Abstract
Background
Adequate physical activity (PA) is essential for preventing sarcopenia in older adults. However, there are insufficient epidemiological data on the intensity of PA needed to prevent age-related sarcopenia. The purpose of this study was to investigate the association of PA intensity with skeletal muscle mass and muscle strength.
Methods
This was a population-based study with a cross-sectional design that was conducted using data from the 2008 − 2011 and 2014 − 2018 Korea National Health and Nutrition Examination Surveys, which included a total of 11,162 participants aged ≥ 60 years. PA was assessed using the results of a questionnaire and organized by intensity, frequency, and duration. The study population was divided into the following groups based on PA intensity: no exercise, walking only, moderate PA, and vigorous PA. To assess sarcopenia, skeletal muscle index (SMI) and hand grip strength (HGS) were measured as indicators of muscle mass and strength, respectively. Logistic regression analysis was used to explore the relationship between PA intensity and sarcopenia.
Results
SMI and HGS were significantly higher in men and women engaged in moderate to vigorous PA than in those who did not exercise. The odds ratios (ORs) for sarcopenia defined based on SMI and HGS were lowest in men engaged in vigorous PA (0.444, 95% confidence interval [CI]: 0.242 − 0.818 and 0.450, 95% CI: 0.228 − 0.890, respectively). In women, the OR for sarcopenia defined based on HGS was the lowest in the group engaged in vigorous PA (0.441, 95% CI: 0.199 − 0.975), while there was no risk reduction for sarcopenia defined based on SMI.
Conclusions
Moderate to vigorous PA was highly correlated with SMI and HGS in men and women. Intensive PA was positively correlated with sarcopenia prevention, which can be monitored using HGS.
Keywords: Aging, Muscle mass, Sarcopenia, Muscle strength, Physical activity
Background
Sarcopenia, a progressive decrease in skeletal muscle mass and function, is associated with poor quality of life, disability, and mortality [1–3]. Sarcopenia has become a serious public health issue in Korea due to the steady increase in the proportion of Koreans aged 65 years or older [3]. Accordingly, nationwide research on sarcopenia is urgently needed. Sarcopenia is diagnosed based on assessments of muscle mass, muscle strength, and physical performance. However, the criteria for diagnosing sarcopenia are inconsistent [4–8], and various parameters, such as muscle mass, appendicular skeletal muscle mass (ASM), and lean muscle mass, have been used in different studies. The most recent and popular criteria, the consensus of the 2019 Asian Working Group for Sarcopenia (AWGS) [9], provided cut-off values for skeletal muscle index (SMI) and hand grip strength (HGS) as measures of muscle mass and strength, respectively.
Various factors such as mitochondrial oxidative stress, apoptosis, and mitophagy and proteins such as myostatin and inflammatory cytokines are involved in the pathogenesis of sarcopenia [4]. Regular physical activity (PA) is recommended as a safe strategy to counter the loss of muscle mass and strength that occurs with aging [10]. Physical activity in the form of aerobic exercise (cycling, dancing, sports), resistance exercise (squats, weightlifting), and a combination of the two have been shown to prevent muscle atrophy and produce beneficial preventive and therapeutic effects via various mechanisms [11–14]. Although PA may indirectly affect other health parameters, it is an important factor associated with muscle strength and mass [15]. A previous meta-analysis on the relationship between sarcopenia and PA in older individuals demonstrated that PA reduces the odds of acquiring sarcopenia in later life (odds ratio [OR] = 0.45; 95% confidence interval [CI]: 0.37 − 0.55) [16]. A subsequent meta-analysis on the effects of nutrition and PA on sarcopenia revealed that PA positively impacted muscle mass and function in healthy participants, with limited effects of nutritional supplements [17].
In the Korea National Health and Nutrition Examination Survey (KNHANES), muscle mass and strength data were measured in different phases (2008 − 2011 for muscle mass data and 2014 − 2018 for muscle strength data). Hence, analyzing these data may provide novel insights into the association between PA and muscle mass and strength. Although numerous studies on the association between sarcopenia and factors such as nutrition and metabolic disease have been performed using KNHANES data [18–20], there is a paucity of studies analyzing the relationship between sarcopenia and PA using the two axes of muscle mass and strength. This could be due to the difficulty of statistical analysis of PA classification and quantification, extensive analysis required for data from different phases, and multiple factors related to the KNHANES. Recently, our study group conducted studies to analyze osteoporosis [21] and metabolic syndrome [22] using KNHANES data by classifying PA according to intensity, frequency, and duration. These studies highlighted the feasibility of analyzing the association between metabolic conditions and PA. Therefore, the purpose of this study was to investigate the relationship between PA amount, which includes intensity, frequency, and duration, and muscle mass and strength in older adults using data from the 2008–2011 and 2014 − 2018 KNHANES.
Methods
Study design and participants
This study used data from the KNHANES datasets from 2008–2011 and 2014 − 2018 produced by the Korea Disease Control and Prevention Agency. KNHANES is a nationwide survey with a cross-sectional design used to evaluate the health and nutritional status of the Korean population through medical history taking, physical examinations, health behavior surveys, and anthropometric and biochemical measurements. The Institutional Review Board of the VHS Medical Center approved the study protocol and waived the requirement for informed consent (IRB No. 2021–05-006) due to the retrospective nature of the study. The study was conducted in compliance with the Declaration of Helsinki.
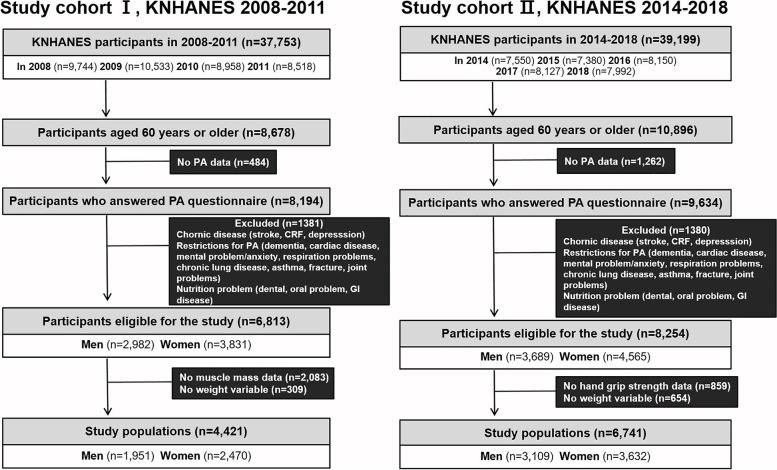
We analyzed data of 8,678 participants aged 60 years or older from the 2008–2011 KNHANES and 10,896 participants aged 60 years or older from the 2014 − 2018 KNHANES (Fig. 1). The exclusion criteria were as follows: missing PA data (n = 484 and n = 1,262, respectively) and conditions that affect muscle condition for exclusion of secondary sarcopenia, including chronic disease, restriction of PA, and/or nutritional issues (n = 1,381 and n = 1,380, respectively). In total, 6,813 participants (2,982 men and 3,831 women) from the 2008–2011 KNHANES and 8,254 participants (3,689 men and 4,565 women) from the 2014 − 2018 KNHANES were eligible for participation in this study. Participants with missing data on muscle parameters (n = 2,083 for muscle mass data, n = 859 for HGS data) and weight variables (n = 309 and n = 654, respectively) were excluded from the analysis. A final total of 4,421 participants (1,951 men and 2,470 women) from the 2008–2011 KNHANES and 6,741 participants (3,109 men and 3,632 women) from the 2014–2018 KNHANES were included in the analyses.
Fig. 1.
Flowchart of participant enrolment according to cohort. The KNHANES 2008 − 2011 and KNHANES 2014 − 2018 obtained data on muscle mass and hand grip strength, respectively. KNHANES, Korea National Health and Nutrition Examination Survey; PA, physical activity
Assessments of skeletal muscle mass and strength
Whole and regional body compositions were measured with dual-energy X-ray absorptiometry (DXA) (QDR4500A; Hologic Inc., Bedford, MA). In the 2008–2011 KNHANES, ASM was calculated as the sum of the mass of the skeletal muscles in the arms and legs measured with DXA, under the assumption that all non-fat and non-bone tissues were skeletal muscles. SMI was calculated by dividing the ASM by height squared (ASM/height2), and this value was used as an indicator of skeletal muscle mass. In the 2014 − 2018 KNHANES, muscle strength was measured as HGS using a digital hand dynamometer (T.K.K 5401, Takei, Tokyo, Japan). With the participant in a standing position and forearm extended in a sideways position away from the body at the thigh level, participants were instructed to exert maximum grip strength three times each with the left and right hands, and the findings for the dominant hand were recorded. A rest interval of at least 30 s was allowed between each measurement. The participants were instructed to squeeze the dynamometer continuously with full force for at least 3 s. The average of the three trials for each hand was recorded. Based on the consensus of the 2019 AWGS [9], low muscle mass for sarcopenia was defined as an SMI < 7.0 kg/m2 for men and < 5.4 kg/m2 for women, and low muscle strength was defined as HGS < 28 kg for men and < 18 kg for women.
