Clinical trials are integral to improve treatment outcomes for patients with hematological malignancies. The approval of drugs to treat hematological malignancies follows a process established by the United States Food and Drug Administration (FDA) to ensure a balance between efficacy and toxicity. The FDA approved more than 50 new drugs to treat various hematological malignancies in the last 5 years, with many of these drugs having been approved for the first time in the USA [1,2].
Early phase (I/II) clinical trials may provide evidence of clinical efficacy; however, the main goal of early phase trials is to assess safety. Results of phase III clinical trials provide the strongest evidence to support the use of new cancer medications. There are multiple concerns that result from the use of early phase clinical trials to support drug approvals [3]. Some examples include the lack of a control group [4], patient heterogeneity [5], short follow-up time due to bias related to early publication [6], and limitation to the generalizability of treatment results [2,7]. Moreover, the chosen surrogate endpoint may not actually predict clinical benefit. It is estimated that two-thirds of the FDA contemporary cancer drug approvals were based on surrogate endpoints [8]. However, up to 86% of approvals based on surrogate endpoints subsequently fail to show gains in overall survival (OS) or have unknown effects on survival [8].
We conducted a retrospective analysis of level of evidence supporting FDA drug approvals for hematological malignancies. Data on product labeling are available publicly at Drugs@FDA. Drugs approved from January 2016 until May 2020 were analyzed. The studied hematologic malignancies include multiple myeloma, leukemias, non-Hodgkin lymphomas, Hodgkin lymphoma, and myelodysplastic syndrome. We calculated the frequency of approvals based on early evidence clinical trials across different types of hematological malignancies.
