Abstract
Afferent innervation of the cochlea by the auditory nerve declines during aging and potentially after sound overexposure, producing the common pathology known as cochlear synaptopathy. Auditory-nerve-fiber loss is difficult to detect with the clinical audiogram and has been proposed to cause ‘hidden hearing loss’ including impaired speech-in-noise perception. While evidence that auditory-nerve-fiber loss causes hidden hearing loss in humans is controversial, behavioral animal models hold promise to rigorously test this hypothesis because neural lesions can be induced and histologically validated. Here, we review recent animal behavioral studies on the impact of auditory-nerve-fiber loss on perception in a range of species. We first consider studies of tinnitus and hyperacusis inferred from acoustic startle reflexes, followed by a review of operant-conditioning studies of the audiogram, temporal integration for tones of varying duration, temporal resolution of gaps in noise, and tone-in-noise detection. Studies quantifying the audiogram show that tone-in-quiet sensitivity is unaffected by auditory-nerve-fiber loss unless neural lesions exceed 80%, at which point large deficits are possible. Changes in other aspects of perception, which were typically investigated for moderate-to-severe auditory-nerve-fiber loss of 50–70%, appear heterogeneous across studies and might be small compared to impairment caused by hair-cell pathologies. Future studies should pursue recent findings that behavioral sensitivity to brief tones and silent gaps in noise may be particularly vulnerable to auditory-nerve-fiber loss. Furthermore, aspects of auditory perception linked to central inhibition and fine neural response timing, such as modulation masking release and spatial hearing, may be productive directions for further animal behavioral research.
Keywords: animal behavior, central gain, cochlear synaptopathy, inner hair cell, operant conditioning, tinnitus
Introduction
Sensorineural hearing loss is a common pathology in humans that involves damage to the delicate hair cells of the cochlear sensory epithelium and reduced innervation density of inner hair cells by auditory-nerve afferent synapses, known as cochlear synaptopathy (Kujawa and Liberman, 2009). While hair-cell damage is generally detectable with a clinical audiogram and causes well-known deficits in real-world listening abilities (Dubno et al., 1982; Halpin and Rauch, 2009), auditory-nerve-fiber loss is clinically difficult to detect (Makary et al., 2011; Schuknecht and Woellner, 1953) and has unclear consequences on perception. Loss of auditory-nerve input to the central nervous system has been proposed to cause real-world listening difficulties including tinnitus and impaired perception of complex sounds in noise (Bharadwaj et al., 2014; Kujawa and Liberman, 2009; Plack et al., 2014; Schaette and McAlpine, 2011), called ‘hidden hearing loss’ because these problems are not readily detected with standard clinical tests including the audiogram. However, despite over a decade of intensifying research on this pathology, it is currently unclear whether auditory-nerve-fiber loss causes hidden hearing loss (Bramhall et al., 2019).
Studies of hidden hearing loss in humans have produced conflicting results, with some finding associations between putative metrics of auditory-nerve health and complex-sound perception or tinnitus (Liberman et al., 2016; Schaette and McAlpine, 2011; Shehorn et al., 2020) and others finding no such relationships (Grose et al., 2017; Johannesen et al., 2019; Le Prell et al., 2018; Marmel et al., 2020; Prendergast et al., 2019, 2017; Yeend et al., 2017). While possibly due to a weak or heterogeneous effect of auditory-nerve-fiber loss on perception (Oxenham, 2016), null results might also reflect other factors. First, most studies focused on young subjects with normal audiograms, who may lack the degree of auditory-nerve synaptic injury necessary to produce a detectable perceptual difference. Second, these studies generally used wave-I amplitude of the auditory brainstem response (ABR), the far-field compound action potential of the auditory nerve, to provide a physiological metric of auditory-nerve status. While reasonably effective in animal models due to close proximity of recording electrodes to auditory-nerve-fiber generators (Yuan et al., 2014), use of ABR wave-I in humans is more difficult due to its lower signal-to-noise ratio and relatively high inter-subject variability even among young normal-hearing individuals with presumably full complements of auditory-nerve fibers/cochlear synapses. More direct measures of auditory-nerve injury (Makary et al., 2011; Viana et al., 2015; Wu et al., 2019) may be needed to rigorously evaluate the possibility of hidden hearing deficits, but note the difficulty of obtaining direct histopathological measures in human subjects for whom controlled measures of real-world listening abilities and tinnitus are also available.
Alternatively, others have proposed that differential measures, for which the outcome is influenced by auditory-nerve-fiber loss to different extents across conditions, might reveal hidden hearing deficits caused by auditory-nerve-fiber loss if they exist (Plack et al., 2016). For example, Prendergast et al. (2017) calculated several differential behavioral measures as the difference in performance between high and low sound levels, based on the assumption that auditory-nerve-fiber loss disproportionately impairs encoding of high-level signals due to greater loss of units with low spontaneous rates (Furman et al., 2013) (but see Cochlear Anatomy and Auditory-Nerve-Fiber Loss, below). Differential measures should theoretically be less influenced by extraneous factors such as central processing and cognition because these factors putatively impact performance at both sound levels; thus, in the differential measure, the effect of extraneous factors is effectively subtracted away. However, differential measures have largely not yet clarified the relationship between auditory-nerve injury and hidden hearing loss in humans (Prendergast et al., 2019, 2017).
Given the limitations inherent to human studies, studies in non-human animal models appear well suited to test the hypothesis that auditory-nerve-fiber loss causes real-world listening difficulties including impaired perception of complex sounds (i.e., hidden hearing loss) and tinnitus. Animal models are advantageous because auditory-nerve injury can be experimentally induced with controlled noise exposure, surgical methods, or neurotoxic drugs, and quantified using well-validated physiological measures and direct histopathological techniques. Furthermore, measurements of behavioral performance can be obtained both before and after auditory-nerve injury, increasing the probability of detecting perceptual deficits if they exist but are small (e.g., within the range of inter-subject variability in control groups). On the other hand, the challenge emerges of quantifying perception in animal subjects that must be trained to report behavioral decisions in response to a seemingly arbitrary acoustic stimuli, a laborious process requiring often extended periods of carefully controlled behavioral data collection and specialized operant-conditioning procedures to maintain animals’ motivation to perform the task.
Here we review existing studies in behavioral animal models on the impact of experimentally induced auditory-nerve-fiber loss on auditory perception and possible hidden hearing loss. A brief overview of the basic anatomy and physiology of the cochlea and auditory nerve is provided first, followed by hypotheses proposed regarding which aspects of auditory perception might be most impacted by auditory-nerve injury, and underlying mechanisms. Thereafter, successive sections review the effects of auditory-nerve-fiber loss on hyperacusis and tinnitus inferred via startle responses and on the audiogram, temporal integration, temporal resolution, and signal detection in noise based on behavioral results in trained animals. Finally, possible directions are introduced for future research.
Cochlear Anatomy and Auditory-Nerve-Fiber Loss
Sound-induced vibrations within the human cochlea are transduced into a receptor potential by ~3,000 inner hair cells arranged in a single row spanning the length of cochlear frequency axis (Fig. 1A). Each inner hair cell receives redundant afferent synaptic contact from 5–15 type-I auditory-nerve fibers in the healthy ear, with greatest innervation density at mid-frequency locations (Spoendlin and Schrott, 1989). Inner-hair-cell synapses are of the ribbon type, specialized for high temporal precision (Nouvian et al., 2006), and exclusive in that each auditory-nerve-fiber neuron makes just one afferent synaptic contact with a single inner hair cell. Auditory-nerve cell bodies are located in the spiral ganglion at the center of the cochlear spiral and send a projection (axon) to the cochlear nucleus carrying acoustic information into the central nervous system in the form of action potentials. Auditory-nerve fibers show V-shaped frequency tuning in response to tones with characteristic frequencies (the frequency of greatest sensitivity) consistent with the cochlear frequency region at which they make synaptic contact with an inner hair cell (Liberman, 1984). Most auditory-nerve fibers in mammals have high spontaneous discharge rates in the absence of sound [greater than 18 spikes/second; ~60% of fibers in cat and gerbil (Liberman, 1978; Schmiedt, 1989)] with remaining fibers having low (<0.5 spikes/second) or medium (0.5–15 spikes/second) spontaneous rates.
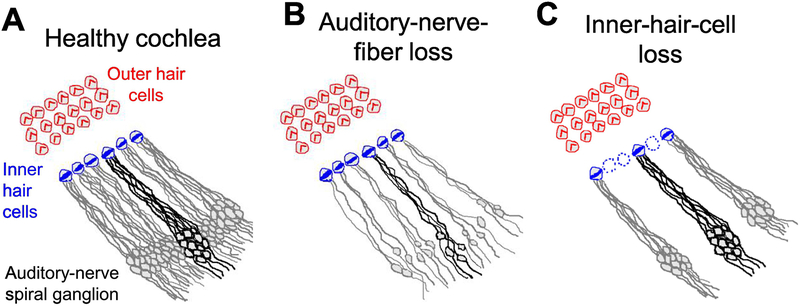
Figure 1.
Illustrations of the mammalian cochlear sensory epithelium showing afferent innervation of inner hair cells by type-I auditory-nerve fibers. Each inner hair cell of the healthy cochlea (A) is innervated by 5–15 auditory-nerve fibers. Auditory-nerve fibers drawn with a black outline all innervate a single inner hair cell. Loss of cochlear afferent innervation, known as cochlear synaptopathy (B), occurs with increasing age and following exposure to noise and neurotoxic agents including ouabain and kainic acid. Inner-hair-cell loss (C) occurs following carboplatin exposure in chinchillas and causes secondary loss of auditory-nerve afferent innervation.
Of ~30,000 total type-I auditory-nerve fibers innervating each human cochlea at birth (Otte et al., 1978), an estimated 7.7% of synapses are lost each decade of life culminating in average auditory-nerve survival of 70% at 40 years of age and 40% at age 80 (Fig. 1B) (Wu et al., 2019). While traditionally considered to be a secondary consequence of inner-hair-cell loss or damage (McFadden et al., 2004; Spoendlin, 1984), recent investigations show that loss of auditory-nerve synapses exceeds inner-hair-cell loss by a factor of 2.6 (Wu et al., 2019) and can even occur in the absence of hair-cell related pathologies (Makary et al., 2011; Viana et al., 2015). Moreover, recent studies in animal models show that sound overexposures cause auditory-nerve synaptic injury even at moderate levels that are insufficient to cause permanent hair-cell damage (Kujawa and Liberman, 2009; Lin et al., 2011). The striking implication of this work is that noise exposures that were previously assumed to be safe based on preservation of the audiogram and outer-hair-cell function might in fact permanently damage auditory-nerve synapses and cause hidden hearing loss. These findings have led to a flurry of research efforts to detect auditory-nerve-fiber loss, reveal possible hidden hearing loss including real-world listening difficulties and tinnitus, determine exposure levels at which auditory-nerve synaptopathy occurs, and even pharmacologically prevent and repair synaptic injury – an intriguing possibility since auditory-nerve neurons survive for sometimes extended time periods in the spiral ganglion despite being functionally disconnected from inner hair cells by initial synaptic breakage due to glutamate excitotoxicity (Liberman and Kujawa, 2017; Young, 2013).