Assessment of physical activity: Intensity, frequency, and duration
The International Physical Activity Questionnaire-Short Form (IPAQ-SF) was used to assess PA. The questionnaires were used to determine the intensity, frequency, and duration of PA performed by the participants, who were then grouped according to our previously reported classification system [21, 22]. In brief, participants were questioned as to whether they had engaged in different types of PA for exercise for at least 10 min over the past week. PAs were categorized as walking only, moderate PA, and vigorous PA, which is the classification system used in our previous studies [21, 22].
Statistical analyses
All analyses were performed using the sample weights from the KNHANES data. When characterizing the participants according to PA intensity, data were expressed as means with standard error (SE) for continuous variables and percentages with SE for categorical variables. Continuous variables were analyzed using the independent t-test or analysis of variance, while categorical variables were analyzed using the Rao–Scott chi-square test. Age, body mass index (BMI), total energy intake, total protein intake, and total fat intake were considered continuous independent variables, whereas, smoking, alcohol intake, monthly household income, education level, diabetes, PA intensity, PA frequency, and PA duration were considered categorical independent variables. Subgroups were compared by applying the post-hoc Bonferroni correction after the t-test.
The following logistic regression models for sarcopenia were sequentially applied: unadjusted; model 1: adjusted for age; model 2: adjusted for age, smoking, drinking, alcohol intake, monthly household income, total energy intake, total protein intake, total fat intake, education level, and diabetes; model 3: adjusted for age and BMI; and model 4: adjusted for age, BMI, smoking, drinking, alcohol intake, monthly household income, total energy intake, total protein intake, total fat intake, education level, and diabetes. In addition, linear regression analysis was performed in model 4 to analyze the trends in SMI or HGS according to PA intensity, frequency, and duration in each PA group. Since adjustment for variables was performed, linear regression analysis was considered more suitable for trend analysis than analysis of variance. Statistical analyses were performed using the R 3.6.3 program (R Foundation, Vienna, Austria) and statistical significance was set at P < 0.05.
Results
Characteristics of the study participants
2008 − 2011 KNHANES: study cohort I
In both men and women, the mean age was lower in the vigorous PA group than in the no exercise group (all P < 0.001, Table 1). In addition, BMI differed by PA group in men (P = 0.012) but not in women (P = 0.958). The proportion of sarcopenia defined based on SMI in all participants was 36.5% for men and 20.8% for women. The no exercise group exhibited the highest sarcopenia ratio (48.9% for men and 23.4% for women). No significant difference in smoking status was observed (P = 0.248), but alcohol consumption and monthly income significantly differed according to PA group in men (P = 0.036 and P < 0.001, respectively). Total energy intake was higher in the vigorous PA group than in the no exercise group, with a trend of marginal significance after adjustment for age in both men and women (P = 0.086 and P = 0.081, respectively). Total protein intake was higher in the vigorous exercise group than in the no exercise group after adjustment for age in men (P = 0.008), but no significant difference was observed in women (P = 0.110). Total fat intake did not differ according to age in the PA groups. The incidence of comorbidities such as hypertension, diabetes, and arthritis did not differ between men and women. A disparity in the duration and frequency of PA was identified between groups according to PA intensity in men, while the frequency of PA was significantly different in the moderate activity group than in the other groups in women.
Table 1.
Baseline characteristics of study subjects of KNHANES 2008–2011 (Study cohort I)
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | no Exercise | Walking-only | Moderate PA | Vigorous PA | Total | no Exercise | Walking-only | Moderate PA | Vigorous PA | |||
| (n = 1,951) | (n = 184) | (n = 866) | (n = 426) | (n = 475) | P | (n = 2,470) | (n = 420) | (n = 1,075) | (n = 567) | (n = 408) | P | |
| Age, years | 68.19 ± 0.167 | 69.18 ± 0.559 | 68.85 ± 0.236 | 68.38 ± 0.339 | 66.49 ± 0.307 | < 0.001 | 69.73 ± 0.174 | 72.59 ± 0.382 | 69.7 ± 0.241 | 69.24 ± 0.329 | 67.45 ± 0.382 | < 0.001 |
| a | a | a | b | a | b | b | c | |||||
| BMI, kg/m2 | 23.44 ± 0.094 | 23.02 ± 0.233 | 23.29 ± 0.124 | 23.42 ± 0.198 | 23.91 ± 0.179 | 0.012 | 24.16 ± 0.083 | 24.12 ± 0.203 | 24.14 ± 0.13 | 24.24 ± 0.157 | 24.15 ± 0.182 | 0.958 |
| a | a | ab | b | |||||||||
| ASM, kg | 20.07 ± 0.09 | 19.08 ± 0.217 | 19.85 ± 0.124 | 20.06 ± 0.161 | 20.83 ± 0.161 | < 0.001 | 13.66 ± 0.052 | 13.27 ± 0.103 | 13.55 ± 0.077 | 13.91 ± 0.092 | 14.06 ± 0.11 | < 0.001 |
| a | b | b | c | a | a | b | b | |||||
| age adjusted | 19.15 ± 0.198 | 19.87 ± 0.118 | 20 ± 0.158 | 20.49 ± 0.161 | < 0.001 | 13.59 ± 0.103 | 13.58 ± 0.072 | 13.9 ± 0.091 | 13.87 ± 0.106 | 0.014 | ||
| age and BMI adjusted | 19.32 ± 0.158 | 19.91 ± 0.106 | 20 ± 0.127 | 20.31 ± 0.12 | < 0.001 | 13.53 ± 0.09 | 13.58 ± 0.061 | 13.88 ± 0.085 | 13.9 ± 0.1 | 0.001 | ||
| SMI, kg/m2 | 7.29 ± 0.028 | 7.06 ± 0.067 | 7.21 ± 0.035 | 7.33 ± 0.05 | 7.5 ± 0.047 | < 0.001 | 5.93 ± 0.018 | 5.86 ± 0.038 | 5.89 ± 0.026 | 6 ± 0.033 | 6.01 ± 0.037 | 0.001 |
| a | ab | b | c | a | a | b | b | |||||
| age adjusted | 7.08 ± 0.063 | 7.21 ± 0.034 | 7.32 ± 0.048 | 7.41 ± 0.047 | < 0.001 | 5.91 ± 0.039 | 5.89 ± 0.025 | 5.99 ± 0.033 | 5.98 ± 0.038 | 0.036 | ||
| age and BMI adjusted | 7.14 ± 0.047 | 7.23 ± 0.026 | 7.32 ± 0.035 | 7.35 ± 0.035 | < 0.001 | 5.88 ± 0.034 | 5.89 ± 0.019 | 5.98 ± 0.03 | 6 ± 0.034 | 0.002 | ||
| Sarcopenia, % | 36.5 (1.44) | 48.9 (4.53) | 40.3 (1.97) | 34.1 (2.95) | 27.2 (2.58) | < 0.001 | 20.8 (1.11) | 23.4 (2.62) | 23.8 (1.63) | 16.1 (2.12) | 15.3 (2.15) | 0.002 |
| Alcohol consumption, % | 0.036 | 0.090 | ||||||||||
| None | 26.3 (1.24) | 33 (4.2) | 28.8 (1.83) | 26.1 (2.75) | 19.5 (2.11) | 61.2 (1.23) | 64.4 (2.91) | 59.9 (1.84) | 65.3 (2.41) | 56.2 (2.98) | ||
