Abstract
Background
To assess the clinical outcomes after endovascular thrombectomy (EVT) in elderly large vessel occlusion (LVO)-related acute ischemic stroke (AIS) patients with atrial fibrillation (AF).
Methods
Between January 2019 and December 2020, consecutive AF patients who received EVT due to anterior-circulation stroke were enrolled. The primary outcome was modified Rankin scale (mRS) score at 90 days. Secondary outcomes included all-cause mortality, the recanalization status after EVT (assessed using modified thrombolysis in cerebral infarction scale, mTICI) and any intracranial hemorrhage (ICH). A multivariate logistic regression model was performed to identify predictors of the functional outcome.
Results
A total of 148 eligible patients were finally enrolled. Among them, 42 were ≥ 80 years old. Compared to their younger counterparts, patients aged ≥80 years had lower likelihood of good functional outcome (mRS score 0–2) at 90 days (26.2% vs. 48.1%, P = 0.015), less satisfied recanalization (mTICI, 2b-3) (78.6% vs. 94.3%, P = 0.004) and higher all-cause mortality rate (35.7% vs. 14.2%, P = 0.003). A multivariable logistic regression analysis showed that age ≥ 80 years at baseline were the significant predictors for a poor functional outcome (OR: 3.72, 95% CI: 1.17–11.89, p = 0.027). Intravenous thrombolysis (IVT) prior to EVT and longer time intervals from onset of symptoms to EVT tended to be associated with poor functional outcome in patients ≥80 years old.
Conclusions
Age ≥ 80 years was a significant predictor of unfavorable outcomes after EVT for AIS patients with AF. An increased risk of adverse events must be balanced against the benefit from EVT in elderly patients with AF.
Keywords: Acute ischemic stroke, Atrial fibrillation, Elderly, Endovascular thrombectomy
Background
Atrial fibrillation (AF) is the most common cause of cardioembolism, accounting for about 20–30% of acute ischemic stroke (AIS) [1]. Approximately one-third of AISs occur in patients aged ≥80 years [2]. Considering that the incidence of AF and the risk of AF-related stroke increases significantly with age, there is a high proportion of elderly AIS patients with AF, especially in patients aged ≥80 years [3].
Since the publication of five major randomized controlled trials (RCTs) [4], endovascular thrombectomy (EVT) has been recommended as a standard treatment applied within 6 h of an AIS in the anterior circulation due to large vessel occlusion (LVO), even in very elderly patients (≥80 years old). Subsequently, for patients within 6–24 window hours who meet the DAWN [5] or DEFUSE 3 [6] eligibility criteria, EVT was also recommended. However, the meta-analysis of the five RCTs showed that at 90 days after AIS, the modified Rankin scale (mRS) scores was significantly higher (lower score indicating better outcome) in the patients aged ≥80 years compared with those at younger ages [7]. Recently, in a ‘real world’ study, EVT carried a higher risk of hemorrhagic complications than medical treatment in elderly patients [8].
Due to their multi-morbidities, declining multiple organ function, poor tolerance to invasive or noninvasive treatment, possible prior use of anticoagulants and high risk of bleeding after intravenous thrombolysis (IVT), elderly AIS patients with AF practically have limited therapeutic options and poor outcomes. In the meantime, the benefit of EVT for LVO-related AIS patients with AF who are ≥80 years was less clearly elucidated.
This study aims to assess the clinical outcomes after EVT in elderly LVO-related AIS patients with AF and their association with previous medical histories and preoperatively available clinical variables.
Methods
Study design and study population
This is a single-center retrospective observational study. From January 2019 to December 2020, consecutive patients with AIS who received EVT were enrolled from the stroke center in this study. All patients suspected to have AIS received post-processed computed tomography angiography (CTA) scan to confirm the occlusion situation of blood vessel in head and neck. Since September 2019, CTA has been replaced by computed tomography perfusion (CTP). The treatment strategies were developed by experienced neurologists and interventional neuro-radiologists after the evaluation of patients and images according to the American Heart Association/American Stroke Association (AHA/ASA) guidelines [9]. In particular, the indication for EVT in this study were as follows: (1) pre-stroke mRS score < 2; (2) AIS due to LVO in the anterior circulation confirmed by CTA or CTP; (3) ≥18 years old; (4) NIHSS score of ≥6; (5) ASPECTS of ≥6; and (6) patients within 6 h of symptom onset or within 6 to 24 h of last known normal who meet the DAWN or DEFUSE 3 eligibility criteria [5, 6, 9].
