Abstract
The effects of various regimens containing combinations of β-lactams, β-lactam inhibitor(s), and rifampin were assessed in a recently described mouse model of Acinetobacter baumannii pneumonia (M. L. Joly-Guillou, M. Wolff, J. J. Pocidalo, F. Walker, and C. Carbon, Antimicrob. Agents Chemother. 41:345–351, 1997). Two aspects of the therapeutic response were studied: the kinetics of the bactericidal effect (treatment was initiated 3 h after intratracheal inoculation, and bacterial counts were determined over a 24-h period) and survival (treatment was initiated 8 h after inoculation, and the cumulative mortality rate was assessed on day 5). Two clinical strains were used: a cephalosporinase-producing strain (SAN-94040) and a multiresistant strain (RCH-69). For SAN-94040 and RCH-69, MICs and MBCs (milligrams per liter) were as follows: ticarcillin, 32, 64, 256, and >256, respectively; ticarcillin-clavulanate, 32, 64, and 512, and >512, respectively; imipenem, 0.5, 0.5, 8, and 32, respectively; sulbactam, 0.5, 0.5, 8, and 8, respectively; and rifampin, 8, 8, 4, and 4, respectively. Against SAN-94040, four regimens, i.e., imipenem, sulbactam, imipenem-rifampin, and ticarcillin-clavulanate (at a 25/1 ratio)-sulbactam produced a true bactericidal effect (≥3-log10 reduction of CFU/g of lung). The best survival rate (i.e., 93%) was obtained with the combination of ticarcillin-clavulanate-sulbactam, and regimens containing rifampin provided a survival rate of ≥65%. Against RCH-69, only regimens containing rifampin and the combination of imipenem-sulbactam had a true bactericidal effect. The best survival rates (≥80%) were obtained with regimens containing rifampin and sulbactam. These results suggest that nonclassical combinations of β-lactams, β-lactamase inhibitors, and rifampin should be considered for the treatment of nosocomial pneumonia due to A. baumannii.
Acinetobacter baumannii is recognized as an increasingly resistant nosocomial pathogen, responsible for pneumonia especially in mechanically ventilated patients (7). Recent isolates of A. baumannii have exhibited antibiotic resistance, making them extremely difficult to treat (13). The majority of clinical isolates of A. baumannii overproduce cephalosporinase and are resistant to aminoglycosides. In addition, strains resistant to virtually all antibiotics, including imipenem, were recently responsible for outbreaks in intensive care unit patients (9). Thus, since there is no “gold standard” for the treatment of nosocomial pneumonia due to multiresistant A. baumannii, new potentially active regimens must be urgently evaluated. We previously demonstrated the enhanced in vitro killing of A. baumannii by β-lactamase inhibitors combined with β-lactams, particularly ticarcillin-clavulanate and sulbactam (14). When we assessed the in vitro activities of rifampin against 30 strains of A. baumannii, the median MIC was 3 mg/liter and was independent of β-lactam resistance (unpublished data). We recently described a new mouse model of A. baumannii pneumonia which offers a reproducible acute course of pneumonia and provides a rigorous test of therapeutic drug efficacy (15). The current study was designed to evaluate the efficacies of various monotherapies and combined regimens including β-lactams, β-lactamase inhibitors, and/or rifampin in treatment of experimental pneumonia caused by A. baumannii.
(This study was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., 15 to 18 September 1996, and the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September to 1 October 1997.)
MATERIALS AND METHODS
Drugs used.
The antimicrobial agents used in this study were obtained from laboratory standard powders and were used immediately after being diluted. The agents and their suppliers were ticarcillin and ticarcillin-clavulanate at ratios of 25/1, 15/1, and 10/1 (SmithKline Beecham, Nanterre, France); sulbactam (Pfizer, Orsay, France); imipenem (Merck Sharp & Dohme, Paris, France); and rifampin (Marion Merrell SA, Puteaux, France).
Bacterial strains.
Two different strains were used. SAN-94040 is a cephalosporinase-overproducing strain resistant to aminoglycosides and fluoroquinolones but susceptible to imipenem, ticarcillin, and sulbactam. It was isolated from the blood culture of a patient with nosocomial pneumonia. RCH-69 is a multiresistant strain with low susceptibility to imipenem and susceptibility only to rifampin. It was isolated from the peritoneal fluid of a patient with postoperative peritonitis.
In vitro tests. (i) MICs and MBCs.
MICs and MBCs were determined by agar dilution and broth dilution methods with geometric twofold serial dilutions in Mueller-Hinton broth (MHB). The final inoculum was 106 CFU/ml. The MIC was determined after incubation at 30°C for 18 h. MBC endpoints were determined by subculturing 100 μl from the first cloudy tube on MHB agar. The MBC was defined as the antibiotic concentration inducing a 99.9% reduction in CFU/ml (<10 CFU/plate) (20). All controls and test samples were run in triplicate.
(ii) In vitro bactericidal effects of β-lactams, β-lactams and β-lactamase inhibitors, and rifampin.