Publicly accessible FDA reviews of 52 clinical trials supporting 49 drug approvals in the 5-year period were available for level of evidence review. Phase III/IV clinical trials supported 60% while earlier phase trials supported 40% of subsequent FDA hematological malignancies approvals. The level of evidence to support FDA approvals improved with time, with 50% of approvals in 2016 and 2017 supported by phase III clinical trials compared to 69% in 2019 (Figure 1). The median number of patients of phase III/IV clinical trials in the studied period was 326 with no significant change with time. Progression-free survival (PFS) was the primary endpoint in 58% and OS was the primary endpoint in 16% of all phase III/IV clinical trials in the studied period. The phase III/IV trials were routinely designed to detect a specified improvement with a false positive rate of at most 5% (p = 0.05) and a false negative rate of 10%–20% (power = 80%–90%).
Figure 1.
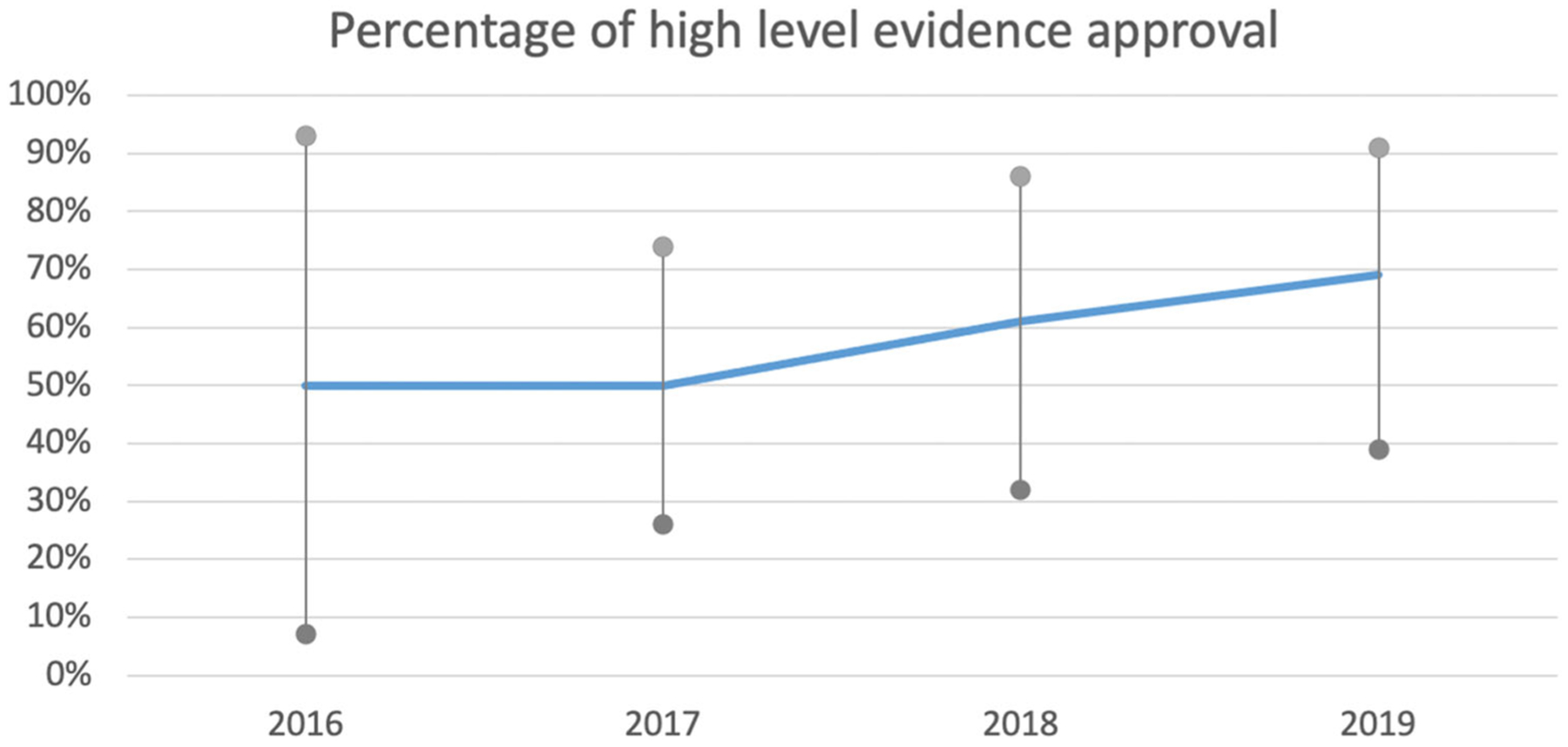
Trend of high-level evidence to support FDA approval of hematological malignancies. Percentage of high-level evidence approval with 95% confidence intervals.
Approvals in the studied period were based on early phase trials in mantle cell lymphoma (100%), diffuse large B-cell lymphoma (100%), chronic myeloid leukemia (75%), classic Hodgkin lymphoma (67%), and acute myeloid leukemia (56%). Eighteen drug approvals (37% of all approvals) were based on 13% of the total number of patients treated in the studied period. Most of the phase II clinical trials were single-arm (i.e. non-randomized). Only 3 of 17 (18%) phase II clinical trials were randomized (Table 1).
Table 1.
Frequencies of analyzed clinical trials.
