Abstract
Objective
Coronavirus disease 2019 (COVID-19) is associated with an increased risk of venous thromboembolism (VTE). Recent studies have characterized racial disparities in the incidence of VTE. The aim of our study was to present a systematic review and meta-analysis to assess the association between race and VTE in patients hospitalized with COVID-19.
Methods
We performed a systematic literature review to evaluate the number of deep vein thrombosis (DVT) and pulmonary embolism (PE) events reported by racial groups in patients hospitalized with COVID-19. For the qualitative analysis, independent reviewers extracted the data from eligible studies, and we used the Newcastle-Ottawa scale to assess the quality of design and content for accurate interpretation. For the quantitative analysis, we pooled the odds ratios with Der Simonian and Laird random effects models.
Results
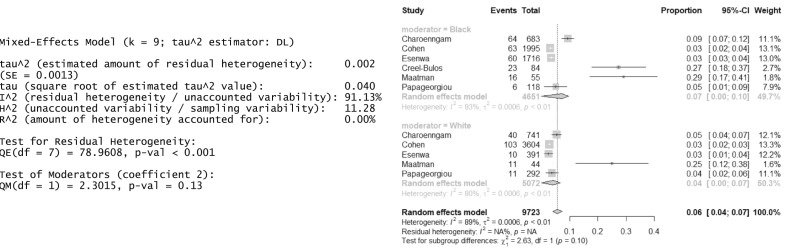
The qualitative analysis included 11 studies, with 6 included in the meta-analysis. All studies were observational, retrospective cohort studies, except for one retrospective case-control study. Six studies were eligible for the meta-analysis owing to the high interstudy heterogeneity; thus, the variable reports of racial groups reduced the cohort to Black/African American and White patients (n = 9723) in the analysis. The estimated proportion for DVT and PE events for Black/African American and White patients was 0.07 (95% confidence interval, 0.00-0.10) and 0.04 (95% confidence interval, 0.00-0.07), respectively. The P value of .13 suggested nonsignificant differences in the VTE rates between Black/African American and White patients.
Conclusions
In our study, the proportion of DVT and PE events between Black/African American and White patients with COVID-19 were comparable. Future COVID-19 studies should include systematic racial group reporting to identify any disparities in the setting of VTE events.
Keywords: COVID-19, Deep vein thrombosis, Pulmonary embolism, Racial disparities, Venous thromboembolism
In March 2020, the World Health Organization declared a global pandemic caused by the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) virus.1 , 2 One of the most serious complications reported has been coagulopathy, with an increased risk of venous thromboembolism (VTE).1 , 3 , 4 Evidence has suggested that patients who develop VTE caused by COVID-19 infection will have poorer clinical outcomes.5 Two systematic reviews and meta-analyses reported a VTE incidence of 26% and 25%, respectively.6 , 7 Within our own hospital system, we reported a VTE rate of 9%.8 Several studies have emphasized the presence of a racial disparity in the incidence of VTE.9, 10, 11 The objective of the present systematic review and meta-analysis was to assess the relationship between race and deep vein thrombosis (DVT) and pulmonary embolism (PE) in hospitalized COVID-19 patients with the aim of improving our current anticoagulation management for each individual patient.