Tubes containing fresh MHB and appropriate amounts of antibiotics were inoculated with an aliquot from a 6-h culture to give a final density of 107 CFU/ml to simulate in vivo conditions at the start of therapy. Shaking cultures of SAN-94040 and RCH-69 strains were incubated at 37°C for 18 h. Aliquots were sampled after 0, 3, 5, 7, and 24 h of incubation and immediately diluted with 10 ml of sterile saline (0.9%) to prevent any drug carryover. CFU were counted on agar plates (20). All controls and test samples were run in duplicate in a single experiment. Bactericidal studies were performed with antibiotics at twice the MIC when tested alone and at the MIC when tested in combination. When MICs were too high to have any clinical relevance, antibiotics were tested at the breakpoint. In order to detect resistant mutants, rifampin was also tested at the MIC.
Animal experiments. (i) Experimental infection.
Six-week-old, specific-pathogen-free, C3H/HeN female mice (20 g) were used. Animals were rendered transiently neutropenic by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally (i.p.) (150 mg/kg of body weight) in a volume of 0.2 ml 4 and 3 days before A. baumannii inoculation (day 0). The mice were anesthetized by i.p. injection of 0.2 ml of 0.65% sodium pentobarbital given before bacterial inoculation. Animals were infected by intratracheal instillation via the mouth as previously described (15). Briefly, the trachea was cannulated with a blunt needle, and 50 μl of a bacterial suspension containing 108 CFU/ml (spectrophotometrically controlled) was instilled. The size of inoculum was confirmed by quantitative cultures. The efficacy of inoculation was systematically tested by quantitation of viable organisms in the lungs removed from two control untreated infected animals, immediately after bacterial inoculation and 3 h later.
(ii) In vivo bactericidal effect of therapy.
In these sets of experiments, the treatment was initiated 3 h after inoculation. At that time, the log CFU (per gram of lung tissue) were 7.6 ± 0.49 for animals infected with SAN-94040 and 7.25 ± 0.71 for animals infected with RCH-69. β-Lactams and β-lactamase inhibitors were administered in four i.p. doses, and rifampin was administered as a single dose. Bacterial counts in lungs were determined every 3 h, over a 12-h period from the start of treatment; 15 animals/regimen were used (three animals/data point). For quantitative bacteriological studies, lungs were removed, weighed, and homogenized in 10 ml of saline. Serial 10-fold dilutions of the homogenates were plated onto Trypticase soy agar (0.1 ml; 9-cm-diameter plates). Results are expressed as the means ± standard deviations (SD) of log10 CFU/gram of lung tissue. The lower limit of detection was 102 CFU/g of lung. The Δlog10 was defined for all regimens as the change in bacterial counts from the onset of treatment to 3 h after the last β-lactam dose.
Regimens tested against SAN-94040.
Four i.p. injections of the following regimens were given every 3 h: ticarcillin (500 mg/kg), imipenem (50 mg/kg), sulbactam (100 mg/kg), ticarcillin-clavulanate at a ratio of 25/1 (500/20 mg/kg), ticarcillin (500 mg/kg)-sulbactam (100 mg/kg), ticarcillin-clavulanate (500/20 mg/kg)-sulbactam (100 mg/kg), ticarcillin-clavulanate at a ratio of 15/1 (500/33 mg/kg)-sulbactam (100 mg/kg), and ticarcillin-clavulanate at a ratio of 10/1 (500/50 mg/kg)-sulbactam (100 mg/kg). A single i.p. dose of rifampin (25 mg/kg) was administered alone or combined with imipenem, sulbactam, or ticarcillin-clavulanate (25/1 ratio)-sulbactam. These doses were chosen according to previously published experimental models which have taken into account human kinetics (2, 3, 15, 23).
Regimens tested against RCH-69.
Four i.p. injections of the following regimens were given every 3 h: imipenem (50 mg/kg), sulbactam (100 mg/kg), imipenem (50 mg/kg)-sulbactam (100 mg/kg), and ticarcillin-clavulanate at a ratio of 25/1 (500/20 mg/kg)-sulbactam (100 mg/kg). A single i.p. dose of rifampin (25 mg/kg) (23) was administered alone or combined with imipenem or ticarcillin-clavulanate (25/1)-sulbactam.
Effect of therapy on survival rate.
In our previously described model, mice were neutropenic only during the first 2 days of infection. Transient leukocytosis was observed on day 3 (12,000/mm3). After day 4, surviving animals cleared bacteria. In these experiments, treatment was initiated 8 h after inoculation, when histological patterns of pneumonia were present (15). At that time, the log CFU (per gram of lung tissue) were 9.3 ± 0.44 for animals infected with the SAN-94040 strain and 8.7 ± 0.7 for animals infected with RCH-69. The same regimens as those administered in the in vivo bactericidal experiments were given for the survival study. β-Lactams and β-lactamase inhibitors were administered every 3 h as five i.p. injections, and rifampin was administered as a single i.p. dose. The observation period was 5 days, a time at which no further deaths occurred. Cumulative survival rates were recorded daily and compared. Controls consisted of infected, untreated animals. Experiments were repeated twice with 15 to 18 animals in each treatment group and 22 to 27 control animals.
Pharmacokinetic parameters.