The exclusion criteria in this study were as follows: (a) patients without AF; (b) patients with in-hospital stroke; (c) loss to follow up or lack of baseline characteristics; (d) patients who only received angiography or intra-arterial thrombolysis; (e) patients with acute vertebrobasilar occlusion. The study was approved by the medical ethics committee of the local university and there is no conflict of interest among all authors.
Endovascular thrombectomy
The procedure detail has been described in our previous report [10]. In brief, local anesthesia supplemented by conscious sedation was performed before the procedure in all patients. A Solitaire AB device (Medtronic, Irvine, California, USA) was used during EVT, combined with aspiration through the corresponding guiding catheter (Envoy, Cordis) or distal access catheter (Navien, ev3). Blood flow recovery was evaluated after each EVT. For the residual stenosis in cases with in situ thrombosis, whether to perform balloon angioplasty and stent placement was at the discretion of the operator. Intra-arterial thrombolysis, or intra-catheter tirofiban administration might be considered as rescue therapies.
Baseline and clinical assessment
The data collected were age, gender, previous medical history including AF, hypertension, diabetes mellitus, coronary atherosclerosis disease (CAD), heart failure, previous stroke or transient ischemic attack (TIA), and valvular heart disease, and previously used antithrombotic drugs. The National Institutes of Health Stroke Scale (NIHSS) score, Alberta Stroke Program Early CT Score (ASPECTS) on admission and CHA2DS2-VASc score (variables age, heart failure, hypertension, diabetes mellitus, vascular disease, stroke and systemic embolism, gender) was assessed right after patients arrived in the hospital. Process time including stroke symptom onset to door, door to puncture, and puncture to reperfusion was recorded. Peripheral blood of each patient was collected for analysis of complete blood count, prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), fibrin, and D-dimer, cardiac troponin T (c-TnT), serum electrolyte, liver and renal function. Patients received scheduled follow-up visits by neurologists or by phone call interview at 90 days post-stroke.
AF diagnosis and effective anticoagulation definition
In this study, AF were diagnosed based on the previous history of AF or electrocardiographic documentation of AF episode on admission. In addition, one or more 24- to 72-h continuous ECG patch monitoring was conducted in patients with no evidence of AF and no obvious stenosis after recanalization of the occluded vessel, which was defined as absence of intracranial atherosclerosis causing ≥50% luminal stenosis in arteries supplying the area of ischemia. Non-paroxysmal AF was defined as permanent or persistent AF. Patients on warfarin with an INR ≥1.7 or last novel oral anticoagulant (NOAC) intake < 48 h before onset of stroke was considered to be effectively anticoagulated [11].
Time to (re)start oral anticoagulants after the procedure of endovascular thrombectomy
When to initiate oral anticoagulants (OACs) was at the discretion of treating doctors. Patient’s age, the severity of postoperative symptoms, the size of cerebral infarction area and the occurrence of hemorrhagic transformation were the most important factors to consider.
Clinical outcomes
The primary outcome was defined as the mRS score at 90 days. The secondary outcome was all-cause mortality at 90 days, the recanalization status after EVT (assessed using modified thrombolysis in cerebral infarction scale, mTICI) and any intracranial hemorrhage (ICH), which included hemorrhagic transformation (HT) [12, 13].
Statistical analysis
Statistical Package for the Social Sciences (SPSS) software 26.0 (IBM, Armonk, NY) was used for all the statistical analyses. Continuous variables were presented as mean ± standard deviation (SD) or median with the interquartile range (IQR). To compare the difference between the patients aged ≥80 years and those aged < 80 years, unpaired Student’s t-test was used if continuous variables were normally distributed, or nonparametric test was used if not normally distributed. Categorical variables were expressed as counts with percentages and compared using Chi-square tests or Fisher’s exact tests. Significance was defined when P < 0.05. Multivariate logistic regression was performed to determine whether age ≥ 80 years and other baseline characteristics had an independent impact on the prognosis of EVT. Variables with a P value < 0.05 in univariate analysis were included in the multivariate logistic regression model. The odds ratios (ORs) and corresponding 95% confidence intervals (Cis) were calculated to assess the association.
