Abstract
Background
Curcumol is a hydrogenated austenitic compound with hemiketal. In this study we evaluated the effects of curcumol on local inflammatory response, cell proliferation, and metastasis in endometriosis, and elucidated the underlying mechanisms.
Material/Methods
Ectopic endometrial stromal cells were treated with increasing doses of curcumol. The MTT assay was used to assess cell viability. FITC-labeled annexin-V/PI double-staining method and flow cytometry were used to determine cell apoptosis. Cell migration was evaluated using a wound healing assay. ELISA kits were used to detect the levels of TNF-α, IL-6, and IL-1β. Western blot assay was used to examine the phosphorylation degree of JAK2 and STAT3 and the expression of Bax, Bcl2, and caspase-3 proteins. Autologous endometrial transplantation was used to establish a rat model to assess the anti-EMS effect of curcumol in vivo.
Results
Curcumol can inhibit the proliferation of ectopic endometrial stromal cells, promote cell apoptosis, and weaken cell migration ability. Curcumol can reduce the expression of Bax and caspase-3 protein and increase the expression of Bcl2 protein. Curcumol also can inhibit the secretion of inflammatory cytokines, including tumor necrosis cytokines (TNF)-α, interleukin (IL)-6, and IL-1β, by ectopic endometrial stromal cells. In addition, curcumol can also inhibit the phosphorylation of JAK2 and STAT3. In vivo experiments also proved that curcumol could inhibit the growth of ectopic lesions in EMS model rats.
Conclusions
Curcumol can inhibit the JAK2/STAT3 pathway, reduce the inflammatory cytokines secreted by ectopic endometrial stromal cells, inhibit cell proliferation and migration, and reduce the volume of ectopic lesions.
Keywords: Curcumol; Endometriosis; JAK2 Protein, Human; STAT Transcription Factors
Background
Endometriosis (EMS) is a common chronic disease characterized by the growth of endometrial glands and stroma outside the uterus, mainly on the pelvic peritoneum and ovaries [1]. This disease affects 10–20% of women of childbearing age, 20–30% of women with infertility, and 40–60% of women with dysmenorrhea [2]. Although EMS is a benign disease, EMS cells are known to have characteristics similar to those of malignant cancer cells, such as continuous proliferation, angiogenesis, migration, and invasion [3]. However, the cellular and molecular mechanisms behind these pathological changes have not been fully defined. Sampson found that endometrial cells are transported through the fallopian tube during menstruation, are implanted into the peritoneal surface, undergo heterotopic implantation, and grow as lesions [4]. However, it is unclear why most women have menstrual blood reflux during menstruation, but only 10% of women develop EMS. It is increasingly recognized that the peritoneal microenvironment plays an important role in the migration, implantation, and invasion of ectopic endometrium, and the severity of the inflammatory microenvironment is related to the development of EMS [5,6]. The disease is characterized by increased production of pro-inflammatory cytokines such as IL-6 and tumor necrosis factor alpha (TNF a), which can promote endometrial cell proliferation and adhesion [7,8].
Janus kinase-signal transducers and activators of transcription (JAK-STAT) is an important pathway of cytokine signal transduction. JAK2/STAT3, as an important signal axis in the JAK-STAT pathway, is an important transduction pathway mediated by a variety of cytokines and growth factors and is involved in cell proliferation, differentiation, apoptosis, migration, and immune cell apoptosis [9,10]. There are many activators of the JAK2/STAT3 signaling pathway in patients with EMS, such as IL-6 and TNF α, and these cytokines can activate the JAK2/STAT3 signaling pathway, phosphorylate STAT3, participate in the expression of target genes, and activate its downstream cytokines such as Bcl2 and caspase-3, which leads to the continuous proliferation of ectopic endometrium and enhances anti-apoptosis ability [8,11].
Curcumol is an active component extracted from a traditional Chinese medicine plant, Curcuma zedoaria. Clinical and experimental research have found that curcumol can inhibit proliferation and promote apoptosis of many kinds of solid tumor cells, such as lung cancer, gastric cancer, and ovarian cancer [12–14]. The results also show that curcumol can not only kill tumor cells directly, but it can also achieve anti-tumor effects by improving the body’s immune function [15]. These effects may be related to inhibition of the JAK2/STAT3 signal transduction pathway by curcumol [16]. We searched the literature on traditional Chinese medicine in the treatment of EMS, and found that Curcuma zedoaria is the most frequently used drug [17]. We also found in previous research that curcumol has a good therapeutic effect on EMS, and its effect is related to the inhibition of local inflammatory response [18,19]. Based on the above results, we speculated that curcumol may affect the EMS process by inhibiting the JAK2/STAT3 signal transduction pathway, but we found no previous research on this topic.
In this research, we conducted cell experiments and animal experiments, from the perspective of anti-inflammatory, promoting apoptosis, and inhibiting proliferation and migration, to explore whether curcumol can block the JAK2/STAT3 signal transduction pathway, reduce local inflammatory response, promote cell apoptosis, inhibit cell proliferation and migration, and inhibit the growth of ectopic lesions.
Material and Methods
Reagents and Antibodies
Curcumol (4871970), purity ≥98%, was purchased from Shanghai Jizhi Biochemical Technology Co., Ltd. Tumor Necrosis Cytokines (TNF-α) Kit (C06PZB), interleukin-6 (IL-6) Kit (C12PDB), and interleukin 1β (IL-1β) Kit (C09PDB) were all purchased from Beijing North Institute of Biotechnology. PCR primer (Shenggong Biological Products Co., Lt. T), Trizol reagent (10296010), and SYBR Green Master Mix (A25742) were purchased from Thermo Fisher Technology (USA). JAK2-specific antagonist α-cyano-(3,4-hydroxy) N-benzylstyrylamine (AG490, 100 mg/piece) was purchased from Santa Cruz Biotechnology (Shanghai) Co. Goat anti-Rabbit IgG TNF-α (ab6671)polyclonal antibody, JAK2 (Rabbit polyclonal antibody, 1: 1000, ab39636), JAK2 (phospho Rabbit polyclonal antibody, 1: 1000, ab32101), STAT3 (Mouse monoclonal antibody, 1: 1000, ab119352), STAT3 phospho Y705 (Rabbit monoclonal antibody, 1: 1000, ab76315), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Mouse monoclonal antibody, 1: 1000, ab8245) were all purchased from Abcam Trading Co.