Methods
Literature search and study selection
We conducted the present study in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines (Supplementary Table I, online only). A medical librarian (T.J.B.) identified the studies using MEDLINE (1946 to the present), Embase (1974 to the present), the Cochrane Central Register of Controlled Trials (1991 to the present), and the Cochrane Database of Systematic Review (2005 to the present; all via the Ovid interface), Scopus (1823 to the Present), Science Citation Index Expanded (1975 to the present), Emerging Sources Citation Index (2015 to the present; via the Web of Science interface), and Epistemonikos databases. The librarian also searched the gray literature resources. We used no limits on language or publication date. We placed filters to exclude pediatric and animal-related studies. The search strategies consisted of a combination of keywords and standardized index terms. The search terms included MeSH, Embase/Emtree terms, and keywords such as COVID-19, SARS-CoV-2, deep vein thrombosis, DVT, pulmonary embolism, PE, African American, Black individuals, and terms associated with racial and ethnic disparities. We searched all databases and gray literature resources on July 22, 2021 (the full search strategies are available at: https://osf.io/cxw2p). The librarian downloaded the results into EndNote, version 9.0 (Clarivate, Philadelphia, PA), a bibliographic database manager. The methods outlined by Bramer et al12 were used to identify and remove any duplicate citations. Two of us (S.B., M.M.H.) independently screened the titles and abstracts for relevance. A third reviewer (S.F.) settled any disagreements. The reviewers assessed the full text of the relevant reports to determine eligibility using the following inclusion criteria: (1) interventional or observational studies in adult humans, (2) studies reporting racial groups for DVT and PE events for patients with COVID-19, and (3) reports written in the English language. Studies with composite outcomes were eligible if the corresponding author could provide the raw dataset for the patient subgroup solely with DVT and/or PE events. The exclusion criteria were as follows: (1) preprint articles; (2) review articles, book sections, congress abstracts, case series, and case reports; and (3) studies reporting postmortem events. A descriptive narrative of ≤10 patients was defined as a case series for our study.
Data extraction and quality assessment
The data extracted from the eligible studies included the study details (eg, first author name, date and country of publication, study design), patient characteristics (eg, number of patients, patient sex, patient age, COVID-19 diagnostic method), use of thromboprophylaxis and/or anticoagulation before the outcome of interest, and VTE events stratified by racial group. Two of us (S.B., M.M.H.) independently extracted the data and resolved any disagreements by consensus.
The primary outcome was the estimated VTE proportion, defined as DVT and PE occurring in patients with COVID-19 stratified by racial group. To assess the quality of the nonrandomized studies in the meta-analysis, we used the Newcastle-Ottawa scale. The Newcastle-Ottawa scale is a star-based system used to evaluate case-control and cohort studies that includes the following sections: selection of study groups, comparability of groups, and ascertainment of exposure or outcome of interest for case-control and cohort studies. Studies allotted a score of seven or greater were considered high-quality research or having a low risk of bias. Two of us (S.B., S.F.) independently assessed the quality of the studies, with consensus used to resolve any disagreements. If necessary, the decision of a third reviewer was considered definitive.
Statistical analysis
We performed a meta-analysis to assess the relationship between race (Black/African American and White) and the occurrence of VTE. Regarding major outcomes, we pooled the odds ratios with Der Simonian and Laird random effects models used for the sensitivity analysis. We calculated the P values to evaluate for statistical significance, with P < .05 indicating a statistically significant difference. All statistical analyses used the absolute values and not proportions. We applied the Hedges estimator and Sidik-Jonkman estimator to assess for the risk of publication bias. We used SAS statistical software, version 9.4 (SAS Institute, Cary, NC), to perform all statistical analysis and forest plots.
