Abstract
Investigations using noninvasive functional magnetic resonance imaging (fMRI) have provided significant insights into the unique functional organization and profound importance of the human default mode network (DMN), yet these methods are limited in their ability to resolve network dynamics across multiple timescales. Electrophysiological techniques are critical to address these challenges, yet few studies have explored the neurophysiological underpinnings of the DMN. Here we investigate the electrophysiological organization of the DMN in a common large-scale network framework consistent with prior fMRI studies. We used intracranial EEG (iEEG) recordings, and evaluated intra- and cross-network interactions during resting-state and its modulation during a cognitive task involving episodic memory formation. Our analysis revealed significantly greater intra-DMN phase iEEG synchronization in the slow-wave (< 4 Hz), while DMN interactions with other brain networks was higher in the beta (12–30 Hz) and gamma (30–80 Hz) bands. Crucially, slow-wave intra-DMN synchronization was observed in the task-free resting-state and during both verbal memory encoding and recall. Compared to resting-state, slow-wave intra-DMN phase synchronization was significantly higher during both memory encoding and recall. Slow-wave intra-DMN phase synchronization increased during successful memory retrieval, highlighting its behavioral relevance. Finally, analysis of nonlinear dynamic causal interactions revealed that the DMN is a causal outflow network during both memory encoding and recall. Our findings identify frequency specific neurophysiological signatures of the DMN which allow it to maintain stability and flexibility, intrinsically and during task-based cognition, provide novel insights into the electrophysiological foundations of the human DMN, and elucidate network mechanisms by which it supports cognition.
1. Introduction
The default mode network (DMN) is a large-scale distributed brain network which plays a critical role in cognition, including episodic memory formation and monitoring internal thoughts (Greicius MD et al. 2003; Buckner RL et al. 2008; Raichle ME 2015). DMN impairments are prominent in psychiatric disorders and this network is particularly sensitive to Alzheimer’s disease pathology and the ensuing loss of episodic memory and related cognitive functions (Greicius MD et al. 2004; Sheline YI et al. 2010; Staffaroni AM et al. 2018). In the past two decades, investigations using noninvasive functional magnetic resonance imaging (fMRI) techniques have provided significant insights into the unique functional organization of the human DMN. However, the electrophysiological basis and network properties of the DMN are poorly understood as fMRI does not have the requisite temporal resolution to address foundational questions in human systems neuroscience: what binds the DMN together as a network and segregates it from other large-scale brain networks? Here, we address this question using depth intracranial EEG (iEEG) recordings and investigate the neurophysiological foundations of the DMN, and its dynamic spectro-temporal properties in resting-state and during cognition.
The DMN consists of a distributed set of brain regions, including the posterior cingulate cortex, medial prefrontal cortex, angular gyrus, anterior prefrontal cortex, lateral temporal cortex and medial temporal lobe (MTL). Investigations of the electrophysiological properties of the DMN have been hampered by challenges of obtaining high-quality iEEG data from large-scale brain networks spanning widely distributed nodes across multiple lobes (Menon V et al. 1996; Freeman WJ et al. 2009; Parvizi J and S Kastner 2018). Furthermore, few electrophysiological investigations of the DMN have examined cortical recordings simultaneously with the MTL, an important constituent node of the DMN (Greicius MD et al. 2003). Here we overcome these limitations using depth iEEG recordings spanning cortical and MTL nodes that constitute the DMN. Critically, the extensive distribution of electrodes allowed us to probe the spectro-temporal organization of the DMN in relation to six other large-scale cortical networks that have been consistently identified using fMRI: dorsal attention, ventral attention, frontoparietal, visual, motor, and limbic (Yeo BT et al. 2011). This approach allowed us to address critical questions regarding the electrophysiological properties of the DMN in relation to fMRI-derived functional architectures of the human brain.
Previous iEEG studies focusing on individual nodes of the DMN have demonstrated suppression of the posterior cingulate cortex during mental arithmetic (Dastjerdi M et al. 2011; Daitch AL and J Parvizi 2018), and activation of the retrosplenial cortex during autobiographical memory retrieval (Foster BL et al. 2013). iEEG studies have also reported resting-state correlations in high-frequency band power fluctuations between the posterior cingulate cortex and angular gyrus (Foster BL et al. 2015), and between the posterior cingulate cortex and medial prefrontal cortex nodes of the DMN (Kucyi A et al. 2018). However, other studies have failed to find such an association (Hacker CD et al. 2017), likely reflecting the small sample sizes and limited electrode coverage in prior studies (Fox KCR et al. 2018). In other related research, resting-state correlations in the ultralow (<0.5 Hz) frequency have been reported in surface electrocorticogram recordings over somatosensory and motor cortex (He BJ et al. 2008), but it is not known whether such a frequency-specific network coupling also extends to the large-scale functional organization of the DMN. The limited electrode coverage in extant iEEG studies has made it impossible to examine connectivity across the distributed nodes that comprise the DMN and identify neurophysiological properties that segregate it from other large-scale brain networks, as identified using whole-brain fMRI.
A different line of research using scalp EEG recordings with concurrent fMRI has also probed the relation between fMRI activity and band-limited EEG fluctuations, but no consensus has emerged about the spectro-temporal organization of the DMN as the frequency-specificity of the correlations has been inconsistent across studies (Laufs H et al. 2003; Moosmann M et al. 2003; Mantini D et al. 2007; Scheeringa R et al. 2008; Jann K et al. 2009; Wu L et al. 2010; Yuan H et al. 2010). Crucially, the use of scalp EEG to characterize intra- and inter-network synchronization of the DMN is highly problematic due to volume conduction (Freeman WJ et al. 2009) and the inability of scalp EEG recordings to capture localized subcortical DMN regions such as the hippocampus.
Beyond the resting-state, little is known about how the large-scale intrinsic spectro-temporal organization and dynamic causal interactions of the DMN is modulated by cognition. Episodic memory is thought to be an important function associated with the DMN (Greicius MD et al. 2003; Greicius MD and V Menon 2004; Buckner RL et al. 2008; Kragel JE and SM Polyn 2015; Raichle ME 2015), and iEEG recordings from individual brain regions have reported theta-band activity in the hippocampus and parahippocampal gyrus during recall of verbal, temporal and spatial information from recently encoded memories (Watrous AJ et al. 2013; Jacobs J et al. 2016; Goyal A et al. 2018). However, the large-scale electrophysiological organization of the DMN and causal network dynamics during memory encoding and retrieval are not known and, crucially, no intracranial studies have probed how the intrinsic organization of the DMN and its interaction with other large-scale brain networks is altered by task-related episodic memory processes. More generally, there is growing evidence from fMRI studies that the DMN has a direct role in cognition across multiple cognitive domains, as revealed by task-related modulation of its posterior cingulate cortex, angular gyrus and middle temporal gyrus nodes (Greicius MD and V Menon 2004; Crittenden BM et al. 2015; Murphy C et al. 2018; Smith V et al. 2018; Sormaz M et al. 2018; Murphy C et al. 2019). A deeper understanding of the role of the DMN during cognition requires clarification of the electrophysiological mechanisms that support task-related network interactions in relation to its intrinsic spectro-temporal organization.
A systematic analysis using depth iEEG recordings spanning distributed nodes of the DMN, including the PCC, MTL, angular gyrus, and anterior temporal cortex, in parallel with other large-scale brain networks is needed to resolve these discrepancies and address fundamental questions regarding the integrity of this critical brain network (Hacker CD et al. 2017). To address this challenge, we used iEEG data from the UPENN-RAM study (Solomon EA et al. 2019) that included depth recordings from 102 participants and 12,780 electrodes, from which we selected 36 participants with 879 electrodes spanning seven fMRI-derived brain networks (Yeo BT et al. 2011). This allowed us to probe intra-DMN connectivity and contrast it with DMN interactions with other brain networks in a common large-scale network framework consistent with prior whole-brain non-invasive fMRI studies.
The first main goal of our study was to probe the intrinsic spectro-temporal organization of the human DMN during a task-free resting-state and determine frequency-specific instantaneous phase synchronization measures that capture linear as well as nonlinear intermittent and nonstationary dynamics observed in iEEG data (Menon V et al. 1996; Lachaux JP et al. 1999). We hypothesized that intra-DMN network interactions would be significantly stronger than inter-network interactions in the slow frequency range (< 4 Hz), based on the hypothesized role of the ultralow (< 0.5 Hz) and delta (0.5–4 Hz) frequency bands in large-scale network synchronization Buzsáki G (2000). The second major goal of our study was to extend our analysis and methodology to investigate DMN phase synchronization during an episodic memory task involving encoding and recall of a list of words. Investigations of intra- and cross-network interactions during verbal episodic memory is particularly relevant in the context of DMN function since the PCC, MTL, angular gyrus, and anterior temporal cortex have each been consistently implicated in verbal episodic and semantic memory (Binder JR and RH Desai 2011; Hasson U et al. 2015). The final goal of our study was to determine how DMN synchrony changes during memory formation, when compared to rest. We hypothesized that intra-DMN network synchronization would increase during memory encoding and recall, when compared to the resting-state, while preserving the overall low-frequency-dependent synchrony of the DMN. Our study provides novel, behaviorally and functionally relevant, insights into the neurophysiological foundations of the human DMN and its role in cognition.