Results
Baseline characteristics
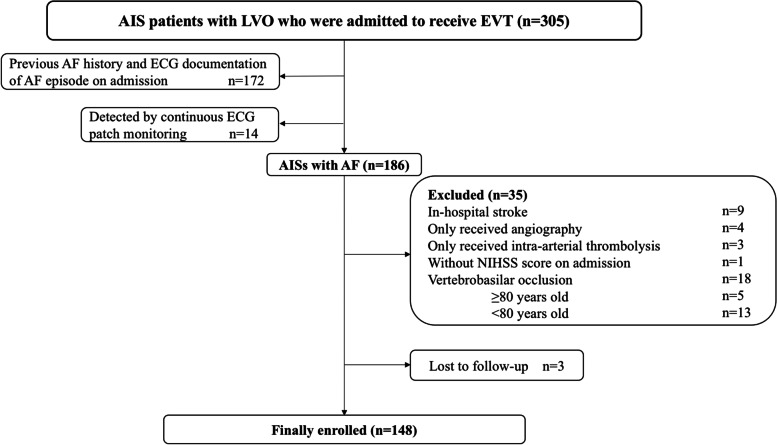
During the study period, a total of 305 AIS patients received EVT. The final analysis included 148 patients with AF who underwent EVT (Fig. 1). There were 14 patients newly detected AF by continuous ECG patch monitoring. Among them, 42 patients (28.4%) were ≥ 80 years old and the remaining 106 patients (71.6%) were < 80 years. Compared to those at younger age, patients aged ≥80 years had lower level of eGFR (73.9 ± 17.2 ml/min vs. 88.4 ± 18.0 ml/min, P < 0.001), less prior IVT (21.4% vs. 41.5%, P = 0.022) and higher CHA2DS2-VASc score (5 [IQR, 4–5] vs. 3 [IQR, 2–4], P < 0.001). There were no significant differences in other baseline clinical characteristics between these two groups (Table 1). The rate of previous anticoagulant use was 7.4% (11/148) in all the enrolled patients, and 2.4% (1/42) in those ≥80 years.
Fig. 1.
Flow chart of patients included in the present study. AF = atrial fibrillation; AIS = acute ischemic stroke; LVO = large intracranial vessel occlusion; EVT = endovascular thrombectomy; ECG = electrocardiogram; NIHSS = National Institute of Health Stroke Scale
Table 1.
Baseline clinical characteristics
| Characteristic | Total (n = 148) | < 80 y (n = 106) | ≥80 y (n = 42) | P value |
|---|---|---|---|---|
| Men, n (%) | 60 (40.5) | 46 (43.4) | 14 (33.3) | 0.261 |
| Age (y) | 73.3 ± 10.2 | 68.9 ± 8.6 | 84.3 ± 2.9 | < 0.001 |
| Type of AF, n (%) | ||||
| Non-paroxysmal AF | 106 (71.6) | 77 (72.6) | 29 (69.0) | 0.662 |
| Pre-stroke mRS | 0.510 | |||
| 0 | 136 (91.9) | 96 (90.6) | 40 (95.2) | |
| 1 | 12 (8.1) | 10 (9.4) | 2 (4.8) | |
| Comorbidities, n (%) | ||||
| Heart failure | 6 (4.1) | 3 (2.8) | 3 (7.1) | 0.352 |
| Hypertension | 106 (71.6) | 74 (69.8) | 32 (76.2) | 0.438 |
| Diabetes | 34 (23.0) | 25 (23.6) | 9 (21.4) | 0.779 |
| Pre-stroke/TIA | 35 (23.6) | 22 (20.8) | 13 (30.1) | 0.188 |
| Coronary artery disease | 17 (11.5) | 10 (9.4) | 7 (16.7) | 0.213 |
| Valvular heart disease, n (%) | ||||
| Rheumatic valvular heart disease | 11 (7.4) | 10 (9.4) | 1 (2.4) | 0.181 |
| Cardiac valve replacement | 5 (3.4) | 5 (4.7) | 0 (0) | 0.322 |
| Antithrombotic, n (%) | ||||
| Anticoagulation | 11 (7.4) | 10 (9.4) | 1 (2.4) | 0.181 |
| warfarin | 4 (2.7) | 4 (3.8) | 0 (0) | 1.000 |
| NOAC | 7 (4.7) | 6 (5.6) | 1 (2.4) | 1.000 |
| Antiplatelet | 31 (20.9) | 20 (18.9) | 11 (26.2) | 0.324 |
| Wake-up stroke, n (%) | 27 (18.2) | 20 (18.9) | 7 (16.7) | 0.755 |
| Clinical scores, median (IQR) | ||||
| Baseline NIHSS score | 16 (12–21) | 15(12–21) | 18 (12–22) | 0.189 |
| CHA2DS2-VASc score | 4 (2–5) | 3(2–4) | 5 (4–5) | < 0.001 |
| Serological indicator, mean ± SD | ||||
| Cardiac troponin T, ng/L | 20.3 ± 21.7 | 19.8 ± 23.3 | 21.3 ± 17.7 | 0.726 |
| D-dimer, mg/L | 2.4 ± 4.4 | 2.6 ± 5.0 | 1.7 ± 1.6 | 0.113 |
| INR | 1.2 ± 0.5 | 1.2 ± 0.6 | 1.1 ± 0.1 | 0.408 |
| Alanine aminotransferase, U/L | 33.2 ± 15.4 | 34.2 ± 15.8 | 30.7 ± 14.6 | 0.212 |
| Aspartate transaminase, U/L | 33.2 ± 12.4 | 33.2 ± 12.3 | 33.3 ± 13.2 | 0.956 |
| eGFR, ml/min | 84.3 ± 18.8 | 88.4 ± 18.0 | 73.9 ± 17.2 | < 0.001 |
| White Blood Cell, 10^9/L | 8.6 ± 3.0 | 8.8 ± 3.0 | 8.2 ± 2.8 | 0.268 |