Results
Study characteristics and quality assessment
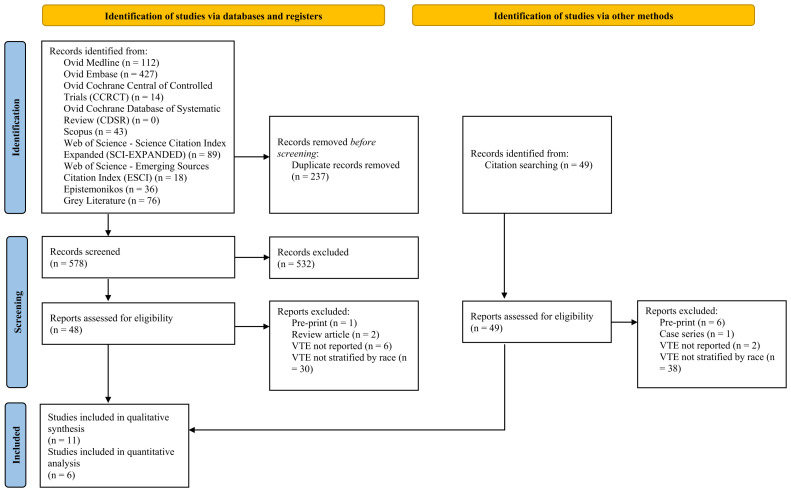
The electronic search yielded 815 studies. After removing the duplicates, we screened 578 titles and abstracts. We also reviewed 48 reports and an additional 49 studies identified from citation searching of the full-text reports to assess for eligibility. Of these studies, 11 fulfilled our inclusion criteria. We excluded 86 reports, which included 7 preprint studies, 2 review studies, 1 case series, 8 studies that had not reported the number of DVT or PE events, and 68 studies that had not reported the DVT and/or PE events by racial group (Fig 1 ). Using the Newcastle-Ottawa scale, four studies (36.4%) had a high risk of bias and seven (63.6%) a low risk of bias13 (Supplementary Table II, online only).
Fig 1.
PRISMA (preferred reporting items for systematic reviews and meta-analysis) flowchart displaying the process for literature selection.
The 11 eligible studies were all observational; 10 were retrospective cohort studies and 1 was a retrospective case-control study. We identified 10 studies that had been conducted in the United States and 1 study that had been conducted in the United Kingdom. The polymerase chain reaction test had been used to determine the COVID-19 status of the patients. The study characteristics are listed in the Table .14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 These 11 studies included a total of 20,023 patients, with a proportion of 27% to 54.2% of female patients. DVT and PE were considered a single outcome termed VTE in five studies. Of those five studies, one had reported VTE and mortality as a composite primary outcome. Two studies had reported DVT and PE as independent events. Four of the retrospective analyses had evaluated either DVT or PE only as the outcome. The documentation of racial groups varied. Ranging from one to five racial groups, Black/African American was the most reported group, followed by White or Caucasian and Asian. Groups termed “other” and “unknown” were the least common. In addition, three studies had used Hispanic and non-Hispanic as ethnic groups, independently of the previously stated racial groups, to report their outcomes.
Table.
Characteristics of 11 included studies
| Investigator | Country | Study design | Patients, No. | Female sex, % | Age,a years | In-hospital thromboprophylaxis or anticoagulation | Incidence/prevalence stratified by race/ethnicityb |
||
|---|---|---|---|---|---|---|---|---|---|
| PE | DVT | VTE | |||||||
| Charoenngam et al,14 2021 | USA | Retrospective cohort, single-center | 1424 | 44.5 | 56.1 ± 17.4 | Yes | Black/African-American, 34/683 (5); White, 17/741 (2.3) | Black/African-American, 30/683 (4.4); White, 23/741 (3.1) | NR |
| Cho et al,15 2021 |
USA | Retrospective cohort, single-center | 158 | 46.2 | 67.4 ± 14.6 | Yes (prophylactic dose LMWH or UFH, n = 148) | NR | Black/African-American, 25/77 (32.5); White, 14/52 (26.9); Asian, 4/7 (57.1); Hispanic,c10/26 (38.5) | NR |
| Cohen et al,16 2021 | USA | Retrospective cohort, single-center | 9407 | 40.7 | >60 (63.8%) | Yes (prophylactic dose, n = 6675; therapeutic dose, n = 1753) | NR | NR | Black/African-American, 63/1995 (3.2); White, 103/3604 (2.9); Asian, 15/812 (1.8); other, 74/2583 (2.9); unknown, 19/413 (4.6) |
| Esenwa et al,17 2021 | USA | Retrospective cohort, multi-center | 4299 | 46.6 | >55 (71.8%) | Yes | Black/African-American, 26/1716 (1.5); White, 4/391 (1); Asian, 1/123 (0.8); Hispanic, 28/1752 (1.6); other, 3/317 (0.9) | Black/African-American, 34/1716 (2); White, 6/391 (1.5); Asian, 4/123 (3.3); Hispanic, 37/1752 (2.1); other, 9/317 (2.8) | NR |