2. Results
2.1. Slow-wave intra-DMN synchronization during resting-state
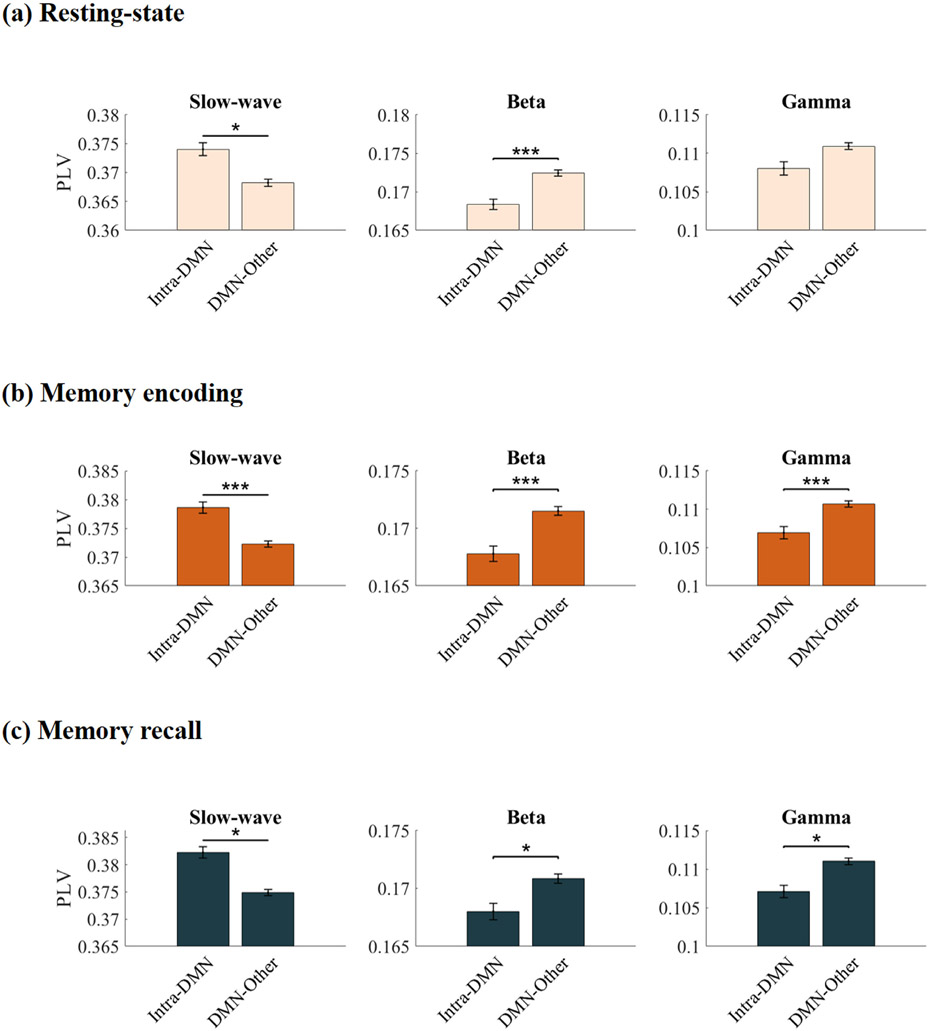
We first examined intrinsic intra-network synchronization of the DMN and contrasted it with cross-network synchronization with six other brain networks, identified using the fMRI-based cortical network atlas (Yeo BT et al. 2011) (Figs. 1a, S1, S2, Tables S1-S4; Methods). iEEG data across the non-DMN networks were combined to limit multiple comparison testing, and more directly address our specific hypotheses (but see Figs. S3-S11 for connectivity matrices between the seven brain networks corresponding to different frequency bands and task conditions). Analysis of instantaneous phase locking values (PLVs) revealed significantly greater intra-DMN phase synchronization, compared to DMN interactions with the other six networks, in the ultralow-delta band (< 4 Hz) (F(1, 7262) = 4.24, p < 0.05) (Fig. 2a). This pattern was reversed in the higher frequency beta band, with stronger cross-network, compared to intra-DMN, synchronization (F(1, 7002) = 20.60, p < 0.001), however intra-DMN phase synchronization did not differ from cross-network synchronization of the DMN in the gamma frequency band (F(1, 6946) = 0.01, p > 0.05) (Fig. 2a). Cross-network phase synchronization of the DMN was also higher than intra-DMN synchronization in the theta frequency band (F(1, 6764) = 43.18, p < 0.001). Intra-DMN phase synchronization did not differ from cross-network DMN synchronization in the alpha frequency band (F(1, 5934) = 0.32, p > 0.05). Direct statistical comparison revealed that intra-DMN phase synchronization in the ultralow-delta band was significantly higher compared to intra-DMN phase synchronization in the theta (F(1, 3210) = 1847.3, p < 0.001), alpha (F(1, 3214) = 1911.3, p < 0.001), beta (F(1, 3214) = 5981.8, p < 0.001), and gamma (F(1, 3212) = 5561.2, p < 0.001) bands. Note the gradual decrease in the PLV values from low to high frequency bands, consistent with the well-known 1/f spectral scaling in EEGs (He BJ et al. 2010).
Fig. 1.
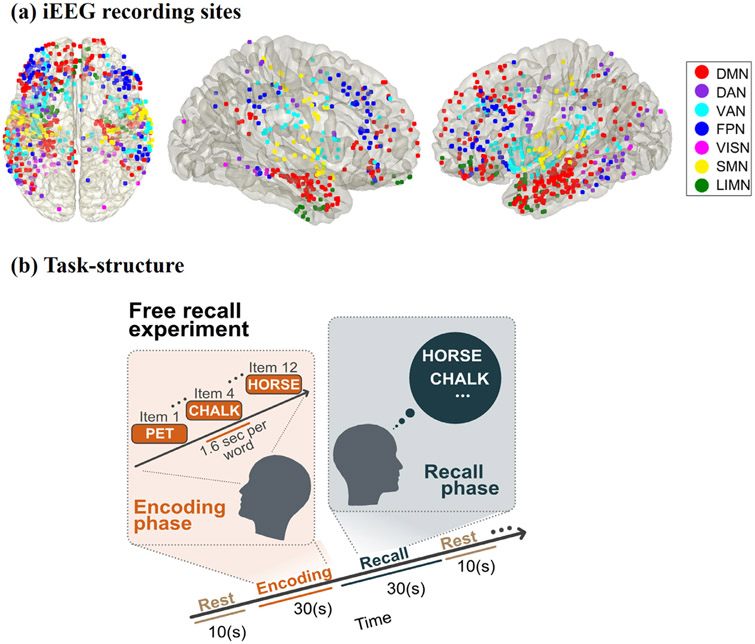
(a) iEEG recording sites for the 7 fMRI-derived brain networks investigated in this study. The Yeo cortical atlas was used to map the default mode (DMN), dorsal attention (DAN), ventral attention (VAN), frontoparietal (FPN), visual (VISN), somatomotor (SMN), and limbic (LIMN) networks. In addition to cortical areas, the DMN also included hippocampal regions determined using the Brainnetome atlas (Figs. S1, S2). (b) Cognitive task structure. Participants performed multiple trials of a “free recall” experiment, where they were first presented with a list of words and later asked to recall as many as possible from the original list (see Methods for details).
Fig. 2.
Intra-DMN phase synchronization during (a) Resting-state, (b) Memory encoding, and (c) Memory recall. In all three conditions, intra-DMN connectivity was characterized by synchronization in the slow-wave frequency band (< 4 Hz) while cross-network interaction of the DMN was dominated by higher frequencies. Intra-DMN denotes phase locking values (PLVs) between DMN electrodes and DMN-Other denotes PLV between DMN electrodes and electrodes in the 6 other brain networks. Data from each trial was analyzed separately and PLVs were averaged across trials for each condition (see Methods for more details). Error bars denote standard error of the mean (SEM) across all pairs of electrodes. *** p < 0.001, * p < 0.05 (FDR-corrected q < 0.05, two-way ANOVA).
These results demonstrate that intra-DMN connectivity is dominated by ultralow-delta (hereafter referred to as slow-wave) synchronization, and that cross-network interaction of the DMN is dominated by beta frequency, thus providing novel evidence for spectral segregation of the DMN in the resting-state.
2.2. Slow-wave intra-DMN synchronization during episodic memory formation
We then extended the above analysis to the memory encoding period of a verbal episodic memory task in which participants were presented with a sequence of words and asked to remember them for subsequent recall (Fig. 1b, Methods). Again, intra-DMN slow-wave phase synchronization was higher when compared to DMN interactions with the other 6 networks (F)1, 7317) = 59.36, p < 0.001) (Fig. 2b). The reverse was true in higher frequency bands: DMN electrodes had higher phase synchronization with electrodes outside the DMN than intra-DMN electrodes in the beta (F(1, 7290) = 18.27, p < 0.001) and gamma (F(1, 7303) = 42.31, p < 0.001) bands (Fig. 2b). DMN electrodes also had higher synchronization with electrodes outside the DMN than intra-DMN electrodes in theta (F(1, 7293) = 9.64, p < 0.01) and alpha (F(1, 7068) = 9.23, p < 0.01) frequency bands. Direct statistical comparison revealed that intra-DMN slow-wave phase synchronization was significantly higher compared to intra-DMN phase synchronization in the theta (F(1, 3211) = 3630.8, p < 0.001), alpha (F(1, 3211) = 3627.6, p < 0.001), beta (F(1, 3213) = 5969.4, p < 0.001), and gamma (F(1, 3211) = 5773, p < 0.001) bands.
Next, we examined phase synchronization during the recall phase of the verbal episodic memory task in which participants recalled the words they had seen during the memory encoding phase. Here again, intra-DMN slow-wave phase synchronization was higher compared to DMN interactions with the other 6 networks (F(1, 7241) = 5.15, p < 0.05) (Fig. 2c). The reverse was again true in higher frequency bands: DMN electrodes had higher phase synchronization with electrodes outside the DMN than intra-DMN electrodes in the beta (F(1, 7091) = 7.35, p < 0.05) and gamma (F(1, 7128) = 4.62, p < 0.05) bands (Fig. 2c). However, intra-DMN phase synchronization was higher than DMN synchronization with the other 6 networks in the theta frequency band (F(1, 6942) = 50.31, p < 0.001). Intra-DMN phase synchronization did not differ from cross-network DMN synchronization in the alpha frequency band (F(1, 6228) = 0.82, p > 0.05). Direct statistical comparison revealed that intra-DMN slow-wave phase synchronization was significantly higher compared to intra-DMN phase synchronization in the theta (F(1, 3214) = 2958, p < 0.001), alpha (F(1, 3212) = 2785.3, p < 0.001), beta (F(1, 3215) = 5801.5, p < 0.001), and gamma (F(1, 3210) = 5665.2, p < 0.001) bands.
These results demonstrate that intra-DMN connectivity is dominated by slow-wave synchronization, and that cross-network interaction of the DMN is dominated by beta and gamma frequencies, during both memory encoding and recall, thus providing evidence that spectral segregation of the DMN, observed intrinsically, is also maintained during memory processing.
2.3. Intra- and cross-network phase synchronization of the DMN sans hippocampus
Most human functional neuroimaging research on the DMN and fMRI-based atlases of brain networks have focused on its cortical nodes. We investigated the extent to which spectral segregation of the DMN was dependent on the hippocampus. We conducted additional analyses probing intra- and cross-network phase synchronization during resting-state, memory encoding, and memory recall conditions excluding hippocampus electrodes from the DMN. This analysis revealed that intra-DMN slow-wave phase synchronization was higher compared to DMN interactions with the other 6 networks in all three conditions (F(1, 5491) = 11.45 for resting-state, F(1, 5520) = 49.45 for memory encoding, and F(1, 5485) = 7.63 for memory recall, ps<0.001) (Figure S12). Cross-network interaction of the DMN was stronger than intra-DMN phase synchronization in the beta and gamma frequency bands during only memory encoding (F(1, 5503) = 5.72 for beta and F(1, 5520) = 13.25 for gamma, ps<0.05), however intra-DMN phase synchronization did not differ from cross-network interactions of the DMN with the other 6 networks in the beta and gamma frequency bands during resting-state (F(1, 5328) = 2.57 for beta and F(1, 5304) = 0.51 for gamma, ps>0.05) and memory recall (F(1, 5429) = 0.0004 for beta and F(1, 5437) = 2.94 for gamma, ps>0.05) periods.
