction could reflect the main species that played the role of activation. After the reaction, the Mn2+/Mnn+ ratios of Mn–Ce–D/AC increased, and the Mn3+/Mnn+ ratio remained almost unchanged, while the Mn4+/Mnn+ ratio decreased obviously. The Mn4+ species played a major role in the adsorption and activation of NO. Mn4+ was preferable for the oxidation reaction over manganese-containing catalysts and could also act as the adsorption site for NH3.26−28 The modification increased the oxidation state of Mn to higher valence states, resulting in the increase of Mn4+ and Mn3+ concentration, which was benefited to improve the performance of NO removal.
Table 2. Relative Percent Content of Catalytic Atom and Element Distribution Percentage.
| Mn 2p3/2 (%) |
Ce 3d
(%) |
O 1s
(%) |
||||||
|---|---|---|---|---|---|---|---|---|
| sample | Mn2+ | Mn3+ | Mn4+ | Ce3+ | Ce4+ | Oα | Oβ | Oγ |
| Mn–Ce/AC | 27.8 | 50.0 | 22.2 | 28.6 | 71.4 | 54.2 | 30.1 | 15.7 |
| 0.08K–Mn–Ce/AC | 73.5 | 26.5 | 40.4 | 59.6 | 64.3 | 20.9 | 14.8 | |
| Mn–Ce–D/AC | 37.1 | 48.3 | 14.6 | 22.1 | 77.9 | 37.5 | 43.5 | 19.0 |
| 0.08K–Mn–Ce–D/AC | 84.6 | 15.4 | 27.1 | 72.9 | 45.4 | 34.9 | 22.7 | |
It could be found from Figure 6b that Ce 3d consisted of Ce 3d3/2 and Ce 3d5/2 spectra. The photoelectron peaks marked as V(881.3 eV), V2(886.6 eV), V3(897.5 eV), U(900.3 eV), U2(907.4 eV), and U3(915.6 eV) have been attributed to Ce4+, and the peaks labeled as V1(884.3 eV) and U1(904.3 eV) were assigned to the Ce3+ species.29,30 The Ce3+ and Ce4+ species were coexisted, and Ce4+ species were the main species on the surface of catalyst. The presence of Ce3+ could contribute to charge imbalance, forming more unstable oxygen holes and oxygen free radicals, which could improve the ability of oxygen migration on the surface of the catalyst and increase the NO removal efficiency. The percentage of the Ce3+ species were calculated by the areas of the Ce 3d components relative to Ce4+ (V, V2, V3, U, U2, U3) and to Ce3+ (V1, U1). The Ce3+/Cen+ and Ce4+/Cen+ of Mn–Ce/AC, 0.08K–Mn–Ce/AC, Mn–Ce–D/AC, and 0.08K–Mn–Ce–D/AC catalysts are listed in Table 2. The Ce3+/Cen+ and Ce4+/Cen+ of Mn–Ce/AC and 0.08K–Mn–Ce/AC catalysts were 28.6 and 40.4%, respectively. After modification, the ratio of Ce3+/Cen+ increased obviously, which benefited the 0.08K–Mn–Ce/AC catalyst, obtaining a high NO conversion.
It could be found from Figure 6c that all O 1s spectra had three characteristic peaks, lattice oxygen (Oα, 530.3 eV), surface oxygen species (Oγ, 531.6 eV) like chemisorbed oxygen or weakly boned oxygen species, and acid radical and/or hydroxyl species (Oβ, 529.3 eV).31−33 The ratio of Oα increased from 54.2% of Mn–Ce/AC to 64.3% of 0.08K–Mn–Ce/AC after modification. The appropriate K had a positive effect for the formation of chemisorbed oxygen Oα on the catalyst surface. Oα was the most active oxygen species and was more reactive than Oβ because of its higher mobility, which could improve the efficiency of the NO oxidation reaction. Especially, Oα could strengthen the adsorption capacity of NO. Therefore, K modification could enhance the adsorption capacity of the catalyst for NO, which may be the main reason for the increase of NO conversion and the improvement of SO2 resistance.