| Koilelat et al,18 2021 | USA | Retrospective case-control, singe-center | 135 | 46.7 | 61 (49-73) | Yes (prophylactic dose LMWH, UFH, apixaban, n = 86; therapeutic dose UFH, DOAC, bivalirudin, n = 23) | NR | Black/African-American, 3/29 (10.3); White, 2/5 (40); Asian, 0/2 (0); Hispanic,c 11/53 (20.7); other, 11/82 (13.4); unknown, 2/17 (11.8) | NR |
| Bilaloglu et al,19 2020 | USA | Retrospective cohort, multicenter | 3334 | 39.6 | 64 (51-75) | Yes | NR | NR | Black/African-American, HR, 0.97 (95% CI, 0.60-1.55); White, reference; Asian, HR, 0.82 (95% CI, 0.46-1.45); Hispanic, HR, 2.01 (95% CI, 0.81-5.00); other, HR, 0.89 (95% CI, 0.61-1.28); unknown, HR, 1.37 (95% CI, 0.8-2.33) |
| Creel-Bulos et al,20 2020 | USA | Retrospective cohort, single-center | 115 | 41 | 64 ± 16 | NR | NR | NR | Black/African-American, 23/84 (27.4) |
| Maatman et al,21 2020 | USA | Retrospective cohort, multi-center | 109 | 43 | 61 ± 16 | Yes (prophylactic UFH, n = 61; prophylactic dose LMWH, n = 40; therapeutic dose, n = 4) | NR | NR | Black/African-American, 16/55 (64); White, 11/44 (25); other, 4/10 (40) |
| Papageorgiou et al,22 2020 | UK | Retrospective cohort, multi-center | 613 | 40 | 70 (57-82) | Yes | NR | NR | Black/African-American, 6/118 (5.1); White, 11/292 (3.8); Asian, 17/203 (8.4) |
| Poyiadji et al,23 2020 | USA | Retrospective cohort, single-center | 328 | 54.2 | NR | Yes (prophylactic dose, n = 122) | Black/African-American, 40/188 (21.3) | NR | NR |
| Xu et al,24 2020 | USA | Retrospective cohort, multi-center | 101 | 27 | 62 ± 15 | Yes | Black/African-American, 33; White, 28; Asian, 17; other, 23 | NR | NR |
CI, Confidence interval; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; HR, hazard ratio; LMWH, low-molecular-weight heparin; NR, not reported; UFH, unfractionated heparin; VTE, venous thromboembolism.
Unless specified otherwise, data presented as mean ± standard deviation or mean (interquartile range).
Outcomes reported as number of events/population at risk (%), stratified by racial group.
Study population stratified by ethnicity as Hispanic, non-Hispanic, and other/unknown, with race not defined for ethnic groups previously reported.
Meta-analysis of DVT and PE events
We conducted a meta-analysis of the six studies that had reported a proportion of the DVT and/or PE rates stratified by race. Owing to the expected heterogeneity, we used the random effects model with inverse variance weights to perform the estimation and accounting for both within- and between-study variances. Because the observed proportions were not skewed, we did not apply transformations to the data. We used the DerSimonian-Laird estimator for our random effect model.25 We could only analyze the primary outcome between the Black/African American and White patients, because the other racial groups had not been reported consistently. The estimated proportion of VTE events for Black/African American and White patients was 0.07 and 0.04, respectively (95% confidence interval, 0.00-0.10 and 0.00-0.07, respectively). The P value of 0.13 suggested a nonsignificant difference in the VTE rates between the Black/African American and White patients. For publication bias purposes, we used both the Hedges estimator and the Sidik-Jonkman estimator, which delivered results consistent with those from the DerSimonian-Laird estimator (Fig 2 ).
Fig 2.
