Abstract
Background
Patients undergoing vascular surgery are a high‐risk population with widespread atherosclerosis, an adverse cardiovascular risk profile and often multiple co‐morbidities. Postoperative cardiovascular complications, including myocardial infarct (MI), are common. Statins are the medical treatment of choice to reduce high cholesterol levels. Evidence is accumulating that patients taking statins at the time of surgery are protected against a range of perioperative complications, but the specific benefits for patients undergoing noncardiac vascular surgery are not clear.
Objectives
We examined whether short‐term statin therapy, commenced before or on the day of noncardiac vascular surgery and continuing for at least 48 hours afterwards, improves patient outcomes including the risk of complications, pain, quality of life and length of hospital stay. We also examined whether the effect of statin therapy on these outcomes changes depending on the dose of statin received.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 7), MEDLINE via Ovid SP (1966 to August 2012), EMBASE via Ovid SP (1966 to August 2012), CINAHL via EBSCO host (1966 to August 2012) and ISI Web of Science (1946 to July 2012) without any language restriction. We used a combination of free text search and controlled vocabulary search. The results were limited to randomized controlled clinical trials (RCTs). We conducted forwards and backwards citation of key articles and searched two clinical trial Websites for ongoing trials (www.clinicaltrials.gov and http://www.controlled‐trials.com).
Selection criteria
We included RCTs that had compared short‐term statin therapy, either commenced de novo or with existing users randomly assigned to different dosages, in adult participants undergoing elective and emergency noncardiac arterial surgery, including both open and endovascular procedures. We defined short‐term as commencing before or on the day of surgery and continuing for at least 48 hours afterwards.
Data collection and analysis
Two authors independently assessed trial quality and extracted data, including information on adverse events. We contacted study authors for additional information. We performed separate analyses for the comparisons of statin with placebo/no treatment and between different doses of statin. We presented results as pooled risk ratios (RRs) with 95% confidence intervals (CIs) based on random‐effects models (inverse variance method). We employed the Chi2 test and calculated the I2 statistic to investigate study heterogeneity.
Main results
We identified six eligible studies in total. The six Included studies were generally of high quality, but the largest eligible study was excluded because of concerns about its validity. Study populations were statin naive, which led to a considerable loss of eligible participants.
Five RCTs compared statin use with placebo or standard care. We pooled results from three studies, with a total of 178 participants, for mortality and non‐fatal event outcomes. In the statin group, 7/105 (6.7%) participants died within 30 days of surgery, as did 10/73 (13.7%) participants in the control group. Only one death in each group was from cardiovascular causes, with an incidence of 0.95% in statin participants and 1.4% in control participants, respectively. All deaths occurred in a single study population, and so effect estimates were derived from one study only. The risk ratio (RR) of all‐cause mortality in statin users showed a non‐significant decrease in risk (RR 0.73, 95% CI 0.31 to 1.75). For cardiovascular death, the risk ratio was 1.05 (95% CI 0.07 to 16.20). Non‐fatal MI within 30 days of surgery was reported in three studies and occurred in 4/105 (3.8%) participants in the statin group and 8/73 (11.0%) participants receiving placebo, for a non‐significant decrease in risk (RR 0.47, 95% CI 0.15 to 1.52). Several studies reported muscle enzyme levels as safety measures, but only three (with a total of 188 participants) reported explicitly on clinical muscle syndromes, with seven events reported and no significant difference found between statin users and controls (RR 0.94, 95% CI 0.24 to 3.63). The only participant‐reported outcome was nausea in one small study,with no significant difference in risk between groups.
Two studies compared different doses of atorvastatin, with a total of 145 participants, but reported data were not sufficient to allow us to determine the effect of higher doses on any outcome.
Authors' conclusions
Evidence was insufficient to allow review authors to conclude that statin use resulted in either a reduction or an increase in any of the outcomes examined. The existing body of evidence leaves questions about the benefits of perioperative use of statins for vascular surgery unanswered. Widespread use of statins in the target population means that it may now be difficult for researchers to undertake the large RCTs needed to demonstrate any effect on the incidence of postoperative cardiovascular events. However, participant‐reported outcomes have been neglected and warrant further study.
Keywords: Adult, Humans, Angioplasty, Atherosclerosis, Atherosclerosis/complications, Atherosclerosis/drug therapy, Atorvastatin, Cardiovascular Diseases, Cardiovascular Diseases/mortality, Cardiovascular Diseases/prevention & control, Cause of Death, Cholestyramine Resin, Cholestyramine Resin/therapeutic use, Heptanoic Acids, Heptanoic Acids/therapeutic use, Hydroxymethylglutaryl‐CoA Reductase Inhibitors, Hydroxymethylglutaryl‐CoA Reductase Inhibitors/therapeutic use, Lovastatin, Lovastatin/therapeutic use, Perioperative Care, Perioperative Care/methods, Postoperative Complications, Postoperative Complications/mortality, Postoperative Complications/prevention & control, Pyrroles, Pyrroles/therapeutic use, Randomized Controlled Trials as Topic, Sitosterols, Sitosterols/therapeutic use, Vascular Surgical Procedures, Vascular Surgical Procedures/adverse effects, Vascular Surgical Procedures/mortality
Plain language summary
Perioperative statin use to improve outcomes during and after noncardiac vascular surgery
Patients undergoing vascular surgery often have widespread atherosclerosis and are at high risk of complications during and after the operation. (Atherosclerosis is a condition in which an artery wall thickens as the result of an accumulation of fatty deposits such as cholesterol.) Statins are widely used to treat raised cholesterol levels but also confer beneficial effects on the inflammatory and circulatory systems. Their long‐term use in patients with vascular disease is well established. Accumulating evidence suggests that short‐term treatment with statins may reduce complications after cardiac surgery, but it is not yet clear whether there are any benefits for vascular surgery patients.