The N 1s spectra of catalysts are shown in Figure 6d. The characteristic peaks included pyridine (N-6, 398.4–399.0 eV); imines, amides, and amines (imines, 399.6–400 eV); pyrrolic acid (N-5, 400.1–400.5 eV); and quaternary nitrogen (N-Q, 401.1–401.7 eV).34,35 The nitrogen-containing functional groups on the surface of the catalyst were mainly quaternary nitrogen type (N-Q), pyridine type (N-6) and pyrrole type (N-5), and pyridine type functional groups accounting for the majority. The nitrogen atom in the pyridine type nitrogen-containing functional group had isolated electrons, which could make the surface of AC show strong alkalinity and strong adsorption affinity for NOx. The mount of basic N-6 groups on 0.08K–Mn–Ce/AC was similar to 0.08K–Mn–Ce–D/AC catalysts, which indicated that NH3 was not adsorbed on the N-6 group at the NH3-SCR process. The adsorption and activation of NH3 occurred on other functional groups, such as −OH. However, the −OH content was limited, indicating that the improvement of the 0.08K–Mn–Ce/AC catalytic activity was not directly related to the increase of NH3 adsorption.
The K 2p XPS spectra of 0.08K–Mn–Ce/AC and 0.08K–Mn–Ce–D/AC are displayed in Figure 6e. The spectra could be resolved into two peaks, which were ascribed to the K–O group. The main one at 292.5 eV for K 2p3/2 being accompanied by satellite peaks at 295.3 eV correspond to the K 2p1/2 level. After the reaction in the presence of SO2, the K species could exist stably. The electron-rich potassium atoms were energetically favorable for dissociation of O2 by charge transfer from potassium to O2. The K modification could promote the dissociative adsorption of O2 and produce higher concentration of Oads species on the surface of catalysts, which increased the NO conversion.
2.7. FT-IR Analysis
The effect of K loading on AC surface functional groups was explored by FT-IR. It could be seen from Figure 7 that the infrared spectra of all samples were similar. There was a broad absorption band in the range of 3700–3200 cm–1, and the maximum value was close to 3435 cm–1, which could belong to the stretching vibration of the O–H.36,37 The adsorption band at 2400–2300 cm–1 could be attributed to the CO2 structure.38,39 The weak band observed near 1632 cm–1 was designated as C=C or C=O stretching vibration.40,41 The absorption band near 1100–1000 cm–1 was related to the C–O or C–O–C stretching vibration, and the peak at 1066 cm–1 was attributed to the C–O functional group.42 There were many kinds of functional groups on the surface of AC and M/AC, and the addition of K did not affect the types of functional groups of AC. The strength of the O–H bond for M/AC at 3435 cm–1 was decreased, which was due to the separation of chemically adsorbed water caused by calcination. After loading K, the peak intensity at 1632 cm–1 decreased, in which it could be assumed that the introduction of metal oxides destroyed the inherent graphite crystallite structures of the carbon material and the metal oxide formed a bond with some unsaturated carbon, leading to the C=C bond breaking and the formation of C–O. A large number of oxygen-containing groups existed on the catalyst surface, which could promote NH3 adsorption and improve the efficiency of NO removal.
Figure 7.
FT-IR spectra of AC and M/AC catalysts. (a) AC, (b) Mn–Ce/AC, (c) 0.06K–Mn–Ce/AC, (d) 0.08K–Mn–Ce/AC, (e) 0.1K–Mn–Ce/AC, (f) 0.12K–Mn–Ce/AC.
2.8. H2-TPR Analysis
The redox performance of the catalyst could be determined by the H2-TPR experiment. It could be seen from Figure 8 that the catalysts had a continuous and wide reduction peak in the range of 500–750 °C, which was a superposition peak formed by the reduction reaction of K, Mn, and Ce metal oxides. It mainly included two redox processes: Mn3O4 ↔ MnO2 and CeO2 ↔ Ce2O3. The reduction peaks of AC, Mn–Ce/AC, and 0.08K–Mn–Ce/AC were at 645, 643, and 604 °C, respectively. The reduction peaks shifted to the left, indicating that the reduction temperature of the metal oxides decreased after K modification. Furthermore, the maximum H2 consumption increased, which was attributed to the fact that the average oxidation state of Mn and Ce in the 0.08K–Mn–Ce/AC catalyst were higher than that of the Mn–Ce/AC catalyst and/or the reduction potential of oxygen covered on the 0.08K–Mn–Ce/AC catalyst surface increased. In addition, this phenomenon could attribute to the increase of active component reduction potential, the formation of more oxygen vacancies, and/or the existence of more surface oxygen species. After modification, the decrease of reduction temperature and the increase of hydrogen consumption suggested that the reducibility of the catalyst was improved, which was beneficial for obtaining high NO conversion in the NH3-SCR reaction at low temperature.