| Platelet, 10^9/L | 173.8 ± 56.2 | 175.7 ± 57.6 | 169.0 ± 53.8 | 0.518 |
| Neutrophil-to-lymphocyte ratio | 6.4 ± 4.1 | 6.4 ± 4.0 | 6.2 ± 4.6 | 0.800 |
| ASPECTS | 7 (6–9) | 7 (6–9) | 7 (6–9) | 0.482 |
| Occlusion site, n (%) | ||||
| Middle cerebral artery | 100 (67.6) | 73 (68.9) | 27 (64.3) | 0.591 |
| M1 | 80 (54.1) | 60 (56.6) | 20 (47.6) | 0.368 |
| M2 | 20 (13.5) | 13 (12.3) | 7 (16.7) | 0.368 |
| Internal carotid artery | 48 (32.4) | 33 (31.1) | 15 (35.7) | 0.591 |
| Prior IVT, n (%) | 53 (35.8) | 44 (41.5) | 9 (21.4) | 0.022 |
| TOAST, n (%) | ||||
| Cardio-embolic | 136 (91.9) | 99 (93.4) | 37 (88.1) | 0.516 |
| Large artery atherosclerosis | 12 (8.1) | 7 (6.6) | 5 (11.9) | 0.516 |
AF atrial fibrillation, mRS modified Rankin Scale, TIA transient ischemic attack, DOAC direct oral anticoagulants, NIHSS National Institute of Health Stroke Scale, CHA2DS2-VASc congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 years, and sex category (female), IVT intravenous thrombolysis, eGFR estimated glomerular filtration rate, ASPECTS Alberta Stroke Program Early CT Score, INR international normalized ratio, IQR interquartile range, SD standard deviation
eGFR = 141 × min (Scr/ĸ, 1)α × max (Scr/ĸ, 1) − 1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black], where Scr is serum creatinine, ĸ is 0.7 for females and 0.9 for males, α is −0.329 for females and − 0.411 for males, min indicates the minimum of Scr/ĸ or 1, and max indicates the maximum of Scr/ĸ or 1
Operation parameters
The median time from puncture to reperfusion in patients ≥80 years was significantly longer than in those at younger age (70 min [IQR, 50–124 min] vs. 54 min [IQR, 40–84 min], P = 0.011). There were no significant differences in other time intervals between the two groups, including onset to door time interval, door to puncture time interval and total procedure duration. Patients aged ≥80 years tended to have more retrieval attempts (2 [IQR, 1–2] vs. 1 [IQR, 1–2], P = 0.064) than those aged < 80 years. Detailed procedural parameters are shown in Table 2.
Table 2.
Operation parameters
| Characteristic | Total (n = 148) | < 80 y (n = 106) | ≥80 y (n = 42) | P value |
|---|---|---|---|---|
| Time intervals (min), median (IQR) | ||||
| Onset to door | 195 (139–278) | 198 (152–264) | 179 (106–315) | 0.550 |
| Door to puncture | 76 (64–98) | 75 (64–97) | 76 (64–99) | 0.467 |
| Puncture to reperfusion (n = 140) | 60 (44–96) | 54 (40–84) | 70 (50–124) | 0.011 |
| Total procedure | 341 (293–455) | 341 (294–442) | 343 (283–477) | 0.798 |
| Procedural features, n (%) | ||||
| Retrieval attempts, median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 0.064 |
| Solitaire | 116 (78.4) | 82 (77.4) | 34 (80.9) | 0.632 |
| Combined with Intra-arterial thrombolysis | 5 (3.4) | 4 (3.7) | 1 (2.4) | 1.000 |
| Balloon dilatation | 7 (4.7) | 5 (4.7) | 2 (4.7) | 1.000 |
IQR interquartile range
Clinical outcomes
During follow-up, 71 (60.2%) patients out of the 118 survivors were prescribed with OACs after EVT. Warfarin was used in 17 patients, rivaroxaban in 18 patients, and dabigatran in 36 patients. Overall, 30 patients (re)started OACs within 14 days after EVT, and another 41 patients received OACs 14 days after EVT.