In July 2012 we searched medical databases for controlled trials of participants who had undergone aortic or arterial surgery and were randomly assigned to either statins or placebo (or standard care). Many vascular surgery patients are already taking statins; therefore we also included trials that randomly assigned participants to different doses of statin. Statin treatment should have been started any time between the decision to operate and performance of the operation and continued for at least 48 hours after the operation. We wanted to investigate the effect of this short‐term statin therapy on the risk of death and cardiovascular events such as heart attack and stroke within 30 days of surgery. We also considered adverse effects of statins such as muscle pain.
We found five studies that compared participants receiving statins with a control treatment or with placebo, but only three of these reported outcomes could be combined in the meta‐analyses. These three studies were of high quality but studied only 178 participants in total. This means that evidence was insufficient to allow review authors to determine whether statins improved patient outcomes after surgery. We were also not able to establish whether any adverse effects such as muscle pain were associated with statin use.
We found that two studies had compared different doses of atorvastatin, but evidence was insufficient to determine whether any benefits or risks were associated with using a higher dose.
Given the limited quantity of data obtained from randomized controlled trials, further studies are required to allow investigators to gather better information about whether prescribing statins around the time of vascular surgery can improve outcomes. However, widespread use of statins in patients before they need surgery may make these studies impracticable.
Summary of findings
Summary of findings for the main comparison. Perioperative statins versus placebo or no treatment for improving outcomes during and after noncardiac vascular surgery.
| Perioperative statins versus placebo or no treatment for improving outcomes during and after noncardiac vascular surgery | ||||||
| Patient or population: participants with improving outcomes during and after noncardiac vascular surgery. Settings: hospital. Intervention: perioperative statins versus placebo or no treatment. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Perioperative statins versus placebo or no treatment | |||||
| All‐cause mortality Follow‐up: 30 days | Moderate1 | RR 0.73 (0.31 to 1.75) | 178 (3 studies) | ⊕⊕⊝⊝ low2,3 | Effect estimate based on 1 study only. | |
| 40 per 1000 | 29 per 1000 (12 to 70) | |||||
| Death from cardiovascular causes Follow‐up: 30 weeks | Moderate1 | RR 1.05 (0.07 to 16.2) | 178 (3 studies) | ⊕⊕⊝⊝ low2,3 | Effect estimate based on 2 events occurring in 1 study. | |
| 30 per 1000 | 31 per 1000 (2 to 486) | |||||
| Myocardial infarction (non‐fatal) Follow‐up: 30 days | Moderate1 | RR 0.47 (0.15 to 1.52) | 178 (3 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 40 per 1000 | 19 per 1000 (6 to 61) | |||||
| Stroke/TIA (non‐fatal) Follow‐up: 30 days | Moderate1 | RR 0.24 (0.03 to 2.25) | 178 (3 studies) | ⊕⊕⊝⊝ low2,3 | Two events only across 3 studies. | |
| 10 per 1000 | 2 per 1000 (0 to 22) | |||||
| Participant‐reported outcomes-not measured | See comment | See comment | Not estimable | ‐ | See comment | Only 1 study measured or reported any participant‐reported outcomes (nausea in STAR VaS). |
| Adverse muscle effects | See comment | See comment | Not estimable | 188 (3) | See comment | Unable to pool. Important differences in assessment of endpoint between studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; and Very low quality: We are very uncertain about the estimate. | ||||||
1 Based on observational data reported by Nowygrod 2006 and Le Manach 2011. 2 Confidence interval crosses no effect and is consistent with increased and decreased risks. 3 Effect estimate from 1 study and/or based on fewer then 5 events.
Background
Description of the condition
Atherosclerosis can affect any major artery in the body, and the clinical consequences depend on the site of atheromatous plaque obstruction. Narrowing or stenosis due to atheroma in the lower limbs or in the abdominal aorta may lead to ischaemia (reduced blood supply) to leg muscles, resulting in muscle pain while walking (claudication). Reduced blood supply to the skin and underlying tissues causes ischaemia, ulceration and ultimately gangrene. Weakening of the arterial wall in the aorta may lead to dilation of the artery and aneurysm formation with risk of rupture. In the carotid arteries, fragments from a plaque (emboli) may cause a stroke or a transient ischaemic attack (TIA). Noncardiac vascular surgical procedures aim to clear, replace or bypass diseased arteries through open surgical dissection or, increasingly, by endovascular approaches. Amputation may be necessary if the circulation cannot be restored to a limb. Using the US National Inpatient Sample from 2003, Nowygrod et al reported 37,726 abdominal aortic aneurysm (AAA) repairs, 139,083 carotid artery revascularizations, 137,019 lower extremity revascularizations and 115,749 amputations (Nowygrod 2006). Data from the UK suggest 3000 to 4000 AAA repairs per year and a similar number of carotid endarterectomies (Sanders 2012; VASGBI 2009).
Patients undergoing these vascular procedures constitute a high‐risk population, with widespread atherosclerosis, an adverse cardiovascular risk profile and often multiple co‐morbidities. The nature of vascular surgery in this population entails a high risk of perioperative complications and mortality. For example, bleeding is the most common perioperative complication, particularly in open procedures. US data indicate that 17.1% of AAA open repairs and 11% of open lower extremity revascularizations were complicated by bleeding (Nowygrod 2006). Cardiac complications are frequent, estimated at 8% in open AAA and 2% to 3% in open carotid and lower extremity procedures (Nowygrod 2006) and are the primary cause of death in the perioperative period (Lee 1999; Le Manach 2005; Le Manach 2011). Postoperative myocardial infarcts (PMI) can be difficult to diagnose, and the widely used universal definition of a myocardial infarct (Thygesen 2007) may not allow detection of all cases (Le Manach 2011). Other investigators found evidence of perioperative myocardial infarction in 24% of 447 vascular surgery participants (Landesberg 2003).
The mechanisms underlying these complications are not fully understood. Stress responses, inflammatory processes and endothelial dysfunction are increased during surgery. Bleeding is known to be an important risk for cardiac events and perioperative mortality, probably acting through effects on oxygen delivery, haemodynamics, sympathetic stress and inflammation and coagulation (Le Manach 2005). For ischaemic events, the relative importance of plaque rupture or embolism and disturbances in blood flow leading to a dynamic mismatch between oxygen supply and demand (Sanders 2011) is unclear. The perioperative period constitutes a high‐risk time for patients undergoing vascular surgery, and optimization strategies to reduce this risk are required.