Shortly after the discovery of noise-induced cochlear synaptopathy, it was reported that low- and medium-spontaneous-rate auditory-nerve fibers were lost at a greater rate than high-spontaneous-rate fibers in guinea pigs following moderate noise overexposure (Furman et al., 2013). In large samples of auditory-nerve-fiber spontaneous activity recorded from 14 control animals and 9 previously exposed to noise, the proportion of low- and medium-spontaneous-rate responses was 47% in the control sample and 29% in the noise-exposed sample. These results suggest that medium and low-spontaneous-rate fibers could be more vulnerable to noise-induced synaptopathy, which is a potentially disconcerting possibility if it is also assumed that these fiber groups play a dominant role in suprathreshold auditory perception (e.g., based on their sometimes wider dynamic range in response to tones of varying level). On the other hand, the role of different auditory-nerve spontaneous-rate groups in auditory perception is controversial (Delgutte, 1996; Sachs and Young, 1979; Young and Sachs, 1979), and a recent study in mice found similar loss of auditory-nerve fibers across spontaneous-rate groups following moderate noise overexposure (Suthakar and Liberman, 2021). Given these uncertainties, it seems unclear whether or how selective loss of low-spontaneous-rate fibers should be included in designing differential measures and predicting perceptual consequences of auditory-nerve injury.
Hypothesized perceptual consequences of auditory-nerve-fiber loss
Auditory-nerve-fiber loss is generally thought not to affect behavioral thresholds for tone detection in quiet (i.e., the clinical audiogram), an assumption consistent with human temporal-bone, retrospective studies (Makary et al., 2011), but rather to impair real-world perception of complex sounds for which integration of auditory-nerve input across a larger number of fibers may be required to support behavioral performance. Tasks requiring fine timing of neural responses might be particularly affected, such as sound localization and processing of temporal fine structure and envelope fluctuations, because auditory-nerve-fiber loss is expected to degrade population-level neural encoding of temporal features based on the volley principle (i.e., pooling across redundant neural inputs) (Bharadwaj et al., 2014; Lopez-Poveda and Barrios, 2013). In contrast, a modeling study based on signal detection theory predicted equal impairment of psychoacoustic performance across a wide range of tasks including tone detection in quiet and in noise, frequency discrimination, level discrimination, and binaural lateralization (Oxenham, 2016). Moreover, the framework predicted small threshold shifts well within the range of normal inter-subject variation for mild-to-moderate cochlear synaptopathy, and just 5 dB of threshold elevation for a profound 90% loss of auditory-nerve fibers.
Perceptual deficits could also arise from specific central processing disorders caused by diminished afferent input to the central auditory pathway. Schaette and McAlpine (2011) described a framework in which homeostatic plasticity acts to stabilize mean neural activity level in central auditory processing centers over long time scales through changes in the strength of excitatory and inhibitory synapses. In response to reduced afferent input, this mechanism appears to cause an increase in ‘central gain’ and/or neural excitability (Chambers et al., 2016; Hickox and Liberman, 2014; Salvi et al., 2017; Wang et al., 2011; Wilson et al., 2021) (Fig. 2) that could amplify spontaneous activity, a potential cause of tinnitus, and adversely impact neural encoding of suprathreshold auditory stimuli (Schaette and McAlpine, 2011). Relationships between hearing damage, tinnitus, and speech comprehension in humans, including the hypothesis that tinnitus is a byproduct of ‘neural noise’ added to the system to improve sensitivity through stochastic resonance, are further discussed by Gollnast and colleagues (Gollnast et al., 2017). Indeed, many fundamental aspects of central processing on which speech perception likely depends are shaped by the balance of excitatory and inhibitory inputs, including frequency selectivity, binaural sensitivity, and amplitude-modulation tuning (Burger and Pollak, 1998; Caspary et al., 2008, 2002; Davis et al., 1999; Palombi and Caspary, 1996; Zhang and Kelly, 2003), and therefore might be degraded by changes in central gain related to auditory-nerve-fiber loss.
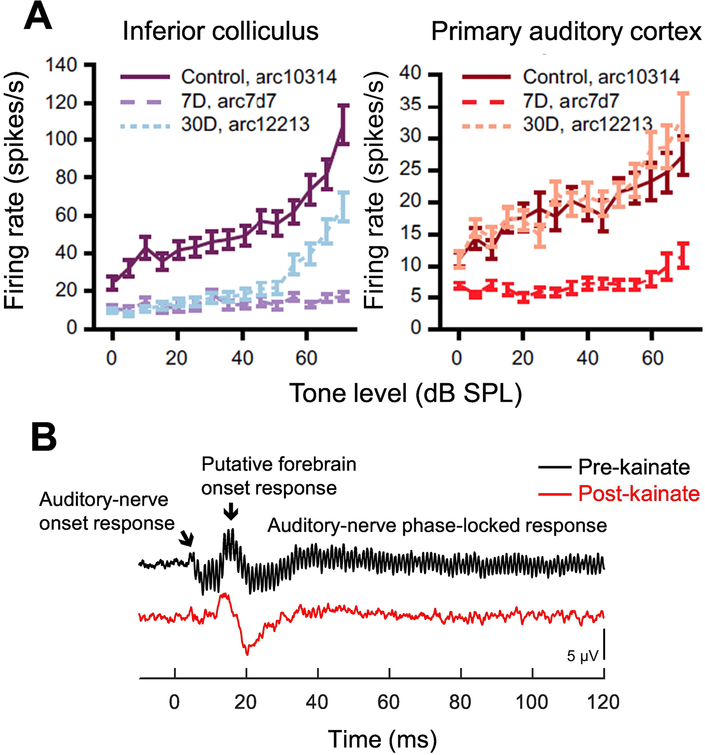
Figure 2.
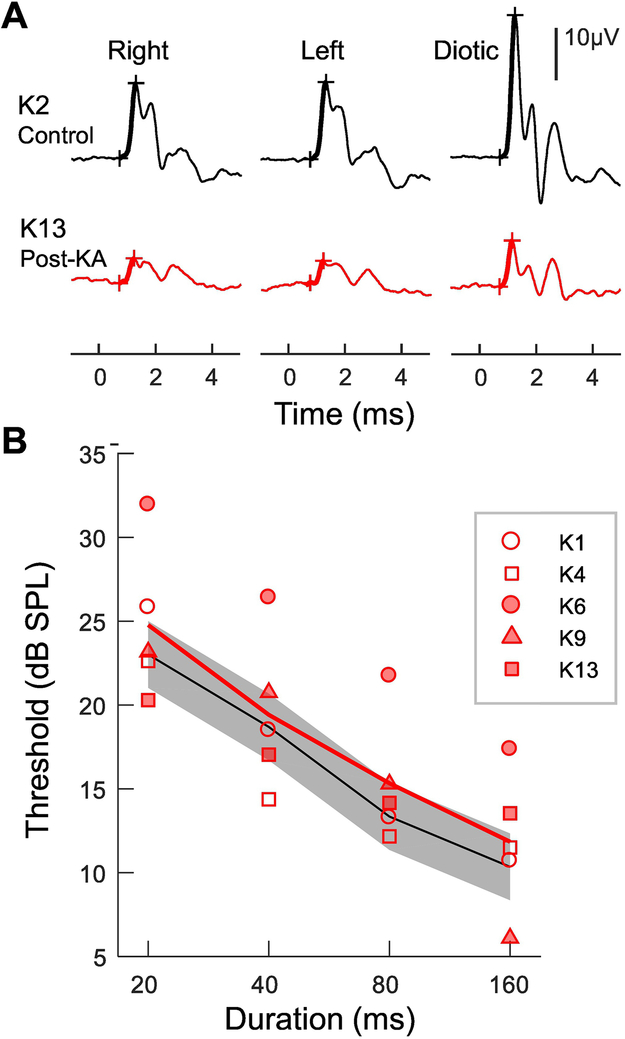
Changes in central gain following auditory-nerve damage. Neural responses to tones in ouabain-exposed mice (A) show transient reduction of firing rate seven days after exposure followed after 30 days by partial recovery at the midbrain level (inferior colliculus; left) and full recovery in primary auditory cortex (right). Central recovery occurs despite permanent depression of auditory-nerve-generated responses, which decrease by 90–95% following ouabain. Adapted from Chambers et al., 2016 (Fig. 2) and reprinted with permission. Far-field responses to sinusoidally amplitude-modulated tones in the budgerigar (B) before and after ~70% loss of auditory-nerve afferent innervation due to kainic acid. Recordings made prior to kainic-acid exposure (pre-kainate) show the onset and sustained response of the auditory nerve and a central onset response putatively generated by the forebrain nucleus Field L. The forebrain response is preserved following kainic-acid exposure (post-kainate) despite permanent reduction of auditory-nerve generated potentials. Adapted from Wilson et al. 2021 (Fig. 1) and reprinted with permission.
Behavioral consequences of cochlear synaptopathy in animal models
Behavioral studies of experimentally induced auditory-nerve injury have been conducted in a variety of nonhuman animal model species including several mammals [mouse (Chambers et al., 2016; Hickox and Liberman, 2014; Resnik and Polley, 2021), rat (Lobarinas et al., 2017), chinchilla (Lobarinas et al., 2020, 2016, 2013), cat (Schuknecht and Woellner, 1953), gerbil (Tziridis et al., 2021)] and the budgerigar, an avian species (Henry and Abrams, 2021; Wong et al., 2019). These studies used diverse methods to induce auditory-nerve damage including bandlimited noise exposures, surgical ablation, and the neurotoxic agents ouabain and kainic acid. This review also includes studies employing carboplatin exposure in chinchillas (Lobarinas et al., 2020, 2016, 2013), for which inner hair cells are lost in addition to auditory-nerve fibers (Fig. 1C). These carboplatin studies are considered because the sensitivity and frequency tuning of surviving auditory-nerve fibers are thought to remain relatively normal unless inner-hair-cell loss is profound (Salvi et al., 2000; Wang et al., 1997), as seems to be the case for pure synaptopathy (Furman et al., 2013). Several studies are first reviewed that used behavioral startle reflexes to provide insight into possible tinnitus and hyperacusis following auditory-nerve injury. Subsequent sections focus on behavioral responses of trained animals assessed using operant-conditioning procedures, to gain insight into the impact of auditory-nerve-fiber loss on different aspects of auditory perception. Perceptual consequences of auditory-nerve-fiber loss are reviewed on the audiogram, temporal integration for tones of varying duration, temporal resolution of silent gaps in noise, and tone-in-noise detection.