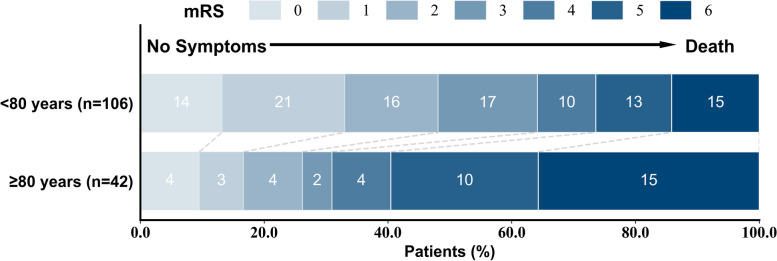
Patients aged ≥80 years undergoing EVT had a lower rate of favorable functional outcome with mRS sore 0–2, compared to those at younger age (26.2% vs. 48.1%, P = 0.015) as shown in Table 3 and Fig. 2. In the multivariate logistic regression model, age ≥ 80 years old (OR: 3.72, 95% CI: 1.17–11.89, P = 0.027), non-paroxysmal AF (OR: 3.74, 95% CI: 1.23–11.38, P = 0.020), higher baseline NIHSS score (OR: 1.17, 95% CI: 1.08–1.27, P < 0.001) and higher c-TnT level (OR: 1.04, 95% CI: 1.01–1.09, P = 0.039) significantly predicted a poor functional outcome of mRS score 3–6 after adjustment (Table 4).
Table 3.
Clinical outcomes
| Outcome | Total (n = 148) | < 80 y (n = 106) | ≥80 y (n = 42) | P value |
|---|---|---|---|---|
| Primary endpoint, n (%) | ||||
| mRS score 0–2 at 90 days | 62 (41.9) | 51 (48.1) | 11 (26.2) | 0.015 |
| Secondary endpoint, n (%) | ||||
| Mortality at 90 days | 30 (20.2) | 15 (14.2) | 15 (35.7) | 0.003 |
| mTICI 2b-3 | 133 (89.9) | 100 (94.3) | 33 (78.6) | 0.004 |
| Intracranial hemorrhage | 48 (32.4) | 34 (32.1) | 14 (33.3) | 0.883 |
mRS modified Rankin Scale, mTICI modified thrombolysis in cerebral infarction
Fig. 2.
Functional outcome on the modified Rankin scale (mRS) at 90 days in patients aged ≥80 years vs. < 80 years
Table 4.
Univariate and multivariate logistic regression to predict unfavorable functional outcome (a Modified Rankin Scale score of 3–6) at 90 days
| Univariate | Multivariate model | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age ≥ 80 years | 2.61 (1.19–5.74) | 0.015 | 3.72(1.17–11.89) | 0.027 |
| Non-paroxysmal AF | 2.39 (1.15–4.94) | 0.019 | 3.74(1.23–11.38) | 0.020 |
| CHA2DS2-VASc score | 1.48 (1.18–1.85) | < 0.001 | ||
| Baseline NIHSS score | 1.19 (1.11–1.28) | < 0.001 | 1.17(1.08–1.27) | < 0.001 |
| Antiplatelet | 3.04 (1.22–7.61) | 0.017 | ||
| c-TnT | 1.05 (1.01–1.09) | 0.008 | 1.04(1.01–1.09) | 0.039 |
| eGFR | 0.98 (0.96–0.99) | 0.013 | ||
| D-dimer | 1.42 (1.08–1.86) | 0.011 | ||
| Neutrophil-to-lymphocyte ratio | 1.14 (1.04–1.25) | 0.006 | ||
Abbreviations as in Table 1
Sex, heart failure, hypertension, diabetes, pre-stroke/TIA, CAD, valvular heart disease, anticoagulation, antiplatelet, wake-up stroke, pre-stroke mRS, time to (re)started OACs, ALT, AST, white blood cell, platelet and several time intervals from onset of symptoms to treatment showed in Table 2 were also conducted univariate analysis and no significant differences were found
In total, 30 patients died during 90 days after EVT. The cause of death was massive cerebral infarction in 11 patients, fatal ICH in 12 patients, serious lung infection in 6 patients and fatal heart failure in 1 patient. Patients aged ≥80 years had a higher all-cause mortality rate compared to those at youngers (35.7% vs. 14.2%, P = 0.003). The rate of good revascularization in patients aged ≥80 years was significantly lower than that in those at younger age (mTICI 2b-3, 78.6% vs. 94.3%, P = 0.004). However, there was no remarkable difference in the proportion of ICH between these two groups (33.3% vs. 32.1%, respectively, P = 0.883) as shown in Table 3. Actually, 10.8% patients had symptomatic ICH in this study. However, the accurate proportion of asymptomatic intracranial hemorrhage was not clear, since not every patient returned to hospital for CT or MRI scan. What’s more, no serious adverse events (neurological deterioration, vascular events, or death) occurred in patients receiving OACs during the 90-day follow-up.