Pharmacokinetic parameters were evaluated for infected mice. Concentrations of antibiotics in lungs and sera were measured after administration of single doses of ticarcillin (500 mg/kg), ticarcillin-clavulanate at a 25/1 ratio, sulbactam (100 mg/kg), and rifampin (25 mg/kg) given 3 h after infection. Animals were killed by exposure to CO2 and exsanguinated by cardiac puncture. Serum was separated and immediately stored at −80°C. The lungs were removed from exsanguinated mice, briefly washed in sterile water, weighed, and cryohomogenized. Sera and lungs were collected from groups of three mice at the following times postinjection: 10, 15, 30, 45, and 60 min and 2 h for ticarcillin, clavulanate, and sulbactam and 10, 30, and 60 min and 2, 6, 12, 24, and 48 h for rifampin. Pharmacokinetic parameters were evaluated by standard methods (10). The parameters were maximal concentration observed (Cmax), time to Cmax, and the elimination half-life calculated by using linear least-squares regression. The inhibitory quotient (IQ) was calculated as follows: IQ = Cmax/MIC. Δt MIC is the time at which the antibiotic level exceeded the MIC in serum or lungs.
Drug assays.
Imipenem concentrations were determined by an agar well microbiological assay with Bacillus subtilis spores (Difco, Detroit, Mich.) as the indicator organism and antibiotic medium 2 (Diagnostics Pasteur, Marnes-la-Coquette, France). Standard samples were prepared in sulfonate buffer, pH 6 (15). The calibration curve was linear from 0.06 to 64 mg/liter, and the limit of sensitivity was 0.06 mg/liter. Variation within replicates was <5%. The method for measuring ticarcillin concentrations was derived from the technique described by Itoh and Yamada (11). Plasma and lung concentrations were determined by reversed-phase high-performance liquid chromatography (RP-HPLC) with UV detection at 205 nm. Chromatographic separation was performed by using a C18 column (Ultrasphere; 250 by 4.6 mm; Beckman) with a mobile phase consisting of PicA-orthophosphoric acid-water-acetonitrile (0.3/0.1/79.5/20%). Proteins were precipitated from the samples with acetonitrile. Limits of quantification were 2 mg/liter and 2 mg/kg for plasma and lung samples, respectively.
The method for dosing clavulanic acid was derived from the technique described by Shah et al (22). Concentrations in plasma and lung were determined by RP-HPLC with UV detection at 311 nm. With a C18 column (μBondapak octadecylsilyl [ODS]; 300 by 3.9 mm; Waters, Guyancourt, France) with a mobile phase consisting of 0.01 M phosphate buffer (pH 3.2), the product was eluted with an acetonitrile gradient (96 to 4%). Proteins were precipitated with acetonitrile, dichloromethane was added for extraction, and the upper aqueous layers were injected onto the column after derivation with triazol. Limits of quantification were 0.1 mg/liter and 0.1 mg/kg for plasma and lung samples, respectively. The method for dosing sulbactam was adapted from the technique reported by Fantin et al. (8). Plasma and lung sulbactam levels were determined by RP-HPLC with UV detection at 313 nm. A C18 column (Hypersil ODS; 250 by 4.6 mm; Shandon, Cergy-Pontoise, France) and a mobile phase consisting of peak A-water-acetonitrile (1/74/25%) were used for extraction. Proteins were precipitated with acetonitrile, and the supernatant was derivatized with triazol at 50°C. Limits of quantification were 1 mg/liter and 1 mg/kg for plasma and lung samples, respectively. The method for dosing rifampin was derived from the technique described by Swart and Papgis (24). Concentrations in plasma and lung were determined by RP-HPLC with UV detection at 342 nm. The product was chromatographically separated with a C18 column (Hypersil ODS) eluted with a mobile phase consisting of 0.05 M citrate buffer (pH 4.3) and acetonitrile (50/50%). Proteins were precipitated with acetonitrile. Limits of quantification were 0.5 mg/liter and 0.5 mg/kg for plasma and lung samples, respectively. Results are expressed in milligrams per liter for sera and milligrams per kilogram for lungs.
Statistical analyses.
All bacterial counts and pharmacokinetic data are presented as means ± SD. Analysis of variance was used to compare intergroup differences between bacterial counts. Survival rate data were analyzed by Student’s t test; P ≤ 0.05 for either test was considered statistically significant.
RESULTS
In vitro studies. (i) MICs and MBCs.
MICs and MBCs (milligrams per liter) for SAN-94040 were as follows: ticarcillin alone, 32 and 64; ticarcillin-clavulanate (ratio, 25/1), 32 and 64; imipenem, 0.5 and 0.5; and sulbactam, 0.5 and 0.5, respectively. The strain exhibited lower susceptibility to rifampin, which had a MIC and an MBC of 8 and 8 mg/liter, respectively. MICs and MBCs (mg/liter) for RCH-69 were as follows: ticarcillin alone, 256 and >256; ticarcillin-clavulanate (ratio, 25/1), 512 and >512; imipenem, 8 and 32; sulbactam, 8 and 8; and rifampin, 4 and 4, respectively.
(ii) In vitro bactericidal studies.
Against the two strains, only two regimens, namely, ticarcillin-clavulanate-sulbactam and rifampin alone, showed a true bactericidal effect (≥3-log decrease at T0 + 7 h). A low bactericidal effect was observed with all other regimens (Table 1). Resistant strains (MIC, 64 mg/liter) were detected after 24 h of SAN-94040 culture containing a rifampin concentration equal to the MIC. With the RCH-69 strain, a regrowth was observed without the emergence of a resistant strain.