Forest plot representing the venous thromboembolism (VTE) proportion between Black/African-American and White patients with coronavirus disease 2019 (COVID-19).
Discussion
From the six studies with 9723 patients included in our meta-analysis, we found that the estimated proportion of DVT and PE between hospitalized Black/African-American and White patients with COVID-19 was comparable. Black/African American patients represented 49.7% of the cohort. Being of either racial group did not increase the risk of DVT or PE. Multiple factors have been associated with an increased the risk of DVT and/or PE for COVID-19 patients, including an abnormal systemic inflammatory response, coupled with endothelial injury.26 Older age, immobilization, invasive mechanical ventilation, and the use of central venous catheters have also been shown to contribute to the prothrombotic state in COVID-19.27 In the present review of racial disparities, the overall proportion of DVT and/or PE events for COVID-19 patients was lower than those reported by other COVID-19 meta-analyses.6 , 7 , 28 , 29
The occurrence of VTE in patients with COVID-19 has bene associated with poor clinical outcomes.26 , 30 However, its true incidence in different racial groups has not been reported consistently. The results from the present study do not support the hypothesis that Black/African American patients have a higher risk of developing DVT or PE. Before the pandemic, several studies had reported a twofold greater VTE event rate for Black/African American patients, which had been attributed to a higher prevalence of comorbidities, greater body mass index, poor educational level, and low socioeconomic status, among others.10 , 11 , 31 It has also been reported that procoagulant proteins such as factor VIII and von Willebrand factor and D-dimer levels have been documented at higher concentrations in Black/African American patients.9 The presence of high interstudy heterogeneity in our systematic review restricted the quantitative analysis. The studies included had reported in various degrees the relevant patient characteristics, including comorbid conditions, clinical severity of infection, relevant laboratory data, the need for oxygen therapy, VTE risk stratification and prophylaxis, and other important outcomes that could have been influenced the outcomes or differed across the racial groups. The inability to adjust for potential confounding bias between the racial groups was a major limitation of the present study. Therefore, we would recommend uniform, systematic reporting standards for future studies related to COVID-19 infection and race. Moreover, higher mortality has been reported for Black patients, probably owing to undiagnosed thrombotic events. Postmortem studies have shown that 22% to 58% of patients will have evidence of DVT and/or PE.32 , 33
Several other limitations included the lack of well-designed studies reporting the racial and social inequalities that the COVID-19 pandemic has exposed. Perhaps, these inequalities contributed to our underpowered study, which would have required a cohort of ∼565 Black/African American patients to demonstrate a racial disparity. Furthermore, the studies included in the meta-analysis represented a small cohort reported from October 2020 to June 2021, limiting the experience. Finally, many hospital practices regarding anticoagulation and prophylaxis for COVID-19 patients have been modified throughout the pandemic, which could have added confounders to the data analysis. The studies included had been performed in the United States and United Kingdom only, further limiting the generalizability of our findings. Another limitation was the lack of standardization and uniformity in terms of reporting for racial differences and disparities, which led to the exclusion from the meta-analysis of at least five other studies. Finally, standardization of DVT and PE risk stratification and prophylaxis was lacking in these studies, especially for this hypercoagulable cohort of patients, which impaired the analysis of the reported data.
Conclusions
The available evidence regarding the proportion of DVT and PE events in Black/African American and White patients demonstrated no significant differences between racial groups. The estimated proportion DVT and PE overall was lower than that from data reported in previous meta-analyses. Further endeavors in the application of uniform racial group definitions and reporting standards should be considered to allow for comparisons, specifically for VTE events.
Author Contributions
Conception and design: SB, YE, DS, YD, TB, PF
Analysis and interpretation: SB, YE, DS, YL, YD, ME, JM, PF
Data collection: SB, SF, MH, TB
Writing the article: SB, YE, SF, YL, MH, TB, PF
Critical revision of the article: SB, YE, DS, YL, YD, ME, JM, PF
Final approval of the article: SB, YE, DS, SF, YL, MH, YD, TB, ME, JM, PF
Statistical analysis: YL
Obtained funding: Not applicable
Overall responsibility: YE
Cynthia K. Shortell, MD, SECTION EDITOR
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvsvenous.org.