Description of the intervention
Statins are the medical treatment of choice for reducing high cholesterol levels. They inhibit 3‐hydroxy‐3‐methyl‐glutaryl (HMG)‐CoA reductase, the rate limiting step in cholesterol synthesis, which leads to upregulation of hepatic low‐density lipoprotein (LDL) receptors and reduction in circulating LDL and very low‐density lipoprotein (VLDL) levels. Meta‐analyses have demonstrated that statins reduce the risk of fatal and non‐fatal cardiac events in the community, both in healthy populations and in those with existing cardiac disease (Mills 2011; Taylor 2011) These meta‐analyses found no evidence for increased risk of rhabdomyolysis or other clinical muscle syndromes in statin users, but there was evidence of increased levels of muscle enzymes (Mills 2011)
Evidence is accumulating that patients taking statins at the time of surgery are protected against a diverse range of perioperative complications, including myocardial infarction, renal failure, delirium and death (Katznelson 2009; Le Manach 2007; Le Manach 2011; Molnar 2011; Noordzij 2007). Systematic reviews of observational studies and randomized controlled trials (RCTs) have indicated that statin users have reduced postoperative cardiac complications compared with participants naive to statins, but the evidence base is weak, particularly for noncardiac surgery (Chopra 2012; Kapoor 2006; Winchester 2010).
How the intervention might work
The lipid‐lowering action of statins is likely to be the primary mechanism involved in preventing the development of cardiovascular disease. However, it is unlikely to be the sole mechanism of protection in the perioperative period as the time scale is too short for any alteration in lipid levels to be meaningful. There is good evidence that statins also have pleiotropic effects that could feasibly influence perioperative outcomes. These include an anti‐inflammatory action, improvement in endothelial function, reduction of oxidative stress, improved organ autoregulation of blood flow, stabilization of atheromatous plaques and inhibition of platelet aggregation (Wang 2008). Although the molecular mechanisms of different effects need to be unravelled, effects on endothelial nitric oxide may be pivotal.
Why it is important to do this review
Many existing studies and one ongoing Cochrane reviews (Liakopoulos 2010;) have focused on perioperative statin use in cardiac surgery to improve outcomes. However, patients undergoing vascular surgery have a particularly high risk of coronary artery disease, and cardiac events are a common postoperative complication in this population. Two existing non‐Cochrane reviews on perioperative statin use have drawn on a range of study designs to examine participants undergoing various surgical and invasive procedures and include two RCTs on participants undergoing vascular surgery (Kapoor 2006; Winchester 2010). A more recent review adds an extra trial and assessment of risk of bias, but does not present results separately for participants undergoing noncardiac vascular surgery. (Chopra 2012). Our review will focus only on trials of participants undergoing vascular surgery; it will include a risk of bias assessment of included studies and will consider outcomes other than cardiovascular events. Our search strategy will include EMBASE, which provides in‐depth drug indexing.
To establish how widely statins should be used in clinical practice, it is important to examine whether statins reduce postoperative morbidity and mortality for procedures other than cardiac surgery. . A systematic review is required to confirm any effect, to estimate effect size and to provide guidance on perioperative use of statins both in clinical trials and in day‐to‐day practice. Guidelines would indicate that many patients with vascular disease should be receiving statins routinely (NICE 2006); however, observational reports indicate that this is often not achieved in practice. In the US, one study found that only 61% of participants undergoing major reconstructive vascular surgery were receiving statins at the time of initial surgical evaluation (Marshall 2009). Only 57% of participants undergoing vascular surgery in the UK were receiving statins (VASGBI 2009). Estimates of the proportion of patients with peripheral vascular disease who are receiving statins have increased from 43.3% in Canada and 56% in the Netherlands in 2004 (Al‐Omran 2008; Hoeks 2008) to 84% in Ireland in 2009 (Coveney 2011). Demonstration of a robust beneficial effect around the time of surgery not only might improve perioperative outcomes but also may lead to longer‐term prescribing.
Evidence suggests a dose‐response effect for statins in primary prevention (Law 2003), with higher doses leading to greater risk reduction. Fewer data are available for a dose‐response relationship in secondary prevention, with some studies describing an effect of duration but not of dosage (Chen 2010). Our review also considered whether any beneficial effect of statins given at the time of surgery is dependent on the dose or duration of statin received.
We anticipated that the evidence base for trials of statin use in vascular surgery could be small. By considering a range of outcomes, including functional measures, we aimed to clarify the endpoints for which evidence of a beneficial or adverse effect has been found and those for which evidence is insufficient to allow researchers to reach a conclusion.
Objectives
We examined whether short‐term statin therapy, commenced before or on the day of noncardiac vascular surgery and continuing for at least 48 hours afterwards, improves patient outcomes including risk of complications, pain, quality of life and length of hospital stay. We also examined whether the effect of statin therapy on these outcomes changes depending on the dose of statin received.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs) including quasi‐randomized and cluster‐randomized trials.
Types of participants
We included all adult participants (older than 18 years of age) who were scheduled for elective and emergency noncardiac arterial vascular surgery, including both open and endovascular procedures. We included participants scheduled for aortic, iliac, peripheral revascularization and carotid procedures. We also included participants scheduled for amputation as the result of complications of the vascular system.
We excluded participants requiring cardiopulmonary bypass.
We included in the review studies restricted to older participants, such as those older than 50 years of age. We also included trials with a wider study population of surgical participants, for instance also including participants undergoing cardiac procedures, provided the noncardiovascular participants were in the majority, or the results are presented separately for this group.
Types of interventions
We considered studies that have prescribed statins of any commercially available type at the time of the study, at any dose, either commenced de novo or with existing users randomly assigned to different dosages. We defined short‐term as commencing before or on the day of surgery and continuing for at least 48 hours afterwards.
Comparison groups were one of:
Placebo; or
No treatment or standard care.