Acquisition of Ectopic Endometrium
Between January 2020 and December 2020, 5 patients with postoperative pathologically confirmed EMS were enrolled at Hebei Provincial Hospital of Traditional Chinese Medicine (according to rAFS: stage III–IV). The patients ranged in age from 24 to 33 years old, with a mean age of 32.0±5.6 (mean±SD) years.
The inclusion criteria were: 1) diagnosis of EMS by pathology; 2) regular menstruation cycle; 3) no any hormonal treatment within 6 months prior to surgery.
The exclusion criteria were: 1) abnormally developed uterus; 2) diagnosis of malignant tumor, hepatic and renal insufficiency, and hematological disease.
The samples were collected during the proliferative period of the menstrual cycle. Written informed consent was obtained from all participants and the study was approved by the local ethics committee (No: YXLL2020004).
Cell Culture
The ectopic endometrium of EMS patients was washed with F-12/DMEM (FD) medium, cut into pieces <1 mm3, and digested with 1% collagenase at 37°C for 60–90 min. After filtration with a cell filter, the cells were gradient-precipitated with 3.0% and 1.5% bovine serum albumin (BSA) solutions, and then the stromal cells were obtained and inoculated in a culture flask. Cell morphology and growth were observed under an inverted microscope.
MTT Cell Viability Assay
Ectopic endometrial stromal cells were seeded in 96-well plates (5×103 cells/well), cultured in medium for 24 h, and then treated with curcumol at the corresponding concentration or at the specified time. After absorbing the supernatant, medium containing 0.5% MTT was added, the culture was continued for 4 h, then DMSO was added. After repeated dissolution of the crystals, the absorbance of each pore was measured at 490 nm by microplate, and the inhibition rate of cell growth was calculated.
Flow Cytometric Analyses of Apoptosis
FITC-labeled annexin-V/PI double-staining method and flow cytometry were used to determine cell apoptosis. The ectopic endometrial stromal cells were treated with curcumol for 48 h. This experiment was carried out according to the operating procedures of the annexin-V-FITC apoptosis detection kit (Nanjing keygen Biotechnology Co., Nanjing, China). Only the fluorescein-positive cells without PI staining were regarded as apoptotic cells, and the percentage was determined by flow cytometry (FACS Calibur flow cytometry, BD, USA). CellQuest software was used to analyze the data.
Wound Healing Assay
The medium was discarded, the cells were washed with PBS 3 times, digested with 0.25% trypsin, counted, and inoculated on 24-well plates at a density of 1×106 cells/ml. After the cells spread to the bottom of the well plate, they were treated with 20 μg/L curcumol. We replaced the supernatant with a serum-free medium, used a pipette tip to make a vertical scratch in the middle of the bottom of the plate, and took pictures under an inverted microscope. Image J software was used to measure the scratch width at 24 h and to assess cell mobility.
Measurements of Cytokines
The cells were incubated with curcumol 2.5–40 μg/L for 48 h, and the supernatant was taken. Different ELISA kits were used to detect the cytokines (TNF-α, IL-6, IL-1β) in the cells or rat peritoneal fluid according to the operating procedures provided by the manufacturer. According to the instructions, the concentration of cytokines was calculated in linear regression.
Western Blot Analysis
The ectopic endometrial stromal cells were treated with curcumol 2.5–40 μg/L for 48 h. The protein was extracted from ectopic endometrial stromal cells or endometrial tissue and quantified. Then, SDS loading buffer was added and denatured by heating. The processed samples were electrophoresed, transferred, and sealed. The antibody of the corresponding protein was added to the membrane and kept overnight at 4°C. The next day, samples were incubated with the corresponding secondary antibody at room temperature for 1 h, and then were assessed by chemiluminescence. The immune complex development was carried out using an enhanced chemiluminescence (ECL) detection kit and the bands were analyzed using the Gel Doc 2000 imaging system. The gray value of the target protein band and the gray value of the internal reference GAPDH protein band were assessed, and the relative expression of each target protein was calculated.
Animal Experiments
The rat EMS model was reproduced by transplantation autoplastic. The protocol was approved by the Animal Ethics Committee of Hebei University of Traditional Chinese Medicine. Sprague-Dawley rats of SPF grade were purchased from the Experimental Animal Center of Hebei Medical University. The rats were fed in the SPF environment, with 23–25°C ambient temperature, and fed standard laboratory rat chow. All possible efforts were made to treat experimental animals humanely and we followed the Guidelines for the Management and Use of Experimental Animals issued by the National Institutes of Health.
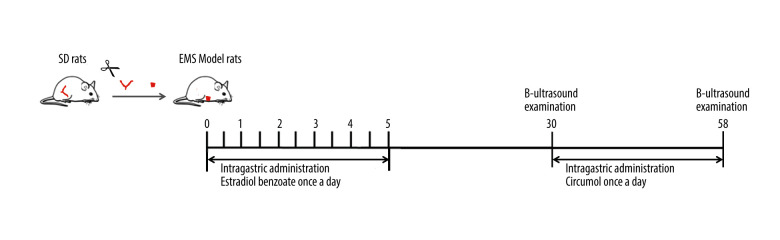
After general anesthesia, rats were laparotomized, and 5×5 mm uterine fragments were cut and sutured into the peritoneum. On day after surgery, rats were given estradiol benzoate at 0.1 mg/kg body weight, once every 5 days for 3 times to promote the growth of ectopic endometrium. Another 10 rats in the sham operation group underwent only a simulated operation. After 30 days, the volume of ectopic lesions was detected by B-ultrasound. The successfully modeled rats were divided into 3 groups according to lesion volume: the model group, the positive control group (intraperitoneal injection of AG490, 4 mg/kg body weight, twice a week), and the experimental group (intragastric administration of curcumol, 20 mg/kg body weight, once a day), with 10 rats in each group. There were 10 rats in the sham operation group. Curcumol was continuously administered for 30 days. On the 28th day of administration, the ectopic lesions of the rats were assessed by B-ultrasound. After the experiment, the long diameter and short diameter of ectopic endometrium were measured by vernier caliper, and we calculated the area. HE staining of ectopic lesions was performed.