In a univariate analysis of patients aged ≥80 years, IVT prior to EVT tended to be associated with worse functional outcomes (OR: 3.48, 95% CI: 0.38–31.63). Shorter time intervals from onset of symptoms to treatment were numerically associated with better functional outcome (Table 5).
Table 5.
Univariate analysis of the association of unfavorable functional outcome (a Modified Rankin Scale score of 3-6) at 90 days with procedural parameters in patients aged ≥80 years
| Univariate | ||
|---|---|---|
| OR (95% CI) | P value | |
| Intravenous rt-PA use | 3.48 (0.38–31.63) | 0.268 |
| Time intervals (per hour) | ||
| Onset to door | 1.13 (0.83–1.53) | 0.442 |
| Onset to puncture | 1.14 (0.85–1.53) | 0.378 |
| Puncture to reperfusion | 1.52 (0.65–3.56) | 0.337 |
| Total procedure | 1.16 (0.88–1.54) | 0.298 |
rt-PA recombinant tissue-Plasminogen Activator, alteplase
Discussion
In this retrospective observational study, we found that age ≥ 80 years was an independent predictor for poor functional outcome at 90 days after EVT in LVO-related AIS patients with AF. In addition, older age might lead to unfavorable recanalization rates and higher all-cause mortality rate. This suggested that the optimization of selection criteria for such elderly patients with AF to undergo EVT was urgently needed to improve their prognosis.
Several single-center studies had shown that patients > 80 years old had worse mRS outcome (90-day mRS 3–6) and higher overall mortality [8, 14]. According to the recent DAWN study and MR CLEAN study, EVT was effective in patients > 80 years old [5, 15]. However, only 25 patients aged ≥80 years who had an NIHSS score of 10 or higher and < 21 mL of infarct volume on imaging were included in DAWN study. A ‘real world’ study found that baseline high NHISS score and the incidence of hemorrhage were the two independent predictors of poor outcome in the elderly patients [8]. However, to the best of our knowledge, the association of age with the prognosis of EVT in patients with AF was less clearly elucidated.
Patients with AF-related stroke always have a heavy thrombus burden, which can easily lead to the occlusion of large intracranial vessels and massive cerebral infarction [16]. AF has been confirmed as an independent predictor for a poor outcome for AIS patients [17]. AIS patients with AF were often at high risk of ICH after IVT, which is associated with larger territories of hypoperfusion and larger infarct volumes [18]. Although EVT is recommended for selected AIS patients, both the subgroup analysis of the MR CLEAN study [15] and a single-center observational study [19] found that compared with those without AF, AIS patients with AF receiving thrombectomy tended to have poor mRS scores at 90 days. In addition, increased risk of ICH after EVT in AIS patients with AF was also found in another study [20].
Elderly patients with AF have poorer blood vessel quality, which may lead to unsuccessful recanalization and ICH. The proportion of ICH after EVT in this study was about 32%, which was similar to the findings in the DIRECT-MT study (33.3%) and subgroup analysis of the BEST study (43.9% in AF group, 27% in non-AF group) [12, 20]. The ENDOSTROKE study emphasized that older age was related to a decrease in clinically successful recanalization in anterior circulation, particularly if over 80 years [21]. Shear force due to abnormal blood flow caused by persistent or permanent AF can damage the cerebrovascular endothelium and promote the formation of artery plaques, atherosclerosis, and even stenosis [22], which may increase the difficulty of recanalization and risk of ICH.