We anticipated that most trials will be restricted to statin‐naive populations, that is, participants who were not taking statins before surgery was scheduled. Many patients with vascular disease will already be on statins in line with current clinical guidelines and will be ineligible for these trials. We therefore, aimed to include any trials that recruited patients already receiving statin therapy but that randomly assigned them to a higher dose. In this patient group, the comparison group was:
A different dose of statin.
We did not include any trials in which participants had existing statin therapy stopped or withdrawn before or immediately after surgery as part of the protocol. Co‐interventions or standard care should have been equivalent in all randomly assigned groups. We included trials where other features of care differ between intervention groups but considered these differences in the risk of bias assessment and performed sensitivity analyses to assess their impact on the results of the review.
Types of outcome measures
Primary outcomes
All‐cause mortality within 30 days of surgery.
Death from cardiovascular causes within 30 days of surgery.
Non‐fatal cardiac events within 30 days of surgery. This endpoint included:
Cardiac arrest;
Myocardial infarction (MI) as defined by the universal definition (Thygesen 2007) and measured by appropriate biomarkers such as troponins (standard or sensitive), electrocardiogram (ECG) readings or clinical signs; and
Myocardial necrosis as measured by increased troponin levels.
ECG evidence of ischaemia alone was not included as a cardiac event.
Secondary outcomes
Non‐fatal stroke or transient ischaemic attack as measured by clinical or neurological deficit.
Incident atrial fibrillation (AF) as measured by ECG readings.
Acute kidney injury or renal failure as measured by increased serum creatine levels, decreased urine output or other biomarker.
Participant‐reported outcomes such as quality of life or pain.
Graft patency as measured by ultrasound or need to return to theatre.
Length of stay: in hospital and intensive care unit.
Clinical muscle syndromes (statin‐induced myopathy) including myositis and rhabdomyolysis.
All outcomes, except length of hospital stay and graft patency, were assessed within 30 days of surgery. Any outcomes reported for periods longer than 30 days after surgery were not included, but those reported for shorter follow‐up times, such as seven days after surgery, were included. Subgroup analyses would have considered length of follow‐up if we had identified sufficient studies.
Outcomes did not form part of the study eligibility assessment, so that studies that met the participant, intervention and comparison criteria were included in the review even if they reported no relevant outcomes. These studies were not included in the data analyses but were reported separately in Characteristics of studies awaiting classification.
Search methods for identification of studies
Electronic searches
We searched for eligible trials in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL,The Cochrane Library, 2012 Issue 7, see Appendix 1), MEDLINE via Ovid SP (1946 to 14 July 2012, see Appendix 2), EMBASE via Ovid SP (1980 to 14 July 2012, see Appendix 3), the CINAHL via EBSCO host (1982 to 14 July 2012, see Appendix 4) and ISI Web of Science (1946 to 14 July 2012, see Appendix 5). The highly sensitive filter for randomized controlled trials was applied in MEDLINE and EMBASE. We also searched trial registers such as clinicaltrials.gov, in February 2012 for ongoing studies. MEDLINE, EMBASE and CENTRAL were searched in March/ April 2012 with an earlier version of the search (Appendix 6), and these results were combined with those from the main search.
No language restrictions were applied. The search strategies were developed in collaboration with a clinical librarian and the CARG Trials Search Co‐ordinator.
Searching other resources
We carried out forward citation on four papers: the two trials identified from the initial searches (DECREASE III; Durazzo 2004) and two review papers (Twine 2011; Winchester 2010). We used the cited reference search facility in Web of Science on 26 March 2012 to identify citing papers. We selected nine papers for backward citation (Biccard 2005; Chopra 2012; Dagher 2007; Hindler 2006; Kapoor 2006; Paraskevas 2011; Singh 1000; Stalenhoef 2009; Winchester 2010)
Data collection and analysis
Selection of studies
Results of the searches were collated and duplicates were removed. The selection of eligible articles took place in two stages.
All titles and abstracts were screened by AN and one of RDS, PA, AFS or medical student assistant to remove studies that were very unlikely to be eligible. This included studies that were clearly of an ineligible design or that included an ineligible study population or intervention. A pilot of 100 titles was reviewed with each pair of screeners before we proceeded with the remaining titles to clarify criteria for discarding articles at this stage. If no abstract was available but the title was possibly relevant, we obtained the full text of the article.
When we had screened all titles and abstracts, the full texts of potentially relevant titles were reviewed by AN or either SRL or RDS and information recorded on the study eligibility form. This form is included in Appendix 7. A pilot of 10 papers was read, and then the investigators discussed results to clarify the criteria for discarding articles at this stage and to modify the form as required. All potentially relevant papers were then read and the results of the two investigators compared. Any differences that we could not resolve were referred to AFS or PA.
Data extraction and management
Data were extracted independently from eligible studies by AN/SRL using a paper data extraction form (Appendix 7). We merged data from multiple eligible publications from the same study into a single data extraction form.
We included the following data items on the data extraction form:
Study design: randomization unit, cluster or participant; sequence generation or other randomization method;
Power calculations with baseline risk and effect size assumed;
Participant group: age, demographic, type of surgical operation;
Intervention: type of statin prescribed, timing and length of course, dosage, standard care given;
Comparison group: placebo, no treatment or different dose of statin;
Outcomes: outcomes and time points (i) collected, (ii) reported. For each outcome: definition, unit of measurement, timing; and
Results: numbers of participants (and number of clusters) assigned to each intervention group. For each outcome: sample size, summary data for each intervention (two‐by‐two table where possible for dichotomous data, means and standard deviations for continuous data), P values and confidence intervals.
AN and SRL met to compare results and prepare a final agreed form for each study. If relevant information or data were not available in the paper, we contacted the lead author to request the additional details.The agreed final data extraction form was entered into RevMan by AN and checked by SRL.
Assessment of risk of bias in included studies
We used the Cochrane risk of bias tool (Higgins 2011a). We considered the following Items:
Whether sequence generation was adequate;
Allocation concealment;
Blinding of participants, personnel and outcomes assessors;
Incomplete outcome data;
Selective outcomes reporting; and
Other potential sources of bias.