TABLE 1.
In vitro bactericidal activities of β-lactams, β-lactamase inhibitors, and rifampin against A. baumannii SAN-94040 and RCH-69a
| Antibiotic(s) | Log CFU of strain/mLb
|
|||
|---|---|---|---|---|
| SAN-94040
|
RCH-69
|
|||
| T0 + 7 h | T0 + 24 h | T0 + 7 h | T0 + 24 h | |
| Imipenem | 4.65 | 7.78 | 5.3 | 8.35 |
| Sulbactam | 4.47 | 4.69 | 4.95 | 6.65 |
| Ticarcillin | 5.37 | 7.7 | NDc | ND |
| Ticarcillin-clavulanate | 4.78 | 7.7 | 6.2d | 9d |
| Rifampin | 2.2 | 2.4 | 1.9 | 5 |
| Imipenem-sulbactam | ND | ND | 5.84 | 8 |
| Ticarcillin-sulbactam | 5 | 9.2 | 4.2 | 7.15 |
| Ticarcillin-clavulanate-sulbactam | 2.2 | 1.4 | 3.69d | 2.3d |
Antibiotics were used at twice the MICs when tested alone and at the MICs when tested in combination.
Control values were 6.86 log CFU of SAN-94040 and 6.3 log CFU of RCH-69 per ml.
ND, not determined.
Ticarcillin was tested at the breakpoint with strain RCH-69.
Pharmacokinetic parameters.
The main pharmacodynamic parameters of each antibiotic are reported in Table 2. For rifampin, high peak concentrations and prolonged half-lives were observed in sera and lungs. Due to differences in MICs, the Cmax/MIC ratios (IQs) and the times above the MICs for the three β-lactams in sera and lungs were much higher against SAN-94040 than against RCH-69. Clavulanate (20 mg/kg) produced peak concentrations in sera and lungs of 35 mg/ml and 17 mg/kg, respectively. The elimination half-lives in sera of the different β-lactams were very short (<0.5 h) compared to that of rifampin (12 h). Although the Δt MICs in sera of the β-lactams differed, they were all much higher for SAN-94040 than for RCH-69. In the lung, the sulbactam Δt MIC was longer than that of imipenem for SAN-94040, and it was the only β-lactam to reach concentrations above the MIC for RCH-69.
TABLE 2.
Pharmacodynamic parameters
| Drug (mg/kg) | Value for sample:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum
|
Lung
|
||||||||||
| Cmaxa (mg/liter) | IQb
|
t1/2 (h)c | Δt MIC (h)
|
Cmaxa (mg/kg) | IQb
|
Δt MIC (h)
|
|||||
| SAN | RCH | SAN | RCH | SAN | RCH | SAN | RCH | ||||
| Ticarcillin (500) | 270 ± 29 | 8.4 | 1.05 | 0.5 | 1.5 | 0.25 | 123 ± 4 | 3.8 | 0.47 | 1.7 | 0 |
| Imipenem (100) | 168 ± 11 | 336 | 10.5 | 0.15 | 2.4 | 0.75 | 10 ± 0.5 | 20 | 0.62 | 2 | 0 |
| Sulbactam (100) | 251 ± 25 | 502 | 31 | 0.35 | >3 | 1.7 | 26 ± 16 | 53 | 3.28 | 4.8 | 1.3 |
| Rifampin (25) | 31 ± 2 | 3.9 | 7.8 | 12 | 30 | 48 | 35 ± 14 | 4.4 | 8.6 | 15 | 21 |
Values are means ± SD for three samples.
IQ = Cmax/MIC calculated with the MICs for the two strains used in experimental infections, SAN-94040 (SAN) and RCH-69 (RCH).
t1/2, elimination half-life.
In vivo efficacy (i) In vivo bactericidal effects against SAN-94040.
Table 3 shows the in vivo bactericidal effects of various regimens against SAN-94040. When the bactericidal effect was assessed at 12 h as the Δlog10, all monotherapies administered, except ticarcillin, significantly reduced the bacterial counts in lungs compared to controls 3 h postinstillation (P < 0.05). However, only two monotherapies, i.e., sulbactam and imipenem, provided true bactericidal effects (≥3-log10 decrease of bacterial count). No significant differences were observed between regimens containing various ratios of clavulanate (data not shown). Only two combinations provided true bactericidal effects, i.e., rifampin-imipenem and ticarcillin-clavulanate (25/1 ratio)-sulbactam, although there was essentially no effect of combinations beyond that of the single most active component.
TABLE 3.