| Moderate | 34.8 (1.5) | 30.1 (4.76) | 34 (2.06) | 33.5 (2.98) | 38.8 (2.62) | 33.9 (1.18) | 31 (2.84) | 35.9 (1.76) | 29.7 (2.26) | 36.6 (2.76) | ||
| Heavy | 38.9 (1.45) | 36.9 (4.05) | 37.1 (1.96) | 40.4 (3.14) | 41.7 (2.65) | 4.9 (0.48) | 4.6 (1.27) | 4.2 (0.75) | 5 (1.02) | 7.2 (1.31) | ||
| Smoking status, % | 0.248 | 0.163 | ||||||||||
| Never | 15.1 (0.96) | 12.2 (2.53) | 13.8 (1.33) | 16.6 (2.04) | 17.3 (2.04) | 89.7 (0.78) | 86.5 (2.18) | 89.2 (1.24) | 92 (1.42) | 91.9 (1.75) | ||
| Ex- | 56.2 (1.38) | 53.6 (4.33) | 56.4 (2.14) | 54.2 (2.87) | 58.3 (2.68) | 5.5 (0.64) | 6.6 (1.73) | 6.6 (1.06) | 3.8 (1.08) | 3.6 (1.1) | ||
| Current | 28.7 (1.28) | 34.3 (4.19) | 29.7 (2.07) | 29.2 (2.62) | 24.4 (2.37) | 4.7 (0.54) | 7 (1.59) | 4.2 (0.76) | 4.3 (0.99) | 4.5 (1.4) | ||
| Monthly household income, % | < 0.001 | 0.053 | ||||||||||
| Lowest | 35.7 (1.42) | 42.3 (4.31) | 41.2 (2.12) | 33.3 (2.78) | 25.5 (2.26) | 47.2 (1.4) | 54.6 (2.98) | 46.7 (2.04) | 46.6 (2.66) | 41.8 (2.93) | ||
| Medium-lowest | 28.6 (1.24) | 27.3 (3.89) | 28.2 (1.92) | 29.1 (2.68) | 29.3 (2.34) | 25.1 (1.07) | 23.2 (2.47) | 23.7 (1.52) | 28.1 (2.45) | 27.2 (2.73) | ||
| Medium-highest | 19.7 (1.03) | 22.6 (3.76) | 17.2 (1.44) | 21.4 (2.53) | 21.7 (2.17) | 16 (0.98) | 13.9 (2.13) | 17.6 (1.49) | 15 (1.98) | 15.3 (2.18) | ||
| Highest | 16 (1.14) | 7.8 (2.35) | 13.4 (1.47) | 16.2 (2.05) | 23.5 (2.59) | 11.7 (0.89) | 8.3 (1.59) | 12.1 (1.33) | 10.3 (1.57) | 15.7 (2.34) | ||
| Education level, % | < 0.001 | < 0.001 | ||||||||||
| ≤ Elementary school | 43.4 (1.68) | 63.3 (4.52) | 42.8 (2.2) | 48.8 (3.09) | 33 (3.07) | 80.7 (1.09) | 89.7 (1.88) | 81.1 (1.47) | 82.9 (1.97) | 67.1 (3.08) | ||
| Middle school | 21.3 (1.28) | 19.1 (3.77) | 23.9 (1.69) | 19.7 (2.84) | 18.7 (2.11) | 8.9 (0.69) | 6.2 (1.35) | 8.5 (0.99) | 8.7 (1.47) | 13.4 (2.16) | ||
| High school | 22.5 (1.14) | 13.4 (2.8) | 22.1 (1.78) | 18 (2.18) | 30.3 (2.65) | 7.9 (0.72) | 3.7 (1.03) | 7.6 (0.92) | 6.2 (1.16) | 15.7 (2.74) | ||
| ≥ College | 12.7 (1.16) | 4.3 (2.07) | 11.2 (1.38) | 13.5 (2.03) | 18 (2.8) | 2.4 (0.42) | 0.3 (0.24) | 2.7 (0.63) | 2.2 (0.73) | 3.9 (1.11) | ||
| Total energy intake, kcal/d | 2012.86 ± 21.097 | 1910.97 ± 67.628 | 1958.17 ± 29.755 | 2049.95 ± 39.798 | 2117.9 ± 39.757 | 0.002 | 1451.24 ± 13.886 | 1418.44 ± 30.895 | 1419.05 ± 20.332 | 1494.17 ± 27.948 | 1524.31 ± 26.831 | 0.004 |
| a | a | ab | b | a | a | ab | b | |||||
| age adjusted | 1927.53 ± 63.721 | 1962.41 ± 28.82 | 2038.84 ± 36.744 | 2047.26 ± 38.99 | 0.086 | 1478.5 ± 30.702 | 1425.18 ± 19.26 | 1491.67 ± 27.285 | 1488.5 ± 28.532 | 0.081 | ||
| Total protein intake, g/d | 68.24 ± 0.976 | 61.57 ± 2.874 | 65.64 ± 1.379 | 69.28 ± 1.849 | 74.48 ± 1.862 | < 0.001 | 46.58 ± 0.602 | 43.46 ± 1.162 | 45.55 ± 0.853 | 48.1 ± 1.257 | 50.9 ± 1.439 | < 0.001 |
| a | a | ab | b | a | ab | bc | c | |||||
| age adjusted | 62.26 ± 2.632 | 65.82 ± 1.365 | 68.82 ± 1.742 | 71.55 ± 1.812 | 0.008 | 46.13 ± 1.147 | 45.82 ± 0.831 | 47.99 ± 1.206 | 49.31 ± 1.497 | 0.110 | ||
| Total fat intake, g/d | 31.08 ± 0.721 | 28.28 ± 2.325 | 29.63 ± 1.015 | 30.38 ± 1.278 | 35.23 ± 1.826 | 0.041 | 18.79 ± 0.41 | 16.71 ± 0.673 | 18.48 ± 0.612 | 19.29 ± 0.776 | 21.24 ± 0.934 | 0.001 |
| ab | a | ab | b | a | ab | ab | b | |||||
| age adjusted | 28.72 ± 2.201 | 29.75 ± 1.011 | 30.08 ± 1.225 | 33.35 ± 1.744 | 0.282 | 18.37 ± 0.678 | 18.65 ± 0.607 | 19.22 ± 0.743 | 20.25 ± 0.986 | 0.461 | ||
| Hypertension, % | 40.7 (1.34) | 28.9 (3.93) | 42.5 (2.02) | 42.8 (3.42) | 40.3 (2.64) | 0.046 | 50 (1.31) | 54.8 (2.8) | 49.7 (2.05) | 49.4 (2.88) | 46.4 (3.01) | 0.276 |
| Diabetes, % | 16.5 (1) | 21.2 (3.9) | 16.1 (1.42) | 15.9 (2.18) | 15.9 (2.14) | 0.553 | 16 (0.96) | 18.7 (2.55) | 16 (1.45) | 14.3 (1.91) | 15.1 (2.26) | 0.552 |
| Arthritis, % | 12.8 (0.88) | 14.9 (3.12) | 12 (1.29) | 13.1 (2.23) | 13.3 (1.81) | 0.829 | 41.6 (1.19) | 40.6 (3.13) | 41.6 (1.84) | 41.4 (2.76) | 42.9 (2.66) | 0.955 |
| Exercise frequency, times/wk | ||||||||||||
| Walking activity | 4.53 ± 0.075 | 0 | 5.32 ± 0.084 | 4.63 ± 0.179 | 4.75 ± 0.142 | < 0.001 | 4.05 ± 0.08 | 0 | 4.89 ± 0.096 | 4.87 ± 0.126 | 4.8 ± 0.148 | 0.873 |
| a | b | b | ||||||||||
| Moderate activity | 1.41 ± 0.071 | 0 | 0 | 4.12 ± 0.139 | 2.3 ± 0.148 | < 0.001 | 1.27 ± 0.06 | 0 | 0 | 4.01 ± 0.11 | 2.78 ± 0.163 | < 0.001 |
| Vigorous activity | 0.91 ± 0.055 | 0 | 0 | 0 | 3.57 ± 0.129 | 0.58 ± 0.044 | 0 | 0 | 0 | 3.65 ± 0.131 | ||
| Exercise duration, min/wk | ||||||||||||
| Walking activity | 430.6 ± 17.5 | 0 | 508.7 ± 27.3 | 393.66 ± 30.7 | 481.7 ± 28.2 | 0.014 | 275.03 ± 10.6 | 0 | 324.8 ± 15.8 | 308.3 ± 20.3 | 375.9 ± 26.9 | 0.103 |
| a | b | a | a | b | a | |||||||
| Moderate activity | 190 ± 15.3 | 0 | 0 | 581.2 ± 43.4 | 290.3 ± 30.9 | < 0.001 | 154.21 ± 11.0 | 0 | 0 | 460.5 ± 33.3 | 371.5 ± 35.0 | 0.060 |
| Vigorous activity | 124.3 ± 10.5 | 0 | 0 | 0 | 489.72 ± 33.4 | 93.11 ± 9.4 | 0 | 0 | 0 | 587.1 ± 46.4 | ||
Data with the same lowercase letters indicate non-specific differences between groups, while those with different letters are statistically different, based on post hoc test
Data are expressed as the mean ± SE or the percentage (SE)
2014 − 2018 KNHANES: study cohort
In both men and women, the mean age was lower in the vigorous PA group than in the no exercise group (all P < 0.001, Table 2). The proportion of sarcopenia defined based on HGS was 19.0% in men and 31.6% in women. The no exercise group had the highest sarcopenia ratio (24.6% in men and 50.5% in women). Alcohol consumption, smoking status, education level, and monthly income differed between men and women according to PA intensity (all P < 0.01). Total energy intake, total protein intake, and total fat intake in men and women were higher in the moderate PA and vigorous PA groups than in the no exercise group after adjustment for age (all P < 0.001). The incidence of comorbidities such as hypertension, diabetes, and arthritis did not differ in men among the PA intensity groups. For women, the incidence of hypertension and diabetes was higher in the no exercise group than in the vigorous PA group (all P < 0.001). Discrepancies in the frequency and duration of PA were identified between the PA groups in men and women, except for the duration of PA in the walking-only group.
Table 2.