Statistical Analysis
For in vitro studies, all experiments were performed in triplicate. The data are expressed as mean values±standard deviation (SD). The quantitative data were tested for normality using the Shapiro-Wilk test, and normally distributed data were evaluated using SPSS19.0 software to perform one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
Curcumol Reduced the Viability of Ectopic Endometrial Cells
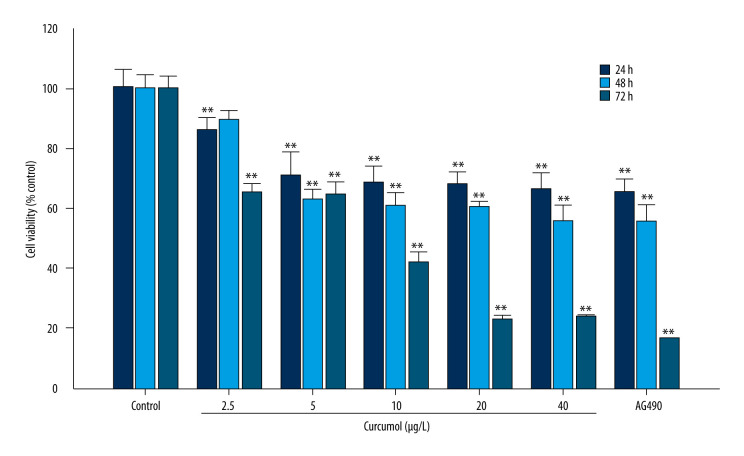
We preliminarily determined that curcumol (Figure 1) has an effect on the activity of ectopic endometrial stromal cells. Cell proliferation was measured by MTT assay. At a concentration of 5–40 μg/L, curcumol has a significant inhibitory effect on the viability of ectopic endometrial cells when it acts for 24, 48, and 72 h (24 h: P=0.000, 48 h: P=0.013, 72 h P=0.000) (Figure 2). Especially when treated with curcumol for 72 h, the cell viability was significantly inhibited, and 20 and 40 μg/L curcumol reduced cell activity to 22% of normal.
Figure 1.
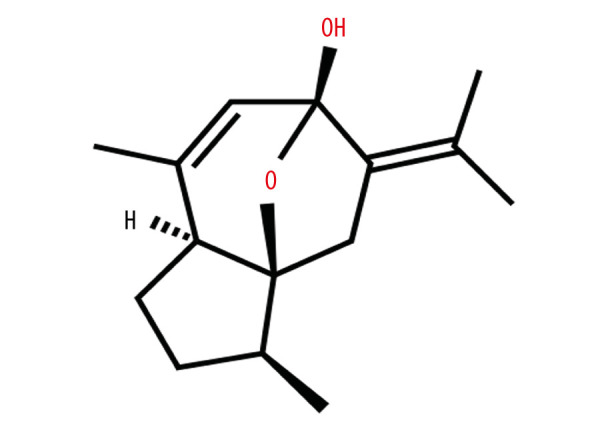
Chemical structure of curcumol.
Figure 2.
The effects of curcumol on cell viability in vitro. MTT cell viability assay shows the dose-dependent growth-inhibitory effects of curcumol on ectopic endometrial stromal cells. The experiments were performed in triplicate, and the results were expressed as mean±SD. Compared with the control group, ** P<0.01.
Curcumol Induced Apoptosis in Ectopic Endometrial Cells
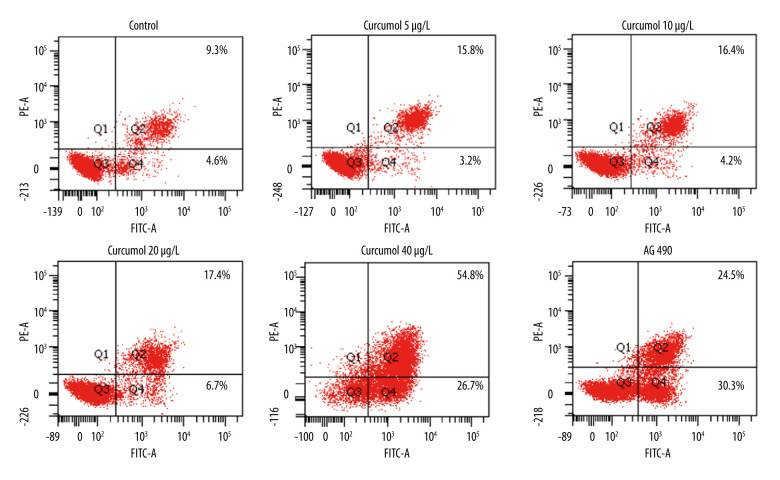
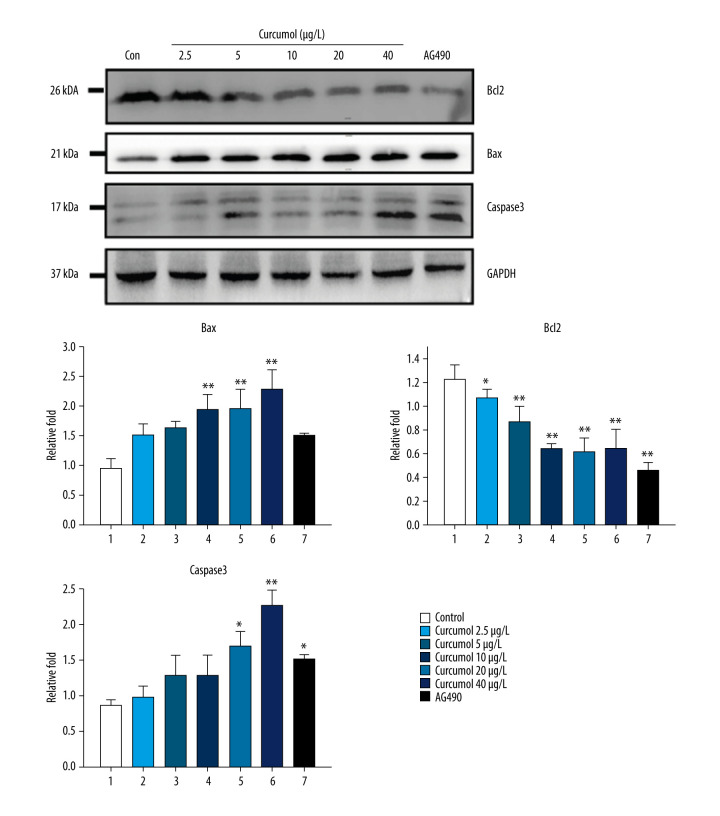