Treatment of experimental pneumonia caused by A. baumannii SAN-94040 or RCH-69 with four doses of β-lactams and one dose of rifampin administered alone or in various combinations
| Treatment group (mg/kg)b | Log CFU/g of lung (mean ± SD)a
|
|
|---|---|---|
| SAN-94040 | RCH-69 | |
| Imipenem (50) | 4.47 ± 0.44c | 4.4 ± 1.1c |
| Sulbactam (100) | 4.31 ± 0.19c | 6.4 ± 1.3 |
| Ticarcillin (500) | 6.70 ± 0.68 | NDd |
| Ticarcillin (500)-clavulanate (25/1 ratio) | 5.79 ± 0.25c | ND |
| Rifampin (25) | 5.02 ± 0.45c | 3.3 ± 0.46c |
| Rifampin (25)-imipenem (50) | 4.3 ± 0.53c | 3.72 ± 0.29c |
| Rifampin (25)-sulbactam (100) | 5.03 ± 0.95c | 4.24 ± 1.1c |
| Imipenem (50)-sulbactam (100) | ND | 4.43 ± 0.7c |
| Ticarcillin (500)-sulbactam (100) | 5.4 ± 0.59c | ND |
| Ticarcillin (500)-clavulanate (25/1 ratio)-sulbactam (100) | 4.25 ± 0.29c | 6.3 ± 0.8 |
| Rifampin (25)-ticarcillin (500)-clavulanate (25/1 ratio)-sulbactam (100) | 5.35 ± 0.87c | 2.55 ± 0.4c |
Bacterial counts were determined 12 h after the first antibiotic dose. Values for controls were 7.6 ± 0.49 log CFU of SAN-94040 and 7.25 ± 0.71 log CFU of RCH-69 per g of lung tissue.
β-Lactams were administered every 3 h, and rifampin was given as a single dose.
P < 0.05 versus control.
ND, not determined.
(ii) Survival in mice infected with SAN-94060.
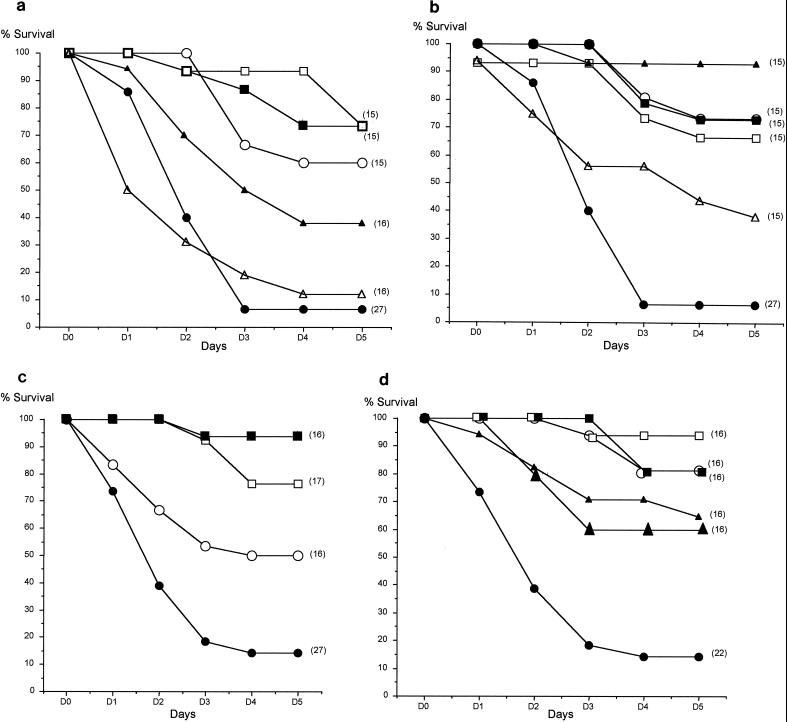
Compared to controls, sulbactam, rifampin, or imipenem alone significantly (P < 0.001) prolonged survival, as did ticarcillin-clavulanate (25/1 ratio) (P = 0.02) (Fig. 1a). Among the regimens administered as combinations, the best survival rate (93%) was obtained with ticarcillin-clavulanate (25/1 ratio)-sulbactam. All combinations containing rifampin yielded a survival rate of at least 65%. Ticarcillin-sulbactam, although less effective, significantly prolonged survival compared to controls (P = 0.02) (Fig. 1b).
FIG. 1.
Cumulative survival rates of treated and control mice challenged with 108 CFU of A. baumannii SAN-94040 (a and b) or RCH-69 (c and d) per mouse. The treatment was initiated 8 h after intratracheal bacterial inoculation (five i.p. doses of β-lactams and one i.p. dose of rifampin). Symbols for monotherapy (a and c): ●, controls; ○, imipenem; □, sulbactam; ▵, ticarcillin; ▴, ticarcillin-clavulanate; ■, rifampin. Symbols for combinations (b and d): ○, rifampin-imipenem; □, rifampin-sulbactam; ▴, imipenem-sulbactam; ▵, ticarcillin-sulbactam; ▴, ticarcillin-clavulanate (25/1 ratio)-sulbactam; ■, rifampin-ticarcillin-clavulanate (25/1 ratio)-sulbactam. The number of mice in each group is indicated in parentheses.
(iii) In vivo bactericidal effects against RCH-69.
Table 3 also shows the in vivo bactericidal effects of the various regimens against RCH-69. All regimens except sulbactam and ticarcillin-clavulanate-sulbactam significantly reduced the bacterial counts in lungs compared to controls 3 h postinstillation (P < 0.05). When the bactericidal effect was assessed at 12 h as the Δlog10, rifampin and imipenem, but not sulbactam, provided true bactericidal effects (≥3-log decrease of bacterial count). Compared to controls 3 h postinstillation, all combined regimens except ticarcillin-clavulanate (25/1 ratio)-sulbactam provided true bactericidal effects. Again, there was essentially no effect of combinations beyond that of the single most active component.