Baseline characteristics of study subjects of KNHANES 2014–2018 (Study cohort II)
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | no Exercise | Walking-only | Moderate PA | Vigorous PA | Total | no Exercise | Walking-only | Moderate | Vigorous | |||
| (n = 3,109) | (n = 595) | (n = 1,684) | (n = 603) | (n = 227) | P | (n = 3,632) | (n = 823) | (n = 2,243) | (n = 469) | (n = 97) | P | |
| Age, years | 69.49 ± 0.129 | 70.21 ± 0.303 | 70.14 ± 0.182 | 68.43 ± 0.236 | 66.21 ± 0.448 | < 0.001 | 69.35 ± 0.13 | 72.63 ± 0.292 | 68.96 ± 0.161 | 67.02 ± 0.288 | 64.52 ± 0.518 | < 0.001 |
| a | a | b | c | a | b | c | d | |||||
| BMI, kg/m2 | 23.87 ± 0.057 | 23.68 ± 0.137 | 23.84 ± 0.081 | 23.97 ± 0.127 | 24.22 ± 0.2 | 0.148 | 24.42 ± 0.063 | 24.72 ± 0.156 | 24.42 ± 0.076 | 24.17 ± 0.161 | 23.39 ± 0.312 | 0.001 |
| a | a | ab | b | |||||||||
| Hand grip strength, right hand, kg | 32.8 ± 0.158 | 31.48 ± 0.36 | 32.17 ± 0.198 | 34.2 ± 0.332 | 36.36 ± 0.467 | < 0.001 | 19.59 ± 0.111 | 17.53 ± 0.236 | 19.84 ± 0.129 | 21.12 ± 0.234 | 22.08 ± 0.443 | < 0.001 |
| a | a | b | c | a | b | c | c | |||||
| age adjusted | 31.8 ± 0.312 | 32.45 ± 0.174 | 33.57 ± 0.306 | 34.52 ± 0.401 | < 0.001 | 18.5 ± 0.211 | 19.6 ± 0.118 | 20.25 ± 0.217 | 20.38 ± 0.419 | < 0.001 | ||
| age and BMI adjusted | 31.92 ± 0.303 | 32.46 ± 0.169 | 33.56 ± 0.303 | 34.48 ± 0.401 | < 0.001 | 18.53 ± 0.204 | 19.63 ± 0.118 | 20.31 ± 0.219 | 20.56 ± 0.429 | < 0.001 | ||
| Hand grip strength, left hand, kg | 31.84 ± 0.152 | 30.78 ± 0.36 | 31.19 ± 0.189 | 33.12 ± 0.318 | 35.32 ± 0.463 | < 0.001 | 18.69 ± 0.108 | 16.97 ± 0.225 | 18.89 ± 0.126 | 20.05 ± 0.211 | 20.83 ± 0.434 | < 0.001 |
| a | a | b | c | a | b | c | c | |||||
| age adjusted | 31.07 ± 0.323 | 31.44 ± 0.168 | 32.54 ± 0.29 | 33.67 ± 0.446 | < 0.001 | 17.88 ± 0.202 | 18.67 ± 0.115 | 19.23 ± 0.198 | 19.23 ± 0.423 | < 0.001 | ||
| age and BMI adjusted | 31.21 ± 0.313 | 31.45 ± 0.163 | 32.54 ± 0.284 | 33.64 ± 0.446 | < 0.001 | 17.92 ± 0.195 | 18.69 ± 0.115 | 19.28 ± 0.2 | 19.36 ± 0.428 | < 0.001 | ||
| Hand grip strength, Dominant hand, kg | 33.69 ± 0.153 | 32.5 ± 0.356 | 33.03 ± 0.189 | 35 ± 0.32 | 37.39 ± 0.468 | < 0.001 | 20.12 ± 0.11 | 18.13 ± 0.23 | 20.36 ± 0.128 | 21.62 ± 0.218 | 22.52 ± 0.425 | < 0.001 |
| a | a | b | c | a | b | c | c | |||||
| age adjusted | 32.81 ± 0.31 | 33.3 ± 0.165 | 34.39 ± 0.293 | 35.62 ± 0.428 | < 0.001 | 19.1 ± 0.205 | 20.13 ± 0.116 | 20.75 ± 0.201 | 20.82 ± 0.407 | < 0.001 | ||
| age and BMI adjusted | 32.94 ± 0.299 | 33.3 ± 0.161 | 34.39 ± 0.288 | 35.58 ± 0.428 | < 0.001 | 19.13 ± 0.198 | 20.15 ± 0.116 | 20.81 ± 0.203 | 20.99 ± 0.414 | < 0.001 | ||
| Sarcopenia, % | 19 (0.83) | 24.6 (2.07) | 21.2 (1.19) | 13.5 (1.59) | 5.2 (1.58) | < 0.001 | 31.6 (1.02) | 50.5 (2.13) | 28.6 (1.2) | 20.1 (2.09) | 13.5 (4.06) | < 0.001 |
| Alcohol consumption, % | < 0.001 | < 0.001 | ||||||||||
| None | 26.5 (0.97) | 31.4 (2.31) | 28 (1.3) | 20.2 (1.81) | 21.5 (3.17) | 55.8 (1.07) | 64.1 (2.16) | 54.5 (1.37) | 50.6 (2.43) | 48.5 (5.83) | ||
| Moderate | 37.5 (1.1) | 30.6 (2.28) | 35.7 (1.41) | 44.5 (2.23) | 46.3 (3.7) | 38.5 (1.03) | 29.8 (2.04) | 39.8 (1.32) | 45.2 (2.42) | 42.2 (5.7) | ||
| Heavy | 36 (1.02) | 37.9 (2.39) | 36.3 (1.36) | 35.2 (2.2) | 32.2 (3.5) | 5.7 (0.43) | 6.1 (1.05) | 5.7 (0.53) | 4.1 (1.05) | 9.3 (3.03) | ||
| Smoking status, % | < 0.001 | 0.003 | ||||||||||
| Never | 19.4 (0.83) | 17.7 (1.85) | 20.7 (1.16) | 17.2 (1.87) | 20 (2.84) | 94.7 (0.5) | 92.8 (1.18) | 94.7 (0.61) | 96.6 (0.86) | 99 (1.01) | ||
| Ex- | 61.3 (1.02) | 55.9 (2.3) | 59.7 (1.45) | 69 (2.24) | 65.3 (3.7) | 3.5 (0.36) | 3.5 (0.79) | 3.7 (0.48) | 2.8 (0.8) | 0 (0) | ||
| Current | 19.3 (0.83) | 26.4 (2.21) | 19.7 (1.17) | 13.8 (1.65) | 14.7 (2.95) | 1.9 (0.35) | 3.7 (0.91) | 1.6 (0.38) | 0.6 (0.32) | 1 (1.01) | ||
| Monthly household income, % | < 0.001 | < 0.001 | ||||||||||
| Lowest | 30.4 (1.02) | 39.2 (2.48) | 33.1 (1.4) | 22.2 (1.95) | 14.2 (2.66) | 39.8 (1.14) | 55.5 (2.24) | 38.1 (1.36) | 27.6 (2.31) | 16 (3.71) | ||
| Medium-lowest | 29.7 (0.91) | 33.1 (2.19) | 29.9 (1.34) | 28.3 (1.95) | 24.9 (3.13) | 26.3 (0.84) | 22.9 (1.7) | 27.6 (1.06) | 25.6 (2.32) | 28.3 (5.08) | ||
| Medium-highest | 21.3 (0.83) | 17.2 (1.74) | 20.4 (1.17) | 25 (2.03) | 26.5 (3.41) | 18.9 (0.83) | 12.7 (1.44) | 19.3 (1.03) | 25.3 (2.52) | 27.7 (5.4) | ||
| Highest | 18.6 (0.91) | 10.5 (1.89) | 16.6 (1.08) | 24.5 (2.08) | 34.5 (3.82) | 14.9 (0.88) | 8.9 (1.22) | 15 (1.11) | 21.5 (2.45) | 28.1 (5.04) | ||
| Education level, % | < 0.001 | < 0.001 | ||||||||||