We considered blinding and incomplete outcome data separately for each outcome. Where possible, we obtained the protocol for the study and compared the methods reported with those outlined in the protocol to identify post hoc changes.
We completed a risk of bias table for each eligible study and outcome using the categories low, high and unclear risk of bias.
Measures of treatment effect
For dichotomous outcomes such as mortality and the occurrence of cardiac events, we entered total and numbers of events, respectively, into RevMan 5.1 (RevMan 5.1) and calculated risk ratios with 95% confidence intervals (CIs). If data had been presented in other forms, such as hazard ratios, and we had been unable to obtain the required tabular data from the study authors, we would have used the generic inverse variance option in RevMan. For continuous measures, such as length of stay, we aimed to calculate weighted mean differences (WMDs) using means and standard deviations. Where these data were unavailable, we reported medians and interquartile range but did not include these results in a meta‐analysis.
Unit of analysis issues
We did not find any cluster‐randomized or cross‐over trials. If any such trials had been included, we would have extracted data directly only if the analysis had properly accounted for the cluster design, using methods such as multilevel modelling or generalized estimating equations. If these adjustments had not been made within the report, we planned to perform approximate analyses by recalculating standard errors or sample sizes based on the design effect (see Section 16.3.6 of Higgins 2011). The resulting effect estimates and their standard errors would have been analysed using the generic inverse variance method in RevMan.
Dealing with missing data
We contacted authors to clarify any missing or unclear follow‐up and outcome data. If missing outcome data remained a concern, we planned to undertake sensitivity analyses to compare the effects of complete case analysis, worst case scenario and last observation carried forward options on the results of any individual study and on any meta‐analysis undertaken.
Assessment of heterogeneity
We anticipated that there might be considerable heterogeneity between studies because of differences in:
Timing and duration of statin before surgery;
Type and dose of statin;
Components of standard care; and
Timing of outcome measurement (e.g. seven days, 28 days).
If we had included sufficient studies in any single meta‐analysis, we planned to study heterogeneity between studies based on participant group, setting and type of intervention using the Chi2 and I2 statistics. Important heterogeneity (Chi2 P < 0.1 and I2 > 50%) would have been investigated, where possible, by subgroup analyses and meta‐regression, particularly for the effect of dosage or duration of statin therapy.
Assessment of reporting biases
Reporting bias may occur within studies, with certain*‐ outcomes not reported. Where a report or the original protocol suggested that data on an outcome were collected but it was not reported in the paper, we contacted the authors and requested the data. If 10 or more studies had been included in any single meta‐analysis, funnel plots would have been examined to visually assess the presence of publication bias and Egger’s test used to test for asymmetry.
Data synthesis
The extent of heterogeneity was considered before any meta‐analysis was attempted. Any I2 values in excess of 80% for any group of studies argued against an overall estimate being presented. Where we had sufficient studies to combine results, differences in study size and between studies in the duration and type of statin, standard care and type of surgery suggested that a random‐effects model would be the most suitable choice. Mantel‐Haenszel risk ratios were used where possible for dichotomous outcomes as the outcomes were not rare.
Subgroup analysis and investigation of heterogeneity
If data had been sufficient we planned to investigate the following subgroups, which could account for heterogeneity between studies:
Type of surgical intervention;
Emergency or elective surgery;
Duration of statin use; and
Timing of outcome measurement.
We planned to assess differences in effect size between subgroups in RevMan, using I2 estimates (Section 9.6.3.1 of Higgins 2011) and to combine results in smaller groups if appropriate.
Sensitivity analysis
If sufficient studies had been found, we aimed to undertake analyses to explore the contributions of:
Unpublished studies;
Risk of bias; and
Risk of missing outcome data
Summary of findings
We used the principles of the GRADE system to assess the quality of the body of evidence associated with the following specific outcomes in our review (Guyatt 2008):
All‐cause mortality within 30 days of surgery;
Death from cardiovascular causes within 30 days of surgery;
Non‐fatal cardiac or stroke events within 30 days of surgery;
Adverse muscle effects; and
Participant‐reported outcomes.
We constructed a 'Summary of findings' (SoF) table using the GRADE software. The GRADE approach appraises the quality of evidence for an outcome and assesses how confident we can be that our estimate of effect or association reflects the real association. The quality measures considered include risk of bias (methodologic quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias. The summary of findings table was completed by AN and checked by SRL. We resolved disagreements by discussion and, if necessary, consultation with AFS or PA.
Results
Description of studies
Results of the search
Our search is summarized in Figure 1.
1.
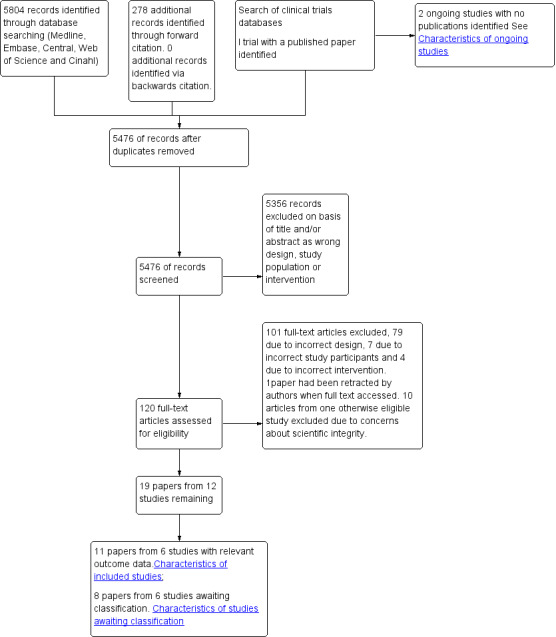
Sudy flow diagram.
We identified 5804 references through searches of electronic databases and 278 from forward citation searching. We removed duplicates, which resulted in 5476 unique references. We found no extra studies via backward citation. We excluded 5356 records on the basis of titles and abstracts and then reviewed 120 full text papers. Of these, 19 were eligible and 101 ineligible. We found three eligible clinical trials by searching trial databases-one of which had an eligible publication (STAR VaS study).