(iv) Survival in mice infected with RCH-69.
Among agents administered as monotherapy (Fig. 1c), the best survival rate was obtained with rifampin (94%). Moreover, rifampin alone at the given dose significantly prolonged survival compared to that for animals treated with imipenem alone (P = 0.01). Although sulbactam was bacteriostatic only in vivo, the survival rate of mice treated with this compound alone reached 76% (Fig. 1c). All combined regimens (Fig. 1d) significantly prolonged survival compared to control values (P < 0.01). The best effect was obtained with those containing rifampin (≥80%). These regimens at the given doses significantly prolonged survival compared to those in animals treated with imipenem-sulbactam (P = 0.03) or ticarcillin-clavulanate-sulbactam (P = 0.05).
DISCUSSION
Faced with the increasing role of A. baumannii in nosocomial pneumonia associated with mechanical ventilation, a mouse model of pneumonia caused by A. baumannii that resembles the human disease (1) was developed (15). Experimental models of acute systemic infection and urinary tract infection with Acinetobacter spp. were first developed in mice in 1985 to examine the virulence factors and the efficacy of therapy with tetracyclines and aminoglycosides (18) or sulbactam (19). These models demonstrated a good correlation between antibiotics that were active in vitro and their 50% effective doses. However, nearly 50% of the strains isolated in France are resistant to all available antibiotics except imipenem and sulbactam. Therefore, treatment of nosocomial infections due to A. baumannii spp. has become extremely complicated, and imipenem is often the only effective treatment that can be prescribed except when imipenem-resistant strains emerge. During the last few years, several outbreaks of imipenem-resistant strains have been described for different intensive care units in Europe and the United States (9, 25). Recently, the emergence of imipenem resistance during treatment was described (5). Imipenem, which is considered to be the gold standard therapy for A. baumannii infections, had a bactericidal effect in the lung against both of our tested strains. However, the magnitude of this effect was relatively low, being around 3 log10 CFU, even after administration of four i.p. doses. The in vivo bactericidal effect observed against RCH-69 may be explained by the high concentrations of imipenem obtained in the lungs and the presence of a postantibiotic effect of long duration (15). This suboptimal effect was associated with a moderate efficacy of mouse survival. It is tempting to draw a parallel between these experimental results and the high mortality rate in patients with ventilator-acquired pneumonia caused by A. baumannii and usually treated with imipenem (7). These results prompted us to evaluate therapeutic alternatives to imipenem in this model. An in vitro study with a killing curve showed a synergistic or additive effect of nonclassical combinations with β-lactamase inhibitors (clavulanate, sulbactam, or tazobactam) and ticarcillin or piperacillin (14). That study pointed out the intrinsic activity of sulbactam, which acts as a true β-lactam against A. baumannii. The in vivo antibacterial activity of sulbactam had already been tested against Acinetobacter calcoaceticus in mouse peritonitis (19). This drug was administered alone or in the ampicillin-sulbactam formulation to treat patients with nosocomial infections caused by imipenem-resistant Acinetobacter strains (4, 26). In the present study, sulbactam produced a bactericidal effect in mice inoculated with the susceptible SAN-94040 strain but not in mice inoculated with RCH-69. The dose used in our experiments was the same as that previously given to mice (3). Three hours after i.p. injection, concentrations in serum were very similar to those obtained in humans after intravenous administration of 1 g (27). In addition, the maximum pulmonary concentrations of sulbactam in mice were also comparable to those measured in the alveolar lining fluid of patients with respiratory infections (27). The Δt MIC appeared to be an important pharmacodynamic determinant of the bactericidal activity of sulbactam in mouse lungs. Indeed, pulmonary sulbactam concentrations were above the MIC for SAN-94040 throughout the entire interval between two doses but during only 43% of the interdose interval in animals challenged with RCH-69. This finding is in accordance with previous experimental data (6). Nonetheless, sulbactam achieved a satisfactory survival rate in animals inoculated with SAN-94040 or RCH-69, despite the differences observed in their in vivo bactericidal effects. These experimental results suggest that sulbactam administration to treat severe pulmonary infections caused by A. baumannii should ensure concentrations in lung tissue above the MIC throughout the entire interval between doses. Thus, short intervals and high doses (e.g., 4 to 6 g), such as those used with a true β-lactam, are recommended. Ticarcillin was not effective against SAN-94040, although its level in the lungs was above the MIC for the strain during 57% of the dosing interval. This result is in accordance with a previous in vitro study in which ticarcillin was less bactericidal than sulbactam against a cephalosporinase-producing strain (14). We used a ticarcillin-clavulanate dosage which provided concentrations in serum within the ranges of those previously reported for mice (2) and humans (12). The ticarcillin-clavulanic acid