| ≤ Elementary school | 34.8 (1.1) | 51.4 (2.36) | 36.7 (1.45) | 22.7 (2.04) | 16 (2.76) | 60.5 (1.1) | 80 (1.85) | 58.7 (1.3) | 42.7 (2.7) | 38.7 (5.65) | ||
| Middle school | 17.3 (0.81) | 18.9 (1.95) | 17.9 (1.1) | 15.1 (1.63) | 15.1 (2.74) | 15.4 (0.74) | 8.3 (1.11) | 16.5 (0.91) | 20.2 (2.08) | 17.9 (5.31) | ||
| High school | 27.1 (0.93) | 20.7 (1.89) | 26.4 (1.24) | 30.9 (2.21) | 36 (3.56) | 16.2 (0.84) | 8.8 (1.39) | 17.4 (1.04) | 20.2 (2.22) | 26.7 (5.37) | ||
| ≥ College | 20.8 (1.01) | 8.9 (1.37) | 19 (1.24) | 31.3 (2.28) | 32.9 (3.75) | 7.9 (0.61) | 2.9 (0.8) | 7.4 (0.69) | 16.9 (2.33) | 16.7 (4.15) | ||
| Total energy intake, kcal/d | 2044.97 ± 17.355 | 1953.63 ± 39.179 | 1987.96 ± 22.029 | 2192.93 ± 37.591 | 2255.67 ± 67.919 | < 0.001 | 1555.62 ± 13.166 | 1421.15 ± 25.589 | 1557.81 ± 16.407 | 1690.6 ± 34.464 | 1888.54 ± 86.786 | < 0.001 |
| a | a | b | b | a | b | c | c | |||||
| age adjusted | 1970.12 ± 39.446 | 2002.58 ± 21.396 | 2160.5 ± 36.994 | 2162.03 ± 71.302 | < 0.001 | 1481.37 ± 26.143 | 1543.29 ± 16.058 | 1636.41 ± 34.057 | 1783.29 ± 84.939 | < 0.001 | ||
| Total protein intake, g/d | 69.38 ± 0.723 | 63.4 ± 1.496 | 66.69 ± 0.924 | 77.33 ± 1.635 | 80.5 ± 2.869 | < 0.001 | 51.51 ± 0.546 | 43.94 ± 1.017 | 51.86 ± 0.668 | 58.98 ± 1.502 | 65.4 ± 3.118 | < 0.001 |
| a | a | b | b | a | b | c | c | |||||
| age adjusted | 64.09 ± 1.496 | 67.31 ± 0.912 | 75.97 ± 1.619 | 76.57 ± 2.998 | < 0.001 | 46.89 ± 1.038 | 51.15 ± 0.633 | 56.32 ± 1.441 | 60.23 ± 3.081 | < 0.001 | ||
| Total fat intake, g/d | 34.38 ± 0.561 | 30.26 ± 1.046 | 32.41 ± 0.706 | 39.78 ± 1.358 | 43 ± 2.603 | < 0.001 | 25.89 ± 0.405 | 20.75 ± 0.779 | 25.82 ± 0.479 | 32.03 ± 1.153 | 37.3 ± 2.552 | < 0.001 |
| a | a | b | b | a | b | c | c | |||||
| age adjusted | 30.72 ± 1.061 | 32.81 ± 0.7 | 38.89 ± 1.35 | 40.4 ± 2.668 | < 0.001 | 22.86 ± 0.806 | 25.31 ± 0.457 | 30.13 ± 1.113 | 33.61 ± 2.557 | < 0.001 | ||
| Hypertension, % | 48.5 (1.04) | 51.5 (2.34) | 49.6 (1.38) | 45.6 (2.33) | 42.7 (3.86) | 0.107 | 49.1 (0.99) | 57.2 (2.04) | 48.9 (1.25) | 39.7 (2.56) | 35.1 (5.64) | < 0.001 |
| Diabetes, % | 20.2 (0.84) | 22.8 (1.97) | 19.8 (1.1) | 20.8 (1.96) | 16.3 (2.62) | 0.274 | 16.8 (0.74) | 20.2 (1.73) | 17.1 (0.93) | 11.2 (1.53) | 10.3 (3.15) | 0.001 |
| Arthritis, % | 11.8 (0.66) | 12.6 (1.53) | 11.9 (0.96) | 11.1 (1.34) | 11.3 (2.71) | 0.914 | 39.7 (0.97) | 43 (2.11) | 38.7 (1.26) | 40.9 (2.59) | 33.5 (5.32) | 0.192 |
| Exercise frequency, times/wk | ||||||||||||
| Walking activity | 3.94 ± 0.062 | 0 | 4.96 ± 0.06 | 4.44 ± 0.116 | 4.55 ± 0.182 | < 0.001 | 3.71 ± 0.06 | 0 | 4.76 ± 0.056 | 4.29 ± 0.119 | 4.78 ± 0.302 | 0.002 |
| a | b | ab | a | b | ab | |||||||
| Moderate activity | 1.05 ± 0.043 | 0 | 0 | 4.16 ± 0.097 | 2.61 ± 0.187 | < 0.001 | 0.57 ± 0.029 | 0 | 0 | 3.84 ± 0.097 | 2.38 ± 0.287 | < 0.001 |
| Vigorous activity | 0.31 ± 0.026 | 0 | 0 | 0 | 3.72 ± 0.135 | 0.09 ± 0.011 | 0 | 0 | 0 | 3.58 ± 0.191 | ||
| Exercise duration, min/wk | ||||||||||||
| Walking activity | 302.4 ± 9.4 | 0 | 377.3 ± 13.5 | 337.7 ± 16.8 | 378.57 ± 33.9 | 0.163 | 246.6 ± 7.8 | 0 | 315.9 ± 10.7 | 283.7 ± 18.6 | 332.9 ± 43.9 | 0.290 |
| Moderate activity | 78.5 ± 4.6 | 0 | 0 | 314.9 ± 17.3 | 189.2 ± 19.5 | < 0.001 | 34.2 ± 2.8 | 0 | 0 | 228.7 ± 16.7 | 157.7 ± 30.2 | 0.043 |
| Vigorous activity | 22.81 ± 2.7 | 0 | 0 | 0 | 275.9 ± 25.7 | 4.71 ± 0.7 | 0 | 0 | 0 | 179.84 ± 19.99 | ||
Data with the same lowercase letters indicate non-specific differences between groups, while those with different letters are statistically different, based on post hoc test
Data are expressed as the mean ± SE or the percentage (SE)
Association between physical activity and skeletal muscle index
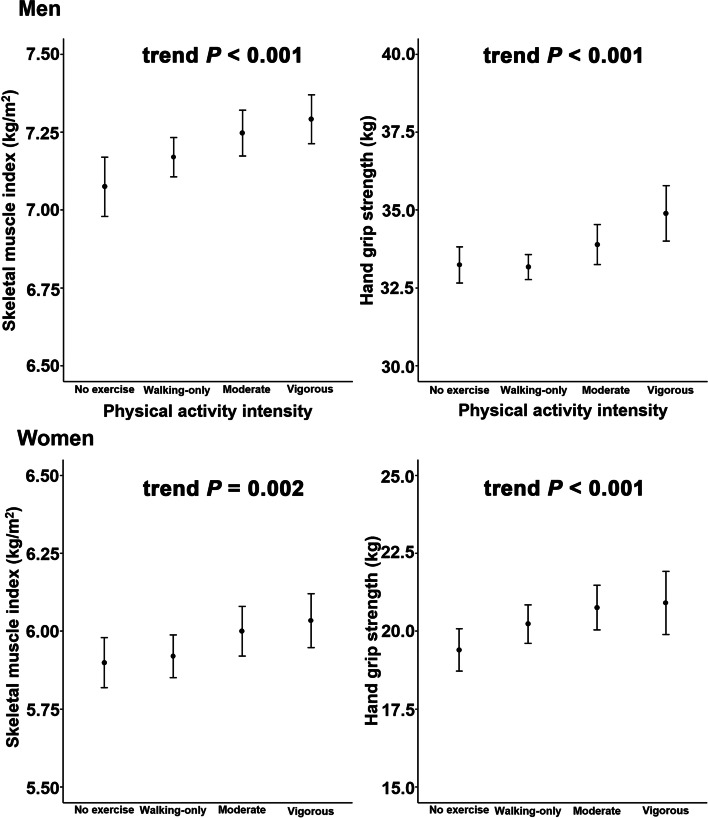
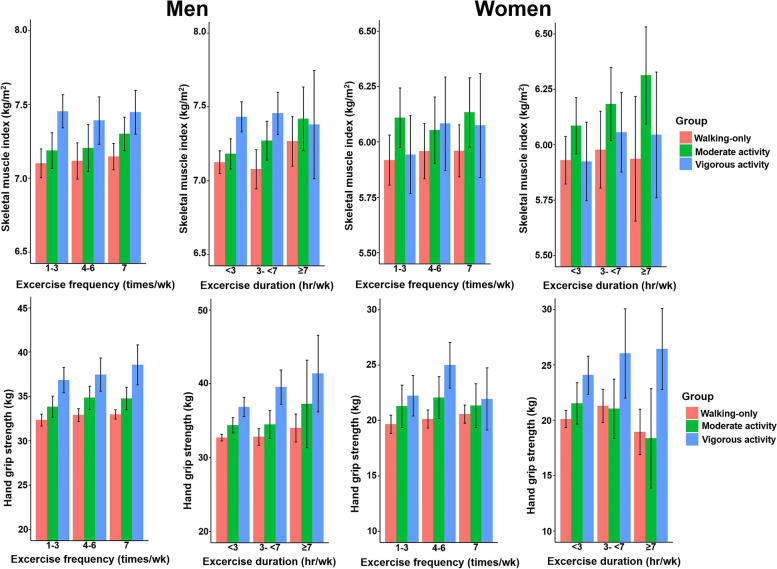
In men and women, both ASM and SMI increased according to PA intensity, and these differences existed even in the models adjusted for age and BMI (all P < 0.05, Table 1). Trend analysis showed that PA intensity was associated with SMI in women and men (P = 0.002 and P < 0.001, respectively; Fig. 2). In men, SMI values based on the frequency and duration of PA did not significantly differ according to PA intensity, except for the duration of PA in the walking-only group (P = 0.013, Table 3 and Fig. 3). In women, SMI values based on the frequency and duration of PA did not differ according to PA intensity, except in the walking-only group (P = 0.001) and the vigorous PA group, which showed significant differences according to exercise duration (P = 0.027).