Figure 8.
H2-TPR profiles of AC, Mn–Ce/AC, and 0.08K–Mn–Ce/AC catalysts.
2.9. NH3-TPD Analysis
The NH3-TPD was used to analyze the properties of acidic sites. In the NH3-SCR reaction, the strength and number of acid sites on the catalyst surface made a significant contribution to the whole low temperature catalytic reaction. The desorption temperature could reflect the strength of acid sites, and the area under the desorption peak could represent the number of acid sites. It could be seen from Figure 9 that the catalysts had two desorption peaks, which were about 95 and 670 °C. The low-temperature desorption peak below 350 °C corresponded to the NH4+ desorbed on Brønsted acid sites, in which the high-temperature desorption peak above 400 °C corresponded to NH3 desorbed on Lewis acid sites. The results showed that the desorption peak of the 0.08K–Mn–Ce/AC catalyst was larger and wider than Mn–Ce/AC, which indicated that the introduction of K could increase the number of acid sites and enhance the acid strength of the catalyst. The relative areas of AC, Mn–Ce/AC, and 0.08K–Mn–Ce/AC peaks around 670 °C were calculated, which were 0.051, 0.122, and 0.163, respectively. It should be noted that the acid content increased significantly in 0.08K–Mn–Ce/AC. K modification exposed more acid active sites and increased NH3 adsorption. Therefore, the increase of acid strength and quantity of acid sites was one of the crucial reasons why the 0.08K–Mn–Ce/AC catalyst had higher catalytic activity than the Mn–Ce/AC catalyst.
Figure 9.
NH3-TPD profiles of AC and M/AC catalysts.
2.10. Summary of Mechanism
Considering the redox reaction and the synergistic effect between the Mn and Ce cations, the reaction mechanism for the catalytic oxidation of NO on xK–Mn–Ce/AC catalyst at low temperature was proposed, as shown in Figure 10. The NH3-SCR of the NO process over xK–Mn–Ce/AC catalysts was a representative heterogeneous catalysis system, which could be classified with six steps.
-
(1)
Reactant gas (NH3, NO, and O2) diffusion from bulk gas to the external surface of xK–Mn–Ce/AC catalysts.
-
(2)
Reactant gas diffusion to the internal surface through AC pores. The AC support had a large specific surface area and abundant pore structure and could reserve vast reactant gas and thereby provide a large amount of reaction units on the catalyst surface.
-
(3)
Reactant gas adsorption on the active sites of the catalyst surface. Oxygen was adsorbed on the surface of AC in the form of active oxygen (Oads/[O]), as shown in eq 1. Oads were conducive to the formation of gaseous NO into NO2-containing species. NO was adsorbed on the catalyst surface as the adsorbed state and then activated by the active sites as the activated state (NOads), as shown in eq 2. The loading of K species exposed more acidic sites, which was conducive to the adsorption of NH3 into activated NH3 or NH4+, as shown in eq 3. Meanwhile, Ce4+ captured an electron from Mn3+ and then became Ce3+ and Mn4+, as shown in eq 4. Oads captured an electron from the adsorbed Ce3+ and Mn4+ and converted it into O–ads. O–ads reacted with NOads to produce NO2–, as shown in eq 5. NO2– reacted with NH4+ to produce N2 and H2O, as shown in eq 6.
-
(4)
Generated gas desorption (N2 and H2O) from the catalyst surface.
-
(5)
Generated gas diffusion to the internal surface through AC pores.