These results suggest that only intra-network phase synchronization properties of the DMN hold independent of the hippocampus, however cross-network properties of the DMN are dependent on the hippocampus.
2.4. Slow-wave increases in intra-DMN phase synchronization during memory encoding and memory recall, compared to resting state
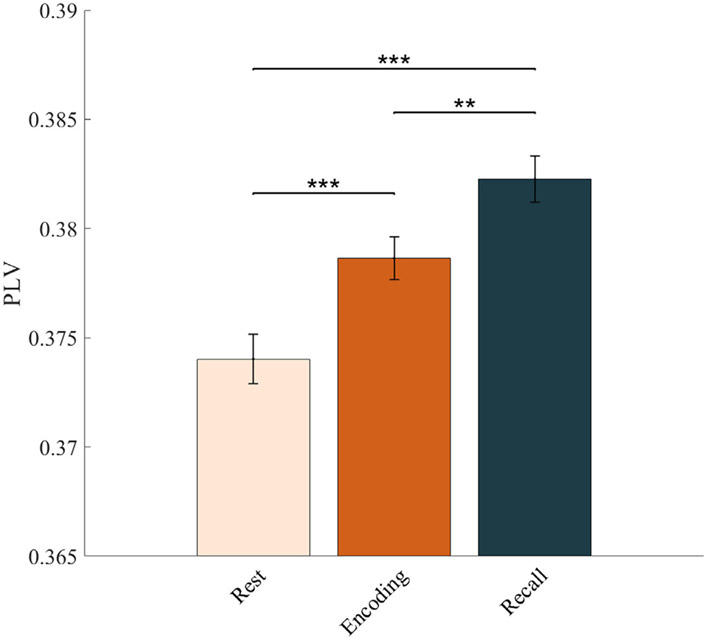
We next investigated intra-DMN synchronization changes during memory encoding and recall, compared to the resting-state. The duration of task and rest epochs were matched to ensure that differences in network dynamics could not be explained by the differences in the duration of the epochs (Methods). Our analysis revealed that intra-network slow-wave phase synchronization of the DMN during both the encoding and recall phases of the verbal episodic memory task was higher than that during rest (F(1, 3210) = 22.66 for encoding and F(1, 3209) = 46.88 for recall, ps<0.001) (Fig. 3). These results demonstrate slow-wave-dependent increases in phase synchronization within the DMN during both memory encoding and recall.
Fig. 3.
Intra-DMN phase synchronization during memory encoding and memory recall, compared to resting-state. Intra-DMN phase synchronization, assessed using phase locking values (PLVs), was higher during both memory encoding and recall compared to resting-state, in the slow-wave frequency band. Intra-DMN phase synchronization was also higher during memory recall compared to memory encoding. The duration of memory encoding and recall, and resting-state epochs were matched to preclude trial-length effects (see Methods for more details). Error bars denote standard error of the mean (SEM) across all pairs of electrodes. *** p < 0.001, ** p < 0.01 (FDR-corrected q < 0.05, two-way ANOVA).
2.5. Increased slow-wave intra-DMN phase synchronization associated with successful vs. unsuccessful memory recall
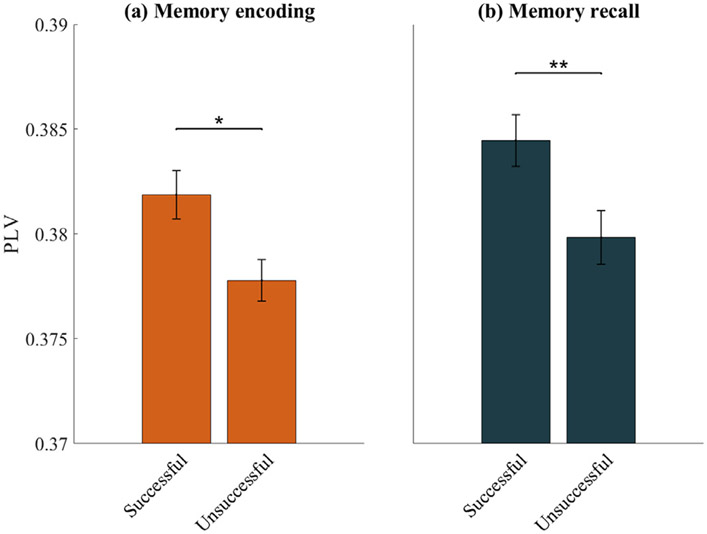
Next, we sought to investigate the behavioral significance of intra-DMN slow-wave phase synchronization. We examined whether intra-DMN phase synchronization differed between trials that were subsequently recalled correctly versus those that were not. During the encoding phase of the memory task, intra-DMN slow-wave phase synchronization was higher for successfully, versus unsuccessfully, recalled words (F(1, 3211) = 4.75, p < 0.05) (Fig. 4a). This finding was replicated in the memory recall phase of the task (F(1, 3211) = 12.02, p < 0.01) (Fig. 4b). These results suggest that spectral integrity of the DMN is associated with successful memory performance.
Fig. 4.
Intra-DMN phase synchronization during (a) successful vs. unsuccessful memory encoding and (b) successful vs. unsuccessful memory recall. Intra-DMN phase synchronization was higher during successful encoding/recall compared to unsuccessful encoding/recall in the slow-wave frequency band. Error bars denote standard error of the mean (SEM) across all pairs of electrodes. ** p < 0.01, * p < 0.05 (FDR-corrected q<0.05, two-way ANOVA).
2.6. Enhanced causal influences from the DMN to other networks during memory encoding and memory recall
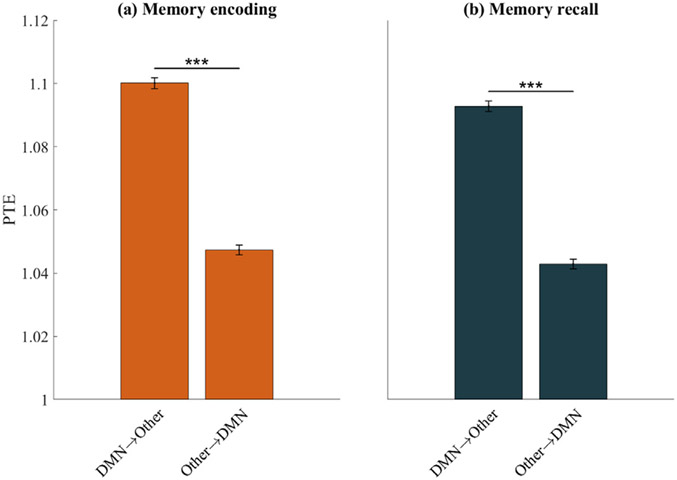
To investigate the role of dynamic causal interactions of the DMN in episodic memory task performance, we used phase transfer entropy (PTE) (Lobier M et al. 2014) to compute the net causal outflow from the DMN to the other six networks (see Methods for details). During both the memory encoding and recall phases, the DMN showed greater net causal outflow to other networks than the reverse (F(1, 11,383) = 781.59 for encoding and F(1, 11,421) = 684.75 for recall, ps<0.001) (Figs. 5a, b). These results demonstrate that the DMN plays an important causal role in interactions with other networks during episodic memory formation.
Fig. 5.
Causal network influences, measured using phase transfer entropy (PTE), during (a) Memory encoding and (b) Memory recall. The DMN showed significantly higher net causal outflow to the 6 other networks, than the reverse. DMN→Other denotes PTE from DMN electrodes to electrodes in the 6 other brain networks; Other→DMN denotes PTE from electrodes in the 6 other networks to the DMN. Error bars denote standard error of the mean (SEM) across all pairs of electrodes. *** p < 0.001 (FDR-corrected q < 0.05, two-way ANOVA).
Finally, comparison of causal outflow from the DMN to other networks across conditions revealed greater causal outflow during both memory encoding and recall compared to resting-state (F(1, 11,420) = 309.9 for encoding and F(1, 11,421) = 365.24 for recall, ps<0.001) (Fig. 6). Causal outflow from the DMN was greater during memory encoding compared to recall (F(1, 11,413) = 21.02, p<0.001) (Fig. 6). These results demonstrate task-specific increases in causal influence of the DMN on other brain networks.
Fig. 6.
Causal network influences of the DMN on other networks during resting-state, memory encoding, and memory recall. Causal outflow from the DMN was higher during both memory encoding and memory recall compared to resting-state. Causal outflow from the DMN was also higher during memory encoding compared to memory recall. The duration of memory encoding and recall, and resting-state epochs were matched to preclude trial-length effects (see Methods for more details). Error bars denote standard error of the mean (SEM) across all pairs of electrodes. *** p < 0.001 (FDR-corrected q<0.05, two-way ANOVA).
2.7. Surrogate data analysis of intra- and cross-network synchronization and causal dynamics of the DMN
We conducted surrogate data analysis to test the significance of the estimated PLV values compared to PLV expected by chance, for intra-DMN and cross-network interactions of the DMN with other networks (Methods). The estimated phases from the Hilbert transform for electrodes from pairs of brain areas were time-shuffled and PLV analysis was repeated on this shuffled data to build a distribution of surrogate PLV values against which the observed PLV was tested. This analysis revealed that phase synchronization for intra- and cross-network interactions of the DMN was significantly higher than those expected by chance in slow-wave, theta, alpha, beta, and gamma frequency bands and across resting-state, memory encoding, and memory recall conditions (p<0.05 in all cases) (Figs. S13, S14). These results demonstrate that all reported phase synchronization effects in this study arise from synchronization that is significantly enhanced above chance levels.
We repeated the surrogate data analysis to test the significance of the estimated PTE values compared to PTE expected by chance for dynamic causal interactions of the DMN with other networks (Methods). Similar to the surrogate analysis for PLV, the estimated phases from the Hilbert transform for electrodes from pairs of brain areas were time-shuffled and PTE analysis was repeated on this shuffled data to build a distribution of surrogate PTE values against which the observed PTE was tested. This analysis revealed that causal information flow from the DMN to the other 6 networks and the reverse were significantly higher than those expected by chance during resting-state, memory encoding, and memory recall conditions, indicating bidirectional causal information flow between the DMN and the 6 other networks (p<0.05 in all cases) (Fig. S15). These results demonstrate that all reported effects in this study arise from causal signaling that is significantly enhanced above chance levels.