Appendix
Additional material for this article may be found online at www.jvsvenous.org.
Appendix (online only)
Supplementary Table I (online only).
PRISMA (preferred reporting items for systematic reviews and meta-analysis) 2020 checklist
| Section and topic | Item No. | Checklist item | Pages |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review | 1 |
| Abstract | |||
| Abstract | 2 | See PRISMA 2020 for abstracts checklist | 2 |
| Introduction | |||
| Rationale | 3 | Describe rationale for review in context of existing knowledge | 4 |
| Objectives | 4 | Provide an explicit statement of the objectives or questions the review addresses | 4 |
| Methods | |||
| Eligibility criteria | 5 | Specify inclusion and exclusion criteria for review and how studies were grouped for synthesis | 4-5 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies; specify the date when each source was last searched or consulted | 4 |
| Search strategy | 7 | Present full search strategies for all databases, registers, and websites, including any filters and limits used | 4-5 |
| Selection process | 8 | Specify methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and, if applicable, details of automation tools used in the process | 5 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process | 5-6 |
| Data items | 10a | List and define all outcomes for which data were sought; specify whether all results that were compatible with each outcome domain in each study were sought (eg, for all measures, time points, analyses) and, if not, the methods used to decide which results to collect | 5 |
| 10b | List and define all other variables for which data were sought (eg, participant and intervention characteristics, funding sources); describe any assumptions made about any missing or unclear information | 5 | |
| Study risk of bias assessment | 11 | Specify methods used to assess risk of bias in included studies, including details of tools used, how many reviewers assessed each study, whether they worked independently, and, if applicable, details of automation tools used in the process | 5-6 |
| Effect measures | 12 | Specify for each outcome, the effect measures (eg, risk ratio, mean difference) used in synthesis or presentation of results | 6 |
| Synthesis methods | 13a | Describe processes used to decide which studies were eligible for each synthesis (eg, tabulating study intervention characteristics and comparing against the planned groups for each synthesis [item 5]) | 6 |
| 13b | Describe any methods required to prepare data for presentation or synthesis, such as management of missing summary statistics or data conversions | 6 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses | 6 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choices; if a meta-analysis was performed, describe the models, methods used to identify the presence and extent of statistical heterogeneity and software packages used | 6 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (eg, subgroup analysis, meta-regression) | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases) | NA |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome | 6 |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram | 6 |
| 16b | Cite studies that might appear to meet the inclusion criteria but were excluded and explain why they were excluded | 6 | |
| Study characteristics | 17 | Cite each included study and present its characteristics | 7; Fig 1 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study | 6; Supplementary Table I |
| Results of individual studies | 19 | For all outcomes, present, for each study: (1) summary statistics for each group (as appropriate); and (2) an effect estimate and its precision (eg, confidence/credible interval), ideally using structured tables or plots | Fig 2 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies | 7 |
| 20b | Present results of all statistical syntheses conducted; if a meta-analysis was performed, present for each, the summary estimate and its precision (eg, confidence/credible interval) and measures of statistical heterogeneity; if comparing groups, describe the direction of the effect | 7 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results | NA | |
| 20d | Present results of all sensitivity analyses conducted to assess robustness of synthesized results | NA | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed | NA |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed | 7 |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence | 8 |
| 23b | Discuss any limitations of the evidence included in the review | 9 | |
| 23c | Discuss any limitations of the review processes used | 9 | |
| 23d | Discuss implications of the results for practice, policy, and future research | 9 | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered | NA |
| 24b | Indicate where the review protocol can be accessed or state that a protocol was not prepared | NA | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol | NA | |
| Support | 25 | Describe sources of financial or nonfinancial support for the review and the role of the funders or sponsors in the review | Application for publication.docx |
| Competing interests | 26 | Declare any competing interests of review authors | Application for publication.docx |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review | Available at: https://osf.io/cxw2p |
NA, Not available.