-
(6)Generated gas diffusion from bulk gas to the external surface of xK–Mn–Ce/AC catalysts. The reaction pathway of NO on the xK–Mn–Ce/AC catalyst mainly followed the L-H mechanism.

1 
2 
3 
4 
5 
6
Figure 10.
The proposed mechanism of the reaction over the xK–Mn–Ce/AC.
The loading of K species increased the chemisorption oxygen on the catalyst surface, and Mn4+/Mnn+ and Ce3+/Cen+ increased slightly. The redox between Mn and Ce ions formed oxygen vacancies and significantly promoted the continuous supplement of gas-phase oxygen, which was conducive to the adsorption of oxygen on the catalyst surface,43,44 and promoted the conversion of NO to NO2. In addition, K+ and basic groups could adjust the acids and alkalis and adsorption position of the catalyst. The loading of K increased the number of pyridine type nitrogen-containing functional groups. The catalyst surface was strongly alkaline and had strong adsorption affinity for NO. A previous study reported that K+ was an electron accelerator, and a small amount of K+ would cause changes in the geometric and electronic structures, promoting the reaction path of NO removal and improving the chemical adsorption of NO.45 The synergistic effect of Mn and Ce ions on NO oxidation was significantly stimulated.
3. Conclusions
The K modified effects on activated carbon supported Mn–Ce catalysts for low-temperature SCR of NO was studied. Compared with the Mn–Ce/AC catalyst, the K, Mn and Ce co-doped trimetallic catalyst had better NH3-SCR performance.
-
(1)
The 0.08K–Mn–Ce/AC catalyst with a K loading of 8% showed the highest catalytic activity, corresponding to 92.1% NO conversion and 92.5% N2 selectivity at 225 °C with a space velocity of 12,000 h–1.
-
(2)
The 0.08K–Mn–Ce/AC catalyst exhibited better resistance to SO2 and H2O than Mn–Ce/AC, which could convert 72.3% and 74.1% of NO at the presence of 5% SO2 and H2O, respectively.
-
(3)
The K modified could improve the ratio of Mn4+/Mnn+ and Ce3+/Cen+ on the surface of the Mn–Ce/AC catalyst and enhance the chemisorbed oxygen and the adsorption performance of NO.
-
(4)
The reduction performance of the catalyst was improved obviously. Both acid strength and quantity of acid sites increased significantly after the K species were introduced in Mn–Ce/AC, which could be attributed to the more exposed active sites of acid.
The reduction performance of the catalyst was improved obviously. Both acid strength and quantity of acid sites increased significantly after the K species were introduced in Mn–Ce/AC, which could be attributed to the more exposed active sites of acid.
4. Experimental Section
4.1. Catalyst Preparation
The experimental activated carbon (AC) was prepared by carbonization and activation 2 h of CO2 in a tube furnace at 900 °C from blue coke as raw material provided from one enterprise in China, Shaanxi province. AC was crushed and sieved into granules ranging from 16 to 20 mesh followed by washing with de-ionized water and drying at 110 °C for 6 h.
Different proportions of (Mn(NO3)2, Ce(NO3)3·6H2O, and K2CO3 were loaded on AC by wet impregnation. The total weight of metal oxide loading was 5 wt % controlled. The impregnated AC was magnetically stirred for 4 h and dried to constant weight in a drying oven at 80 °C. The obtained samples were calcined under a N2 atmosphere at 400 °C for 4 h. The obtained catalysts were marked as M/AC; M represents Mn/Ce/K, where the mass ratio of Mn/Ce was 7:3. The content of K was controlled to be 6, 8, 10, and 12 wt %.
4.2. Catalyst Performance
A continuous flow fixed bed was used to test the SCR performance of the catalysts under the following conditions: 500 ppm of NH3, 500 ppm of NO, 13% O2, CO2 7%, and N2 as balance at a total flow rate of 500 mL/min. The amount of catalyst added was 0.4 g, corresponding to the gas hourly space velocities (GHSV) of 12,000 h–1. The outlet concentration of gas was measured by a flue gas analyzer, and the data were recorded after the system remained stable for 20 min at each desired temperature. The NO conversion and N2 selectivity were calculated by the following equations:
| 7 |
| 8 |
where the subscripts in and out indicated the inlet and outlet concentrations at the steady state, respectively.