2.8. Power spectral density of DMN and other networks during rest
To examine whether intra-DMN synchronization and DMN synchronization with other networks was driven by differences in the amplitude of iEEG fluctuations, we compared power spectral density (see Methods for details) in the DMN and the other 6 large-scale networks. This analysis revealed that power in the DMN was higher than the power in the other six networks in the slow-wave frequency band (p<0.05), however power in the DMN did not differ from power in the other six networks in beta and gamma frequency bands (ps>0.05). These results suggest that DMN synchronization with other networks is not driven by differences in the amplitude of iEEG fluctuations.
2.9. Power spectral density of DMN and other networks during memory processing
Similar to our analysis of resting-state iEEG, we compared power spectral density in the DMN and the other 6 networks during memory encoding, and separately during memory recall phases of the episodic memory task. This analysis revealed that power in the DMN was higher than the power in the other six networks in the slow-wave frequency band during memory encoding (p < 0.01), however power in the DMN did not differ from power in the other six networks in beta and gamma frequency bands during memory encoding (ps>0.05). Furthermore, power in the DMN was lower than the power in the other six networks in the slow-wave frequency band during memory recall (p < 0.01), however power in the DMN did not differ from power in the other six networks in beta and gamma frequency bands during memory recall (ps>0.05). Together, these results suggest that DMN synchronization with other networks is not driven by differences in the amplitude of iEEG fluctuations during memory processing.
2.10. Spectral power density during memory processing does not differ from rest
We next compared the power spectral density during memory encoding or recall with power spectral density during resting-state in the slow-wave (< 4 Hz) frequency band. As with previous analyses, we randomly selected epochs from the resting-state periods to match their duration to those from the memory encoding or recall periods, thereby ensuring that differences in network dynamics could not be explained by the differences in the duration of the epochs (Methods). For the DMN, power during memory encoding or recall did not differ from power during rest (ps>0.05). This indicates that the increased phase synchronization during memory encoding/recall is not driven by differences in the amplitude of iEEG fluctuations.
2.11. Spectral power density during successful memory encoding/recall does not differ from unsuccessful memory encoding/recall
We compared the power spectral density during successful memory encoding/recall with the power spectral density during unsuccessful memory encoding/recall in the slow-wave (< 4 Hz) frequency band for the DMN. This analysis revealed that power during successful memory encoding/recall did not differ from power during unsuccessful memory encoding/recall (ps>0.05). This indicates that the increased intra-DMN phase synchronization during successful encoding/recall compared to unsuccessful encoding/recall in the slow-wave frequency band is not driven by differences in the amplitude of iEEG fluctuations.
2.12. Intra- and cross-network correlation of the DMN in high-gamma band amplitude fluctuations
Previous studies have suggested that low-frequency fluctuations in the high-gamma band (80–160 Hz) are correlated with fMRI BOLD signals (Leopold DA et al. 2003; Mantini D et al. 2007; Schölvinck ML et al. 2010; Hutchison RM et al. 2015; Lakatos P et al. 2019). Our analysis (Methods) revealed that intra-DMN correlation in high-gamma band fluctuations was higher than that of cross-network correlations of the DMN with other networks during memory encoding. However, intra-DMN and cross-network correlations of the DMN with other networks in high-gamma band fluctuations did not differ from each other in the resting-state and memory recall conditions (ps>0.05).
2.13. Elevated slow-wave intra-DMN phase synchronization is not related to inter-electrode distance
We performed additional control analysis to rule distance out as a potential factor for the higher intra-DMN phase synchronization in the slow-wave frequency band. We calculated the Euclidean distance between all pairs of intra-DMN electrodes and for all pairs with one electrode in the DMN and the second in one of the six other networks. The distance of intra-DMN electrodes was, in fact, higher than the cross-DMN electrodes distance (p < 0.001) (Figure S16). To further rule out the possibility that low-frequency synchronization may arise from greater inter-electrode distance within the DMN, we examined the relation between PLV and distance in resting-state iEEG. We found no significant correlation between the two (r = 0.02, p > 0.34, Pearson) (Figure S17). These results suggest that the intra-DMN spectral integration in the slow-wave frequency band is a reflection of the strong instantaneous phase coupling among the intra-DMN brain regions as captured by the phase locking values.
2.14. Intra- vs. cross-network synchronization of the six other networks
Finally, we conducted additional control analyses of intra- vs. cross-network phase synchronization of the other 6 networks: dorsal attention, ventral attention, frontoparietal, visual, motor, and limbic (Figures S18-S23 respectively). No other network showed any consistent intra-network phase synchronization in the slow-wave frequency band and cross-network synchronization with other networks in the beta and gamma frequency bands, across the resting-state, memory encoding, and memory recall conditions (ps > 0.05). These control analyses suggest that the pattern of stronger intra-network phase synchronization in the slow-wave frequency band and increased cross-network synchronization in the beta and gamma frequency bands across resting-state, memory encoding, and memory recall conditions is not consistently observed in the other six large-scale brain networks (Table S5).
3. Discussion
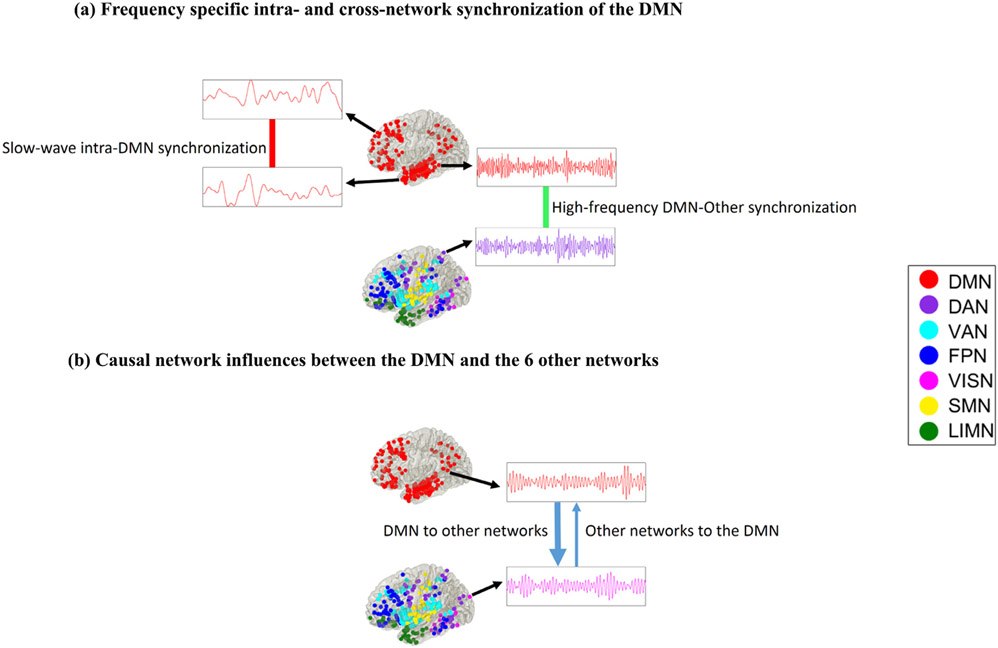
Using electrophysiological recordings spanning seven large-scale brain networks, we addressed the following key questions to probe DMN function in a common network framework with fMRI studies: (1) What foundational electrophysiological properties of the human DMN allow it to function as a coherent network? (2) What are the mechanisms underlying synchronization and causal interactions of the DMN with other networks during stimulus-driven cognition? To probe the spectro-temporal dynamics of the DMN, we examined phase locking and transfer entropy measures which are better suited to capturing nonlinear and nonstationary synchronization as well as causal dynamics associated with intra- and cross-network interactions (Menon V et al. 1996; Lachaux JP et al. 1999; Bruns A 2004; Sporns O et al. 2004; Lopour BA et al. 2013; Lobier M et al. 2014; Hillebrand A et al. 2016). Fig. 7 shows a schematic visualization of our main findings. We report three main findings: (1) Greater slow wave (<4 Hz) synchronization within the DMN compared to interactions between DMN and other networks. Interactions between DMN and other networks showed greater synchronization compared to intra-DMN connectivity in higher frequency bands (beta and gamma). These findings were observed across both rest and task conditions. Surrogate data analysis further revealed that phase synchronization for intra- and cross-network interactions of the DMN was significantly higher than those expected by chance in slow-wave, theta, alpha, beta, and gamma frequency bands and across resting-state, memory encoding, and memory recall conditions. (2) Slow-wave intra-DMN phase synchronization was higher during memory encoding and recall compared to rest. Within task conditions, slow wave phase synchronization within the DMN was also greater during encoding and recall for successful versus unsuccessful retrieval. (3) The DMN showed greater causal outflow to other networks relative to inflow. Our findings provide novel evidence for frequency-specific segregation and integration of the DMN from other brain networks during rest and its maintenance during episodic memory task. More generally, our findings advance knowledge of the neurophysiological foundations of the DMN, and clarify dynamic neural mechanisms underlying its role in task-based cognition.
Fig. 7.
Visualization of the main results reported in this study. (a) Intra-DMN connectivity was dominated by synchronization in the slow-wave frequency band (< 4 Hz) while cross-network interaction of the DMN was dominated by beta and gamma frequencies. Phase locking value (PLV) was used determine frequency-specific instantaneous phase synchronization that capture linear as well as nonlinear intermittent and nonstationary dynamics observed in iEEG data. (b) Causal network influences between the DMN and the 6 other networks was characterized by significantly higher net causal outflow from the DMN to the 6 other networks, than the reverse. Phase transfer entropy (PTE), which provides a robust and powerful measure for characterizing nonlinear information flow between brain regions based on phase coupling, was used to estimate causal network interactions. (See Results and Methods for details).