combination at a 25/1 ratio was moderately active, providing a weak bactericidal effect in lungs (<2 log10 CFU) and a mortality rate of 60% in animals infected with SAN-94040. In addition, no further benefit was obtained by increasing the clavulanic acid dose within the combination. The absence of clavulanic acid activity against cephalosporinase-producing strains could explain the poor results observed in our model. At present, we do not know why the ticarcillin-clavulanate-sulbactam combination yielded better activity, as previously observed in vitro (14), and why the combination of ticarcillin and sulbactam appeared to be antagonistic compared to sulbactam alone. Given the limited number of available antibiotics active against multiresistant strains, it is crucial to evaluate new potentially effective regimens. As a matter of fact, the in vitro bactericidal effect of rifampin observed against both strains led us to test this antibiotic alone and in combination in our model. Rifampin was very effective, providing a strong bactericidal effect in lungs against RCH-69 and a high survival rate in animals inoculated with either strain. These findings may reflect the very long time during which rifampin concentrations in both sera and lungs are above the MICs for these strains. The dose of 25 mg/kg was selected on the basis of a previously published model of experimental murine brucellosis (23) in which the maximal concentration of rifampin in serum was 28 mg/liter. In another study, Cmax was 11 mg/liter after a 10-mg/kg dose (17). Thus, although the concentrations observed in our study are very similar to those reported in previous experimental models, they are somewhat higher than those usually observed in humans. However, the relationship between the peak levels in serum and the size of the dose is nonlinear, since larger doses resulted in greater-than-proportional peak levels. Thus, after a 1,200-mg dose, the peak level in serum in patients is usually >30 mg/liter (16). Considering the rifampin MIC against susceptible A. baumannii strains, rifampin should certainly be administered in high doses, like those given for severe staphylococcal infections. Moreover, because of the high risk of development of resistant mutants, rifampin should always be administered in combination therapy. Indeed, in our model, all combinations containing rifampin were effective. Although we did not observe the emergence of resistant mutants in vivo, this phenomenon was detected in vitro after 24 h of culture containing a rifampin concentration equal to the MIC for SAN-94040 (8 mg/liter).
A. baumannii is an opportunistic bacterium with a high degree of resistance to various antibiotics. As a consequence, the antibiotic effect is more often bacteriostatic than bactericidal. These characteristics could explain the discrepancies sometimes observed between a drug’s bactericidal effect and its efficacy in survival, as we observed in the present study for sulbactam and RCH-69. Interference with some other host parameters was probably responsible for these discrepancies. Therefore, both the survival rate and in vivo bactericidal effects in lungs must be taken into consideration for the analysis and interpretation of the global antibiotic activity in this model. This approach has also been applied in a mouse model of pneumonia due to Streptococcus pneumoniae (21).
In conclusion, this study pointed out (i) the relatively low in vivo bactericidal effect of imipenem on A. baumannii isolates, even against a susceptible strain, and (ii) the provision of a good bactericidal effect against a sulbactam-susceptible strain by this drug, which acts as a true β-lactam. However, the best in vivo effect was obtained with the triple combination of ticarcillin-clavulanate-sulbactam. Furthermore, the high efficacy of rifampin suggested that this antibiotic could be used in combination to treat pneumonia caused by strains with reduced susceptibility to imipenem and when the MIC is not >4 mg/liter.
Nonclassical combinations, containing antibiotics such as sulbactam, rifampin, and ticarcillin-clavulanate, could be effective alternative treatments for A. baumannii infections and warrant clinical evaluation.
ACKNOWLEDGMENTS
This work was supported by a grant from SmithKline Beecham, France.
We are indebted to Janet Jacobson for technical assistance in the preparation of the manuscript.
REFERENCES
- 1.Anstey N M, Curry B J, Withnall K M. Community-acquired Acinetobacter pneumonia in the northern territory of Australia. Clin Infect Dis. 1992;14:83–91. doi: 10.1093/clinids/14.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Boon R J, Beale A, Sutherland R. Bactericidal effects of ticarcillin-clavulanic acid against β-lactamase-producing bacteria in vivo. Antimicrob Agents Chemother. 1986;29:838–844. doi: 10.1128/aac.29.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang-Xiao L, Jia-Rong W, Yi-Li L. Pharmacokinetics of sulbactam and ampicillin in mice and in dogs. Eur J Pharmacol. 1990;183:1859–1860. [Google Scholar]
- 4.Corbella X, Ariza J, Ardanuy C, Vuelta M, Tubeau F, Sora M, Pujol M, Gudiol F. Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother. 1998;42:793–802. doi: 10.1093/jac/42.6.793. [DOI] [PubMed] [Google Scholar]
- 5.Costa S F, Wodcock J, Child J, Caiaffa H H, Gill M, Wise R, Levin A S. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Characterization of the β-lactamase and outer-membrane proteins of imipenem-resistant Acinetobacter baumannii clinical isolates from Brazil, abstr. C123; p. 56. [Google Scholar]