4.3. Catalyst Characterization
The pore structure parameters (specific surface area, pore volume, and pore size) of the samples were determined by the JW-BK222 N2 adsorption apparatus. The specific surface area of the samples was calculated according to the BET equation, and the pore volume and pore size of the samples were obtained by the BJH model.
The X-ray diffraction (XRD) patterns of samples were measured with a BRUKER D8 ADVANCE diffractometer and recorded between a 2θ range of 10–80° with scan speed of 5°/min.
X-ray photoelectron spectroscopy (XPS) was performed to investigate the surface chemical states of Mn, Ce, O, and N species in Mn–Ce/AC and xK–Mn–Ce/AC with a Thermo ESCALAB250Xi. Additionally, the obtained binding energies were referenced to the C 1s line at 284.8 eV.
Fourier transform infrared (FT-IR) analysis was recorded using a Nicolet Is50. The samples were mixed with KBr and then ground into powder to form a slice. The spectral range was 4000–400 cm–1 and the resolution was 4 cm–1.
H2-TPR measurements were performed on an AutoChem II TPR/TPD 2920 apparatus. For each experiment, a 100 mg sample was pretreated at 300 °C for 1 h followed by cooling to 50 °C under an Ar atmosphere. The sample was then heated to 700 °C at a constant heating rate of 10 °C/min under a flow of H2 (10%)/Ar (50 mL/min).
The outlet exhaust detected by NH3-TPD was conducted to be similar to that of H2-TPR. The samples were exposed to a 500 ppm NH3 flow in N2 for 1 h. Then, the samples were heated to 650 °C at a constant heating rate of 10 °C/min under a flow of 100 mL/min N2.
Acknowledgments
The present work was financially supported by Natural Science Basic foundation of China (program no. 52174325), the Shanxi Province Key Research and Development Plan (Grant No. 2020GY-166), and China Postdoctoral Science Found (grant no. 2019M663932XB). The authors gratefully acknowledge their support.
The authors declare no competing financial interest.
References
- Damma D.; Boningari T.; Ettireddy P. R.; Reddy B. M.; Smirniotis P. G. Direct decomposition of NOx over TiO2 supported transition metal oxides at low temperatures. Ind. Eng. Chem. Res. 2018, 57, 16615–16621. 10.1021/acs.iecr.8b03532. [DOI] [Google Scholar]
- Lei Z.; Han B.; Yang K.; Chen B. H. Influence of H2O on the low-temperature NH3-SCR of NO over V2O5/AC catalyst: An experimental and modeling study. Chem. Eng. J. 2013, 215, 651–657. 10.1016/j.cej.2012.11.011. [DOI] [Google Scholar]
- He Y.; Ford M. E.; Zhu M.; Liu Q.; Tumuluri U.; Wu Z.; Wachs I. E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B. 2016, 193, 141–150. 10.1016/j.apcatb.2016.04.022. [DOI] [Google Scholar]
- Xie X.; Lu J.; Hums E.; Huang Q.; Lu Z. Study on the deactivation of V2O5-WO3/TiO2 selective catalytic reduction catalysts through transient kinetics. Energy Fuels 2015, 29, 3890–3896. 10.1021/acs.energyfuels.5b01034. [DOI] [Google Scholar]
- Tomas Da Rocha L.; Chung B. J.; Jung S. M. Formation and reduction of NO from the combustion of the fuels used in the sintering process of iron ore in the presence of additives. J. Sustainable Metall. 2021, 7, 377–390. 10.1007/s40831-021-00368-w. [DOI] [Google Scholar]