The results of flow cytometry showed that curcumol could induce apoptosis of ectopic endometrial stromal cells. At a concentration of 5–40 μg/L, when curcumol acts on ectopic endometrial stromal cells for 48 h, the apoptosis rate is significantly increased in a concentration-dependent manner. The number of apoptotic cells was found to be significantly increased by curcumol treatment with 9.3% in the control group and 54.8% at 40 μg/L (Figure 3). In addition, we also observed that curcumol at the concentrations of 10, 20, and 40 μg/L significantly upregulated the expression of Bax in ectopic endometrial cells (P=0.026). The protein expression of Bcl2 was decreased at 5–40 μg/L (P=0.002). The expression of caspase-3 was increased at 20 and 40 μg/L (P=0.004) (Figure 4).
Figure 3.
The results of flow cytometry showed that curcumol could induce apoptosis of ectopic endometrial stromal cells. The experiments were performed in triplicate.
Figure 4.
Western blot shows that curcumol resulted in increased expression of Bax and caspase-3, reduced expression of Bcl2. The experiments were performed in triplicate, and the results were expressed as mean±SD. Compared with control group, * P<0.05, ** P<0.01.
Curcumol Inhibits Migration of Ectopic Endometrial Cells
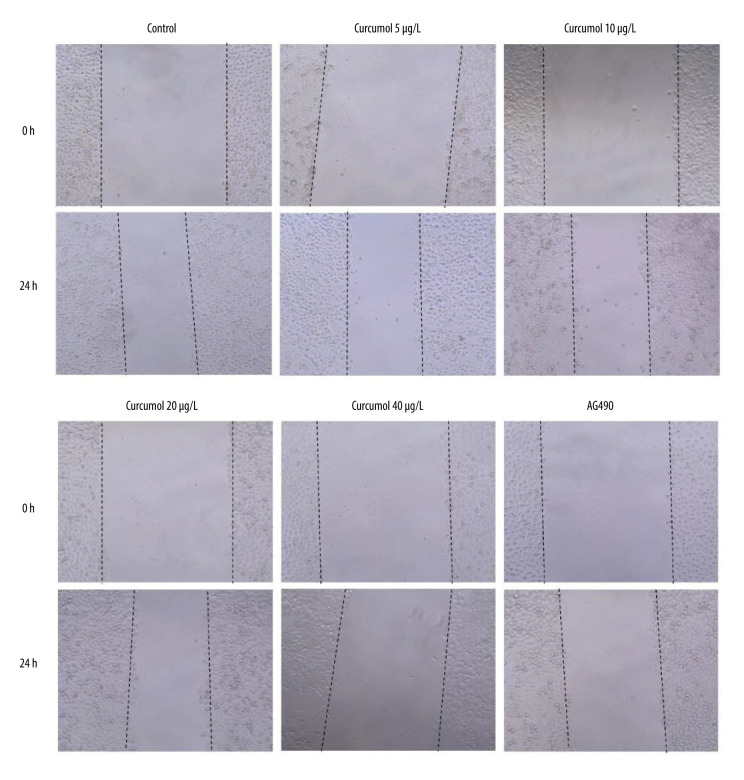
Cell wound healing assay proved the effect of curcumol on the migration of ectopic endometrial cells. The results showed that curcumol could significantly inhibit the migration of ectopic endometrial stromal cells at the concentrations of 5, 10, 20, and 40 μg/L for 24 h, which was measured as the wound width (Figure 5).
Figure 5.
Effect of curcumol on ectopic endometrial stromal cell migration. Cell wound healing assay proved the inhibited effect of curcumol on the migration of ectopic endometrial stromal cells. The experiments were performed in triplicate.