Studies of hyperacusis and tinnitus
Hyperacusis and tinnitus are common problems in individuals with sensorineural hearing loss and indicate abnormal discomfort in response to loud sounds and ‘ringing of the ears’ in the absence of sound, respectively. Several studies have investigated the potential contribution of auditory-nerve injury to hyperacusis and tinnitus using startle reflexes, based on the hypothesis that auditory-nerve-fiber loss causes abnormal elevation of central gain that leads to these perceptual phenomena. Hickox and Liberman (2014) induced auditory-nerve injury in mice through moderate bilateral overexposure to bandlimited noise (8–16 kHz; 100 dB SPL; 2 h), which caused approximately 40% reduction of tone-evoked ABR wave-I amplitude and a concomitant loss of auditory-nerve afferent synapses in the 32-kHz region of the cochlea (the region withstanding the greatest synaptic injury) assessed 11 weeks after the noise exposure (Hickox and Liberman, 2014). Hair cells showed no permanent damage post-exposure except in the extreme base, where scattered loss of outer hair cells was found. The amplitude of acoustic startle reflexes and the extent to which startle amplitude could be inhibited by preceding sounds were compared over a 10-week test period, starting one day after noise overexposure, between noise-exposed mice and an age-matched control group. Mice with noise-induced auditory-nerve damage showed evidence of post-exposure hyperacusis including enhanced acoustic startle reflexes in noise (Fig. 3A) and greater inhibition of acoustic startle reflexes by an immediately preceding low-level tone (heightened pre-pulse inhibition; Fig. 3B). On the other hand, similar inhibition of acoustic startle responses by a preceding silent gap suggested no role of a tinnitus sensation “filling in the gap” in noise-exposed animals though the validity of this behavioral tinnitus phenotype is an area of active debate and investigation (Zeng et al., 2020). In summary, acoustic startle results in mice with noise-induced auditory-nerve injury appeared consistent with possible hyperacusis but perhaps not tinnitus.
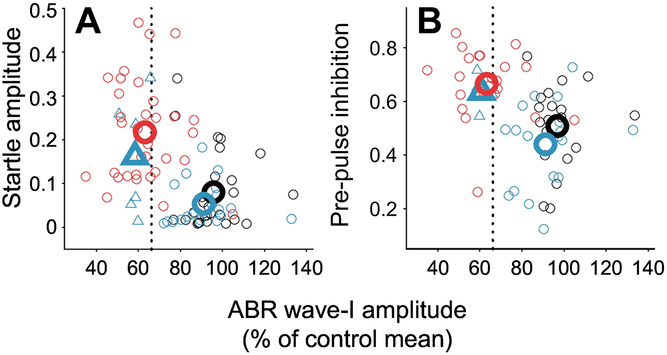
Figure 3.
Correlations of sound-evoked startle amplitude (A) and the extent of startle inhibition provided by a preceding low-level tone (pre-pulse inhibition; B) with wave-I amplitude of the auditory brainstem response (ABR) in mice. Stimulus frequency is 32 kHz in all cases. Red symbols indicate results from mice with ~40% noise-induced cochlear synaptopathy in the 32-kHz frequency region of the cochlea. Black and blue symbols indicate results from unexposed mice and mice exposed to a lower noise level that did not induce synaptopathy, respectively. Large symbols indicate group means. Mice with lower wave-I amplitude, suggesting greater synaptopathy, show stronger startle responses and greater inhibition of startle responses by preceding low-level tones, consistent with possible hyperacusis. Adapted from Hickox and Liberman 2014 (Figs 4 and 7) and reprinted with permission.
Chambers at al. (2016) found quite different results in mice with profound unilateral auditory-nerve injury induced through application of 1-mM ouabain solution to the round window membrane. Pharmacological induction of auditory-nerve damage has the advantage of producing cochlear neural lesions that are larger and more diffuse across cochlear frequency regions compared to the moderate focal lesions produced by bandlimited noise, and thus more closely resemble the neuropathy commonly observed in aging human ears (Makary et al., 2011). The extent of auditory-nerve-fiber loss in the ouabain-exposed ear, assessed through reduction of ABR wave-I amplitude one week and 30 days after exposure and validated histologically, was profound (>95%) yet with complete preservation of both inner and outer hair cells (Chambers et al., 2016). Intriguingly, though still able to behaviorally detect low-level tones presented to the exposed ear several weeks post-exposure as described below (see Behavioral Audiograms), acoustic startle reflexes for suprathreshold tones presented to the exposed ear were completely abolished (yet preserved in the control ear). Elimination of acoustic startle reflexes, in contrast to enhancement observed in mice exposed to bandlimited noise at 100 dB SPL (Hickox and Liberman, 2014), appears inconsistent with hyperacusis in ouabain-exposed mice and could be due to the fact the auditory-nerve injury was unilateral and/or far more pronounced. On the other hand, startle reflexes were clearly dissociated from behavioral measures of tone perception in ouabain-exposed mice, and therefore might not provide the best index of abnormal loudness perception. Indeed, hyperactivity of cortical responses in ouabain-exposed mice (Chambers et al., 2016; see also Salvi et al., 2017) suggest a possible neural substrate for hyperacusis in these animals.
Startle reflexes have also been used in rats to evaluate possible auditory perceptual changes following auditory-nerve damage. Lobarinas et al. (2017) exposed rats to bandlimited noise and found modest but persistent reduction of ABR wave-I amplitude two weeks post-exposure with no accompanying change in hair-cell generated otoacoustic emissions, consistent with selective auditory-nerve injury. Inhibition of air-puff startle reflexes by narrowband signals in noise was evaluated before and after auditory-nerve damage to gain insight into possible perceptual changes caused by this neuropathy. Rats showed less inhibition of air-puff startle reflexes by the acoustic signal two weeks following auditory-nerve damage, a finding at odds with the apparent hyperacusis observed in mice (Hickox and Liberman, 2014) with a similar degree of neural loss. Rather than hyperacusis, reduced startle inhibition by narrowband signals in noise was interpreted as possible evidence of reduced hearing-in-noise ability, though note the indirect relationship between pre-pulse inhibition of startle reflexes and conditioned responses of behaviorally trained animals (Behrens and Klump, 2016, 2015; Greene et al., 2018). Whether different results in rats and mice reflect use of different elicitors for the startle reflex (air puff vs. sound) or perhaps a species difference remains unclear. Indeed, even different inbred mouse strains can show substantial differences in startle reflexes (Paylor and Crawley, 1997).
Finally, in gerbil, Tziridis and colleagues (2021) evaluated the potential role of auditory-nerve-fiber loss in tinnitus using a moderate-level tone exposure intended to damage auditory-nerve-fiber synapses without causing much permanent hearing loss. Change in the extent to which a preceding silent gap in narrowband noise inhibited acoustic startle reflexes, between time points before and 13 days after exposure, was used as a behavioral correlate of tinnitus based on the previously mentioned framework that tinnitus ‘fills in the gaps’ in the stimulus. Hearing loss was evaluated with ABR thresholds measured before and 3–5 days after acoustic overexposure, and ranged from none up to 10–15 dB. Auditory-nerve injury was assessed at the conclusion of behavioral testing. In contrast to Hickox and Liberman’s (2014) findings in mice, for which no correlate of tinnitus was found, gerbils showed startle-based evidence of tinnitus following acoustic overexposure that was positively associated with the extent of auditory-nerve-fiber injury yet unrelated to the degree of apparent (minor) hearing loss (Tziridis et al., 2021). Notable differences between these studies include the choice of species and the use of a differential behavioral response measure gerbils (i.e., based on pre- and post-exposure assessment of startle reflexes), thereby potentially reducing the impact of cross-animal variability.
Behavioral audiograms
The behavioral audiogram, pure-tone detection thresholds in quiet as a function of frequency, is the standard clinical tool for detecting sensorineural hearing loss in humans and is particularly sensitive to hair-cell pathologies due to the primary role of outer hair cells in amplifying sound induced-cochlear vibrations. The effects of auditory-nerve-fiber loss on the behavioral audiogram have been examined in a number of animal models, all of which show minimal impact of this pathology except in cases where neuropathy was profound. In an early study of cats, Schuknecht and Woellner (1953) induced auditory-nerve injury through partial surgical lesions of the auditory-nerve trunk near where it intersects the brainstem. Behavioral audiograms were determined before and from 2–7 months after surgical damage using a shock-avoidance paradigm, and the density of spiral ganglion neurons was evaluated (i.e., percent of average normal) at the conclusion of behavioral testing to quantify the extent and pattern of cochlear neural injury. The behavioral results in cats showed that strikingly, greater than 80% loss of auditory-nerve innervation was required to produce significant elevation of pure-tone thresholds in quiet (Schuknecht and Woellner, 1953).
More recently, Lobarinas et al. (2013) quantified the impact of inner hair cell loss from carboplatin on behavioral audiograms evaluated using a shock-avoidance paradigm in chinchillas. Single injections of 75 mg/kg carboplatin caused inner-hair-cell lesions ranging from 15–100% across animals and cochlear frequency regions based on histological analyses conducted at the conclusion of behavioral testing (Fig. 4A). Behavioral audiograms were assessed before carboplatin exposure and over a five-day period beginning 21–28 days after the exposure. Strikingly, greater than 80–90% loss of inner hair cells was required to produce significant elevation of behavioral pure-tone detection thresholds in quiet (Fig. 4B). Lesions greater than 90% were associated with heterogeneous outcomes with some animals showing normal performance and others showing threshold elevation of up to 80 dB (Lobarinas et al., 2013).
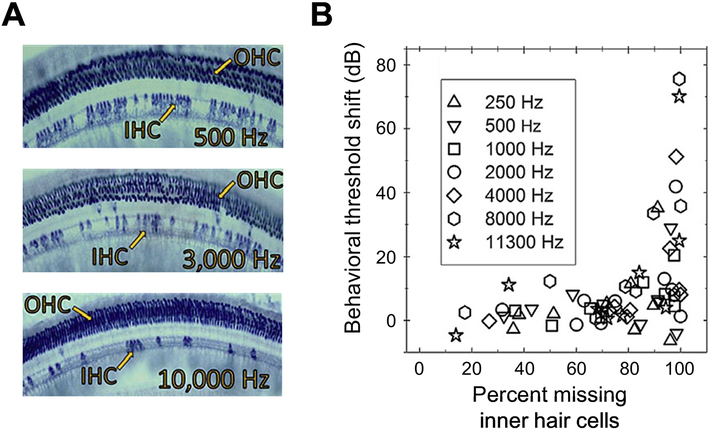
Figure 4.