- Wang M.; Liu H.; Huang Z.-H.; Rang F. Activated carbon fibers loaded with MnO2 for removing NO at room temperature. Chem. Eng. J. 2014, 256, 101–106. 10.1016/j.cej.2014.06.108. [DOI] [Google Scholar]
- Fang D.; Xie J.; Hu H.; Yang H.; He F.; Fu Z. Identification of MnOx species and Mn valence states in MnOx/TiO2 catalysts for low temperature SCR. Chem. Eng. J. 2015, 271, 23–30. 10.1016/j.cej.2015.02.072. [DOI] [Google Scholar]
- Jiang L.; Liu Q.; Ran G.; Kong M.; Ren S.; Yang J.; Li J. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem. Eng. J. 2019, 370, 810–821. 10.1016/j.cej.2019.03.225. [DOI] [Google Scholar]
- Zhu Z.; Liu Z.; Liu S.; Niu H.; Hu T.; Liu T.; Xie Y. NO reduction with NH3 over an activated carbon-supported copper oxide catalysts at low temperatures. Appl. Catal. B. 2000, 26, 25–35. 10.1016/S0926-3373(99)00144-7. [DOI] [Google Scholar]
- Yang W.; Liu F.; Xie L.; Lian Z.; He H. Effect of V2O5 additive on the SO2 resistance of a Fe2O3/AC catalyst for NH3-SCR of NOx at low temperatures. Ind. Eng. Chem. Res. 2016, 55, 2677–2685. 10.1021/acs.iecr.5b04974. [DOI] [Google Scholar]
- Zheng H.; Song W.; Zhou Y.; Ma S.; Deng J.; Li Y.; Liu J.; Zhao Z. Mechanistic study of selective catalytic reduction of NOx with NH3 over Mn-TiO2: A combination of experimental and DFT study. J. Phys. Chem. C 2017, 121, 19859. 10.1021/acs.jpcc.7b06715. [DOI] [Google Scholar]
- Wu H. M.; Wang X. B.; Gui K. T. Performance of SCR denitration of impregnated catalysts using activated carbon as support. J Southeast Univ.:Nat Sci Ed. 2013, 43, 814–818. [Google Scholar]
- Wang C. Z.; Zhao Y. G.; Zhang C.; Yan X.; Cao P. Effect of iron doping on SO2 and H2O resistance of honeycomb cordierite-based Mn-Ce/Al2O3 catalyst for NO removal at low temperature. Res. Chem. Intermediate 2018, 44, 3135–3150. 10.1007/s11164-018-3297-0. [DOI] [Google Scholar]
- Zhou Y.; Su B.; Ren S.; Chen Z.; Su Z.; Yang J.; Chen L.; Wang M. Nb2O5-modified Mn-Ce/AC catalyst with high ZnCl2 and SO2 tolerance for low-temperature NH3-SCR of NO. J. Environ. Chem. Eng. 2021, 9, 106323. 10.1016/j.jece.2021.106323. [DOI] [Google Scholar]
- Wang X.; Wu S. G.; Zou W.; Yu S.; Gui K.; Dong L. Fe-Mn/Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chinese J. Catal. 2016, 37, 1314–1323. 10.1016/S1872-2067(15)61115-9. [DOI] [Google Scholar]
- Cao F.; Su S.; Wang P.; Hu S.; Sun L. S.; Zhang A. The activity and mechanism study of Fe-Mn-Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. 10.1016/j.fuel.2014.08.060. [DOI] [Google Scholar]
- Jin R.; Liu Y.; Wang Y.; Cen W.; Wu Z.; Wang H.; Weng X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B. 2014, 148-149, 582–588. 10.1016/j.apcatb.2013.09.016. [DOI] [Google Scholar]
- Li J.; Chang H.; Ma L.; Hao J.; Yang R. T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. 10.1016/j.cattod.2011.03.034. [DOI] [Google Scholar]
- Pacultova K.; Bilkova T.; Klegova A.; Karaskova K.; Fridrichova D.; Jiratova K.; Kiska T.; Balabanova J.; Kostejn M.; Kotarba A.; Kaspera W.; Stelmachowski P.; Slowik G.; Obalova L. Co-Mn-Al mixed oxides promoted by K for direct NO decomposition: effect of preparation parameters. Catalysts 2019, 9, 593. 10.3390/catal9070593. [DOI] [Google Scholar]