3.1. Intrinsic spectro-temporal network organization of the DMN
The first goal of our study was to characterize the intrinsic spectro-temporal organization of the DMN. Analysis of the spectral properties of the human DMN has been necessarily constrained by limited placement of iEEG electrodes, consequently previous work has mainly focused on activation and deactivation in isolated nodes of the network (Ossandon T et al. 2011; Lopour BA et al. 2013; Foster BL et al. 2015; Hacker CD et al. 2017; Daitch AL and J Parvizi 2018; Solomon EA et al. 2019). It is currently impossible to acquire iEEG data across the entire human brain with resolutions matching those of fMRI. This limitation motivated our approach of using the network atlas of Yeo et al. (Yeo BT et al. 2011), which allowed us to fill critical gaps in our knowledge of the neurophysiological underpinnings of the human DMN vis-a-vis the dorsal attention, ventral attention, frontoparietal, visual, motor, and limbic networks. Importantly, for the purposes of the present study, this 7-network atlas was coarse-grained enough to allow sampling of a large number of participants who had simultaneous iEEG recordings spanning the DMN as well as the six other cortical networks. Furthermore, such atlases have been widely used to investigate resting-state and task-based interactions using fMRI (Menon V 2015). Note that in contrast to previous iEEG studies (Dastjerdi M et al. 2011; Foster BL et al. 2015; Daitch AL et al. 2016; Daitch AL and J Parvizi 2018; Kucyi A et al. 2018; Raccah O et al. 2018; Kucyi A et al. 2020), we took an unbiased approach for assigning electrodes to individual brain networks and we did not select electrodes based on arbitrary task activation or deactivation profiles. Our approach thus allowed us to more directly probe the electrophysiological foundations of the DMN as identified in fMRI studies, in line with our study goals. Crucially, compared to subdural recordings, bipolar depth recordings in the UPENN-RAM dataset provide a more effective way to capture local iEEG signals (Ekstrom AD and AJ Watrous 2014; Jacobs J et al. 2016; Goyal A et al. 2018). These advances allowed us to probe intra- and cross-network dynamics of the DMN in a common framework with fMRI studies.
We evaluated inter-regional synchronization of iEEG time series using phase-locking values (PLVs), based on the notion that two connected brain areas generate signals whose instantaneous phases evolve together (Lopour BA et al. 2013; Lobier M et al. 2014). We contrasted intra- and cross-network phase synchronization of the DMN and found greater PLV among DMN electrodes in a combined slow-wave frequency band that encompasses the delta (0.5–4 Hz) and a lower ultralow (< 0.5 Hz) band. Control analysis revealed that this elevated slow-wave intra-DMN phase synchronization was not related to inter-electrode distance. As the length of each verbal memory trial was ~1.6 s (see Methods), which did not allow us to analyze the ultralow band separately, we combined these frequency bands into a single ultralow-delta (< 4 Hz) ‘slow-wave’ band (Dalal SS et al. 2011). Our findings are consistent with previous reports of synchronized delta frequency oscillations correlated with resting-state fMRI (Lu H et al. 2007), and with slow-wave resting-state correlations observed in electrocorticogram recordings over somatosensory and motor cortex (He BJ et al. 2008) and in iEEG recordings from the anterior insula and anterior cingulate cortex nodes of the salience network (Das A and V Menon 2020).
In contrast to slow-wave dominated iEEG synchronization within the DMN, we found that cross-network interactions of the DMN were dominated by the beta (12–30 Hz) frequency band. Based on these observations, we suggest that stronger intra-network phase synchronization in the slow-wave and stronger cross-network phase synchronization in the beta frequency band may enable the DMN to maintain a frequency-specific balance between stability and flexibility in its interactions with other large-scale brain networks.
3.2. Phase synchronization, rather than spectral power density, separates the DMN from other large-scale networks
Phase locking values as used in the present study provide a robust measure of instantaneous synchronization between electrode pairs (Menon V et al. 1996; Lachaux JP et al. 1999; Lopour BA et al. 2013; Lobier M et al. 2014). Previous findings using multielectrode array recordings in both humans and animal models have established that phase, rather than amplitude, is responsible for both spatial and temporal encoding of information in the brain (Lachaux JP et al. 1999; Kayser C et al. 2009; Siegel M et al. 2009; Lopour BA et al. 2013; Ng BS et al. 2013). In line with this computation, we found no differences in overall power between the DMN and any of the other six cortical networks in beta and gamma frequency bands. These results suggest that high frequency phase synchronization of the DMN with other networks is not driven by differences in the amplitude of iEEG fluctuations. Rather, the spectro-temporal segregation of the DMN is a reflection of frequency-specific instantaneous phase as accurately captured by PLV measures.
3.3. DMN phase synchronization and spectral integrity is preserved during memory encoding and recall
The next goal of our study was to investigate the spectral integrity of the DMN during task. Episodic memory is one of the putative functions ascribed to the DMN (Greicius MD et al. 2003; Greicius MD and V Menon 2004; Buckner RL et al. 2008). Previous studies and theoretical viewpoints have suggested that the DMN is integral for autobiographical memory, thinking about episodic events from the past, and planning the future (Buckner RL et al. 2008; Binder JR and RH Desai 2011). A related line of research has shown that the posterior cingulate cortex, angular gyrus, and anterior temporal lobe nodes of the DMN are also involved in semantic associations that modulate the formation of new episodic associations (Long NM and MJ Kahana 2017). Despite considerable evidence for the involvement of individual DMN nodes in memory formation and recall, to our knowledge, no studies have examined network-level neurophysiological organization of the DMN and its interaction with other large-scale brain networks during memory processing.
To address this, we first examined frequency-specific iEEG phase synchronization during a verbal episodic memory task in which participants had to subsequently recall a list of visually-presented words (Solomon EA et al. 2019). We found that intra-DMN slow-wave phase synchronization was significantly greater than DMN interactions with the six other large-scale networks. In contrast, the reverse was true with respect to cross-network interactions of the DMN, which showed greater synchronization of the DMN in the beta and gamma bands with other networks when compared to intra-DMN synchronization. We repeated this analysis with iEEG acquired during recall of the previously encoded words and uncovered the same pattern of frequency-specific synchronization: greater intra-DMN slow-wave synchronization and greater cross-network synchronization in higher frequencies (beta and gamma).
Taken together, findings demonstrate that intra-DMN connectivity is dominated by slow-wave synchronization during both memory encoding and recall. Task-related phase locking patterns thus recapitulate and extend features observed with resting-state iEEG and points to a pattern of DMN organization that is stable under perturbations induced by episodic memory. In sum, our analysis demonstrates that the frequency-specific spectral integrity of the DMN and its segregation from other large-scale networks is at least partly maintained during task. We suggest that a unique combination of frequency-specific synchronization and desynchronization may facilitate formation and break-up of functionally relevant neuronal assemblies associated with the DMN (Ahn S and LL Rubchinsky 2013).
3.4. DMN phase synchronization increases during memory formation, compared to rest, and is behaviorally relevant
The final goal of our study was to determine how DMN synchrony changes during episodic memory encoding and recall, when compared to rest. To address this, we compared slow-wave intra-DMN synchronization during memory processing and the resting-state using PLVs. We found that intra-DMN slow-wave synchronization was significantly higher during both memory encoding and recall, compared to the resting-state. Crucially, these increases were not related to overall iEEG amplitude, as spectral power during both memory tasks did not differ from rest. This provides novel electrophysiological evidence that the DMN plays a critical role in episodic memory formation. Consistent with this view, analysis of phase transfer entropy revealed enhanced causal interactions from DMN to other large-scale networks during both memory encoding and recall.
The lack of concurrent recordings spanning multiple brain areas has limited our ability to characterize frequency-specific synchronization associated with large-scale brain networks such as the DMN. Previous investigators focusing on regional response during verbal memory recall have reported increased iEEG activity in multiple frequency bands along with increased synchronization within the MTL (Long NM and MJ Kahana 2017; Solomon EA et al. 2019). Greater synchronization has also been reported between the hippocampus and multiple lateral prefrontal cortex areas during spatial memory retrieval (Watrous AJ et al. 2013; Ekstrom AD and AJ Watrous 2014; Neuner I et al. 2014) and between the lateral temporal and fronto-parietal cortices during inward directed attention (Kam JWY et al. 2019). Extending these findings specifically to the DMN organization at a network-level, we found greater phase synchronization during both the encoding and retrieval phases of the verbal episodic memory task when compared to the resting-state.
Crucially, memory-related increase in DMN phase synchronization was behaviorally relevant. Phase synchronization was higher for words that were subsequently recalled correctly, and these increases were observed during both the memory encoding and recall phases of the task. Importantly, increased intra-DMN phase synchronization during successful encoding/recall compared to unsuccessful encoding/recall was not driven by differences in the amplitude of iEEG fluctuations. Memory success-related changes were observed only in the slow-wave spectral range, consistent with a previous report of frequency-specific network connectivity during spatial memory retrieval (Watrous AJ et al. 2013). Our findings are also consistent with reports of higher phase synchronization in the delta band associated with successful memory retrieval (Jacobs J et al. 2007; Fell J et al. 2008) and memory-related teleportation (Vass LK et al. 2016), although these recordings were solely confined to the hippocampus. In an advance over prior studies, frequency-specific increases in our analysis are based on iEEG recordings from the major cortical and MTL regions of the entire DMN providing novel evidence of network level signatures of successful memory retrieval in the human DMN. Findings support the view that network-wide synchronization is a key feature of memory formation and emphasize the role of the DMN in this process (Hebscher M and JL Voss 2020).
3.5. Causal dynamics and directionality of information flow from the DMN
Finally, we examined the directionality of information flow between the DMN and other networks as regions whose activities are not instantaneously synchronized may interact via time-delayed causal influences. Granger causal analyses of fMRI time series suggest that multiple DMN nodes, including the posterior cingulate cortex and the ventromedial prefrontal cortex may exert influence on lateral frontoparietal cortex (Uddin LQ et al. 2009) rather than the other way around. However, it is unclear whether these results are an artifact of regional variations in hemodynamic response in fMRI which confound Granger causal measures, or whether they truly reflect an underlying neuronal process. Critically, previous iEEG studies have not examined causal interaction of the DMN with other large-scale brain networks during cognition. To address this question, we used phase transfer entropy (PTE), which provides a robust and powerful measure for characterizing information flow between brain regions based on phase coupling (Lobier M et al. 2014; Hillebrand A et al. 2016; Wang MY et al. 2017). PTE has several advantages over Granger causal analysis (Barnett L and AK Seth 2011) and transfer entropy (Schreiber T 2000; Lobier M et al. 2014), as it can capture nonlinear interactions, is more accurate than transfer entropy, and it estimates causal interactions based on phase, rather than amplitude, coupling. Taking advantage of the 1000 Hz sampling rate of the UPENN-RAM cohort iEEG data, we examined causal outflow from the DMN to the other six networks and contrasted it with inflow into the DMN from the other networks.