Fig. 2.
Mean skeletal muscle index and hand grip strength by physical activity intensity using trend analysis. Trend P using a linear regression model after adjusting for age, body mass index, smoking, drinking, monthly income, total energy intake, total protein intake, total fat intake, education level, and diabetes. Error bars indicate 95% confidence intervals
Table 3.
Adjusted mean values of skeletal muscle index and hand grip strength according to the frequency or duration of physical activities in men and women
| Men | Women | |||
|---|---|---|---|---|
| Skeletal muscle index | Hand grip strength | Skeletal muscle index | Hand grip strength | |
| Walking-only group | n = 866 | n = 1684 | n = 1075 | n = 2243 |
| Frequency | ||||
| 1-3 | 7.1 ± 0.05 | 32.35 ± 0.343 | 5.92 ± 0.058 | 19.65 ± 0.424 |
| 4-6 | 7.12 ± 0.062 | 32.9 ± 0.372 | 5.96 ± 0.064 | 20.13 ± 0.417 |
| everyday | 7.15 ± 0.045 | 32.97 ± 0.272 | 5.96 ± 0.06 | 20.57 ± 0.418 |
| P | 0.639 | 0.237 | 0.553 | 0.001 |
| Duration | ||||
| <3 | 7.03 ± 0.048 | 5.93 ± 0.057 | 32.08 ± 0.32 | 19.82 ± 0.414 |
| 3- <7 | 7.14 ± 0.052 | 5.93 ± 0.065 | 32.96 ± 0.345 | 20.43 ± 0.419 |
| ≥7 | 7.18 ± 0.046 | 5.95 ± 0.061 | 33.36 ± 0.28 | 20.53 ± 0.441 |
| P | 0.013 | 0.878 | 0.001 | 0.008 |
| Moderate PA group | n = 426 | n = 603 | n = 567 | n = 469 |
| Frequency | ||||
| 1-3 | 7.19 ± 0.062 | 33.85 ± 0.605 | 6.11 ± 0.068 | 21.29 ± 0.976 |
| 4-6 | 7.21 ± 0.081 | 34.87 ± 0.66 | 6.05 ± 0.076 | 22.07 ± 0.969 |
| everyday | 7.3 ± 0.058 | 34.78 ± 0.645 | 6.13 ± 0.08 | 21.33 ± 1.014 |
| P | 0.273 | 0.218 | 0.488 | 0.152 |
| Duration | ||||
| <3 | 7.16 ± 0.073 | 6.06 ± 0.07 | 33.85 ± 0.577 | 21.01 ± 0.916 |
| 3- <7 | 7.26 ± 0.058 | 6.07 ± 0.072 | 34.46 ± 0.729 | 21.96 ± 0.961 |
| ≥7 | 7.24 ± 0.061 | 6.2 ± 0.082 | 35.57 ± 0.725 | 22.04 ± 1.025 |
| P | 0.424 | 0.072 | 0.073 | 0.030 |
| Vigorous PA group | n = 475 | n = 227 | n = 408 | n = 97 |
| Frequency | ||||
| 1-3 | 7.45 ± 0.057 | 36.84 ± 0.73 | 5.94 ± 0.09 | 22.23 ± 0.932 |
| 4-6 | 7.39 ± 0.082 | 37.47 ± 0.947 | 6.08 ± 0.108 | 24.99 ± 1.051 |
| everyday | 7.45 ± 0.076 | 38.55 ± 1.153 | 6.07 ± 0.119 | 21.94 ± 1.429 |
| P | 0.748 | 0.369 | 0.058 | 0.011 |
| Duration | ||||
| <3 | 7.41 ± 0.076 | 5.76 ± 0.09 | 36.54 ± 0.688 | 24.15 ± 0.851 |
| 3- <7 | 7.43 ± 0.058 | 6.06 ± 0.096 | 37.1 ± 0.853 | 23.00 ± 1.248 |
| ≥7 | 7.47 ± 0.07 | 6.08 ± 0.095 | 39.57 ± 1.124 | 24.69 ± 1.389 |
| P | 0.792 | <0.001 | 0.027 | 0.404 |
Linear regression analysis adjusted with age, BMI, smoking, alcohol intake, total energy intake, total protein intake, total fat intake, monthly household income, education level and diabetes
Fig. 3.
Mean skeletal muscle index and hand grip strength by physical activity frequency and duration. Linear regression model after adjusting for age, body mass index, smoking, drinking, monthly income, total energy intake, total protein intake, total fat intake, education level, and diabetes. Error bars indicate 95% confidence intervals
Association between physical activity and hand grip strength
HGS of the right, left, and dominant hands increased according to PA intensity, and these differences persisted after adjustment for age and BMI (all P < 0.001, Table 2). Trend analysis revealed that PA intensity was associated with HGS in both men and women (P < 0.001 and P < 0.001, respectively; Fig. 2). In men, HGS values based on the frequency and duration of PA did not significantly differ according to PA intensity, except for the duration of PA in the vigorous PA group (P < 0.001, Table 3 and Fig. 3). In women, the frequencies of PA based on HGS were significantly different in the walking-only and vigorous PA groups (P = 0.001, P = 0.011, respectively), while the PA duration as associated with HGS differed in the walking-only and moderate PA groups (P = 0.008, P = 0.030, respectively).
Logistic regression model for physical activity
For men engaged in vigorous PA, the ORs of sarcopenia as defined based on SMI were 0.468 (95% CI: 0.298 − 0.734) in model 1, 0.529 (95% CI: 0.326 − 0.858) in model 2, 0.450 (95% CI: 0.250 − 0.808) in model 3, and 0.444 (95% CI: 0.242 − 0.818) in model 4 (Table 4). Men engaged in moderate PA also exhibited a lower risk of sarcopenia as defined based on SMI (OR = 0.559, 95% CI: 0.354 − 0.883 in model 1; OR = 0.606, 95% CI: 0.374 − 0.984 in model 2; OR = 0.505, 95% CI: 0.287 − 0.888 in model 3; and OR = 0.512, 95% CI: 0.289 − 0.907 in model 4); however, in women, there was no risk reduction for sarcopenia as defined based on SMI according to PA intensity.
Table 4.
Odds ratio for sarcopenia according to physical activities intensity
| no Exercise | Walking-only | Moderate PA | Vigorous PA | P | |
|---|---|---|---|---|---|
| Men | |||||
| Sarcopenia (Skeletal muscle index) | n = 184 | n = 866 | n = 426 | n = 475 | |
| Unadjusted | 1 | 0.706 (0.485–1.029) | 0.542 (0.351–0.837)† | 0.390 (0.254–0.601)† | < 0.001 |
| Model 1 | 1 | 0.710 (0.479–1.052) | 0.559 (0.354–0.883)* | 0.468 (0.298–0.734)† | < 0.001 |
| Model 2 | 1 | 0.748 (0.485–1.152) | 0.606 (0.374–0.984)* | 0.529 (0.326–0.858)* | 0.003 |
| Model 3 | 1 | 0.671 (0.419–1.075) | 0.505 (0.287–0.888)* | 0.450 (0.250–0.808)† | 0.005 |
| Model 4 | 1 | 0.644 (0.395–1.049) | 0.512 (0.289–0.907)* | 0.444 (0.242–0.818)† | 0.010 |
| Sarcopenia (Hand grip strength) | n = 595 | n = 1684 | n = 603 | n = 227 | |
| Unadjusted | 1 | 0.824 (0.636–1.068) | 0.479 (0.337–0.680)† | 0.166 (0.083–0.333)† | < 0.001 |
| Model 1 | 1 | 0.831 (0.624–1.107) | 0.636 (0.438–0.924)* | 0.283 (0.143–0.563)† | < 0.001 |
| Model 2 | 1 | 0.957 (0.729–1.257) | 0.865 (0.595–1.259) | 0.431 (0.216–0.861)* | 0.040 |
| Model 3 | 1 | 0.865 (0.646–1.158) | 0.672 (0.460–0.982)* | 0.293 (0.149–0.576)† | < 0.001 |
| Model 4 | 1 | 0.996 (0.752–1.319) | 0.912 (0.624–1.332) | 0.450 (0.228–0.890)* | 0.070 |
| Women | |||||
| Sarcopenia (Skeletal muscle index) | n = 420 | n = 1075 | n = 567 | n = 408 | |
| Unadjusted | 1 | 1.021 (0.737–1.414) | 0.630 (0.433–0.918)* | 0.593 (0.388–0.904)* | < 0.001 |
| Model 1 | 1 | 1.186 (0.848–1.658) | 0.745 (0.507–1.095) | 0.770 (0.499–1.187) | 0.018 |
| Model 2 | 1 | 1.216 (0.858–1.724) | 0.785 (0.526–1.172) | 0.795 (0.508–1.245) | 0.034 |
| Model 3 | 1 | 1.213 (0.814–1.809) | 0.726 (0.470–1.122) | 0.649 (0.390–1.082) | 0.005 |
| Model 4 | 1 | 1.304 (0.861–1.975) | 0.791 (0.510–1.227) | 0.694 (0.413–1.164) | 0.009 |
| Sarcopenia (Hand grip strength) | n = 823 | n = 2243 | n = 469 | n = 97 | |
| Unadjusted | 1 | 0.393 (0.327–0.472)† | 0.246 (0.181–0.336)† | 0.153 (0.076–0.307)† | < 0.001 |
| Model 1 | 1 | 0.566 (0.466–0.689)† | 0.446 (0.323–0.616)† | 0.383 (0.183–0.800)* | < 0.001 |
| Model 2 | 1 | 0.632 (0.516–0.775)† | 0.541 (0.387–0.754)† | 0.463 (0.210–1.021) | < 0.001 |
| Model 3 | 1 | 0.566 (0.464–0.690)† | 0.440 (0.318–0.608)† | 0.368 (0.176–0.771)† | < 0.001 |
| Model 4 | 1 | 0.628 (0.510–0.773)† | 0.534 (0.382–0.747)† | 0.441 (0.199–0.975)* | < 0.001 |
Unadjusted: no adjustment; model 1: adjusted by age; model 2: age, smoking, alcohol intake, total energy intake, total protein intake, total fat intake, monthly household income, education level and diabetes; model 3: age and BMI; model 4: age, BMI, smoking, alcohol intake, total energy intake, total protein intake, total fat intake, monthly household income, education level and diabetes
*: indicate, if P < 0.05, †: indicate, if P < 0.01
Men engaged in vigorous PA also showed a lower risk of sarcopenia as defined based on HGS (OR = 0.283, 95% CI: 0.143 − 0.563 in model 1; OR = 0.431, 95% CI: 0.216 − 0.861 in model 2; OR = 0.293, 95% CI: 0.149–0.576 in model 3; and OR = 0.450, 95% CI: 0.228 − 0.890 in model 4) (Table 4). For men engaged in moderate PA, the ORs of sarcopenia as defined based on HGS were significant only in models 1 (0.636, 95% CI: 0.438 − 0.924) and 3 (0.672, 95% CI: 0460 − 0.982). Women in the vigorous PA group also demonstrated a lower risk of sarcopenia as defined based on HGS (OR = 0.383, 95% CI: 0.183 − 0.800 in model 1; OR = 0.368, 95% CI: 0.176 − 0.771 in model 3; and OR = 0.441, 95% CI: 0.199 − 0.975 in model 4). For women in the moderate PA group, the ORs of sarcopenia as defined based on HGS were 0.446 (95% CI: 0.323 − 0.616) in model 1, 0.541 (95% CI: 0.387 − 0.754) in model 2, 0.440 (95% CI: 0.318 − 0.608) in model 3, and 0.534 (95% CI: 0.382 − 0.747) in model 4. In women, risk reduction was observed in those engaged in walking only, whereby ORs of sarcopenia as defined based on HGS were 0.566 (95% CI: 0.466 − 0.689) in model 1, 0.632 (95% CI: 0.516 − 0.775) in model 2, 0.566 (95% CI: 0.464 − 0.690) in model 3, and 0.628 (95% CI: 0.510 − 0.773) in model 4.