Supplementary Table II (online only).
Risk of bias assessment of cohort and case-control studies using Newcastle-Ottawa scale
| Investigator | Study design | Score by reviewers 1 and 2a |
|||
|---|---|---|---|---|---|
| Selection | Comparability | Exposure or outcome | Risk of bias | ||
| Charoenngam et al, 2021 | Retrospective cohort, single-center | 3 | 2 | 2 | Low |
| Cho et al, 2021 | Retrospective cohort, single-center | 4 | 1 | 2 | Low |
| Cohen et al, 2021 | Retrospective cohort, single-center | 4 | 1 | 2 | Low |
| Esenwa et al, 2021 | Retrospective cohort, multicenter | 4 | 1 | 2 | Low |
| Koleilat et al, 2021 | Retrospective case-control, single-center | 3 | 1 | 2 | High |
| Bilaloglu et al, 2020 | Retrospective cohort, multicenter | 1 | 1 | 2 | High |
| Creel-Bulos et al, 2020 | Retrospective cohort, single-center | 3 | 2 | 2 | Low |
| Maatman et al, 2020 | Retrospective cohort, multicenter | 4 | 1 | 2 | Low |
| Papageorgiou et al, 2020 | Retrospective cohort, multicenter | 4 | 2 | 2 | Low |
| Poyiadji et al, 2020 | Retrospective cohort, single-center | 3 | 1 | 2 | High |
| Xu et al, 2020 | Retrospective cohort, multicenter | 2 | 1 | 2 | High |
Numerical values represent stars awarded for each section evaluated.
References
- 1.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohamadian M., Chiti H., Shoghli A., Biglari S., Parsamanesh N., Esmaeilzadeh A. COVID-19: virology, biology and novel laboratory diagnosis. J Gene Med. 2021;23:e3303. doi: 10.1002/jgm.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashraf O., Young M., Malik K.J., Cheema T. Systemic complications of COVID-19. Crit Care Nurs Q. 2020;43:390–399. doi: 10.1097/CNQ.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 4.Han H., Yang L., Liu R., Liu F., Wu K., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 5.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C., Shen L., Le K.J., Pan M.M., Kong L.C., Gu Z.C., et al. Incidence of venous thromboembolism in hospitalized coronavirus disease 2019 patients: a systematic review and meta-analysis. Front Cardiovasc Med. 2020;7:151. doi: 10.3389/fcvm.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erben Y., Franco-Mesa C., Gloviczki P., Stone W., Quinones-Hinojoas A., Meltzer A.J., et al. Deep vein thrombosis and pulmonary embolism among hospitalized coronavirus disease 2019-positive patients predicted for higher mortality and prolonged intensive care unit and hospital stays in a multisite healthcare system. J Vasc Surg Venous Lymphat Disord. 2021;9:1361–13670.e1. doi: 10.1016/j.jvsv.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakai N.A., McClure L.A. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9:1877–1882. doi: 10.1111/j.1538-7836.2011.04443.x. [DOI] [PubMed] [Google Scholar]
- 10.White R.H., Keenan C.R. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123:S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 11.Folsom A.R., Basu S., Hong C.P., Heckbert S.R., Lutsey P.L., Rosamond W.D., et al. Reasons for differences in the incidence of venous thromboembolism in black versus white Americans. Am J Med. 2019;132:970–976. doi: 10.1016/j.amjmed.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bramer W, Holland L, Mollema J, Hannon T, Bekhuis T. Removing duplicates in retrieval sets from electronic databases 2015. Available at: http://www.iss.it/ibnary/eahi/cont/57_Bramer_Wichor_slides_EAHIL_2014.pdf. Accessed April 2, 2022.