Our analysis revealed that the DMN showed significantly greater net causal outflow to other networks, rather than the reverse, during both memory encoding and recall. Surrogate data analysis further revealed that causal information flow from the DMN to the six other networks and the reverse were significantly higher than those expected by chance during resting-state, memory encoding, and memory recall conditions, indicating bidirectional causal information flow between the DMN and the other networks. Our findings are consistent with and extend findings based on transcranial magnetic stimulation suggesting a causal role of key DMN nodes during episodic memory formation (Warren KN et al. 2019). Our causal analysis also clarifies the question previously raised in fMRI studies hinting that the DMN exerts causal influences in the resting-state (Uddin LQ et al. 2009). We found that the DMN exerts strong causal influences on other brain networks intrinsically and causal outflow from the DMN to the other networks was greater during both memory encoding and recall compared to the resting-state. Together, these findings suggest that causal influences of the DMN may not be specific to task-induced memory encoding and recall processes, and that they may also underlie internal mental processes such as recall of autobiographical information and self-monitoring as hypothesized previously (Greicius MD et al. 2003; Greicius MD and V Menon 2004; Buckner RL et al. 2008; Raichle ME 2015). This pattern of causal network interactions may enable the DMN to broadcast signals to other networks and thereby function at the apex of a neural and cognitive representational hierarchy (Margulies DS et al. 2016).
3.6. Limitations
Two of the key nodes of the DMN, the PCC/precuneus and the ventromedial PFC had limited sampling (Fig. 1a). These are sites where electrodes are not typically implanted in patients undergoing surgery for intractable epilepsy. Another limitation is that the 7-network atlas that we used to demarcate brain networks may be too coarse grained. Further research with denser sampling of electrodes in multiple cortical and subcortical regions, and a wider range of experimental tasks, are necessary to further probe the electrophysiological properties of the DMN and its interactions with other brain networks in internal and stimulus-driven cognition.
3.7. Conclusions
Our study advances foundational knowledge of the neural mechanisms underlying the large-scale functional organization of the human DMN. Findings provide new insights into the neurophysiological basis and intrinsic spectro-temporal organization of the DMN as well as its integrity and cross-network dynamic interactions during episodic memory formation. Findings further demonstrate that the DMN supports external stimulus-driven cognition, and not just internal mental processes (Sormaz M et al. 2018; Turnbull et al., 2019; Turnbull A, HT Wang, JW Schooler, et al. 2019). More generally, our study provides a deeper understanding of the electrophysiological mechanisms underlying operation of the DMN during cognition and its behavioral relevance. Extensions of this work with denser iEEG recordings and more fine-grained brain parcellations will further help advance knowledge of its operating mechanisms and inform the neural basis of DMN function, aberrations in which impact almost all psychiatric and neurological disorders including Alzheimer’s disease, schizophrenia, epilepsy, anxiety, depression, autism, and ADHD Menon V (2011).
4. Methods
4.1. UPENN-RAM iEEG recordings
We examined iEEG recordings from 102 patients shared by Kahana and colleagues at the University of Pennsylvania (UPENN) (obtained from the UPENN-RAM public data release under release ID “Release_20171012”, released on 12 October, 2017) (Jacobs J et al. 2016).
Patients with pharmaco-resistant epilepsy underwent surgery for removal of their seizure onset zones. iEEG recordings of these patients were downloaded from a UPENN-RAM consortium hosted data sharing archive (http://memory.psych.upenn.edu/RAM). Prior to data collection, research protocols and ethical guidelines were approved by the Institutional Review Board at the participating hospitals and informed consent was obtained from the participants and guardians (Jacobs J et al. 2016). Details of all the recordings sessions and data preprocessing procedures are described by Kahana and colleagues (Jacobs J et al. 2016). Briefly, iEEG recordings were obtained using subdural grids and strips (contacts placed 10 mm apart) or depth electrodes (contacts spaced 5–10 mm apart) using recording systems at each clinical site. iEEG systems included DeltaMed XlTek (Natus), Grass Telefactor, and Nihon-Kohden EEG systems. Electrodes located in brain lesions or those which corresponded to seizure onset zones or had significant interictal spiking or had broken leads, were excluded from analysis (Ezzyat Y et al. 2018).
Anatomical localization of electrode placement was accomplished by co-registering the postoperative computed CTs with the postoperative MRIs using FSL (FMRIB (Functional MRI of the Brain) Software Library), BET (Brain Extraction Tool), and FLIRT (FMRIB Linear Image Registration Tool) software packages. Preoperative MRIs were used when postoperative MRIs were not available. The resulting contact locations were mapped to MNI space using an indirect stereotactic technique and OsiriX Imaging Software DICOM viewer package. We used the Yeo cortical atlas (Yeo BT et al. 2011) in volumetric space (MNI volumetric coordinates) for mapping electrodes to the default mode, dorsal attention, ventral attention, frontoparietal, visual, motor, and limbic networks. We used the Brainnetome atlas (Fan L et al. 2016) to demarcate the hippocampus and incorporated electrodes from this region into the DMN, as the hippocampus is an important constituent subcortical node of the DMN (Greicius MD et al. 2003). Out of 102 individuals and 12,780 electrodes, data from 36 individuals and 879 electrodes were used for subsequent analysis based on electrode placement in these networks of interest. For intra-DMN connectivity analysis, at least one pair of electrodes needed to be available per patient for the given pair of DMN nodes (for example, hippocampus-PCC/precuneus). In the DMN, the range was 2–338, mean was 60. Similarly, for inter-DMN connectivity analysis, at least one pair of electrodes needed to be available per patient for the given pair of brain networks (for example, DMN-DAN). For inter-DMN, the range was 1–240, mean was 46. In addition, we required that data be available from at least 5 participants involving each pair of brain regions (Table S3). iEEG signals were sampled at 1000 Hz. The two major concerns when analyzing interactions between closely spaced intracranial electrodes are volume conduction and confounding interactions with the reference electrode (Burke JF et al. 2013). Hence bipolar referencing was used to eliminate confounding artifacts and improve the signal-to-noise ratio of the neural signals, consistent with previous studies using UPENN-RAM iEEG data (Burke JF et al. 2013; Ezzyat Y et al. 2018). Signals recorded at individual electrodes were converted to a bipolar montage by computing the difference in signal between adjacent electrode pairs on each strip, grid, and depth electrode and the resulting bipolar signals were treated as new virtual electrodes originating from the midpoint between each contact pair. Among the electrodes in the DMN, ~81% were depth electrodes, ~10% were grid electrodes, and ~9% were strip electrodes. For the non-DMN electrodes, these numbers were ~71%, ~17%, and ~12% respectively, indicating that the distributions of the type of electrodes in the DMN and non-DMN brain regions were similar. Line noise (60 Hz) and its harmonics were removed from the bipolar signals and finally each bipolar signal was Z-normalized by removing mean and scaling by the standard deviation. A fourth order two-way zero phase lag Butterworth filter was used in all spectral analyses.
4.2. iEEG verbal memory encoding and recall, and resting-state conditions
Patients performed multiple trials of a free recall experiment, where they were presented with a list of words and subsequently asked to recall as many as possible from the original list (Fig. 1) (Solomon EA et al. 2017; Solomon EA et al. 2019). The task consisted of three periods: encoding, delay, and recall. During encoding, a list of 12 words was visually presented for ~30 sec. Words were selected at random, without replacement, from a pool of high frequency English nouns (http://memory.psych.upenn.edu/Word_Pools). Each word was presented for a duration of 1600 msec, followed by an inter-stimulus interval of 800 to 1200 msec. After a 20 sec post-encoding delay, participants were instructed to recall as many words as possible during the 30 sec recall period. We analyzed iEEG epochs from the encoding and recall periods of the free recall task. For the recall periods, iEEG recordings 1600 msec prior to the vocal onset of each word were analyzed (Solomon EA et al. 2019).
Resting-state epochs consisted of intervals prior to each encoding block in which participants were asked to fixate on the screen and alerted to the upcoming task. This choice of resting-state epochs is consistent with previous iEEG and fMRI studies (Garrison KA et al. 2015; Kamp T et al. 2018; Smith V et al. 2018; Betzel RF et al. 2019; Das A and V Menon 2020). Moreover, in a previous study we showed that these epochs accurately reproduced findings from conventional resting-state iEEG data acquired from an independent cohort (Das A and V Menon 2020). For the resting-state epochs, we extracted 10-second iEEG recordings (epochs) prior to the beginning of each encoding block. To reduce boundary and carry over effects, we discarded 3 seconds each of iEEG data from the beginning and end of each epoch, resulting in multiple 4 s epochs. Data from each epoch was analyzed separately and specific measures were averaged across trials. For comparison of memory encoding and recall with the resting-state (Figs. 3 and 6), the duration of memory encoding and recall, and resting-state epochs were matched to preclude trial-length effects.
4.3. iEEG analysis of intra- and cross-network phase synchronization
Intra-network phase synchronization was assessed between all electrode pairs across distinct brain regions within the network. To minimize potential bias from sampling of electrodes with a specific brain region, we did not include electrode pairs within individual brain regions which were determined using the Brainnetome atlas (Fan L et al. 2016) as noted above. For example, for intra-DMN analysis, we computed phase synchronization between the PCC/precuneus and hippocampus electrodes but not between electrodes within the PCC/precuneus or the hippocampus. Cross-network phase synchronization was assessed between all electrode pairs across networks.
We used phase locking value (PLV) to compute phase synchronization between two time-series (Lachaux JP et al. 1999). We first calculated the instantaneous phases of the two signals by using the analytical signal approach based on the Hilbert transform Bruns A (2004). Given time-series x(t), t = 1, 2, … M, its complex-valued analytical signal z(t) can be computed as
| (1) |
where i denotes the square root of minus one, is the Hilbert transform of x(t), and Ax(t) and Φx(t) are the instantaneous amplitude and instantaneous phase respectively and can be given by
| (2) |
The Hilbert transform of x(t) was computed as
| (3) |
where PV denotes the Cauchy principal value. MATLAB function “hilbert” was used to calculate the Hilbert transform in our analysis. Given two time-series x(t) and y(t), where t = 1, 2, …, M, the PLV (zero-lag) can be computed as
| (4) |
where Φy(t) is the instantaneous phase for time-series y(t), ∣ · ∣ denotes the absolute value operator, E[·] denotes the expectation operator with respect to time t, and idenotes the square root of minus one. PLVs were then averaged across trials to estimate the final PLV for each pair of electrodes.
4.4. iEEG analysis of power spectral density
To calculate average power, we first filtered the iEEG time-series in the frequency band of interest and power, after removing the linear trend, was calculated as the sum of the squares of the amplitudes of the iEEG time-series divided by the length of the time-series.
4.5. Correlation analysis of high-gamma band amplitude fluctuations
We investigated whether intra-DMN interactions showed a signature in the high-gamma band (80–160 Hz) amplitude fluctuations. iEEG signals were filtered between 80–90, 90–100, …, 150–160 Hz and the amplitude of each narrowband signal was calculated by taking the absolute value of the analytic signal obtained from the Hilbert transform (Anderson KL et al. 2010; Foster BL et al. 2015). Each narrowband amplitude was then normalized with respect to the mean amplitude, i.e., expressed as fraction of the mean, to correct for 1/f decay. Normalized amplitude time-series from each band were then filtered in the slow-wave (< 4 Hz) band to generate a time series reflecting high-gamma band fluctuations in each electrode. The resulting time series was used to investigate intra- and inter-DMN interactions using Pearson correlations.