Discussion
Our study showed a positive correlation between PA intensity and both SMI and HGS in men and women aged ≥ 60 years. Men engaged in moderate-to-vigorous PA had a lower risk of sarcopenia as defined based on SMI than in those who did not exercise, although this relationship was not observed in women. However, PA intensity was associated with a significant reduction in the risk of sarcopenia as defined based on HGS in both men and women.
It is well established that PA improves physical function and quality of life, thereby reducing the burden of chronic disease. Indeed, PA influences key drivers of aging, including chronic inflammation, oxidative damage, and reduced insulin-like growth factor signaling [23, 24]. Our results are similar to the results of a meta-analysis that recommended the use of regular vigorous intensity resistance training rather than walking alone to prevent sarcopenia in older adults [16]. Several studies have reported that resistance training mitigates sarcopenia via satellite cell proliferation and increases muscle hypertrophy [25, 26]. Although a decrease in daily PA due to the decline in muscle function with age is common, it remains unclear whether PA intensity can prevent muscle aging. In our study, PA intensity was associated with skeletal muscle mass, including SMI and ASM, which was consistent with a previous study on skeletal muscle mass in older women in Japan [27]. A recent study demonstrated that the risk of sarcopenic obesity due to active PA was decreased by 45% in men and 29% in women [28].
Muscle strength measurement is relatively simpler and less expensive than muscle mass measurement. HGS is a measure of muscle strength that is widely used for the evaluation of myopathy [29]. Poor HGS is independently associated with a high risk of falls in older adults [30]. A previous study on risk factors associated with low HGS using a similar cohort [31] reported that a low HGS was associated with various factors including alcohol consumption, exercise, education, and BMI. In our study, PA amount was classified according to its intensity, frequency, and duration, and cut-off values of HGS < 28 kg for men and < 18 kg for women were used according to the 2019 AWGS [9]. In contrast, in the previous study, the group engaged in 150 min or more of exercise was defined as a PA group, and cut-off values of 28.9 kg for men and 16.8 kg for women were used to define sarcopenia [31]. In our study, logistic regression analysis revealed a strong relationship between PA intensity and HGS, but the relationship between PA intensity and SMI did not exhibit a protective effect in women. This result differs from that of a recent study demonstrating that regular PA in older women promotes the maintenance of muscle mass and prevents sarcopenia [32]. This discrepancy could be due to 1) the definition for sarcopenia in women based on SMI being strict (20.8%) whereas that based on HGS is more conservative and sensitive (31.6%) in the AWGS criteria, and 2) differences in muscle mass and strength, which may be due to physiological differences between women and men, hormonal changes, and aging mechanisms [33]. This suggests that HGS in women more strongly reflects the effects of PA than SMI.
The International Exercise Recommendations in Older Adults (ICFSR) consensus guidelines were developed in a study that evaluated PA and exercise for health promotion in older adults and provided various strategies based on intended outcomes for lifestyle integration [34]. PA volume, intensity, and modality-specific adaptations should be considered during the prescription of PA/exercise for health. Hence, individualized PA/exercise programs would be desirable based on the intended outcomes. In this regard, our study is meaningful in that it summarizes the amount of PA/exercise in older Korean adults, which may be reflected in the ICFSR consensus guidelines. A standard approach in IPAQ-SF is an analytical method based on metabolic equivalents (MET) [35]. However, a systematic review revealed that the IPAQ-SF has a low validity, although correlations of IPAQ-SF score have been observed with amount of vigorous activity and walking [36, 37]. A previous study on a similar cohort that used MET-min per week to determine the relationship between HGS and total PA amount yielded results that are comparable to ours [31]. However, in routine clinical practice, exercise is prescribed in terms of its type and intensity rather than according to MET, and we believe our use of these variables to be a strength of our study [36].
Other strengths of this study are that it includes a large representative population with weighted data that reflects nationwide prevalence estimates, uses recent criteria for sarcopenia, and categorizes PA based on intensity, duration, and frequency [21, 22]. In addition, instead of constructing an exercise program to analyze its effectiveness, we classified exercise patterns based on the validity of the IPAQ-SF in Koreans. Nevertheless, this study has several limitations. First, this was a cross-sectional study. Thus, we were not able to identify causal relationships. Furthermore, we cannot rule out reverse causation: good muscle mass and muscle strength may lead to increased PA in older adults. Although such an interpretation cannot be excluded, the following should be considered: Aerobic exercise induces ATP production in the mitochondria in skeletal muscles and improves aerobic capacity and muscle protein synthesis [38]. In addition, aerobic exercise influences mRNA expression of myostatin and autophagy protein [39, 40]. Resistance exercise is an important strategy for preventing muscle atrophy and increasing muscle strength and mass [11, 12]. Given the limitations of our study and the importance of preventing sarcopenia, further studies are warranted to conclude that the intensity of exercise impacts muscle mass and strength. Second, obtaining both SMI and HGS data from a single cohort would provide better results and enable more complex analyses. However, since the KNHANES was conducted for multidisciplinary purposes, two tests with a similar purpose might not be performed concurrently in a cohort. In addition, comparison of different cohorts provided insight into relevant clinical objectives. Nevertheless, using different definitions of sarcopenia based on muscle strength and mass makes the interpretation of the true impact of PA difficult, and additional research with trend analysis is needed to address these issues. Third, as all information was obtained through self-reported health surveys, there is the potential for recall or acquiescence bias, which could lead to misclassification. Fourth, the relationship between PA amount and sarcopenia might have been estimated incorrectly in our analyses, which were predominantly based on PA intensity. Similar results may be obtained in retrospective studies; therefore, confirmation of our findings through prospective studies is warranted. Fifth, there may be a potential for selection bias or data missing not at random, since all data on missing exposures and outcomes were removed from the analyses. The data on muscle mass and strength of some older adults might have been missing because they were too old and physically weak to go out. To address these issues, missing demographics were ascertained. The mean age of the 2,942 missing individuals was 79 years, and the mean age of the study participants was 69 years, with no significant differences between the two. As a result, we believe these concerns to be minor.