Effects of inner-hair-cell loss on behavioral sensitivity to tones in quiet in chinchillas. Cochlear sections stained with succinate dehydrogenase (A) show diffuse loss off inner hair cells following carboplatin exposure without any accompanying loss of outer hair cells. Behavioral threshold shifts (B) following carboplatin exposure as a function of the degree of inner-hair-cell loss. Threshold shifts are minor for inner-hair-cell losses up to 80%, beyond which large threshold shifts are possible but not observed in all experimental animals. Symbols indicate different test frequencies. Adapted from Lobarinas et al. 2013 (Figs 3 and 7) and reprinted with permission.
In mice, Chambers et al. (2016) assessed the impact of unilateral auditory-nerve-fiber loss induced with 1-mM ouabain on behavioral detection of 8-kHz tones by animals trained using a shock-avoidance paradigm. Behavioral testing was conducted three weeks after ouabain exposure. Perhaps surprisingly, given the loss of the acoustic startle reflex and the profound extent of neural injury (>95% synaptic loss), most (3/4) ouabain-exposed mice showed normal behavioral sensitivity to 8-kHz tones presented to the lesioned ear across the full range of sound levels tested (10–90 dB SPL). On the other hand, one animal was unable to behaviorally detect tones with the lesioned ear, suggesting heterogenous outcomes for >90% synaptic loss. Preservation of behavioral tone sensitivity was perhaps possible due to a compensatory increase in central gain that restored the magnitude of neural responses to these relatively simple stimuli following ouabain exposure. Indeed, extracellular multi-unit neural recordings in primary auditory cortex, and to a lesser extent in the inferior colliculus, showed remarkable recovery/plasticity of suprathreshold neural responses over 30 days following the ouabain exposure (Fig. 2A), consistent with downregulation of inhibitory input to these processing centers and a concomitant increase in central gain (Chambers et al., 2016).
Finally, Wong et al. (2019) found no effect of auditory-nerve damage from kainic acid on the behavioral audiogram in budgerigars. The budgerigar is a small parrot species with the ability to mimic speech. Much is known about their auditory perceptual abilities (Dooling et al., 2000), some of which is summarized here since this species has been used in several recent behavioral studies of auditory-nerve damage. Budgerigar behavioral performance is similar to that of humans on a variety of simple and complex listening tasks including tone detection in quiet and in noise (Dooling and Saunders, 1975; Henry et al., 2020; Okanoya and Dooling, 1987; Saunders et al., 1978), amplitude modulation detection (Carney et al., 2013; Dooling and Searcy, 1981; Henry et al., 2016), gap detection (Dooling et al., 2000), and frequency discrimination of tones and synthetic vowel formants (Dent et al., 2000; Henry et al., 2017a, 2017b). On the other hand, the cochlea in budgerigars and other avians is uncoiled and shorter than in mammals, with up to 30 hair cells across its width (Manley et al., 1993; Takasaka and Smith, 1971). Rather than having distinct inner and outer hair cells, as in mammals, avian hair-cell shape changes gradually across the width of the sensory epithelium from tall to short, with tall hair cells located closest to the auditory-nerve ganglion and receiving primarily afferent synaptic innervation with up to four synapses per hair cell. Short hair cells are thought to supply cochlear amplification and receive primarily efferent synaptic input (Gleich, 1989; Köppl et al., 2000; Smolders et al., 1995). Despite these anatomical differences, the avian cochlea is tonotopic and avian auditory-nerve fibers show fundamentally similar response properties to those of mammals, including V-shaped frequency tuning curves, limited dynamic range of rate-level functions, and phase-locking up to a maximum frequency of several kHz (Gleich, 1989; Manley et al., 1985; Sachs et al., 1974; Salvi et al., 1992). Furthermore, while budgerigars and other avians differ from mammals in that they can regenerate hair cells within several weeks after noise trauma or exposure to ototoxic drugs (Corwin and Cotanche, 1988; Ryals et al., 2013), birds nonetheless lack the ability to regenerate auditory-nerve soma following neuropathy as in mammals (Henry and Abrams, 2018; Ryals et al., 1989; Sun et al., 2001) and even show age-related irreversible decline of cochlear auditory-nerve innervation (Ryals and Westbrook, 1988)
Auditory-nerve damage in budgerigars was induced bilaterally through intracochlear infusion of kainic acid (Wong et al., 2019), the glutamate analog that damages auditory-nerve afferent synapses in mammals and birds due to excitotoxicity followed, after several weeks or months, by irreversible degeneration of neuron cell bodies in the auditory-nerve ganglion (Bledsoe et al., 1981; Juiz et al., 1989; Sun et al., 2001). Bilateral infusions of 1-mM kainic acid solution in budgerigars caused permanent, 40–70% reduction of ABR wave-I amplitude across animals (Fig. 5A) with no accompanying change in hair-cell generated otoacoustic emissions, consistent with moderate-to-severe auditory-nerve-fiber loss (Wong et al., 2019). Behavioral audiograms were measured over several months beginning 5–40 weeks after auditory-nerve injury in animals trained to detect tones using seed reinforcement and time outs during which the house light was turned off. Pure-tone detection thresholds in the animal group exposed to kainic acid were as sensitive as those in age-matched controls across the full range of frequencies tested (0.25–8 kHz), indicating little or no impact of up to 70% auditory-nerve-fiber loss on the behavioral audiogram (Wong et al., 2019).
Figure 5.
Effects of auditory-nerve-fiber loss on behavioral temporal integration in trained budgerigars. ABRs evoked by high-level clicks (A) show a dramatic reduction of wave-I amplitude generated by the auditory nerve (thick lines and crosses) in animals exposed to kainic acid (KA; red). Temporal integration functions (B) show pure-tone detection thresholds in quiet as a function of stimulus duration. Animals with 40–70% auditory-nerve-fiber loss from kainic acid show similar improvement of thresholds with increasing stimulus duration to an unexposed control group, suggesting no change in the capacity of the system for temporal integration. The black line and gray band indicate the mean and standard deviation of behavioral thresholds in an unexposed control group. Open and closed symbols show results from individual animals with moderate (40–50%) and severe (65–70%) auditory-nerve-fiber loss, respectively. Adapted from Wong et al. 2020 (Figs 3 and 8) and reprinted with permission.
Taken together, studies of the behavioral audiogram in animal models show that up to 70–80% of cochlear afferent innervation can be lost prior to substantial elevation of pure-tone thresholds in quiet. Greater loss of auditory-nerve input to the central nervous system may yield heterogeneous results, with some individual animal subjects exhibiting impaired behavioral performance but others showing essentially normal behavioral tone-detection thresholds in quiet (Chambers et al., 2016; Lobarinas et al., 2013; Schuknecht and Woellner, 1953).
Temporal integration
Temporal integration of acoustic signals over time is a fundamental aspect of hearing that results in the well-known improvement of auditory detection and discrimination thresholds for stimuli of increasing duration. For the simple case of tone detection in quiet, human thresholds decrease (i.e., become more sensitive) by approximately 3–4 dB for each doubling of stimulus duration due to temporal integration, with no further improvement observed for tones longer than a few hundred ms (Green et al., 1957; Plomp and Bouman, 1959; Watson and Gengel, 1969). Similar behavioral patterns of auditory temporal integration have been found in a wide range of nonhuman animal species (Heil et al., 2017), suggesting that temporal integration is a general property of auditory processing shared widely across taxa. A deficit in the ability of the auditory system to integrate signals over time, if it were to result from auditory-nerve-fiber loss, could cause pronounced difficulties for perception of steady-state features of speech and other complex sounds under real-world listening conditions (e.g., tracking vowel fundamental and formant frequencies).
Only one published animal behavioral study has investigated the impact of auditory-nerve damage on temporal integration, using tones of varying duration in budgerigars exposed to kainic acid (Wong et al., 2019). This study tested the hypothesis that auditory-nerve-fiber loss impairs temporal integration, thereby reducing the improvement of pure-tone detection thresholds typically observed for stimuli of longer duration. Temporal integration of a 2-kHz pure-tone stimulus was quantified using operant-conditioning procedures for stimulus durations ranging from 20–160 ms, over a period of several months beginning 5–40 weeks after kainic acid exposure. Contrary to the expected impairment, temporal-integration functions of budgerigars with estimated 40–70% auditory-nerve-fiber loss were indistinguishable from those of an age-matched control group, decreasing by 4–5 dB for each doubling of stimulus duration in all animals, regardless of auditory-nerve status (Fig. 5B) (Wong et al., 2019). Incidentally, unpublished observations in carboplatin-exposed chinchillas show no impact of diffuse inner-hair-cell loss on temporal integration (Escabi C, Martin J, Lobarinas E. 2018. Gap detection and temporal summation deficits in chinchillas with selective inner hair cell loss [PS 383]. Association for Research in Otolaryngology Abstracts 41, 252).
The finding that up to 70% loss of afferent cochlear innervation in budgerigars does not impact behavioral temporal integration of tones (Wong et al., 2019) is interesting because impaired (flatter) temporal integration functions are commonly observed with sensorineural hearing damage in humans and in behaviorally trained animals (Gerken et al., 1990, 1983; Solecki and Gerken, 1990; Watson and Gengel, 1969; Wright, 1968), including avians (Lauer et al., 2007). A potential implication of this difference is that impaired temporal integration with sensorineural hearing loss might be caused more by outer-hair-cell pathologies than by accompanying loss of auditory-nerve synapses (i.e., the neural component of sensorineural hearing loss). Finally, it should be noted that budgerigars showed no recovery of auditory-nerve-generated gross potentials throughout behavioral testing (Wong et al., 2019), consistent with permanent neural injury (Ryals et al., 1989; Sun et al., 2001).