- 6.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 7.Fagon J Y, Chastre J, Domart Y, Trouillet J L, Gibert C. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species; assessment by quantitative culture of samples obtained by a protected specimen brush. Clin Infect Dis. 1996;23:538–542. doi: 10.1093/clinids/23.3.538. [DOI] [PubMed] [Google Scholar]
- 8.Fantin B, Pierre J, Castéla-Papin N, Saint-Julien L, Drugeon H, Farinotti R, Carbon C. Importance of penicillinase production for activity of penicillin alone or in combination with sulbactam in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1219–1224. doi: 10.1128/aac.40.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go E S, Urban C, Burns J, Kreiswirth J, Elsner W, Mariano N, Mosinka-Snipas K, Rahal J J. Clinical and molecular epidemiology of Acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet. 1994;344:1329–1332. doi: 10.1016/s0140-6736(94)90694-7. [DOI] [PubMed] [Google Scholar]
- 10.Greenblatt D J, Koch-Weser J. Clinical pharmacokinetics. N Engl J Med. 1975;297:702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- 11.Itoh T, Yamada H. Diastereomeric beta-lactam antibiotics: analytical methods, isomerization and stereo selective pharmacokinetic. J Chromatogr. 1995;694:195–208. doi: 10.1016/0021-9673(94)00932-y. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs R F, Trang J M, Kearns G L, Warren R H, Brown A L, Underwodd F L, Kluza R B. Ticarcillin-clavulanic acid pharmacokinetics in children and young adults with cystic fibrosis. J Pediatr. 1985;106:1001–1007. doi: 10.1016/s0022-3476(85)80258-4. [DOI] [PubMed] [Google Scholar]
- 13.Joly-Guillou M L. Acinetobacter baumannii: sensibilité actuelle aux antibiotiques. Mécanismes de résistance. Fréquence Lett Infectiol. 1997;9:399–404. [Google Scholar]
- 14.Joly-Guillou M L, Decré D, Herrman J L, Bourdelier E, Bergogne-Berezin E. Bactericidal in-vitro activity of β-lactams and β-lactamase inhibitors, alone or associated, against clinical strains of Acinetobacter baumannii: effect of combination with aminoglycosides. J Antimicrob Chemother. 1995;36:619–629. doi: 10.1093/jac/36.4.619. [DOI] [PubMed] [Google Scholar]
- 15.Joly-Guillou M L, Wolff M, Pocidalo J J, Walker F, Carbon C. Use of a new model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob Agents Chemother. 1997;41:345–351. doi: 10.1128/aac.41.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucers A, Crowe S, Grayson M L, Hoy J. Rifampicin (rifampin) In: Kucers A, Crowe S, Grayson M L, Hoy J, editors. The use of antibiotics. A comprehensive review with clinical emphasis. 5th ed. Oxford, United Kingdom: William Heinemann Medical Books; 1997. pp. 676–708. [Google Scholar]
- 17.Nordmann P, Kerestedjian J J, Ronco E. Therapy of Rhodococcus equi disseminated infections in nude mice. Antimicrob Agents Chemother. 1992;36:1244–1248. doi: 10.1128/aac.36.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obana Y, Nishino T, Tanino T. In-vitro and in-vivo activities of antimicrobial agents against Acinetobacter calcoaceticus. J Antimicrob Chemother. 1985;15:441–448. doi: 10.1093/jac/15.4.441. [DOI] [PubMed] [Google Scholar]
- 19.Obana Y, Nishino T. In-vitro and in-vivo activities of sulbactam and YTR 830H against Acinetobacter calcoaceticus. J Antimicrob Chemother. 1990;26:677–682. doi: 10.1093/jac/26.5.677. [DOI] [PubMed] [Google Scholar]
- 20.Pearson R R, Steigbeigel R T, Davis H T, Chapman S W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauve C, Azoulay-Dupuis E, Moine P, Darras-Joly C, Rieux V, Carbon C, Bédos J P. Efficacies of cefotaxime and ceftriaxone in a mouse model of pneumonia induced by two penicillin- and cephalosporin-resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:2829–2834. doi: 10.1128/aac.40.12.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah A J, Adlard M W, Stride J D. A sensitive assay for clavulanic acid and sulbactam in biological fluids by high performance liquid chromatography and pre-column derivatization. J Pharm Biomed Anal. 1990;8:437–443. doi: 10.1016/0731-7085(90)80072-w. [DOI] [PubMed] [Google Scholar]
- 23.Shasha B, Lang R, Rubinstein E. Therapy of experimental murine brucellosis with streptomycin, cotrimoxazole, ciprofloxacin, ofloxacin, pefloxacin, doxycycline, and rifampin. Antimicrob Agents Chemother. 1992;36:973–976. doi: 10.1128/aac.36.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swart K J, Papgis M. Automated high performance liquid chromatographic method for determination of rifampicin in plasma. J Chromatogr. 1992;593:21–24. doi: 10.1016/0021-9673(92)80260-2. [DOI] [PubMed] [Google Scholar]
- 25.Tankovic J, Legrand P, de Gatines G, Chemineau V, Brun-Buisson C, Duval J. Characterization of a hospital outbreak of imipenem-resistant Acinetobacter baumannii by phenotypic and genotypic typing methods. J Clin Microbiol. 1994;32:2677–2681. doi: 10.1128/jcm.32.11.2677-2681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urban C, Go E, Mariano N, Berger B J, Avraham I, Rubin D, Rahal J J. Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J Infect Dis. 1993;167:448–451. doi: 10.1093/infdis/167.2.448. [DOI] [PubMed] [Google Scholar]
- 27.Valcke Y J, Roosel M T, Pauwells R A, Bogaert M G, van der Straeten M E. Penetration of ampicillin and sulbactam in the lower airways during respiratory infections. Antimicrob Agents Chemother. 1990;34:958–961. doi: 10.1128/aac.34.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]