Conclusions
PA intensity was positively correlated with SMI and HGS in men and women aged ≥ 60 years. Logistic regression analysis revealed a strong relationship between PA intensity and SMI and HGS, suggesting that high intensity PA may have protective effects against sarcopenia. In men, the effects of PA are clearly observed in muscle mass and strength. In contrast, in women, the effects of PA are reflected in HGS rather than SMI, and further studies are warranted to investigate this difference.
Acknowledgements
The authors thank the Korea National Health and Nutrition Examination Surveys (KNHANES) (https://knhanes.kdca.go.kr/knhanes/eng/index.do)
Abbreviations
- ASM
Appendicular skeletal muscle mass
- AWGS
Asian Working Group for Sarcopenia
- BMI
Body mass index
- CI
Confidence interval
- DXA
Dual-energy X-ray absorptiometry
- HGS
Hand grip strength
- ICFSR
International Exercise Recommendations in Older Adults
- IPAQ-SF
International Physical Activity Questionnaire-Short Form
- KNHANES
Korea National Health and Nutrition Examination Surveys
- OR
Odds ratio
- MET
Metabolic equivalent
- PA
Physical activity
- SE
Standard error
- SMI
Skeletal muscle index
Authors' contributions
JHS designed the study concept and design. JHS and YL contributed to the acquisition, analysis, or interpretation of data. YL performed statistical analysis and provided administrative, technical, or material support. JHS wrote and revised the manuscript. JHS supervised the study. All authors read and approved the final manuscript.
Funding
This study was supported by a Veterans Health Service Medical Center Research Grant (grant no. VHSMC21052).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the Korea National Health & Nutrition Examination Survey (KNHANES) official website. (https://knhanes.kdca.go.kr/knhanes/eng/index.do).
Declarations
Ethics approval and consent to participate
The Institutional Review Board of the VHS Medical Center approved the study protocol and waived the requirement for informed consent (IRB No. 2021–05-006), and the study was conducted in compliance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, Guralnik JM, Ferrucci L. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64(3):377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Tsuda Y, Kimura M, Hayashida I, Kusabiraki T, Kono K. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch Gerontol Geriatr. 2012;55(2):e9–13. doi: 10.1016/j.archger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M. Asian Working Group for S: Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2016;17(8):767. doi: 10.1016/j.jamda.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12(6):403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 11.Sousa N, Mendes R, Silva A, Oliveira J. Combined exercise is more effective than aerobic exercise in the improvement of fall risk factors: a randomized controlled trial in community-dwelling older men. Clin Rehabil. 2017;31(4):478–486. doi: 10.1177/0269215516655857. [DOI] [PubMed] [Google Scholar]
- 12.Johnston AP, De Lisio M, Parise G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl Physiol Nutr Metab. 2008;33(1):191–199. doi: 10.1139/H07-141. [DOI] [PubMed] [Google Scholar]
- 13.Konopka AR, Douglass MD, Kaminsky LA, Jemiolo B, Trappe TA, Trappe S, Harber MP. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci. 2010;65(11):1201–1207. doi: 10.1093/gerona/glq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bori Z, Zhao Z, Koltai E, Fatouros IG, Jamurtas AZ, Douroudos II, Terzis G, Chatzinikolaou A, Sovatzidis A, Draganidis D, et al. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol. 2012;47(6):417–424. doi: 10.1016/j.exger.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breen L, Phillips SM. Interactions between exercise and nutrition to prevent muscle waste during ageing. Br J Clin Pharmacol. 2013;75(3):708–715. doi: 10.1111/j.1365-2125.2012.04456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steffl M, Bohannon RW, Sontakova L, Tufano JJ, Shiells K, Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:835–845. doi: 10.2147/CIA.S132940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, Reginster JY, Chapurlat R, Chan DC, Bruyere O, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang S, Moon MK, Kim W, Koo BK. Association between muscle strength and advanced fibrosis in non-alcoholic fatty liver disease: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2020;11(5):1232–1241. doi: 10.1002/jcsm.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Shin S, Hong N, Rhee Y. Low Serum Vitamin E Level Associated with Low Hand Grip Strength in Community-Dwelling Adults: Korean National Health and Nutrition Examination Survey (KNHANES VII) 2016-2018. Nutrients. 2021;13(5):1598. doi: 10.3390/nu13051598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong J, Shin WK, Lee JW, Kim Y. Relationship Between Protein Intake and Sarcopenia in the Elderly with Nonalcoholic Fatty Liver Disease Based on the Fourth and Fifth Korea National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2021;19(8):452–459. doi: 10.1089/met.2021.0011. [DOI] [PubMed] [Google Scholar]
- 21.Kim YA, Lee Y, Lee JH, Seo JH. Effects of physical activity on bone mineral density in older adults: Korea National Health and Nutrition Examination Survey, 2008–2011. Arch Osteoporos. 2019;14(1):103. doi: 10.1007/s11657-019-0655-5. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Lee Y, Seo JH, Kim YA. Association between Exercise and Metabolic Syndrome in Koreans. J Obes Metab Syndr. 2018;27(2):117–124. doi: 10.7570/jomes.2018.27.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenzuela PL, Castillo-Garcia A, Morales JS, Izquierdo M, Serra-Rexach JA, Santos-Lozano A, Lucia A. Physical Exercise in the Oldest Old. Compr Physiol. 2019;9(4):1281–1304. doi: 10.1002/cphy.c190002. [DOI] [PubMed] [Google Scholar]
- 24.Izquierdo M, Morley JE, Lucia A. Exercise in people over 85. BMJ. 2020;368:m402. doi: 10.1136/bmj.m402. [DOI] [PubMed] [Google Scholar]
- 25.Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci. 2003;58(10):M918–922. doi: 10.1093/gerona/58.10.M918. [DOI] [PubMed] [Google Scholar]
- 26.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64(3):332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakayama S, Fujita Y, Fujii K, Sasaki T, Yuine H, Hotta K. Skeletal Muscle Mass and Higher-Level Functional Capacity in Female Community-Dwelling Older Adults. Int J Environ Res Public Health. 2021;18(13):6692. doi: 10.3390/ijerph18136692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Son J, Yu Q, Seo JS. Sarcopenic obesity can be negatively associated with active physical activity and adequate intake of some nutrients in Korean elderly: Findings from the Korea National Health and Nutrition Examination Survey (2008–2011) Nutr Res Pract. 2019;13(1):47–57. doi: 10.4162/nrp.2019.13.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohannon RW. Grip Strength: An Indispensable Biomarker For Older Adults. Clin Interv Aging. 2019;14:1681–1691. doi: 10.2147/CIA.S194543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neri SGR, Lima RM, Ribeiro HS, Vainshelboim B. Poor handgrip strength determined clinically is associated with falls in older women. J Frailty Sarcopenia Falls. 2021;6(2):43–49. doi: 10.22540/JFSF-06-043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CR, Jeon YJ, Jeong T. Risk factors associated with low handgrip strength in the older Korean population. PLoS One. 2019;14(3):e0214612. doi: 10.1371/journal.pone.0214612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edholm P, Veen J, Kadi F, Nilsson A. Muscle mass and aerobic capacity in older women: Impact of regular exercise at middle age. Exp Gerontol. 2021;147:111259. doi: 10.1016/j.exger.2021.111259. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107(2):123–136. doi: 10.1016/S0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 34.Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, Aubertin-Leheudre M, Bernabei R, Cadore EL, Cesari M, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021;25(7):824–853. doi: 10.1007/s12603-021-1665-8. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa-Takata K, Tabata I, Sasaki S, Rafamantanantsoa HH, Okazaki H, Okubo H, Tanaka S, Yamamoto S, Shirota T, Uchida K, et al. Physical activity level in healthy free-living Japanese estimated by doubly labelled water method and International Physical Activity Questionnaire. Eur J Clin Nutr. 2008;62(7):885–891. doi: 10.1038/sj.ejcn.1602805. [DOI] [PubMed] [Google Scholar]
- 36.Kim YA. Association between Exercise and Metabolic Syndrome in Koreans (J Obes Metab Syndr 2018;27:117–24) J Obes Metab Syndr. 2018;27(4):264–266. doi: 10.7570/jomes.2018.27.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erlich AT, Tryon LD, Crilly MJ, Memme JM, Moosavi ZSM, Oliveira AN, Beyfuss K, Hood DA. Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr Med Res. 2016;5(3):187–197. doi: 10.1016/j.imr.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko IG, Jeong JW, Kim YH, Jee YS, Kim SE, Kim SH, Jin JJ, Kim CJ, Chung KJ. Aerobic exercise affects myostatin expression in aged rat skeletal muscles: a possibility of antiaging effects of aerobic exercise related with pelvic floor muscle and urethral rhabdosphincter. Int Neurourol J. 2014;18(2):77–85. doi: 10.5213/inj.2014.18.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012;40(3):159–164. doi: 10.1097/JES.0b013e3182575599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the Korea National Health & Nutrition Examination Survey (KNHANES) official website. (https://knhanes.kdca.go.kr/knhanes/eng/index.do).