Curcumol Inhibits the Secretion of Inflammatory Cytokines in Ectopic Endometrial Cells
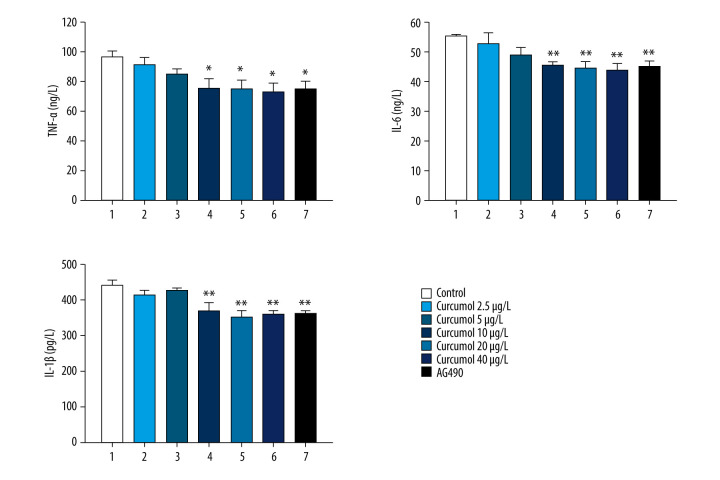
The inhibitory effect of curcumol on the secretion of inflammatory cytokines by interstitial cells was evaluated. We measured the levels of inflammatory cytokines (TNF-α, IL-6, and IL-1 β) in the supernatant of ectopic endometrial cells treated with curcumol by ELISA. The levels of TNF-α, IL-6, and IL-1 β in the supernatant of curcumol at 10, 20, and 40 μg/L were significantly decreased (TNF-α: P=0.047, IL-6: P=0.013, IL-1 β: P=0.001). These data indicate that curcumol can inhibit the secretion of inflammatory cytokines in ectopic endometrial cells at relatively low concentrations (Figure 6).
Figure 6.
Effects of curcumol on levels of inflammatory cytokines (TNF-α, IL-6, and IL-1 β) in the supernatant of ectopic endometrial stromal cells. The experiments were performed in triplicate, and the results were expressed as mean±SD. Compared with control group, * P<0.05, ** P<0.01.
Curcumol Interferes with JAK2/STAT3 Signal in Ectopic Endometrial Stromal Cells
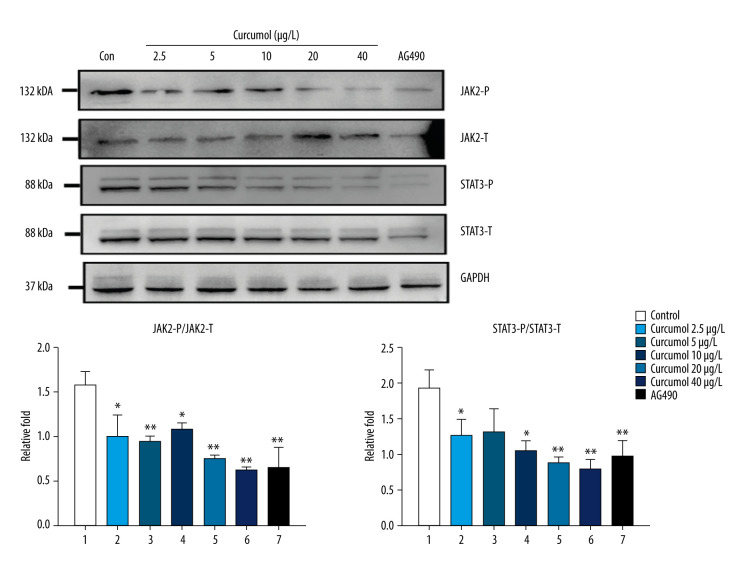
Because the phosphorylation level of JAK2/STAT3 in ectopic lesions of EMS patients is increased, we studied the effect of curcumol on JAK2/STAT3 signaling in ectopic endometrial cells. As expected, similar to AG490, an inhibitor of JAK2/STAT3 signaling, curcumol can inhibit the phosphorylation of JAK2 and STAT3 (JAK2: P=0.006, STAT3: P=0.033) (Figure 7).
Figure 7.
Western blot results show that curcumol is associated with a dose-dependent decrease in the phosphorylation levels of JAK2 and STAT3 in ectopic endometrial stromal cell. The experiments were performed in triplicate, and the results were expressed as mean±SD. Compared with the control group, * P<0.05, ** P<0.01.
Curcumol Can Inhibit the Growth of Ectopic Lesions in EMS Model Rats
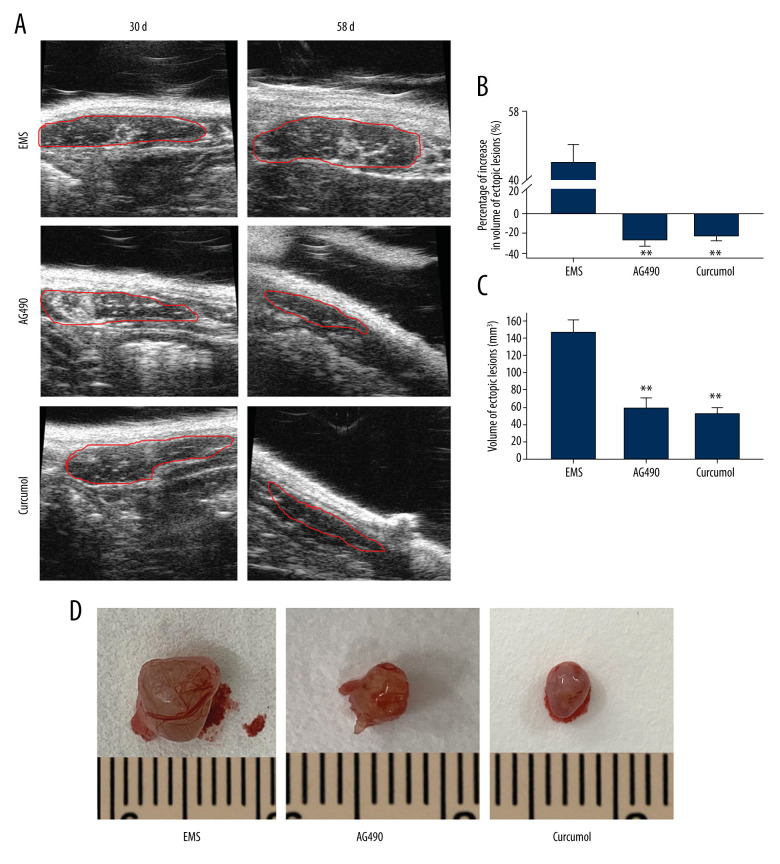
We used the method of autologous endometrial transplantation to construct the rat model of EMS. After the formation of ectopic lesions, rats were continuously fed with curcumol for 28 days (Figure 8). The results showed that curcumol and AG490 could inhibit the growth of ectopic lesions. By using B-ultrasound detection and caliper measurement, we found that the volume of ectopic lesions in the treatment group was significantly smaller than that in the model group (B-ultrasound detection: P=0.000, caliper measurement: P=0.000) (Figure 9).