4.6. iEEG analysis of phase transfer entropy (PTE) and causal dynamics
Causal interactions between networks was assessed using phase transfer entropy (PTE) between all electrode pairs across networks. PTE is a nonlinear measure of the directionality of information flow between time-series (Lobier M et al. 2014). Given two time-series {xi} and {yi}, where i = 1, 2, …, M, instantaneous phases were first extracted using the Hilbert transform. Let and , where i = 1, 2, …, M, denote the corresponding phase time-series. If the uncertainty of the target signal at delay τ is quantified using Shannon entropy, then the PTE from driver signal to target signal can be given by
| (5) |
where the probabilities can be calculated by building histograms of occurences of singles, pairs, or triplets of instantaneous phase estimates from the phase time-series (Hillebrand A et al. 2016). For our analysis, the number of bins in the histograms was set as 3.49 × ST D × M−1/3 and delay τ was set as 2M / M±, where ST D is average standard deviation of the phase time-series and and M± is the number of times the phase changes sign across time and channels (Hillebrand A et al. 2016). Note that PTE is robust against the choice of the delayτ and the number of bins for forming the histograms and variations in these parameters do not change the results much (Hillebrand A et al. 2016). For PTE estimation, we used the broadband signal rather than the filtered signal, since causality estimation is very sensitive to filtering (see (Barnett L and AK Seth 2011) for a detailed discussion on this).
4.7. Statistical analysis
Statistical analysis of intra- and cross-network interactions was conducted using mixed effects analysis with the lmerTest package (Kuznetsova A et al. 2017) implemented in R software (version 4.0.2, R Foundation for Statistical Computing). Mixed effects models are now the recommended procedure for iEEG studies (Das A and V Menon 2021; Hoy CW et al. 2021;Salamone PC et al. 2021). Because PLV data were not normally distributed, we used BestNormalize (Peterson RA and JE Cavanaugh 2018) which contains a suite of transformation-estimating functions that can be used to optimally normalize data. The resulting normally distributed data were subjected to mixed effects analysis with the following model: PLV ~ Condition + (1∣Subject), where Condition models the fixed effects (condition differences) and (1∣Subject) models the random repeated measurements within the same participant. Analysis of variance (ANOVA) was used to test the significance of findings with FDR-corrections for multiple comparisons (p < 0.05). For intra- and cross-network comparisons of phase synchronization of DMN for different frequency and task conditions, p-values were corrected for 15 comparisons. Similarly, for comparison of PLV/PTE for task vs. rest conditions, p-values were corrected for 3 comparisons. For phase synchronization comparison related to successful vs. unsuccessful memory encoding and recall, p-values were corrected for 2 comparisons. For comparison of PTE for DMN→Other and Other→DMN interactions, p-values were corrected for 2 comparisons. Similar mixed effects statistical analysis procedures were used for analysis of PTE and power spectral density across task conditions.
Finally, we conducted surrogate analysis to test the significance of the estimated PLV values (Hillebrand A et al. 2016). The estimated phases from the Hilbert transform for electrodes from a given pair of brain areas were time-shuffled so that the synchronization between the two time-series is destroyed, and PLV analysis was repeated on this shuffled data to build a distribution of surrogate PLV values against which the observed PLV was tested (p < 0.05). Similar surrogate analysis was conducted to test the significance of the estimated PTE values. The estimated phases from the Hilbert transform for electrodes from a given pair of brain areas were time-shuffled so that the predictability of one time-series from another is destroyed, and PTE analysis was repeated on this shuffled data to build a distribution of surrogate PTE values against which the observed PTE was tested (p<0.05).
Supplementary Material
Acknowledgements
We are grateful to members of the UPENN-RAM consortia for generously sharing their unique iEEG data. We thank Drs. Paul A. Wanda, Michael V. DePalatis, Youssef Ezzyat, Richard Betzel, and Leon A. Davis for assistance with the UPENN-RAM dataset. We thank Dr. Byeongwook Lee for assistance with the figures. We thank Drs. Matteo Fraschini and Arjan Hillebrand for generously sharing their MATLAB code for phase transfer entropy analysis. We acknowledge the computational resources and support provided by the Stanford Research Computing Center. This research was supported by NIH grants NS086085 and EB022907.
Footnotes
Data and code availability statement
iEEG recordings used in this study can be downloaded from a UPENN-RAM consortium hosted data sharing archive (http://memory.psych.upenn.edu/RAM). MATLAB codes used in this study are available through our lab GitHub (https://github.com/scsnl/Das_NeuroImage_2022).
Credit_author_statement
Anup Das: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Roles/Writing - original draft; Writing - review & editing.
Carlo de los Angeles: Methodology; Resources; Software; Visualization.
Vinod Menon: Conceptualization; Funding acquisition; Investigation; Project administration; Supervision; Roles/Writing - original draft; Writing - review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2022.118927.
References
- Ahn S, Rubchinsky LL, 2013. Short desynchronization episodes prevail in synchronous dynamics of human brain rhythms. Chaos 23, 013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M, 2010. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex 20, 1604–1612. [DOI] [PubMed] [Google Scholar]
- Barnett L, Seth AK, 2011. Behaviour of Granger causality under filtering: Theoretical invariance and practical application. J. Neurosci. Methods 201, 404–419. [DOI] [PubMed] [Google Scholar]
- Betzel RF, Medaglia JD, Kahn AE, Soffer J, Schonhaut DR, Bassett DS., 2019. Structural, Geometric and Genetic Factors Predict Interregional Brain Connectivity Patterns Probed By Electrocorticography. Nat Biomed Eng. 3 (11), 902–916. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH., 2011. The neurobiology of semantic memory. Trends Cogn. Sci 15, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns A, 2004. Fourier-, Hilbert- and wavelet-based signal analysis: are they really different approaches? J. Neurosci. Methods 137, 321–332. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL., 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann. N Y Acad. Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Burke JF, Zaghloul KA, Jacobs J, Williams RB, Sperling MR, Sharan AD, Kahana MJ., 2013. Synchronous and asynchronous theta and gamma activity during episodic memory formation. J. Neurosci 33, 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, 2000. Rhythms of the Brain. Oxford University Press, New York. [Google Scholar]
- Crittenden BM, Mitchell DJ, Duncan J, 2015. Recruitment of the default mode network during a demanding act of executive control. Elife 4, e06481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch AL, Foster BL, Schrouff J, Rangarajan V, Kaşikçi I, Gattas S, Parvizi J., 2016. Mapping human temporal and parietal neuronal population activity and functional coupling during mathematical cognition. Proc. Natl. Acad. Sci. USA 113, E7277–e7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch AL, Parvizi J, 2018. Spatial and temporal heterogeneity of neural responses in human posteromedial cortex. Proc Natl Acad Sci U S A. 115, 4785–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Vidal JR, Hamame CM, Ossandon T, Bertrand O, Lachaux JP, Jerbi K, 2011. Spanning the rich spectrum of the human brain: slow waves to gamma and beyond. Brain Struct. Funct 216, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Menon V, 2020. Spatiotemporal integrity and spontaneous nonlinear dynamic properties of the salience network revealed by human intracranial electrophysiology: a multicohort replication. Cereb Cortex 30 (10), 5309–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Menon V, 2021. Asymmetric frequency-specific feedforward and feedback information flow between hippocampus and prefrontal cortex during verbal memory encoding and recall. J. Neurosci 41 (40), 8427–8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi M, Foster BL, Nasrullah S, Rauschecker AM, Dougherty RF, Townsend JD, Chang C, Greicius MD, Menon V, Kennedy DP, Parvizi J, 2011. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc. Natl. Acad. Sci 108, 3023–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Watrous AJ., 2014. Multifaceted roles for low-frequency oscillations in bottom-up and top-down processing during navigation and memory. Neuroimage. 85 Pt 2, 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzyat Y, Wanda PA, Levy DF, Kadel A, Aka A, Pedisich I, Sperling MR, Sharan AD, Lega BC, Burks A, Gross RE, Inman CS, Jobst BC, Gorenstein MA, Davis KA, Worrell GA, Kucewicz MT, Stein JM, Gorniak R, Das SR, Rizzuto DS, Kahana MJ., 2018. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat. Commun 9, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T, 2016. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 26, 3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Rosburg T, Axmacher N, Elger CE., 2008. Phase-locking within human mediotemporal lobe predicts memory formation. Neuroimage 43, 410–419. [DOI] [PubMed] [Google Scholar]
- Foster BL, Kaveh A, Dastjerdi M, Miller KJ, Parvizi J, 2013. Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. J. Neurosci 33, 10439–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Rangarajan V, Shirer WR, Parvizi J, 2015. Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex. Neuron 86, 578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Foster BL, Kucyi A, Daitch AL, Parvizi J, 2018. Intracranial Electrophysiology of the Human Default Network. Trends Cogn. Sci 22, 307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WJ, Ahlfors SP, Menon V, 2009. Combining fMRI with EEG and MEG in order to relate patterns of brain activity to cognition. Int. J. Psychophysiol 73, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Zeffiro TA, Scheinost D, Constable RT, Brewer JA., 2015. Meditation leads to reduced default mode network activity beyond an active task. Cogn. Affect. Behav. Neurosci 15, 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Miller J, Watrous AJ, Lee SA, Coffey T, Sperling MR, Sharan A, Worrell G, Berry B, Lega B, Jobst BC, Davis KA, Inman C, Sheth SA, Wanda PA, Ezzyat Y, Das SR, Stein J, Gorniak R, Jacobs J, 2018. Electrical stimulation in hippocampus and entorhinal cortex impairs spatial and temporal memory. J. Neurosci 38, 4471–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V, 2003. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci 100, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V, 2004. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J. Cogn. Neurosci 16, 1484–1492. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V, 2004. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. USA 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Snyder AZ, Pahwa M, Corbetta M, Leuthardt EC, 2017. Frequency-specific electrophysiologic correlates of resting state fMRI networks. Neuroimage 149, 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Chen J, Honey CJ, 2015. Hierarchical process memory: memory as an integral component of information processing. Trends Cogn. Sci 19, 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME., 2008. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci 105, 16039–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Zempel JM, Snyder AZ, Raichle ME., 2010. The temporal structures and functional significance of scale-free brain activity. Neuron 66, 353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebscher M, Voss JL., 2020. Testing network properties of episodic memory using non-invasive brain stimulation. Curr. Opin. Behav. Sci 32, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Tewarie P, van Dellen E, Yu M, Carbo EW, Douw L, Gouw AA, van Straaten EC, Stam CJ., 2016. Direction of information flow in large-scale resting-state networks is frequency-dependent. Proc. Natl. Acad. Sci. USA 113, 3867–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy CW, Steiner SC, Knight RT., 2021. Single-trial modeling separates multiple overlapping prediction errors during reward processing in human EEG. Commun. Biol 4, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Hashemi N, Gati JS, Menon RS, Everling S, 2015. Electrophysiological signatures of spontaneous BOLD fluctuations in macaque prefrontal cortex. Neuroimage 113, 257–267. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I, 2007. Brain oscillations control timing of single-neuron activity in humans. J. Neurosci 27, 3839–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Miller J, Lee SA, Coffey T, Watrous AJ, Sperling MR, Sharan A, Worrell G, Berry B, Lega B, Jobst BC, Davis K, Gross RE, Sheth SA, Ezzyat Y, Das SR, Stein J, Gorniak R, Kahana MJ, Rizzuto DS., 2016. Direct electrical stimulation of the human entorhinal region and hippocampus impairs memory. Neuron 92, 983–990. [DOI] [PubMed] [Google Scholar]