Temporal resolution of gaps in noise
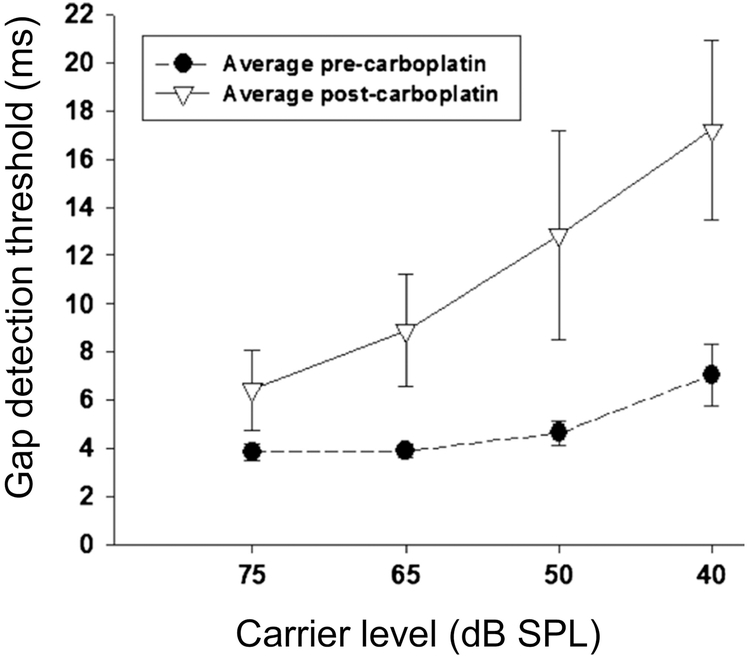
Effective auditory processing of speech and other complex sounds requires not only temporal integration but also sensitivity to transient events lasting only a few milliseconds, such as plosive consonants. Another fundamental property of auditory processing is the temporal resolution or minimum time interval over which acoustic events can be resolved, which is sometimes evaluated using a gap-detection paradigm. The impact of auditory-nerve-fiber loss on gap detection has been evaluated in a single study in chinchillas, for which 75 mg/kg carboplatin was used to produce diffuse inner-hair-cell loss accompanied by loss of associated afferent auditory-nerve synapses (Lobarinas et al., 2020). Animals were trained with a shock-avoidance paradigm and showed ~70% inner-hair-cell loss across cochlear frequency regions, while preserving outer hair cells and behavioral thresholds for tone detection in quiet (see also Lobarinas et al., 2013). Behavioral sensitivity to silent gaps in a wideband noise carrier was evaluated at sound levels from 40–75 dB SPL to assess the possible impact of carboplatin treatment on temporal resolution. The testing procedures estimated the shortest silent gap duration detectable by trained animals, both before carboplatin exposure and after a 21-day recovery period.
Chinchilla behavioral gap-detection thresholds prior to carboplatin were ~4 ms for moderate-to-high stimulus levels and increased slightly for lower stimulus levels (i.e., to 7 ms for a noise level of 40 dB SPL) (Lobarinas et al., 2020), similar to prior results in this and other species, including humans. Thresholds increased significantly following carboplatin exposure (Fig. 6), with considerable variability across animals and greater impairment observed for lower stimulus levels (i.e., increasing from 4 to 7 ms at 75 dB SPL, yet from 7 to 17 ms at 40 dB SPL; Fig. 6). These results in chinchilla are significant because, first, they demonstrate a hidden hearing loss for which impairment of suprathreshold temporal resolution occurs despite preservation of audiometric thresholds (Lobarinas et al., 2020). Second, the chinchilla behavioral results are similar to those found in human listeners with sensorineural hearing loss, who exhibit impaired gap detection at low sound levels (and at comfortable levels) but relatively normal thresholds for high-level carrier signals (Fitzaibbons and Wightman, 1982; Glasberg et al., 1998; Nelson and Thomas, 1997).
Figure 6.
Effects of inner-hair-cell loss of behavioral sensitivity to gaps in noise in chinchillas. Minimum detectable gap durations (gap thresholds) are plotted as a function of the noise carrier level before and after induction of inner-hair-cell loss with carboplatin exposure. Gap thresholds increase dramatically following carboplatin exposure, especially at low sound levels, while sensitivity to tones in quiet remains unchanged indicating a hidden hearing loss. Adapted from Lobarinas et al. 2020 (Fig. 3) and reprinted with permission.
Tone-in-noise detection
Finally, three recent behavioral studies have investigated the impact of auditory-nerve-fiber loss on tone detection in noise, providing a potentially more direct test of whether this pathology impairs real-world listening abilities in noisy backgrounds, as is commonly expected. One study in chinchillas examined the impact of inner-hair-cell loss due to carboplatin ototoxicity (Lobarinas et al., 2016), while studies in the budgerigar (Henry and Abrams, 2021) and mouse (Resnik and Polley, 2021) used targeted auditory-nerve lesions from kainic acid and ouabain, respectively.
In chinchillas, Lobarinas et al. (2016) assessed tone thresholds in noise using shock-avoidance conditioning before and after induction of 40–80% inner-hair-cell loss from a single injection of 75 mg/kg carboplatin. Behavioral results were collected before carboplatin exposure and after 21 days of recovery over a test period of 4–6 weeks. Tones were 500 ms in duration presented in continuous background noise. While tone thresholds in quiet were unaffected by carboplatin, consistent with other reports in the same behavioral animal model (Lobarinas et al., 2020, 2013), thresholds in 50-dB SPL wideband noise increased significantly, by 6–11 dB at all test frequencies (250–11,300 Hz), demonstrating a hidden hearing-in-noise deficit under real-world noisy listening conditions (Lobarinas et al., 2016). Behavioral thresholds were also evaluated in 70-dB SPL narrowband noise for tone frequencies ranging from several octaves below to several octaves above the center frequency of the noise band. Noise center frequencies were 500, 2000, and 4000 Hz and the bandwidth of the noise was 100 Hz in all cases. Threshold in narrowband noise prior to carboplatin treatment were highest for tone frequencies within the noise band and considerably lower (more sensitive) for higher and lower test frequencies, consistent with the well-known auditory filtering process of sound that occurs within the cochlea (i.e., frequency decomposition of sound input into bandlimited processing channels). Tone thresholds in narrowband noise increased following carboplatin treatment consistent with the wideband tone-in-noise results, but surprisingly, threshold elevation was greater for test frequencies above and below the noise band compared to test frequencies falling within the noise band (Lobarinas et al., 2016). This result was unexpected because it suggests broadened cochlear frequency tuning, yet the outer hair cells responsible for sharp cochlear frequency tuning were unaffected by the carboplatin treatment.
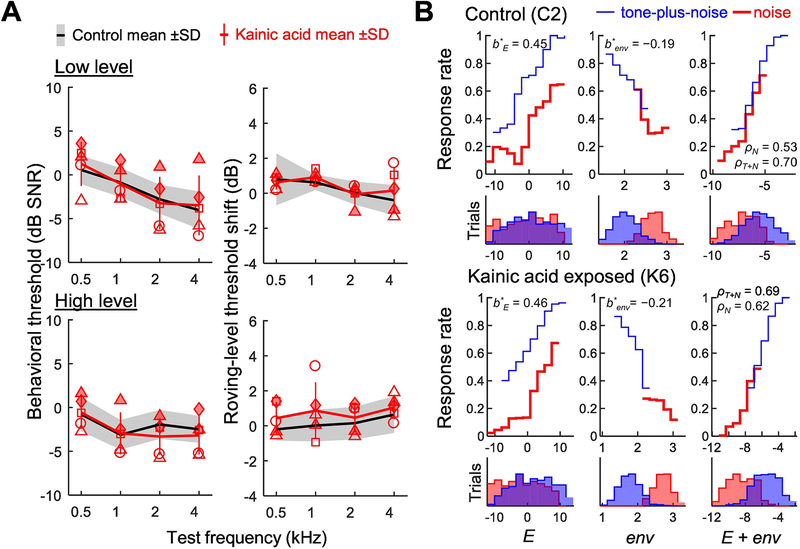
In contrast to the results of Lobarinas and colleagues (2016), a recent study in budgerigars found no impact of bilateral 40–70% auditory-nerve damage from kainic acid excitotoxicity on behavioral thresholds for tone-in-noise detection or on the acoustic cues used by animals to perform the behavioral task (Henry and Abrams, 2021). Behavioral experiments were conducted beginning 36–77 weeks after induction of auditory-nerve injury across animals, over a period of several months. The budgerigar study examined tone sensitivity in narrowband noise at octave spaced frequencies from 500–4,000 Hz using the same operant conditioning procedures and seed-reward paradigm described previously. The noise band was log-centered on the tone frequency in all cases, one-third octave in bandwidth, and presented concurrently with the tone signal (300-ms duration; simultaneously gated) at 45 or 85 dB SPL. Furthermore, a challenging roving level condition was included with unpredictable changes in noise level (+/− 10 dB) from trial to trial, to increase the complexity of the task and help evaluate the extent to which disruption of single-channel energy cues might adversely impact behavioral performance. Envelope cues for tone-in-noise detection (e.g., ‘flattening’ of the normalized temporal envelope upon addition of the tone) are unaffected by the roving-level manipulation whereas energy cues are degraded; hence, a threshold shift in roving-level noise compared to the fixed-level condition suggests that animals use single-channel energy cues to perform the task (Wang et al., 2021).
Surprisingly despite 40–70% estimated auditory-nerve synaptic loss based on permanent reduction of ABR wave-I amplitude, tone-in-noise detection thresholds of kainic acid exposed animals were statistically indistinguishable (0.1 +/−1.0 dB; mean difference +/− SE) from those of an unexposed control group across stimulus conditions (Fig. 7A, left) (Henry and Abrams, 2021). Moreover, all animals regardless of kainic-acid exposure showed minimal threshold shifts under the roving-level condition (Fig. 7A, right) suggesting use of cues other than or in addition to energy by animals to perform the task. Finally, decision-variable correlation analyses (Sebastian et al., 2017; Sebastian and Geisler, 2018) (Fig. 7B) were performed to quantify the extent to which trial-by-trial differences in envelope cues and energy cues could explain behavioral choices made by the animals during testing. Notably, all animals relied on both cues with slightly stronger perceptual weighting of the energy cue (Henry and Abrams, 2021). These results in budgerigars suggest that the impact of auditory-nerve-fiber loss on hearing in noise may be less than is generally expected, even under a challenging roving level condition known to impair performance in human listeners with sensorineural hearing loss (Leong et al., 2020).
Figure 7.
Effects of auditory-nerve-fiber loss on behavioral tone-in-noise sensitivity in trained budgerigars. Tone-in-noise detection thresholds (A, left) and the impact of roving stimulus level on tone-in-noise sensitivity (A, right) are similar between animals with 40–70% auditory-nerve-fiber loss from kainic acid and an unexposed control group. Symbols identify individual animals. The roving-level condition has +/− 10 dB random variation in stimulus level across trials, increasing the complexity of the task and discounting the value of single-channel energy cues to perform the task. Decision-variable-correlation analyses of acoustic cues for tone-in-noise detection (B) show variation in the probability of subject responses that the tone was present as a function of the value of the energy cue (E), the value of the envelope cue (env), and the value of a decision variable combining weighted energy and envelope cues (E + env). Budgerigars show slightly stronger normalized weighting of energy (b*E) than the envelope cue (b*env). The correlation of the combined decision variable to trial-by-trial behavioral responses (rhoN: noise trials; rhoT+N: tone-plus-noise trials) ranged from 0.5–0.7 (i.e., explaining 25–50% of the variance in behavioral responses). No differences in cues used for tone-in-noise detection were found between control animals (top) and those with 40–70% auditory-nerve-fiber loss from kainic acid (bottom). Adapted from Henry and Abrams 2021 (Figs 5–7) and reprinted with permission.