- Franken T.; Palkovits R. Investigation of potassium doped mixed spinels CuxCO3-xO4 as catalysts for an efficient N2O decomposition in real reaction conditions. Appl. Catal. 2015, 176, 298–305. 10.1016/j.apcatb.2015.04.002. [DOI] [Google Scholar]
- Xu Z. C.; Li Y. R.; Guo J. X.; Xiong J.; Lin Y. T.; Zhu T. Y. An efficient and sulfur resistant K-modified activated carbon for SCR denitrification compared with acid- and Cu-modified activated carbon. Chem. Eng. J. 2020, 395, 125047. [Google Scholar]
- Kim D. H.; Mudiyanselage K.; Szanyi J.; Kwak J. H.; Zhu H. Y.; Peden C. H. F. Effect of K loadings on nitrate formation/decomposition and on NOx storage performance of K-based NOx storage-reduction catalysts. Appl. Catal. B. 2013, 142, 472–478. 10.1016/j.apcatb.2013.05.063. [DOI] [Google Scholar]
- Takahashi N.; Matsunaga S.; Tanaka T.; Sobukawa H.; Shinjoh H. New approach to enhance the NOx storage performance at high temperature using basic MgAl2O4 spinel support. Appl. Catal. B. 2007, 77, 73–78. 10.1016/j.apcatb.2007.07.007. [DOI] [Google Scholar]
- Wang Y. X.; Wang G. C. A systematic theoretical study of water gas shift reaction on Cu(111) and Cu(110): potassium effect. ACS Catal. 2019, 9, 2261–2274. 10.1021/acscatal.8b04427. [DOI] [Google Scholar]
- Chen L.; Li J.; Ge M. The poisoning effect of alkali metals doping over nano V2O5-WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. 10.1016/j.cej.2010.11.020. [DOI] [Google Scholar]
- Cheng C.; Mao L.; Shi J.; Xue F.; Zong S.; Zheng B.; Guo L. NiCo2O4 nanosheet as a novel oxygen-evolution-reaction cocatalyst in-situ bonded on g-C3N4 photocatalyst for excellent overall water splitting. J. Mater. Chem. A 2021, 9, 12299–12306. 10.1039/D1TA00241D. [DOI] [Google Scholar]
- Wan Y.; Zhao W.; Tang Y.; Li L.; Wang H.; Cui Y.; Gu J.; Li Y.; Shi J. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B. 2014, 148, 114–122. 10.1016/j.apcatb.2013.10.049. [DOI] [Google Scholar]
- Liu C.; Xue L.; He H. Influence of alkaline earth metals on cobalt-cerium composite oxide catalysts for N2O decomposition. Acta Phys.-Chim. Sin. Acta. 2009, 25, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Cheng C.; Dong C.-L.; Shi J.; Mao L.; Huang Y.-C.; Kang X.; Zong S.; Shen S. Regulation on polymerization degree and surface feature in graphitic carbon nitride towards efficient photocatalytic H2 evolution under visible-light irradiation. J. Mater. Sci. Technol. 2022, 98, 160–168. 10.1016/j.jmst.2021.05.019. [DOI] [Google Scholar]
- Yang J.; Ren S.; Zhang T.; Su Z.; Long H.; Kong M.; Yao L. Iron doped effects on active sites formation over activated carbon supported Mn-Ce oxide catalysts for low-temperature SCR of NO. Chem. Eng. J. 2020, 379, 122398. 10.1016/j.cej.2019.122398. [DOI] [Google Scholar]
- Zhang Y.; Shi J.; Huang Z.; Guan X.; Zong S.; Cheng C.; Zheng B.; Guo L. Synchronous construction of CoS2 in-situ loading and S doping for g-C3N4: Enhanced photocatalytic H2-evolution activity and mechanism insight. Chem. Eng. J. 2020, 401, 126135. 10.1016/j.cej.2020.126135. [DOI] [Google Scholar]
- Niu Y.; Shang T.; Hui S.; Zhang X.; Lei Y.; Lv Y.; Wang S. Synergistic removal of NO and N2O in low-temperature SCR process with MnOx/Ti based catalyst doped with Ce and V. Fuel 2016, 185, 316–322. 10.1016/j.fuel.2016.07.122. [DOI] [Google Scholar]