Included studies
We found nine papers from five eligible studies reporting outcomes(APVS study; Durazzo 2004; Rahman 1995; Ramo 1995; STAR VaS study). We also found 10 papers reporting on seven studies that were eligible (RCTs studying perioperative statin used in participants undergoing vascular surgery ) but had reported no relevant outcome data (ATROCAP study; Crisby 2001; Cuccurollo 2006; Evans 2007; Kajimoto 2009; Martin Ventura 2005; MAPS study). These studies are mainly small and focused on cellular outcomes or biomarkers in the vessel wall. We contacted the authors to clarify design and duration of statin therapy and to ask whether clinical outcomes were available.The investigators of the MAPS study supplied clinical outcome data, and so this study has been added as an eligible study-giving a total of 11 papers from six eligible studies.These remaining six eligible studies with no outcome data are summarized in the Characteristics of studies awaiting classification table. The six eligible studies are summarised in Characteristics of included studies.
Participants
Five study populations were exclusively participants who had undergone vascular surgery either a mixture of procedures, including abdominal aortic aneurysm (AAA) repair, carotid endarterectomy (CEA) and lower limb arterial surgery or amputation (APVS study; Durazzo 2004), or a single procedure-open AAA repair (Rahman 1995), CEA (MAPS study) or lower limb angioplasty (Ramo 1995). The Star VaS study included a minority of nonvascular participants (STAR VaS study), but we were able to obtain outcome data on participants undergoing vascular surgery only. Participants were not selected on the basis of lipid level, and in all studies, participants were required not to be on statins before enrolment in the study. In some studies, this led to a considerable loss of eligible participants. For instance, during recruitment for the STAR VaS study, 815 out of 1037 potential subjects were excluded because of statin use. We found no studies in which existing statin users were randomly assigned to different dosages.
Interventions / comparisons
Atorvastatin was the intervention drug in five studies, with doses varying from 80 mg/day ( APVS study; MAPS study; Rahman 1995; STAR VaS study) to 20 mg/day (APVS study; Durazzo 2004) or 10 mg/day (MAPS study). Ramo 1995 used 20 mg/day lovastatin. Three studies compared statin with a placebo ( Durazzo 2004; Rahman 1995; STAR VaS study) and one compared statin with no additional treatment (Ramo 1995). Two studies compared high‐dose (80 mg/day) atorvastatin with low‐dose (20 mg/day) atorvastatin in the APVS study or 10 mg/day in the MAPS study. The MAPS study also had a control group of cholestyramine and sitosterol, so this study is entered in both comparisons, with the two dosage groups of atorvastatin combined for comparison of statin versus placebo/control.
In most studies, the statin intervention was started three to four weeks before surgery. In the angioplasty study-when the procedure was often urgent-statins were commenced on average two days before the procedure (Ramo 1995). The STAR Vas study had two intervention arms and compared both statin therapy commenced seven days before (AA group) and on the day of surgery (PA group) with placebo therapy (PP group). The AA and PA groups were combined in our analyses.
Ramo et al, studying angioplasty participants, prescribed aspirin to both intervention and control groups (Ramo 1995). In other studies, the use of co‐interventions was left to the discretion.of the clinical team (Durazzo 2004; STAR VaS study).
Excluded studies
We reviewed 101 studies in full text that were ineligible or excluded. Mostof these were editorials, reviews or reports from observational data. We have summarized only 19 publications from randomized trials that were excluded in the Characteristics of excluded studies table. The most important exclusion is the 10 articles related to the DECREASE III study. After careful consideration and consultation with CARG Co‐ordinating Editors (Nathan Pace and Ann Møller), we decided to exclude this study because of the dismissal of principal investigator Professor Polderman for breaches of academic integrity for recurrent and systematic problems in his research activity (Erasmus 2011). We excluded the Heart Protection Study (HPS) as it reported the effects of statin therapy on outcomes in peripheral arterial disease (PAD) participants considered long‐term not perioperative therapy (HPS 2007). Other excluded studies examined PAD participants who were not undergoing surgery (Blann 2001; Hagenaars 2001). In two studies, both intervention and control groups received statins (ELIMIT 2007; Martin‐Ventura 2008). For clarity, we have listed some of the observational studies on vascular surgery participants that we excluded on the basis of title and abstract but that have been reported in other reviews (Hindler 2006; Kapoor 2006; Stalenhoef 2009).
Ongoing studies
We found three studies in our review of clinical trials databases. The STAR VaS study has published results and is considered with included studies. We were able to obtain from the authors outcome data on vascular surgery participants. The LOAD study has just commenced and expects to complete recruitment in 2014. It includes vascular surgery as well other types of surgery in high-cardiovascular risk participants. The Clinical Utility of Endothelial Dysfunction in PAD study is focused on endothelial outcomes but is a randomized trial of statin use in participants undergoing vascular surgery(Vita 2012).
Risk of bias in included studies
Our risk of bias assessments for each included study are summarized in Figure 2 and as percentages across all studies in Figure 3. Details and reasons for each assessment are listed in Characteristics of included studies.
2.