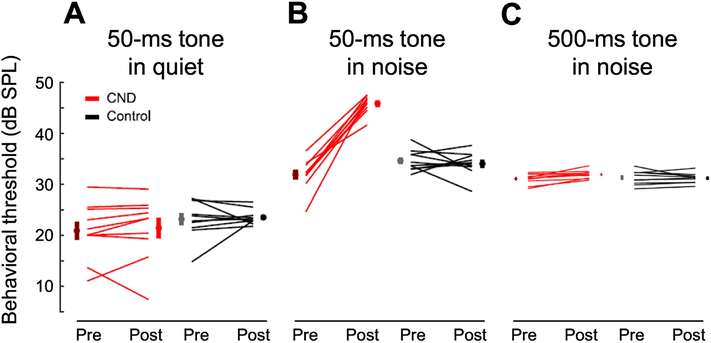
Finally, Resnik and Polley (2021) tested for hidden hearing deficits in mice by examining the impact of ~70% ouabain-induced auditory-nerve injury on behavioral tone-in-noise sensitivity. Mice were trained to detect a 12-kHz tone in quiet and in continuous 50-dB SPL background noise using operant-conditioning procedures and a water-reinforcement paradigm. Tones were 50-ms long for the quiet condition and either 50 or 500 ms for wideband noise. Behavioral experiments were conducted before ouabain exposure and over a 10-day test period beginning 20 days post-ouabain. Notably, all treated mice showed a dramatic ~15-dB increase in thresholds for detecting 50-ms tones in noise following ouabain, but no change in behavioral thresholds for tones in quiet or for the 500-ms tone duration in noise (Fig. 8; see supplemental figures in Resnik and Polley, 2021). Impaired behavioral sensitivity to short tones in noise following ouabain exposure was accompanied by changes in the response properties of cortical excitatory and inhibitory neurons that served to increase hyperactivity and neural gain, potential correlates of hyperacusis and tinnitus, while also decreasing neural adaptation to background noise. Furthermore, highly correlated bursts of spontaneous activity were observed that coincided with missed behavioral responses to target stimuli, suggesting that increased cortical noise may underlie this manifestation of hidden hearing loss (Resnik and Polley, 2021).
Figure 8.
Effects of auditory-nerve damage on behavioral tone-in-noise sensitivity in mice. Thresholds for detection of 50-ms tone are unaffected by ~70% auditory-nerve-fiber loss due to ouabain exposure in quiet (A) but increase by ~15 dB in background noise indicating hidden hearing loss (B). On the other hand, sensitivity to 500-ms tones in noise appears to be unaffected by auditory-nerve damage (C), suggesting that hidden hearing-in-noise deficits may be limited to brief signals. Red lines connect behavioral thresholds measured before and after ouabain exposure while black lines connect thresholds before and after sham exposures in a control group. Adapted from Resnik and Polley 2021 (Figs 1 and S1) and adapted with permission.
While seemingly at odds with the results of Henry and Abrams in budgerigars (2021), note that tone-in-noise deficits in ouabain-exposed mice were restricted to 50-ms signals, a duration substantially shorter than the 300-ms duration used in the budgerigar tone-in-noise study. For longer 500-ms stimuli in mice, for which the median behavioral reaction time was ~200 ms, similar to budgerigar reaction times to 300-ms signals (Wong et al., 2019), mice exhibited no impairment of tone-in-noise detection thresholds, similar to the findings in kainic-acid exposed budgerigars. The possibility was also raised that birds might not show the same forms of central plasticity thought to underlie tone-in-noise detection deficits in mice, based on reports that European starlings show minimal reorganization of auditory forebrain regions following death and regeneration of cochlear hair cells from aminoglycoside ototoxicity (Irvine et al., 2009). On the other hand, following auditory-nerve injury the far-field neural potentials in budgerigars suggest a substantial increase in central gain that maintains the amplitude of forebrain generated middle-latency responses, despite persistent 70% reduction of compound auditory-nerve responses (Wilson et al., 2021) (Fig. 2B).
The reasons for different results from Lobarinas et al. (2016) in carboplatin exposed chinchillas are unclear (i.e., impaired behavioral sensitivity to long tones in noise), but several possible factors are worth noting. First, the degree of auditory-nerve-fiber loss was not directly quantified in carboplatin-exposed chinchillas, and could in fact have been greater than the degree of inner-hair-cell loss if some ‘primary’ neural injury occurred in addition to the secondary loss expected following hair-cell death. Consistent with this idea, the amplitude of compound auditory-nerve action potentials following carboplatin injections is lower than would be expected based on the extent of inner-hair-cell loss alone (Trautwein et al., 1996; Wang et al., 1997). Second, carboplatin injection can in some cases disrupt the physiological response properties of surviving inner hair cells (inferred from auditory-nerve recordings), particularly when inner-hair-cell survival drops below 20% (Salvi et al., 2000; Wang et al., 1997). Impaired tone-in-noise detection in chinchillas (and perhaps gap detection as well; Lobarinas et al., 2020) might therefore partly reflect abnormal response properties of surviving inner hair cells.
Finally, differences in the timeline of behavioral testing exist between these three studies that could contribute to different results. Whereas chinchillas were tested over 4–6 weeks beginning 21 days after carboplatin injection (Lobarinas et al., 2016) and mice were tested for 10 days beginning 20 days after ouabain exposure (Resnik and Polley, 2021), budgerigar behavioral testing commenced several months after auditory-nerve injury and lasted for 3–4 months, giving animals ampler opportunity to adapt to the peripheral injury.
Conclusions
In summary, behavioral studies of auditory-nerve damage in animal models show that sensitivity to tones in quiet is relatively unaffected by this pathology unless synaptic loss exceeds 80%, at which point large threshold shifts are possible but not always observed (Chambers et al., 2016; Lobarinas et al., 2013; Schuknecht and Woellner, 1953; Wong et al., 2019). Behavioral studies of temporal processing suggest possible impairment of temporal resolution by auditory-nerve injury (Lobarinas et al. 2020; in a study of inner-hair-cell damage) but not temporal integration (Wong et al., 2019), whereas studies of tone-in-noise detection have produced varying results. Specifically, a study in ouabain-exposed mice found impaired behavioral sensitivity to 50-ms tones in noise (Resnik and Polley, 2021). In contrast, behavioral sensitivity to longer tones in noise appears unaffected by auditory-nerve damage in mice (Resnik and Polley, 2021) and budgerigars (Henry and Abrams, 2021), but is adversely impacted by inner-hair-cell loss in carboplatin-exposed chinchillas (Lobarinas et al., 2016). One possible interpretation is that auditory-nerve-fiber loss adversely impacts perception of short target signals in noise, but not longer signals for which there is ampler opportunity for temporal integration.
Future animal behavioral research might benefit from focusing on tasks linked to central inhibition, which appears to decrease following auditory-nerve injury, such as modulation detection, modulation masking, and forward masking. Aspects of perception that rely on especially fine timing of neural responses, such as sensitivity to interaural time differences and sound localization, might also be productive directions for future research since separation of target signals from maskers based on spatial cues is an important aspect of auditory perception under noisy, real-world listening conditions.
With the small number of behavioral studies conducted so far in a fairly wide range of animal models, the extent to which divergent results reflect species differences rather than differences in stimuli and experimental design remains unknown. Further testing with the same stimuli in different species will be needed to help resolve this open question. Other factors that could influence study outcomes include the timing and duration of behavioral testing, which began several weeks following auditory-nerve injury in some studies (Chambers et al., 2016; Lobarinas et al., 2020, 2017, 2016, 2013; Resnik and Polley, 2021) and several months post-exposure in others (Henry and Abrams, 2021; Schuknecht and Woellner, 1953). These details likely impact the extent of compensatory changes in central processing present at the time of behavioral testing (Chambers et al., 2016; Salvi et al., 2017). Future studies would benefit from tracking behavioral performance over a longer timeframe, beginning as soon as possible after auditory-nerve injury and extending for several months thereafter to evaluate longitudinal changes in auditory perception caused by this common cochlear pathology.
Highlights.
Auditory-nerve-fiber loss occurs with age and following sound overexposure
Nerve-fiber loss in animal models can enhance or suppress acoustic startle reflexes
Behavioral tone-in-quiet sensitivity is unaffected unless fiber loss exceeds 80–90%
Other perceptual changes also appear small compared to effects of hair-cell damage
Perception of brief targets in noise, modulation, and spatial hearing need further study
Acknowledgements
Laurel Carney proofread and provided helpful comments on a previous version of the manuscript.
Funding
Preparation of this article was funded by the National Institute on Deafness and Communication Disorders Grant R01-DC-17519.