Figure 8.
We used the method of autologous endometrial transplantation to copy the rat model of EMS.
Figure 9.
Effect of curcumol on growth of ectopic lesions in EMS model rats. (A) The volume of ectopic lesions was detected by B-ultrasound (the lesions are marked by red line). (B) Percentage of volume proliferation of ectopic lesions calculated from B-mode ultrasound results. (C) The ectopic lesion volume calculated according to caliper measurement. (D) The appearance of ectopic lesions. The experiments were performed in duplicate, and the results were expressed as mean±SD. Compared with control group, ** P<0.01.
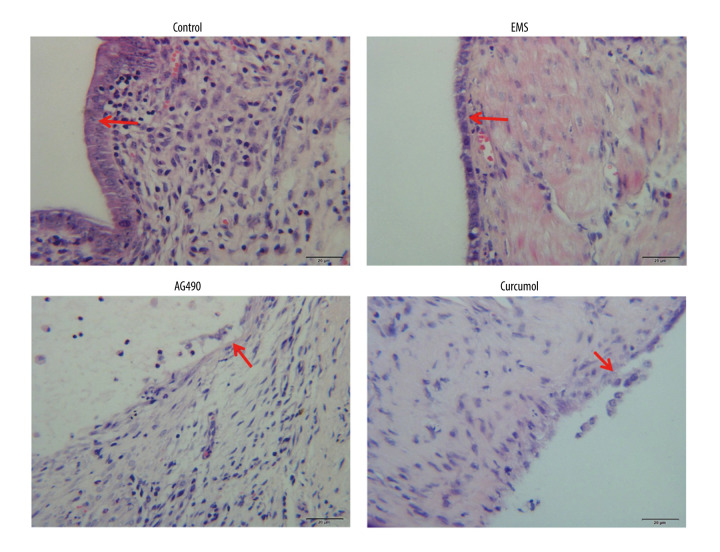
HE staining (Figure 10) showed that the histomorphology of ectopic lesions was similar to that of eutopic uterus, the surface layer was composed of neatly arranged flat columnar epithelial cells, and glands were seen in the interstitium. After treatment, the epithelial cells, which were originally neat and dense, became incomplete.
Figure 10.
HE staining was used to observe the pathological morphology (epithelial cells are marked by red arrows). The experiments were performed in triplicate.
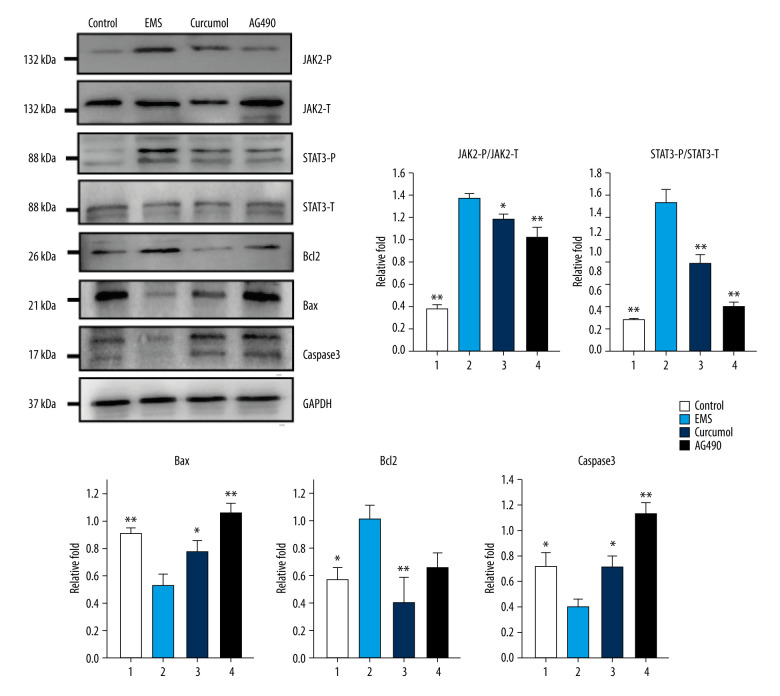
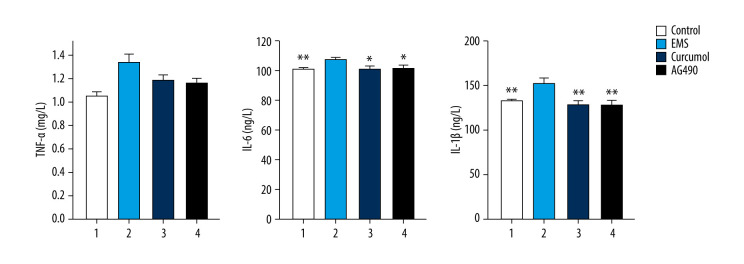
The results of the western blot experiment show that, similar to the JAK2/STAT3 inhibitor AG490, curcumol can also inhibit the phosphorylation of JAK2 and STAT3 (JAK2: P=0.000, STAT3: P=0.000). After the signal transduction of JAK2/STAT3 is inhibited, the protein expression levels of Bax and caspase-3 in the ectopic lesion tissue were significantly increased, while the protein expression level of Bcl2 was significantly reduced (Bax: P=0.005, caspase-3: P=0.003, Bcl2: P=0.044) (Figure 11). Detecting the levels of inflammatory cytokines in the rat’s peritoneal fluid showed that after treatment, the levels of TNF-α, IL-6 and IL-1β had decreased significantly (TNF-α: P=0.002, IL-6: P=0.036, IL-1 β: P=0.001) (Figure 12). In conclusion, curcumol has an obvious therapeutic effect on EMS rats.