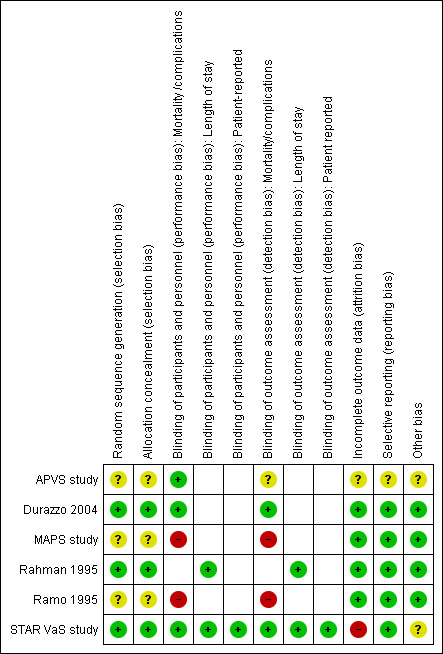
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
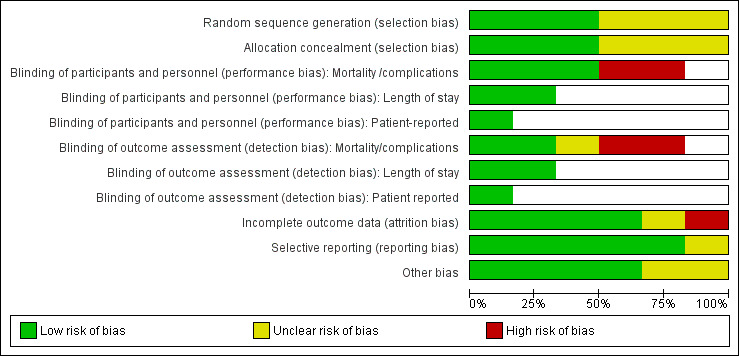
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three studies gave sufficient details about their methods for random sequence generation and allocation concealment to be assessed as at low risk of selection bias (Durazzo 2004; Rahman 1995; STAR VaS study).Three studies described the allocation as randomly assigned but gave insufficient detail to allow assessment of either sequence generation or allocation concealment (APVS study; MAPS study; Ramo 1995). In general, the distribution of baseline characteristics and the type of operation between intervention and control groups were similar, suggesting effective randomization. However in Ramo et al, where no details of randomization method were given, the intervention group's clinical condition baseline was worse, with fewer run‐off vessels. The effect of this bias would be to decrease any protective effect of statins (Ramo 1995), so we did not assess this as high risk.
Blinding
We have separated these assessments by type of outcome as the impact of staff or participant knowledge of allocation may vary across different outcomes.
Of the six studies reporting clinical endpoints (death, non‐fatal cardiovascular (CV) events or graft patency), we assessed two studies as being at low risk of performance or detection bias with good descriptions of the procedures for double‐blinding (Durazzo 2004; STAR VaS study). The clinical endpoints (death, CV events) reported in these studies were assessed to recognised standard definitions by staff unaware of allocation. The APVS study, which has been reported in only two abstracts, is described as double‐blind but gave no details of how the CV endpoints were assessed. Ramo's study (Ramo 1995) of graft patency was assessed as being at high risk of performance and detection bias because the control group received no placebo and there is no mention of blinding. Although repeat angiograms were assessed by radiologists blinded to allocation, the decision to initiate a repeat angiogram before one‐year follow‐up was made by clinicians who knew allocation.The MAPS study was also classified as at high risk of performance and detection bias because no description of blinding is provided in the publication. The trial registration on clinicaltrials.gov states that the trial was single‐blind (user).
Length of stay was measured in two studies (Rahman 1995; STAR VaS study). Both studies were blinded, but we have no details about discharge criteria and who made the decision that participants were fit for discharge.
Incomplete outcome data
Two studies gave few details of numbers included in analyses (APVS study; Rahman 1995), so completeness of outcome reporting could not be assessed. In two studies, the analyses in published papers included participants who had not undergone surgery and therefore were not eligible for the trial (Durazzo 2004; STAR VaS study). Additional correspondence with authors of Durazzo 2004 allowed us to establish that participants who had not undergone surgery had not suffered any outcomes. For this study, we decided to exclude these 10 participants who had not undergone surgery from all outcomes except adverse muscle effects. We considered that these were postrandomization exclusions and were not eligible as they had not undergone the surgery that posed the increased risk event. However, they were included for outcomes related to adverse effects of statin therapy. The denominator for the STAR VaS study includes only participants who underwent vascular surgery and about whom we were able to obtain additional information from the authors.
Selective reporting
All studies reported the outcomes described in the methods section or the trial register entry if available (APVS study; STAR VaS study). Preliminary reports from APVS have reported only a combined endpoint (APVS study). Although studies reported what they measured, it is notable that only one study measured any participant‐reported outcomes, and only two measured length of stay.
Other potential sources of bias
Funding sources. Two studies list non‐commercial funding sources (Durazzo 2004; STAR VaS study), and three give no information about study funding (APVS study; Rahman 1995; Ramo 1995). MAPS study was funded in part by Pfizer with an unrestricted educational grant.
Effects of interventions
See: Table 1
Comparison of statin with no treatment/ placebo
Five studies reported the effects of statin compared with placebo (Rahman 1995; Durazzo 2004; STAR VaS study), no additional treatment (Ramo 1995) or control treatment (MAPS study).
Primary outcomes
All‐cause mortality within 30 days of surgery (Analysis 1.1); was reported in three studies with a total of 178 participants. 7/105 (6.7%) participants in the statin group died within 30 days of surgery, as did 10/73 (13.7%) participants in the control group. No perioperative deaths were reported in the STAR VaS study or the MAPS study, and the estimate from Durazzo 2004 shows a statistically non‐significant decrease in risk (risk ratio (RR) 0.73, 95% confidence interval (CI) 0.31 to 1.75). Mortality rates were high in this study, approaching 20%, possibly reflecting the poor state of health of participants (Figure 4).
1.1. Analysis.
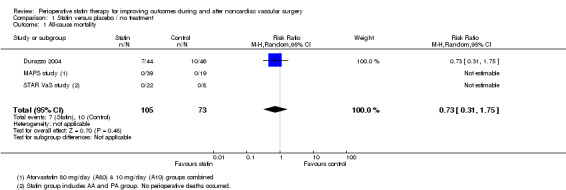
Comparison 1 Statin versus placebo / no treatment, Outcome 1 All‐cause mortality.
4.
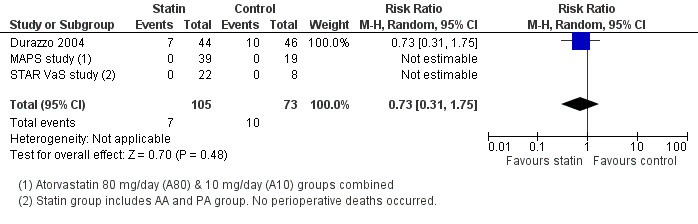
Forest plot of comparison: 1 Statin versus placebo / no treatment, outcome: 1.2 All‐cause mortality.