Footnotes
conflicts of interest
The authors declare no competing conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Behrens D, Klump GM, 2016. Comparison of mouse minimum audible angle determined in prepulse inhibition and operant conditioning procedures. Hear. Res. 333, 167–178. 10.1016/j.heares.2016.01.011 [DOI] [PubMed] [Google Scholar]
- Behrens D, Klump GM, 2015. Comparison of the sensitivity of prepulse inhibition of the startle reflex and operant conditioning in an auditory intensity difference limen paradigm. Hear. Res. 321, 35–44. 10.1016/j.heares.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG, 2014. Cochlear neuropathy and the coding of supra-threshold sound. Front. Syst. Neurosci. 8, 26. 10.3389/fnsys.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Bobbin RP, Chihal DM, 1981. Kainic acid: an evaluation of its action on cochlear potentials. Hear. Res. 4, 109–20. [DOI] [PubMed] [Google Scholar]
- Bramhall N, Beach EF, Epp B, Le Prell CG, Lopez-Poveda EA, Plack CJ, Schaette R, Verhulst S, Canlon B, 2019. The search for noise-induced cochlear synaptopathy in humans: Mission impossible? Hear. Res. 377, 88–103. 10.1016/j.heares.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Burger RM, Pollak GD, 1998. Analysis of the role of inhibition in shaping responses to sinusoidally amplitude-modulated signals in the inferior colliculus. J. Neurophysiol. 80, 1686–701. [DOI] [PubMed] [Google Scholar]
- Carney LH, Ketterer AD, Abrams KS, Schwarz DM, Idrobo F, 2013. Detection thresholds for amplitude modulations of tones in budgerigar, rabbit, and human. Adv. Exp. Med. Biol. 787, 391–8. 10.1007/978-1-4614-1590-9_43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF, 2008. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J. Exp. Biol. 211, 1781–1791. 10.1242/jeb.013581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF, 2002. GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hear. Res. 168, 163–73. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB, 2016. Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron 89, 867–879. 10.1016/j.neuron.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA, 1988. Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–4. [DOI] [PubMed] [Google Scholar]
- Davis KA, Ramachandran R, May BJ, 1999. Single-Unit Responses in the Inferior Colliculus of Decerebrate Cats II. Sensitivity to Interaural Level Differences. 10.1152/jn.1999.82.1.164 82, 164–175. [DOI] [PubMed] [Google Scholar]
- Delgutte B, 1996. Physiological models for basic auditory percepts, in: Hawkins H, McMullen T, Fay R (Eds.), Auditory Computation. Springer, New York, pp. 157–220. 10.1007/978-1-4612-4070-9_5 [DOI] [Google Scholar]
- Dent ML, Dooling RJ, Pierce AS, 2000. Frequency discrimination in budgerigars (Melopsittacus undulatus): effects of tone duration and tonal context. J. Acoust. Soc. Am. 107, 2657–2664. 10.1121/1.428651 [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Lohr B, Dent ML, 2000. Hearing in Birds and Reptiles, in: Dooling RJ, Fay RR, Popper AN (Eds.), Comparative Hearing: Birds and Reptiles. Springer, New York, pp. 308–359. [Google Scholar]
- Dooling RJ, Saunders JC, 1975. Hearing in the parakeet (Melopsittacus undulatus): absolute thresholds, critical ratios, frequency difference limens, and vocalizations. J. Comp. Physiol. Psychol. 88, 1–20. 10.1037/h0076226 [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Searcy MH, 1981. Amplitude modulation thresholds for the parakeet (Melopsittacus undulatus). J. Comp. Physiol. A 143, 383–388. 10.1007/BF00611177 [DOI] [Google Scholar]
- Dubno JR, Dirks DD, Langhofer LR, 1982. Evaluation of hearing-impaired listeners using a Nonsense-syllable Test. II. Syllable recognition and consonant confusion patterns. J. Speech Hear. Res. 25, 141–148. [DOI] [PubMed] [Google Scholar]
- Fitzaibbons PJ, Wightman FL, 1982. Gap detection in normal and hearing-impaired listeners. J. Acoust. Soc. Am. 72, 761–765. 10.1121/1.388256 [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC, 2013. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol. 110, 577–586. 10.1152/jn.00164.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken GM, Bhat VKH, Hutchison-Clutter M, 1990. Auditory temporal integration and the power function model. J. Acoust. Soc. Am. 88, 767–778. 10.1121/1.399726 [DOI] [PubMed] [Google Scholar]
- Gerken GM, Gunnarson AD, Allen CM, 1983. Three Models of Temporal Summation Evaluated Using Normal-Hearing and Hearing-Impaired Subjects. J. Speech Lang. Hear. Res. 26, 256. 10.1044/jshr.2602.256 [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BCJ, Bacon SP, 1998. Gap detection and masking in hearing-impaired and normal-hearing subjects. J. Acoust. Soc. Am. 81, 1546. 10.1121/1.394507 [DOI] [PubMed] [Google Scholar]
- Gleich O, 1989. Auditory primary afferents in the starling: correlation of function and morphology. Hear. Res. 37, 255–67. [DOI] [PubMed] [Google Scholar]
- Gollnast D, Tziridis K, Krauss P, Schilling A, Hoppe U, Schulze H, 2017. Analysis of audiometric differences of patients with and without tinnitus in a large clinical database. Front. Neurol. 8, 1. 10.3389/FNEUR.2017.00031/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Birdsall TG, Tanner WP, 1957. Signal Detection as a Function of Signal Intensity and Duration. J. Acoust. Soc. Am. 29, 523–531. 10.1121/1.1908951 [DOI] [Google Scholar]
- Greene NT, Anbuhl KL, Ferber AT, DeGuzman M, Allen PD, Tollin DJ, 2018. Spatial hearing ability of the pigmented Guinea pig (Cavia porcellus): Minimum audible angle and spatial release from masking in azimuth. Hear. Res. 365, 62–76. 10.1016/J.HEARES.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Buss E, Hall JW, 2017. Loud Music Exposure and Cochlear Synaptopathy in Young Adults: Isolated Auditory Brainstem Response Effects but No Perceptual Consequences. Trends Hear. 21, 233121651773741. 10.1177/2331216517737417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin C, Rauch SD, 2009. Clinical implications of a damaged cochlea: Pure tone thresholds vs information-carrying capacity. Otolaryngol. - Head Neck Surg. 140, 473–476. 10.1016/j.otohns.2008.12.021 [DOI] [PubMed] [Google Scholar]
- Heil P, Matysiak A, Neubauer H, 2017. A probabilistic Poisson-based model accounts for an extensive set of absolute auditory threshold measurements. Hear. Res. 353, 135–161. 10.1016/J.HEARES.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Henry KS, Abrams KS, 2021. Normal Tone-In-Noise Sensitivity in Trained Budgerigars despite Substantial Auditory-Nerve Injury: No Evidence of Hidden Hearing Loss. J. Neurosci. 41, 118–129. 10.1523/JNEUROSCI.2104-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Abrams KS, 2018. Persistent Auditory Nerve Damage Following Kainic Acid Excitotoxicity in the Budgerigar (Melopsittacus undulatus). J. Assoc. Res. Otolaryngol. 19, 435–449. 10.1007/s10162-018-0671-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Abrams KS, Forst J, Mender MJ, Neilans EG, Idrobo F, Carney LH, 2017a. Midbrain Synchrony to Envelope Structure Supports Behavioral Sensitivity to Single-Formant Vowel-Like Sounds in Noise. JARO - J. Assoc. Res. Otolaryngol. 18, 165–181. 10.1007/s10162-016-0594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Amburgey KN, Abrams KS, Carney LH, 2020. Identifying cues for tone-in-noise detection using decision variable correlation in the budgerigar (Melopsittacus undulatus). J. Acoust. Soc. Am. 147, 984–997. 10.1121/10.0000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Amburgey KN, Abrams KS, Idrobo F, Carney LH, 2017b. Formant-frequency discrimination of synthesized vowels in budgerigars (Melopsittacus undulatus) and humans. J. Acoust. Soc. Am. 142, 2073–2083. 10.1121/1.5006912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Neilans EG, Abrams KS, Idrobo F, Carney LH, 2016. Neural correlates of behavioral amplitude modulation sensitivity in the budgerigar midbrain. J. Neurophysiol. 115, 1905–1916. 10.1152/jn.01003.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC, 2014. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J. Neurophysiol. 111, 552–564. 10.1152/jn.00184.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DRF, Brown M, Kamke MR, Rubel EW, 2009. Effects of restricted basilar papillar lesions and hair cell regeneration on auditory forebrain frequency organization in adult European starlings. J. Neurosci. 29, 6871–6882. 10.1523/JNEUROSCI.5513-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen PT, Buzo BC, Lopez-Poveda EA, 2019. Evidence for age-related cochlear synaptopathy in humans unconnected to speech-in-noise intelligibility deficits. Hear. Res. 374, 35–48. 10.1016/j.heares.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Juiz JM, Rueda J, Merchán JA, Sala ML, 1989. The effects of kainic acid on the cochlear ganglion of the rat. Hear. Res. 40, 65–74. 10.1016/0378-5955(89)90100-7 [DOI] [PubMed] [Google Scholar]
- Köppl C, Wegscheider A, Gleich O, Manley GA, 2000. A quantitative study of cochlear afferent axons in birds. Hear. Res. 139, 123–143. 10.1016/S0378-5955(99)00178-1 [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC, 2009. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 29, 14077–14085. 10.1523/JNEUROSCI.2845-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Dooling RJ, Leek MR, Poling K, 2007. Detection and discrimination of simple and complex sounds by hearing-impaired Belgian Waterslager canaries. J. Acoust. Soc. Am. 122, 3615–3627. 10.1121/1.2799482 [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Siburt HW, Lobarinas E, Griffiths SK, Spankovich C, 2018. No reliable association between recreational noise exposure and threshold sensitivity, distortion product otoacoustic emission amplitude, or word-in-noise performance in a college student population. Ear Hear. 39, 1057–1074. 10.1097/AUD.0000000000000575 [DOI] [PubMed] [Google Scholar]
- Leong U-C, Schwarz DM, Henry KS, Carney LH, 2020. Sensorineural Hearing Loss Diminishes Use of Temporal Envelope Cues: Evidence From Roving-Level Tone-in-Noise Detection. Ear Hear. 41, 1009–1019. 10.1097/aud.0000000000000822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, 1984. Single-neuron labeling and chronic cochlear pathology. I. Threshold shift and characteristic-frequency shift. Hear Res 16, 33–41. [DOI] [PubMed] [Google Scholar]
- Liberman MC, 1978. Auditory-nerve response from cats raised in a low-noise chamber. J. Acoust. Soc. Am. 63, 442–455. 10.1121/1.381736 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF, 2016. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 11, 1–15. 10.1371/journal.pone.0162726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Kujawa SG, 2017. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 349, 138–147. 10.1016/j.heares.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC, 2011. Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. JARO - J. Assoc. Res. Otolaryngol. 12, 605–616. 10.1007/s10162-011-0277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D, 2020. Gap Detection Deficits in Chinchillas with Selective Carboplatin-Induced Inner Hair Cell Loss. J. Assoc. Res. Otolaryngol. 2020 216 21, 475–483. 10.1007/S10162-020-00744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D, 2016. Selective Inner Hair Cell Dysfunction in Chinchillas Impairs Hearing-in-Noise in the Absence of Outer Hair Cell Loss. JARO - J. Assoc. Res. Otolaryngol. 17, 89–101. 10.1007/s10162-015-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D, 2013. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear. Res. 302, 113–120. 10.1016/j.heares.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Spankovich C, Le Prell CG, 2017. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear. Res. 349, 155–163. 10.1016/j.heares.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Barrios P, 2013. Perception of stochastically undersampled sound waveforms: a model of auditory deafferentation. Front. Neurosci 7. 10.3389/FNINS.2013.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary C. a., Shin J, Kujawa SG, Liberman MC, Merchant SN, 2011. Age-related primary cochlear neuronal degeneration in human temporal bones. JARO - J. Assoc. Res. Otolaryngol. 12, 711–717. 10.1007/s10162-011-0283-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Gleich O, Leppelsack HJ, Oeckinghaus H, 1985. Activity patterns of cochlear ganglion neurones in the starling. J. Comp. Physiol. A. 157, 161–181. 10.1007/BF01350025 [DOI] [PubMed] [Google Scholar]