Figure 11.
Effects of curcumol on phosphorylation levels of JAK2, STAT3 and expression levels of Bax, Bcl2, caspase-3 in ectopic lesions as shown by western blot analysis. The experiments were performed in triplicate, and the results were expressed as mean±SD. Compared with control group, * P<0.05, ** P<0.01.
Figure 12.
Curcumol can decrease the levels of inflammatory cytokines (TNF a, IL-6, and IL-1 b) in rat peritoneal fluid. The experiments were performed in duplicate, and the results were expressed as mean±SD. Compared with the control group, * P<0.05, ** P<0.01.
Discussion
In recent years, the incidence rate of EMS has increased year by year. It is often accompanied by chronic pelvic pain, adhesions, and infertility, which seriously affect the quality of life and reproductive capacity of patients, thus causing widespread concern in the field of gynecology. At present, the methods for treating EMS are surgery and ovarian inhibitors, including GnRH agonists, progestins, oral contraceptives, and androgen drugs. However, hormone therapy is associated with adverse effects such as delayed conception [20]. More seriously, EMS increases the risk of epithelial ovarian cancer by 50% [21]. Therefore, it is still necessary to find effective drugs for the treatment of EMS. The findings of the present study showed that curcumol, an important component of the herbal medicine compound derived from Curcuma zedoaria, exerted growth-inhibitory effects on ectopic lesions in EMS. We also found in previous research that curcumol’s inhibition of ectopic lesion growth is associated with anti-inflammatory effects [18]. Therefore, we continue the discussion of the relevant mechanisms in the present article.
In the present study, we found that curcumol decreased ectopic endometrial stromal cell viability in a dose-dependent manner. Flow cytometry assay showed that curcumol triggered apoptosis in cells. Increased apoptosis was associated with upregulation of the expression of apoptosis-related proteins Bax and caspase-3 and downregulation of Bcl2 by curcumol. The expression of cleaved caspase-3 was considerably increased after treatment of the ectopic endometrial stromal cells with curcumol, indicating an increase in apoptosis. Bax and Bcl2 are considered to be important biomarkers of apoptosis, and an increase in the Bax/Bcl2 ratio promotes apoptosis [22].
In this research, curcumol treatment was also shown to reduce the secretion of inflammatory cytokines TNF-α, IL-6, and IL-1 β in ectopic endometrial cells. Research has shown that TNF-α and IL-1β are necessary for chronic inflammation, and their activation of the JAK2/STAT3 signaling pathway curcumol is closely related to the development of EMS [23,24]. IL-6 can also cooperate with other cytokines, such as TGF-β, to induce epithelial–mesenchymal transition, thus promoting the proliferation, movement, and invasion of endometrial cells [25]. Research has shown that stimulation of the JAK2/STAT3 signal transduction pathway can also promote the proliferation and migration of human endometrial cells, and it can inhibit apoptosis and promote inflammatory reaction in EMS model rats [26,27]. Increasing evidence also shows that blocking JAK2/STAT3 signaling not only inhibits the proliferation of endometrial cells, but also reduces the inflammatory response in the microenvironment [28]. Previous research has confirmed that curcumol can inhibit the JAK2/STAT3 pathway [29]. The findings of this study show that the effect of curcumol is similar to that of the JAK2/STAT3 signal inhibitor AG490 in reducing the phosphorylation of JAK2 and STAT3 in ectopic endometrial stromal cells.
In animal experiments, curcumol showed a therapeutic effect equivalent to that of AG490, and curcumol can reduce the volume of ectopic lesions and atrophy of the endometrium of EMS model rats. During this experiment, we also observed an increase in the phosphorylation of JAK2 and STAT3 in the ectopic lesion tissue of the EMS model rats. The expression of apoptotic protein decreased, while the expression of protective protein Bcl2 increased significantly.
Here, we report that curcumol can inhibit the growth of EMS ectopic lesions by affecting JAK2/STAT3 signaling and reduction of inflammatory response, suggesting that curcumol could prove to be an important component for the treatment of EMS. However, curcumol requires further investigation to determine whether it can be developed into a lead molecule for the treatment of EMS. The lack of observation of the effects of curcumol on normal endometrial mesenchymal cells is the most important limitation of our study. In the future, we will also continue to assess the role of curcumol in normal endometrial stromal cells overexpressing JAK2 or STAT3 to clarify the therapeutic effect of curcumol on EMS.
Conclusions
Our current research shows that curcumol can inhibit the proliferation and migration of ectopic endometrial stromal cells, reduce the secretion of inflammatory cytokines, and attenuate endometriosis. These effects may be related to inhibition of the JAK2/STAT3 pathway.