To explore whether the procedure-related parameters have an impact on 90-day prognosis in elderly patients with AF, we performed a subgroup analysis of 42 elderly patients. The time intervals were not meaningful predictors in the univariate analysis, which may be due to the small sample size of the present study. However, reducing time intervals in total procedure tended to improve the functional outcome. Compared to those with stroke due to cervical carotid atherosclerosis, patients with AF-related stroke have less extensive collateral circulation. Previous studies have shown that the degree of intracranial vascular stenosis is related to the establishment of collateral circulation, and severe vascular stenosis can promote extensive collateral circulation [23]. AF-related stroke occurs when a cardiogenic thrombus breaks off. Therefore, collateral circulation fails to establish timely in this circumstance. From a practical perspective, the data from the present study suggest that earlier recanalization is important in elderly patients.
The present study also showed that compared with direct thrombectomy, bridging therapy with intravenous alteplase use might increase the risk of poor functional outcome. As a matter of fact, the DIRECT-MT study have demonstrated that with regard to functional outcome, EVT alone was noninferior to EVT combined with prior intravenous alteplase administered within 4.5 h after symptom onset in acute ischemic stroke from LVO [12]. Therefore, direct thrombectomy may have more beneficial effect on functional outcome in elderly AIS patients with AF.
In addition to older age, the present study suggests that non-paroxysmal AF, higher baseline NIHSS score and higher level of baseline c-TnT was associated with poor clinical outcomes at 90 days after EVT in elderly AIS patients with AF. These variables have been identified as the risk factors predicting the poor outcomes after stroke in prior studies [7, 24–27]. In elderly patients with these risk factors, the benefit and risk of EVT needs to be taken into consideration before the decision to perform the procedure is made.
The use of anticoagulants can effectively prevent stroke in elderly patients with AF [11]. In the present study, few elderly people with AF were effectively anticoagulated before the onset of AIS. Considering the poor prognosis of thrombectomy in elderly AF patients, it is urgent to emphasis the importance of primary thromboprophylaxis using anticoagulants in this population.
This study has some limitations. First, it was a single-center retrospective analysis with small sample size. Second, only available preoperative and interventional data were analyzed, while many other variables including cardiac structural and functional parameters measured using 2-dimensional echocardiography, level of B-type natriuretic peptide (BNP) and some other serological indicators were not assessed. Therefore, further larger-scale studies are needed to clarify the treatment effect of EVT in elderly patients with AF.
Conclusions
Age ≥ 80 years was a significant predictor of unfavorable outcomes after EVT for AIS patients with AF. An increased risk of adverse events must be balanced against the benefit from EVT in elderly patients with AF. More efforts are needed to improve the use of anticoagulation in elderly AF patients for stroke prevention.
Acknowledgements
None.
Abbreviations
- EVT
Endovascular thrombectomy
- AF
Atrial fibrillation
- LVO
Large vessel occlusion
- IVT
Intravenous thrombolysis
- mTICI
modified thrombolysis in cerebral infarction scale
- mRS
Modified Rankin Scale
- ICH
Intracranial hemorrhage
- NIHSS
National Institutes of Health Stroke Scale
- NOAC
Novel oral anticoagulant
Authors’ contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
The Key Clinical Study Project of Jiangsu Province, China (ID: BE2017750).
Availability of data and materials
The datasets used and/or analyzed during the current investigation are available upon reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2014-SR-113). The patients/participants provided their written informed consent to participate in this study. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
All of the authors have approved the contents of this paper and have agreed to agree to BMC Neurology submission policies.
Competing interests
All authors listed have no conflict of interest, financial or otherwise.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jincheng Jiao and Sheng Liu contributed equally to this work.
Contributor Information
Minglong Chen, Email: chenminglong@njmu.edu.cn.