- Manley GA, Schwabedissen G, Gleich O, 1993. Morphology of the basilar papilla of the budgerigar,Melopsittacus undulatus. J. Morphol. 218, 153–165. 10.1002/jmor.1052180205 [DOI] [PubMed] [Google Scholar]
- Marmel F, Cortese D, Kluk K, 2020. The ongoing search for cochlear synaptopathy in humans: Masked thresholds for brief tones in Threshold Equalizing Noise. Hear. Res. 392. 10.1016/j.heares.2020.107960 [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding DL, Jiang H, Salvi R, 2004. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 997, 40–51. 10.1016/j.brainres.2003.10.031 [DOI] [PubMed] [Google Scholar]
- Nelson PB, Thomas SD, 1997. Gap Detection as a Function of Stimulus Loudness for Listeners With and Without Hearing Loss 40, 1387–1394. 10.1044/JSLHR.4006.1387 [DOI] [PubMed] [Google Scholar]
- Nouvian R, Beutner D, Parsons TD, Moser T, 2006. Structure and Function of the Hair Cell Ribbon Synapse. J. Membr. Biol. 2006 2092 209, 153–165. 10.1007/S00232-005-0854-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ, 1987. Hearing in passerine and psittacine birds: a comparative study of absolute and masked auditory thresholds. J. Comp. Psychol. 101, 7–15. [PubMed] [Google Scholar]
- Otte J, Schuknecht HF, Kerr AG, 1978. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope 88, 1231–1246. 10.1288/00005537-197808000-00004 [doi] [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, 2016. Predicting the Perceptual Consequences of Hidden Hearing Loss. Trends Hear. 20, 233121651668676. 10.1177/2331216516686768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM, 1996. GABA Inputs Control Discharge Rate Primarily Within Frequency Receptive Fields of Inferior Colliculus Neurons. J. Neurophysiol. 75, 2211–2219. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN, 1997. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacol 1997 1322 132, 169–180. 10.1007/S002130050333 [DOI] [PubMed] [Google Scholar]
- Plack CJ, Barker D, Prendergast G, 2014. Perceptual consequences of “hidden” hearing loss. Trends Hear. 18, 1–11. 10.1177/2331216514550621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack CJ, Léger A, Prendergast G, Kluk K, Guest H, Munro KJ, 2016. Toward a Diagnostic Test for Hidden Hearing Loss. Trends Hear 20. 10.1177/2331216516657466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp R, Bouman MA, 1959. Relation between Hearing Threshold and Duration for Tone Pulses. J. Acoust. Soc. Am. 31, 749–758. 10.1121/1.1907781 [DOI] [Google Scholar]
- Prendergast G, Couth S, Millman RE, Guest H, Kluk K, Munro KJ, Plack CJ, 2019. Effects of Age and Noise Exposure on Proxy Measures of Cochlear Synaptopathy 23, 1–16. 10.1177/2331216519877301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Millman RE, Guest H, Munro KJ, Kluk K, Dewey RS, Hall DA, Heinz MG, Plack CJ, 2017. Effects of noise exposure on young adults with normal audiograms II: Behavioral measures. Hear. Res. 356, 74–86. 10.1016/j.heares.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik J, Polley DB, 2021. Cochlear neural degeneration disrupts hearing in background noise by increasing auditory cortex internal noise. Neuron 109, 984–996.e4. 10.1016/J.NEURON.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Dent ML, Dooling RJ, 2013. Return of function after hair cell regeneration. Hear. Res. 297, 113–120. 10.1016/j.heares.2012.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Ten Eyck B, Westbrook EW, 1989. Ganglion cell loss continues during hair cell regeneration. Hear. Res. 43, 81–90. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Westbrook EW, 1988. Ganglion cell and hair cell loss in Coturnix quail associated with aging. Hear. Res. 36, 1–8. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Young ED, 1979. Encoding of steady-state vowels in the auditory nerve: Representation in terms of discharge rate. J. Acoust. Soc. Am. 66, 470–479. 10.1121/1.383098 [DOI] [PubMed] [Google Scholar]
- Sachs MB, Young ED, Lewis RH, 1974. Discharge patterns of single fibers in the pigeon auditory nerve. Brain Res. 70, 431–447. [DOI] [PubMed] [Google Scholar]
- Salvi R, Ding D, Wang J, Jiang H, 2000. A review of the effects of selective inner hair cell lesions on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise Heal. 2, 9–26. [PubMed] [Google Scholar]
- Salvi R, Saunders SS, Powers NL, Boettcher FA, 1992. Discharge patterns of cochlear ganglion neurons in the chicken. J. Comp. Physiol. A. 170, 227–41. [DOI] [PubMed] [Google Scholar]
- Salvi R, Sun W, Ding DL, Chen G-D, Lobarinas E, Wang J, Radziwon KE, Auerbach BD, 2017. Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Front. Neurosci. 10, 1–14. 10.3389/fnins.2016.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC, Denny RM, Bock GR, 1978. Critical bands in the parakeet (Melopsittacus undulatus). J. Comp. Physiol. A 125, 359–365. 10.1007/BF00656871 [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D, 2011. Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. J. Neurosci. 31, 13452–13457. 10.1523/JNEUROSCI.2156-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, 1989. Spontaneous rates, thresholds and tuning of auditory-nerve fibers in the gerbil: Comparisons to cat data. Hear. Res. 42, 23–35. 10.1016/0378-5955(89)90115-9 [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC, 1953. Hearing losses following partial sectioning of the cochlear nerve. Laryngoscope 63, 441–465. 10.1288/00005537-195306000-00001 [DOI] [PubMed] [Google Scholar]
- Sebastian S, Abrams J, Geisler WS, 2017. Constrained sampling experiments reveal principles of detection in natural scenes. Proc. Natl. Acad. Sci. 114, E5731–E5740. 10.1073/pnas.1619487114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian S, Geisler WS, 2018. Decision-variable correlation. J. Vis. 18, 1–19. 10.1167/18.4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehorn J, Strelcyk O, Zahorik P, 2020. Associations between speech recognition at high levels, the middle ear muscle reflex and noise exposure in individuals with normal audiograms. Hear. Res. 392. 10.1016/j.heares.2020.107982 [DOI] [PubMed] [Google Scholar]
- Smolders JW, Ding-Pfennigdorff D, Klinke R, 1995. A functional map of the pigeon basilar papilla: correlation of the properties of single auditory nerve fibres and their peripheral origin. Hear. Res. 92, 151–69. [DOI] [PubMed] [Google Scholar]
- Solecki JM, Gerken GM, 1990. Auditory temporal integration in the normal-hearing and hearing-impaired cat. J. Acoust. Soc. Am. 88, 779–785. 10.1121/1.399727 [DOI] [PubMed] [Google Scholar]
- Spoendlin H, 1984. Factors inducing retrograde degeneration of the cochlear nerve. Ann. Otol. Rhinol. Laryngol. Suppl. 112, 76–82. [DOI] [PubMed] [Google Scholar]
- Spoendlin H, Schrott A, 1989. Analysis of the human auditory nerve. Hear. Res. 43, 25–38. 10.1016/0378-5955(89)90056-7 [DOI] [PubMed] [Google Scholar]
- Sun H, Hashino E, Ding DL, Salvi R, 2001. Reversible and irreversible damage to cochlear afferent neurons by kainic acid excitotoxicity. J. Comp. Neurol. 430, 172–181. [DOI] [PubMed] [Google Scholar]
- Suthakar K, Liberman MC, 2021. Auditory-Nerve Responses in Mice with Noise-Induced Cochlear Synaptopathy. J. Neurophysiol. 10.1152/JN.00342.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaka T, Smith C, 1971. The Structure and Innervation of the Pigeon ‘ s Basilar Papilla 1. J. Ultrastruct. Res. 35, 20–65. [DOI] [PubMed] [Google Scholar]
- Trautwein P, Hofstetter P, Wang J, Salvi R, Nostrant A, 1996. Selective inner hair cell loss does not alter distortion product otoacoustic emissions. Hear. Res. 96, 71–82. [DOI] [PubMed] [Google Scholar]
- Tziridis K, Forster J, Buchheidt-Dörfler I, Krauss P, Schilling A, Wendler O, Sterna E, Schulze H, 2021. Tinnitus development is associated with synaptopathy of inner hair cells in Mongolian gerbils. Eur. J. Neurosci. 54, 4768–4780. 10.1111/ejn.15334 [DOI] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC, 2015. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear. Res. 327, 78–88. 10.1016/j.heares.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM, 2011. Inhibitory neurotransmission in animal models of tinnitus: Maladaptive plasticity. Hear. Res. 279, 111–117. 10.1016/j.heares.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R, 1997. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear. Res. 107, 67–82. 10.1016/S0378-5955(97)00020-8 [DOI] [PubMed] [Google Scholar]
- Wang Y, Abrams KS, Carney LH, Henry KS, 2021. Midbrain-level neural correlates of behavioral tone-in-noise detection: dependence on energy and envelope cues. J. Neurosci. 41, JN-RM-3103–20. 10.1523/jneurosci.3103-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Gengel RW, 1969. Signal Duration and Signal Frequency in Relation to Auditory Sensitivity. J. Acoust. Soc. Am. 46, 989–997. 10.1121/1.1911819 [DOI] [PubMed] [Google Scholar]
- Wilson JL, Abrams KS, Henry KS, 2021. Effects of Kainic Acid-Induced Auditory Nerve Damage on Envelope-Following Responses in the Budgerigar (Melopsittacus undulatus) 22, 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SJSJ, Abrams KS, Amburgey KN, Wang Y, Henry KS, 2019. Effects of selective auditory-nerve damage on the behavioral audiogram and temporal integration in the budgerigar. Hear. Res. 374, 24–34. 10.1016/j.heares.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright HN, 1968. The Effect of Sensori-Neural Hearing Loss on Threshold-Duration Functions. J. Speech Hear. Res. 11, 842–852. 10.1044/jshr.1104.842 [DOI] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, Gruttola V. De, Malley JTO, Liberman MC, 2019. Primary Neural Degeneration in the Human Cochlea : Evidence for Hidden Hearing Loss in the Aging Ear. Neuroscience 407, 8–20. 10.1016/j.neuroscience.2018.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeend I, Beach EF, Sharma M, Dillon H, 2017. The effects of noise exposure and musical training on suprathreshold auditory processing and speech perception in noise. Hear. Res. 353, 224–236. 10.1016/j.heares.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Young ED, 2013. Which neurons survive the glutamate storm? J. Neurophysiol. 110, 575–576. 10.1152/jn.00292.2013 [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB, 1979. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory- nerve fibers. J. Acoust. Soc. Am. 66, 1381–1403. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shi F, Yin Y, Tong M, Lang H, Polley DB, Liberman MC, Edge ASB, 2014. Ouabain-induced cochlear nerve degeneration: Synaptic loss and plasticity in a mouse model of auditory neuropathy. JARO - J. Assoc. Res. Otolaryngol. 15, 31–43. 10.1007/s10162-013-0419-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Richardson M, Turner K, 2020. Tinnitus Does Not Interfere with Auditory and Speech Perception. J. Neurosci. 40, 6007–6017. 10.1523/JNEUROSCI.0396-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kelly JB, 2003. Glutamatergic and GABAergic regulation of neural responses in inferior colliculus to amplitude-modulated sounds. J. Neurophysiol. 90, 477–90. 10.1152/jn.01084.2002 [DOI] [PubMed] [Google Scholar]