| Disease | Number of trials | Number of patients | Range of patient numbers enrolled in all studies | Median (range) of number of patients enrolled on high-level evidence trials | Number (%) of early phase studies that supported approval |
|---|---|---|---|---|---|
| Leukemia | 26 | 7262 | 34–717 | 389 (80–717) | 11 (42) |
| Chronic lymphoid leukemia | 8 | 3094 | 106–535 | 432 (310–535) | 1 (13) |
| Chronic myeloid leukemia | 4 | 854 | 51–487 | 487 (n/a) | 3 (75) |
| Acute lymphoid leukemia | 4 | 922 | 75–405 | 366 (326–405) | 2 (50) |
| Acute myeloid leukemia | 9 | 2312 | 34–717 | 290 (247–717) | 5 (56) |
| Hairy cell leukemia | 1 | 80 | n/a | 80 (n/a) | 0 (0) |
| Lymphoma | 18 | 5212 | 80–1334 | 321 (83–1334) | 9 (50) |
| Hodgkin lymphoma | 3 | 1639 | 95–1334 | 1334 (n/a) | 2 (67) |
| Diffuse large B-cell lymphoma | 3 | 281 | 80–108 | n/a | 3 (100) |
| Mantle cell lymphoma | 2 | 210 | 86–124 | n/a | 2 (100) |
| Follicular lymphoma | 6 | 2300 | 83–1202 | 321 (83–1202) | 1 (17) |
| Primary mediastinal B-cell lymphoma | 1 | 53 | n/a | n/a | 1 (100) |
| T-cell lymphoma | 3 | 729 | 131–372 | 226 (131–372) | 0 (0) |
| Multiple myeloma | 7 | 3543 | 122–1085 | 515 (263–1085) | 1 (14) |
| Myelodysplastic syndrome | 1 | 229 | n/a | 229 (n/a) | 0 (0) |
| Total | 52 | 16,246 | 34–1334 | 34–1334 | 21 (40) |
n/a: not applicable.
Our study has limitations. First, we included only clinical trials that led to FDA approval. In addition, some of the hematological malignancies included are rare, which can limit the ability to perform large phase III clinical trials. Nonetheless, even for more frequent diseases, such as acute myeloid leukemia, with 19,940 new cases expected to occur in 2020 [9], only 44% of new drug approval were based on high-level evidence clinical trials in the last 5 years. The remaining, early phase-based drug approvals were based on small patient sample sizes.
In conclusion, evidence to support drug approvals in hematological malignancies was based on early phase trials data in more than a third of the times. Although early phase studies are appropriate to assess safety signals, further clinical activity assessment should be done to support the use of new drugs to treat hematological malignancies, as previous successful early phase studies have at times failed to show clinical activity in subsequent phase III studies. Although the use of newly approved drugs based on early phase studies evidence may be necessary (especially when the targeted diseases are rare), patients and healthcare providers should be aware of these caveats when using newly approved medications.
Acknowledgments
Part of this manuscript was presented at the American Society of Hematology 2020 Annual meeting.
Funding
Samer Al Hadidi reports salary support by National Heart, Lung, and Blood Institute grant No. 5T32HL092332-18.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Lls.org [Internet]. New York: Leukemia and Lymphoma Society, 2020; [cited 2020 Oct 22]. Available from: https://www.lls.org/blog/recent-drug-approvals-for-blood-cancer-mark-significant-progress-for-patients [Google Scholar]
- [2].Al Hadidi S, Mims M, Miller-Chism CN, et al. Participation of African American persons in clinical trials supporting U.S. Food and Drug Administration approval of cancer drugs. Ann Intern Med. 2020;173(4):320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Walter RB, Appelbaum FR, Tallman MS, et al. Shortcomings in the clinical evaluation of new drugs: acute myeloid leukemia as paradigm. Blood. 2010;116(14):2420–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walter RB, Estey EH. The power of comparative studies. Leuk Res. 2009;33(5):610–612. [DOI] [PubMed] [Google Scholar]
- [5].Barnes CN, Rai SN. Modeling heterogeneity in phase II clinical trials. Am J Biostat. 2010;6(1):9–16. [Google Scholar]
- [6].Rowe JM, Yao X, Cassileth PA, et al. The pitfalls of early publication of data in acute myeloid leukemia: a report from the Eastern Cooperative Oncology Group (ECOG) [abstract]. Blood. 2008;112(11):1952–1952. [Google Scholar]
- [7].Joseph G, Dohan D. Diversity of participants in clinical trials in an academic medical center: the role of the ‘Good Study Patient?’ Cancer. 2009;115(3):608–615. [DOI] [PubMed] [Google Scholar]
- [8].Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992–1994. [DOI] [PubMed] [Google Scholar]
- [9].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]