- Zhao B.; Ran R.; Guo X.; Cao L.; Xu T.; Chen Z.; Wu X.; Si Z.; Weng D. Nb-modified Mn/Ce/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperature. APPL CATAL A-GEN. 2017, 545, 64–71. 10.1016/j.apcata.2017.07.024. [DOI] [Google Scholar]
- Cheng C.; Shi J.; Wen L.; Dong C.-L.; Huang Y.-C.; Zhang Y.; Zong S.; Diao Z.; Shen S.; Guo L. Disordered nitrogen-defect-rich porous carbon nitride photocatalyst for highly efficient H2 evolution under visible-light irradiation. Carbon 2021, 181, 193–203. 10.1016/j.carbon.2021.05.030. [DOI] [Google Scholar]
- Lin Y.; Li Y.; Xu Z.; Xiong J.; Zhu T. Transformation of functional groups in the reduction of NO with NH3 over nitrogen-enriched activated carbons. Fuel 2018, 223, 312–323. 10.1016/j.fuel.2018.01.092. [DOI] [Google Scholar]
- Zheng J.; Xing X.; Pang Z.; Wang S.; Du Y.; Lv M. Effect of Na2CO3, HF, and CO2 treatment on the regeneration of exhausted activated carbon used in sintering flue gas. ACS Omega 2021, 6, 25762–25771. 10.1021/acsomega.1c04182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyathi T. M.; Fadlalla M. I.; Fischer N.; York A. P. E.; Olivier E. J.; Gibson E. K.; Wells P. P.; Claeys M. Support and gas environment effects on the preferential oxidation of carbon monoxide over Co3O4 catalysts studied in situ. Appl. Catal. B. 2021, 120450. 10.1016/j.apcatb.2021.120450. [DOI] [Google Scholar]
- Zhang Y.; Huang Z.; Dong C.-L.; Shi J.; Cheng C.; Guan X.; Zong S.; Luo B.; Cheng Z.; Wei D.; Huang Y.-c.; Shen S.; Guo L. Synergistic effect of nitrogen vacancy on ultrathin graphitic carbon nitride porous nanosheets for highly efficient photocatalytic H2 evolution. Chem. Eng. J. 2022, 431, 134101. 10.1016/j.cej.2021.134101. [DOI] [Google Scholar]
- Morterra C.; Low M. J. D. IR studies of carbons—II: The vacuum pyrolysis of cellulose. Carbon 1983, 21, 283–288. 10.1016/0008-6223(83)90092-1. [DOI] [Google Scholar]
- Yan Z.; Liu L.; Zhang Y.; Liang J.; Wang J.; Zhang Z.; Wang X. Activated semi-coke in SO2 removal from flue gas: selection of activation methodology and desulfurization mechanism study. Energy Fuels 2013, 27, 3080–3089. 10.1021/ef400351a. [DOI] [Google Scholar]
- Xing X.; Du Y.; Zheng J.; Wang S.; Ren S.; Ju J. Isothermal carbothermal reduction of FeTiO3 doped with MgO. JOM 2021, 73, 1328–1336. 10.1007/s11837-021-04628-8. [DOI] [Google Scholar]
- Yang C.; Wang Y.; Fan H.; de Falco G.; Yang S.; Shangguan J.; Bandosz T. J. Bifunctional ZnO-MgO/activated carbon adsorbents boost H2S room temperature adsorption and catalytic oxidation. Appl. Catal. B. 2020, 266, 118674. 10.1016/j.apcatb.2020.118674. [DOI] [Google Scholar]
- Wu Z.; Jin R.; Liu Y.; Wang H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. 10.1016/j.catcom.2008.05.001. [DOI] [Google Scholar]
- Boningari T.; Ettireddy P. R.; Somogyvari A.; Liu Y.; Vorontsov A.; McDonald C. A.; Smirniotis P. G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. 10.1016/j.jcat.2015.03.002. [DOI] [Google Scholar]
- Pecchi G.; Cabrera B.; Buljan A.; Delgado E. J.; Gordon A. L.; Jimenez R. Catalytic oxidation of soot over alkaline niobates. J. Alloys Compd. 2013, 551, 255–261. 10.1016/j.jallcom.2012.10.015. [DOI] [Google Scholar]