Footnotes
Conflict of interest: None declared
Ethics Statement
The human experiment was approved by the Medical Ethics Committee of Hebei University of Chinese Medicine (No: YXLL2020004), and animal research was approved by the Animal Ethics Committee of Hebei University of Chinese Medicine.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This project was supported in part by the National Natural Science Foundation of China (81803774), the National Natural Science Foundation of Hebei Province (H2020423222), and the Medical Science Research Project of Hebei Provincial Health Commission in 2020 (20201383)
References
- 1.Agostinis C, Balduit A, Mangogna A, et al. Immunological basis of the endometriosis: the complement system as a potential therapeutic target. Front Immunol. 2020;11:599117. doi: 10.3389/fimmu.2020.599117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theobald PV, Cottenet J, Iacobelli S, et al. Epidemiology of endometriosis in France: A large, nation-wide study based on hospital discharge data. Biomed Res Int. 2016;11:3260952. doi: 10.1155/2016/3260952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Qu PP. Factors associated with ovarian endometriosis malignancy and its recurrence in Chinese women. J Obstet Gynaecol. 2019;39(8):1148–53. doi: 10.1080/01443615.2019.1603209. [DOI] [PubMed] [Google Scholar]
- 4.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endo-metrial tissues into the peritoneal cavity. Am J Obstet Gynaecol. 1927;14(4):422–69. [Google Scholar]
- 5.Miller JE, Lingegowda H, Symons LK, et al. Interleukin-33 activates group 2 innate lymphoid cell expansion and modulates endometriosis. JCI Insight. 2021;6(23):e149699. doi: 10.1172/jci.insight.149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao FY, Liu XS, Guo SW. Platelets and regulatory T cells may induce a type 2 immunity that is conducive to the progression and fibrogenesis of endometriosis. Front Immunol. 2020;11:610963. doi: 10.3389/fimmu.2020.610963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simsek MS, Ozkan ZS, Deveci D, et al. Investigation of plasma cytokine levels and endometrial tissue leukocytes in recurrent pregnancy loss. J Infertil Reprod Biol. 2015;3(3):192–98. [Google Scholar]
- 8.Kotlyar AM, Mamillapalli R, Flores VA, et al. Tofacitinib alters STAT3 signaling and leads to endometriosis lesion regression. Mol Hum Reprod. 2021;27(4):gaab016. doi: 10.1093/molehr/gaab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gritsina G, Xiao F, O’Brien SW, et al. Targeted blockade of JAK/STAT3 signaling inhibits ovarian carcinoma growth. Mol Cancer Ther. 2015;14(4):1035–47. doi: 10.1158/1535-7163.MCT-14-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An M, Li D, Yuan M, et al. Interaction of macrophages and endometrial cells induces epithelial-mesenchymal transition-like processes in adenomyosis. Biol Reprod. 2017;96(1):46–57. doi: 10.1095/biolreprod.116.144071. [DOI] [PubMed] [Google Scholar]
- 11.Han SJ, Lee JE, Cho YJ, et al. Genomic function of estrogen receptor β in endometriosis. Endocrinology. 2019;160(11):2495–516. doi: 10.1210/en.2019-00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Li XM, Bai Z, et al. Curcumol induces cell cycle arrest in colon cancer cells via reactive oxygen species and Akt/GSK3β/cyclin D1 pathway. J Ethnopharmacol. 2018;210(1):1–9. doi: 10.1016/j.jep.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 13.Zang SL, Tang QL, Dong FR, et al. Curcumol inhibits the proliferation of gastric adenocarcinoma MGC-803 cells via downregulation of IDH1. Oncol Rep. 2017;38(6):3583–91. doi: 10.3892/or.2017.6028. [DOI] [PubMed] [Google Scholar]
- 14.Zhao N, Han FJ, Liu Y, et al. Curcumol induced apoptosis of human ovarian cancer SKOV3 cells and its effect on PI3K-Akt signaling pathway related proteins. J Emerg Trad Chin Med. 2020;29(8):1411–14. [Google Scholar]
- 15.Tang QL, Guo JQ, Wang QY, et al. Curcumol induces apoptosis in SPC-A-1 human lung adenocarcinoma cells and displays anti-neoplastic effects in tumor bearing mice. Asian Pac J Cancer Prev. 2015;16(6):2307–12. doi: 10.7314/apjcp.2015.16.6.2307. [DOI] [PubMed] [Google Scholar]
- 16.Tang X, Han FJ, Li W, et al. [Effect of Curcumol on JAK2/STAT3 signaling pathway in human ovarian cancer SKOV3 cell line]. Chin J Obstet Gynecol. 2013;14(1):43–46. [in Chinese] [Google Scholar]
- 17.Wu YJ. Modern literature study on TCM treatment of endometriosis. Beijing: Beijing University of Chinese Medicine; 2013. [Google Scholar]
- 18.Nie XB, Ma YK, Zhao N, et al. [Effects of Curcumol on inflammatory cytokines in endometriosis model rats]. Tianjin Med J. 2019;47(9):913–17. [in Chinese] [Google Scholar]
- 19.Han LL, Zhang WX, Liu J. [Improvement effect of Zedoary on endometriosis in rats]. Chinese J Clin Pharm. 2019;35(19):2328–31. [in Chinese] [Google Scholar]
- 20.Ata B, Telek SB. Assisted reproductive technology for women with endometriosis, a clinically oriented review. Curr Opin Obstet Gynecol. 2021;33(3):225–31. doi: 10.1097/GCO.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 21.Brunty S, Mitchell B, Nadim BZ, et al. Endometriosis and ovarian cancer risk, an epigenetic connection. Ann Transl Med. 2020;8(24):1715. doi: 10.21037/atm-20-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oltval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74(4):609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 23.Chadchan SB, Cheng M, Parnell LA, et al. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum Reprod. 2019;34(6):1106–16. doi: 10.1093/humrep/dez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosette MK. The expression of β-Catenin in the epithelial cells and stromal cells of endometriosis and normal endometrial cells. Journal of Infertility and reproductive Biology. 2014;2(3):70–76. [Google Scholar]
- 25.Sharma D, Saxena NK, Vertino PM, et al. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–40. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Tian J, Lv Y, et al. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 2009;100:389–95. doi: 10.1111/j.1349-7006.2008.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117(12):3846–56. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou HX, Jin Y, Wang Z, et al. Curcumol inhibits the expression of programmed cell death-ligand 1 through crosstalk between hypoxia-inducible factor-1α and STAT3 (T705) signaling pathways in hepatic cancer. J Ethnopharmacol. 2020;257:112835. doi: 10.1016/j.jep.2020.112835. [DOI] [PubMed] [Google Scholar]