- 13.Peterson J., Welch V., Losos M., Tugwell P. 2011. The Newcastle-Ottawa scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute. [Google Scholar]
- 14.Charoenngam N., Ilori T.O., Holick M.F., Hochberg N.S., Apovian C.M. Self-identified race and COVID-19-associated acute kidney injury and inflammation: a retrospective cohort study of hospitalized inner-city COVID-19 patients. J Gen Intern Med. 2021;36:3487–3496. doi: 10.1007/s11606-021-06931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho E.S., McClelland P.H., Cheng O., Kim Y., Hu J., Zenilman M.E., et al. Utility of D-dimer for diagnosis of deep vein thrombosis in coronavirus disease-19 infection. J Vasc Surg Venous Lymphat Disord. 2021;9:47–53. doi: 10.1016/j.jvsv.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S.L., Gianos E., Barish M.A., Chatterjee S., Kohn N., Lesser M., et al. Prevalence and predictors of venous thromboembolism or mortality in hospitalized COVID-19 patients. Thromb Haemost. 2021;121:1043–1053. doi: 10.1055/a-1366-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esenwa C., Unda S.R., Altschul D.J., Patel N.K., Malaviya A., Seiden J., et al. The effect of race on composite thrombotic events in patients with COVID-19. Thromb Res. 2021;199:10–13. doi: 10.1016/j.thromres.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koleilat I., Galen B., Choinski K., Hatch A.N., Jones D.B., Billett H., et al. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2021;9:36–46. doi: 10.1016/j.jvsv.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creel-Bulos C., Liu M., Auld S.C., Gaddh M., Kempton C.L., Sharifpour M., et al. Trends and diagnostic value of D-dimer levels in patients hospitalized with coronavirus disease 2019. Medicine (Baltimore) 2020;99:e23186. doi: 10.1097/MD.0000000000023186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maatman T.K., Jalali F., Feizpour C., Douglas A., II, McGuire S.P., Kinnaman G., et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48:e783–e790. doi: 10.1097/CCM.0000000000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papageorgiou N., Providencia R., Saberwal B., Sohrabi C., Tyrlis A., Atieh A.E., et al. Ethnicity and COVID-19 cardiovascular complications: a multi-center UK cohort. Am J Cardiovasc Dis. 2020;10:455–462. [PMC free article] [PubMed] [Google Scholar]
- 23.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K., et al. Acute pulmonary embolism and COVID-19. Radiology. 2020;297:E335–E338. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H., Martin A., Singh A., Narasimhan M., Lau J., Weinberg M., et al. Pulmonary embolism in patients hospitalized with COVID-19 (from a New York health system) Am J Cardiol. 2020;133:148–153. doi: 10.1016/j.amjcard.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Thondapu V., Montes D., Rosovsky R., Dua A., McDermott S., Lu M.T., et al. Venous thrombosis, thromboembolism, biomarkers of inflammation, and coagulation in coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2021;9:835–844. doi: 10.1016/j.jvsv.2020.11.006. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan S.S., Radford S., Kow C.S., Zaidi S.T.R. Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta-analysis. J Thromb Thrombolysis. 2020;50:814–821. doi: 10.1007/s11239-020-02235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y.F., Pan L.Y., Zhang W.W., Cheng F., Hu S.S., Zhang X., et al. A meta-analysis of the incidence of venous thromboembolic events and impact of anticoagulation on mortality in patients with COVID-19. Int J Infect Dis. 2020;100:34–41. doi: 10.1016/j.ijid.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakai N.A., McClure L.A., Judd S.E., Safford M.M., Folsom A.R., Lutsey P.L., et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129:1502–1509. doi: 10.1161/CIRCULATIONAHA.113.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzek A., Schädler J., Dietz E., Ron A., Gerling M., Kammal A.L., et al. Prospective postmortem evaluation of 735 consecutive SARS-CoV-2-associated death cases. Sci Rep. 2021;11:19342. doi: 10.1038/s41598-021-98499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]