Mingfang Li, Email: mingfangli@njmu.edu.cn.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 2.Sweid A, Weinberg JH, Xu V, Shivashankar K, Alexander TD, Khalife J, et al. Mechanical Thrombectomy in acute ischemic stroke patients greater than 90 years of age: experience in 26 patients in a large tertiary care center and outcome comparison with younger patients. World Neurosurg. 2020;133:e835–ee41. doi: 10.1016/j.wneu.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GYH. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147(1):109–119. doi: 10.1378/chest.14-0321. [DOI] [PubMed] [Google Scholar]
- 4.Mokin M, Rojas H, Levy EI. Randomized trials of endovascular therapy for stroke--impact on stroke care. Nat Rev Neurol. 2016;12(2):86–94. doi: 10.1038/nrneurol.2015.240. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 8.Alawieh A, Chatterjee A, Feng W, Porto G, Vargas J, Kellogg R, et al. Thrombectomy for acute ischemic stroke in the elderly: a 'real world' experience. J Neurointerv Surg. 2018;10(12):1209–1217. doi: 10.1136/neurintsurg-2018-013787. [DOI] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 10.Hang Y, Jia ZY, Zhao LB, Cao YZ, Huang H, Shi HB, et al. Effect of "drip-and-ship" and "drip-and-drive" on endovascular treatment of acute ischemic stroke with large vessel occlusion: a single-center retrospective study. Acta Radiol. 2021;2841851211006897. 10.1177/02841851211006897. [DOI] [PubMed]
- 11.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular Thrombectomy with or without intravenous Alteplase in acute stroke. N Engl J Med. 2020;382(21):1981–1993. doi: 10.1056/NEJMoa2001123. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19(2):115–122. doi: 10.1016/S1474-4422(19)30395-3. [DOI] [PubMed] [Google Scholar]
- 14.Kleine JF, Boeckh-Behrens T, Prothmann S, Zimmer C, Liebig T. Discrepancy between early neurological course and mid-term outcome in older stroke patients after mechanical thrombectomy. J Neurointerv Surg. 2016;8(7):671–676. doi: 10.1136/neurintsurg-2015-011702. [DOI] [PubMed] [Google Scholar]
- 15.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 16.Freeman WD, Aguilar MI. Prevention of cardioembolic stroke. Neurotherapeutics. 2011;8(3):488–502. doi: 10.1007/s13311-011-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamel H, Healey JS. Cardioembolic stroke. Circ Res. 2017;120(3):514–526. doi: 10.1161/CIRCRESAHA.116.308407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbik F, Alawieh A, Cawley CM, Howard BM, Tong FC, Nahab F, et al. Differential effect of mechanical thrombectomy and intravenous thrombolysis in atrial fibrillation associated stroke. J Neurointerv Surg. 2021;13(10):883-8. [DOI] [PMC free article] [PubMed]
- 19.Giray S, Ozdemir O, Bas DF, Inanc Y, Arlier Z, Kocaturk O. Does stroke etiology play a role in predicting outcome of acute stroke patients who underwent endovascular treatment with stent retrievers? J Neurol Sci. 2017;372:104–109. doi: 10.1016/j.jns.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Huang K, Zha M, Gao J, Du J, Liu R, Liu X. Increased intracranial hemorrhage of mechanical thrombectomy in acute ischemic stroke patients with atrial fibrillation. J Thromb Thrombolysis. 2021;51(2):536–544. doi: 10.1007/s11239-020-02269-3. [DOI] [PubMed] [Google Scholar]
- 21.Singer OC, Haring HP, Trenkler J, Nolte CH, Bohner G, Reich A, et al. Age dependency of successful recanalization in anterior circulation stroke: the ENDOSTROKE study. Cerebrovasc Dis. 2013;36(5–6):437–445. doi: 10.1159/000356213. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Korantzopoulos P, Liu T. Carotid atherosclerosis in patients with atrial fibrillation. Curr Atheroscler Rep. 2019;21(12):55. doi: 10.1007/s11883-019-0808-4. [DOI] [PubMed] [Google Scholar]
- 23.Guglielmi V, LeCouffe NE, Zinkstok SM, Compagne KCJ, Eker R, Treurniet KM, et al. Collateral circulation and outcome in atherosclerotic versus Cardioembolic cerebral large vessel occlusion. Stroke. 2019;50(12):3360–3368. doi: 10.1161/STROKEAHA.119.026299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kornej J, Zeynalova S, Buttner P, Burkhardt R, Bae YJ, Willenberg A, et al. Differentiation of atrial fibrillation progression phenotypes using troponin T. Int J Cardiol. 2019;297:61–65. doi: 10.1016/j.ijcard.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Diederichsen SZ, Haugan KJ, Brandes A, Graff C, Krieger D, Kronborg C, et al. Incidence and predictors of atrial fibrillation episodes as detected by implantable loop recorder in patients at risk: from the LOOP study. Am Heart J. 2020;219:117–127. doi: 10.1016/j.ahj.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Healey JS, Amit G, Field TS. Atrial fibrillation and stroke: how much atrial fibrillation is enough to cause a stroke? Curr Opin Neurol. 2020;33(1):17–23. doi: 10.1097/WCO.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 27.Cao YZ, Zhao LB, Liu S, Liu QH, Jiang L, Zhou CG, et al. Prognostic value of elevated high-sensitivity cardiac troponin T levels in patients with acute ischemic stroke treated with endovascular thrombectomy. J Clin Neurosci. 2019;64:145–149. doi: 10.1016/j.jocn.2019.03.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current investigation are available upon reasonable request from the corresponding author.