- Jann K, Dierks T, Boesch C, Kottlow M, Strik W, Koenig T, 2009. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. Neuroimage 45, 903–916. [DOI] [PubMed] [Google Scholar]
- Kam JWY, Lin JJ, Solbakk AK, Endestad T, Larsson PG, Knight RT., 2019. Default network and frontoparietal control network theta connectivity supports internal attention. Nat. Hum. Behav 3, 1263–1270. [DOI] [PubMed] [Google Scholar]
- Kamp T, Sorger B, Benjamins C, Hausfeld L, Goebel R, 2018. The prestimulus default mode network state predicts cognitive task performance levels on a mental rotation task. Brain Behav. 8, e01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Montemurro MA, Logothetis NK, Panzeri S, 2009. Spike-phase coding boosts and stabilizes information carried by spatial and temporal spike patterns. Neuron 61, 597–608. [DOI] [PubMed] [Google Scholar]
- Kragel JE, Polyn SM., 2015. Functional interactions between large-scale networks during memory search. Cereb Cortex 25, 667–679. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Daitch A, Raccah O, Zhao B, Zhang C, Esterman M, Zeineh M, Halpern CH, Zhang K, Zhang J, Parvizi J, 2020. Electrophysiological dynamics of antagonistic brain networks reflect attentional fluctuations. Nat. Commun 11, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Schrouff J, Bickel S, Foster BL, Shine JM, Parvizi J, 2018. Intracranial electrophysiology reveals reproducible intrinsic functional connectivity within human brain networks. J. Neurosci 38, 4230–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB., 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Software 82, 1–26. [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ, 1999. Measuring phase synchrony in brain signals. Hum. Brain Mapp 8, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Gross J, Thut G., 2019. A new unifying account of the roles of neuronal entrainment. Curr. Biol 29, R890–r905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A, 2003. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc. Natl. Acad. Sci. USA 100, 11053–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y, Logothetis NK., 2003. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb. Cortex 13, 422–433. [DOI] [PubMed] [Google Scholar]
- Lobier M, Siebenhühner F, Palva S, Palva JM, 2014. Phase transfer entropy: a novel phase-based measure for directed connectivity in networks coupled by oscillatory interactions. Neuroimage 85, 853–872. [DOI] [PubMed] [Google Scholar]
- Long NM, Kahana MJ., 2017. Modulation of task demands suggests that semantic processing interferes with the formation of episodic associations. J. Exp. Psychol. Learn Mem. Cogn 43, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopour BA, Tavassoli A, Fried I, Ringach DL., 2013. Coding of information in the phase of local field potentials within human medial temporal lobe. Neuron 79, 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA, 2007. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc. Natl. Acad. Sci. USA 104, 18265–18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M, 2007. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA 104, 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, Bezgin G, Eickhoff SB, Castellanos FX, Petrides M, Jefferies E, Smallwood J, 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. USA 113, 12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Menon V, 2015. Large-Scale functional brain organization. In: Toga AW (Ed.), Brain Mapping Waltham. Academic Press, pp. 449–459. [Google Scholar]
- Menon V, Freeman WJ, Cutillo BA, Desmond JE, Ward MF, Bressler SL, Laxer KD, Barbaro N, Gevins AS., 1996. Spatio-temporal correlations in human gamma band electrocorticograms. Electroencephalogr. Clin. Neurophysiol 98, 89–102. [DOI] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A, 2003. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage 20, 145–158. [DOI] [PubMed] [Google Scholar]
- Murphy C, Jefferies E, Rueschemeyer SA, Sormaz M, Wang HT, Margulies DS, Smallwood J, 2018. Distant from input: evidence of regions within the default mode network supporting perceptually-decoupled and conceptually-guided cognition. Neuroimage 171, 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Wang HT, Konu D, Lowndes R, Margulies DS, Jefferies E, Smallwood J, 2019. Modes of operation: a topographic neural gradient supporting stimulus dependent and independent cognition. Neuroimage 186, 487–496. [DOI] [PubMed] [Google Scholar]
- Neuner I, Arrubla J, Werner CJ, Hitz K, Boers F, Kawohl W, Shah NJ, 2014. The default mode network and EEG regional spectral power: a simultaneous fMRI-EEG study. PLoS One 9, e88214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng BS, Logothetis NK, Kayser C, 2013. EEG phase patterns reflect the selectivity of neural firing. Cereb Cortex 23, 389–398. [DOI] [PubMed] [Google Scholar]
- Ossandon T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, Jung J, Minotti L, Bertrand O, Kahane P, Lachaux JP, 2011. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J. Neurosci 31, 14521–14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi J, Kastner S, 2018. Promises and limitations of human intracranial electroencephalography. Nat. Neurosci 21, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RA, Cavanaugh JE., 2018. Ordered quantile normalization: a semiparametric transformation built for the cross-validation era. J. Appl. Statist 82, 2312–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccah O, Daitch AL, Kucyi A, Parvizi J, 2018. Direct cortical recordings suggest temporal order of task-evoked responses in human dorsal attention and default networks. J. Neurosci 38, 10305–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME., 2015. The brain’s default mode network. Ann. Rev. Neurosci 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Salamone PC, Legaz A, Sedeño L, Moguilner S, Fraile-Vazquez M, Campo CG, Fittipaldi S, Yoris A, Miranda M, Birba A, Galiani A, Abrevaya S, Neely A, Caro MM, Alifano F, Villagra R, Anunziata F, Okada de Oliveira M, Pautassi RM, Slachevsky A, Serrano C, García AM, Ibañez A, 2021. Interoception primes emotional processing: multimodal evidence from neurodegeneration. J. Neurosci 41, 4276–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P, 2008. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int. J. Psychophysiol 67, 242–251. [DOI] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA., 2010. Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci 107, 10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber T, 2000. Measuring information transfer. Phys. Rev. Lett 85, 461–464. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA, 2010. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry 67, 584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Warden MR, Miller EK., 2009. Phase-dependent neuronal coding of objects in short-term memory. Proc. Natl. Acad. Sci. USA 106, 21341–21346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V, Mitchell DJ, Duncan J, 2018. Role of the default mode network in cognitive transitions. Cereb Cortex 28, 3685–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon EA, Kragel JE, Sperling MR, Sharan A, Worrell G, Kucewicz M, Inman CS, Lega B, Davis KA, Stein JM, Jobst BC, Zaghloul KA, Sheth SA, Rizzuto DS, Kahana MJ., 2017. Widespread theta synchrony and high-frequency desynchronization underlies enhanced cognition. Nat. Commun 8, 1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon EA, Stein JM, Das S, Gorniak R, Sperling MR, Worrell G, Inman CS, Tan RJ, Jobst BC, Rizzuto DS, Kahana MJ., 2019. Dynamic theta networks in the human medial temporal lobe support episodic memory. Curr. Biol 29, 1100–1111 e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormaz M, Murphy C, Wang HT, Hymers M, Karapanagiotidis T, Poerio G, Margulies DS, Jefferies E, Smallwood J, 2018. Default mode network can support the level of detail in experience during active task states. Proc. Natl. Acad. Sci. USA 115, 9318–9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC, 2004. Organization, development and function of complex brain networks. Trends Cogn. Sci 8, 418–425. [DOI] [PubMed] [Google Scholar]
- Staffaroni AM, Brown JA, Casaletto KB, Elahi FM, Deng J, Neuhaus J, Cobigo Y, Mumford PS, Walters S, Saloner R, Karydas A, Coppola G, Rosen HJ, Miller BL, Seeley WW, Kramer JH., 2018. The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J. Neurosci 38, 2809–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull A, Wang HT, Murphy C, Ho NSP, Wang X, Sormaz M, Karapanagiotidis T, Leech RM, Bernhardt B, Margulies DS, Vatansever D, Jefferies E, Smallwood J, 2019a. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nat. Commun 10, 3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull A, Wang HT, Schooler JW, Jefferies E, Margulies DS, Smallwood J, 2019b. The ebb and flow of attention: Between-subject variation in intrinsic connectivity and cognition associated with the dynamics of ongoing experience. Neuroimage 185, 286–299. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP., 2009. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp 30, 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass LK, Copara MS, Seyal M, Shahlaie K, Farias ST, Shen PY, Ekstrom AD., 2016. Oscillations go the distance: low-frequency human hippocampal oscillations code spatial distance in the absence of sensory cues during teleportation. Neuron 89, 1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Wang J, Zhou J, Guan YG, Zhai F, Liu CQ, Xu FF, Han YX, Yan ZF, Luan GM, 2017. Identification of the epileptogenic zone of temporal lobe epilepsy from stereo-electroencephalography signals: A phase transfer entropy and graph theory approach. Neuroimage Clin. 16, 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KN, Hermiller MS, Nilakantan AS, Voss JL., 2019. Stimulating the hippocampal posterior-medial network enhances task-dependent connectivity and memory. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Tandon N, Conner CR, Pieters T, Ekstrom AD., 2013. Frequency-specific network connectivity increases underlie accurate spatiotemporal memory retrieval. Nat. Neurosci 16, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Eichele T, Calhoun VD, 2010. Reactivity of hemodynamic responses and functional connectivity to different states of alpha synchrony: a concurrent EEG-fMRI study. Neuroimage 52, 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL., 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Liu T, Szarkowski R, Rios C, Ashe J, He B, 2010. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: an EEG and fMRI study of motor imagery and movements. Neuroimage 49, 2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.