Death from cardiovascular causes within 30 days of surgery (Analysis 1.2); was reported in three studies but with only two events in one study. Mortality from cardiovascular causes occurred in 1/105 (0.95%) participants receiving statins and 1/73 (1.4%) control participants. The estimate from Durazzo 2004 is not informative because confidence intervals were wide (RR 1.05, 95% CI 0.07 to 16.20).
Nonfatal cardiac events within 30 days of surgery: No studies reported cardiac arrest outcomes. Only one study reported the occurrence of two cases of myocardial necrosis in the AA group based on a troponin rise to above the 99th percentile, but these cases did not meet criteria for MI (STAR VaS study). Non‐fatal MI within 30 days of surgery was reported in three studies and occurred in 4/105 (3.8%) participants in the statin group and 8/73 (11.0%) participants receiving placebo (RR 0.47, 95% CI 0.15 to 1.52) (Figure 5).
1.2. Analysis.
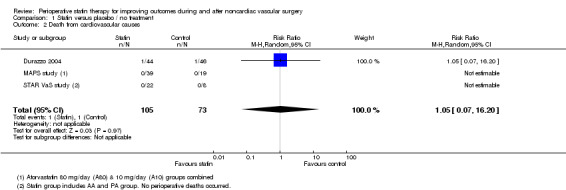
Comparison 1 Statin versus placebo / no treatment, Outcome 2 Death from cardiovascular causes.
5.
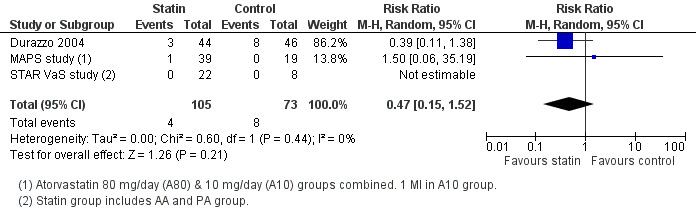
Forest plot of comparison: 1 Statin versus placebo/no treatment, outcome: 1.4 Myocardial infarction (non‐fatal).
Secondary outcomes
Non‐fatal stroke/TIA (Analysis 1.4); Stroke events were rare, with only two events reported and no statistically significant difference between intervention and placebo groups (RR 0.24, 95% CI 0.03 to 2.25).
Acute atrial fibrillation (Analysis 1.5): The STAR VaS study reported that 2/22 (9.1%) participants receiving statins developed new atrial fibrillation within seven days of surgery compared with 0/8. This is a much higher reported incidence than that reported in the MAPS study, which may reflect differences in detection (Holter used in the STAR VaS study) (pooled RR 1.73, 95% CI 0.20 to 14.84).
Acute kidney injury: No studies reported this outcome.
Participant‐reported outcomes: Only one study described participant‐reported outcomes in unpublished data. In the STAR VaS study, 3/16 participants in the group-1/7 in the PA group and 2/8 in the PP group-reported nausea. The differences were not statistically significant.
Graft patency (Analysis 1.7): One small study reported this outcome with a non‐significant reduction in restenosis after lower limb arterial angioplasty; 4/18 (22%) participants receiving statins and aspirin required repeat angioplasty within one year compared with 8/19 (42%) in the control group receiving aspirin only (RR 0.53, 95% CI 0.19 to 1.45) (Ramo 1995). Two participants in the statin group and four in the aspirin only group required amputation during follow‐up. In the STAR VaS study, one participant who had received statins before peripheral vascular disease (PVD) required toe amputation, although the graft remained patent.
Length of stay: Length of stay was reported in Rahman's study of open AAA repair (Rahman 1995) and in the STAR VaS study (Analysis 1.6). No statistically significant differences in total hospital stay or stay in the high‐dependency unit were noted between statin and placebo groups.
Clinical muscle syndromes (Analysis 1.8): Several studies reported muscle enzyme levels as a safety measure, but only three (with a total of 188 participants) reported explicitly on clinical syndromes, with seven events reported. No statistically significant differences were noted between statin and placebo groups. Given differences in the ascertainment and definition of outcome, we decided not to pool these estimates.
1.4. Analysis.
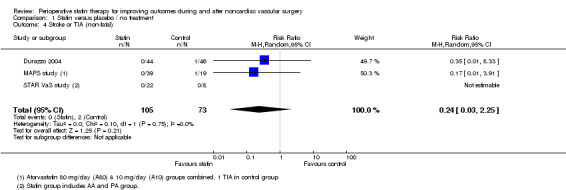
Comparison 1 Statin versus placebo / no treatment, Outcome 4 Stroke or TIA (non‐fatal).
1.5. Analysis.
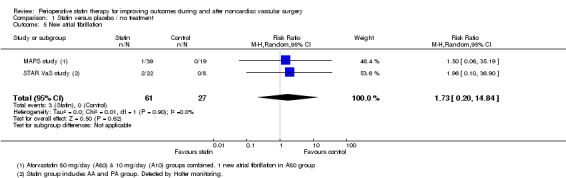
Comparison 1 Statin versus placebo / no treatment, Outcome 5 New atrial fibrillation.
1.7. Analysis.
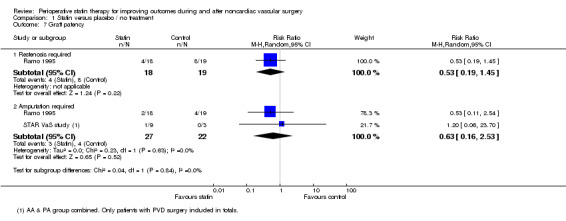
Comparison 1 Statin versus placebo / no treatment, Outcome 7 Graft patency.
1.6. Analysis.
Comparison 1 Statin versus placebo / no treatment, Outcome 6 Length of stay.
| Length of stay | |||
|---|---|---|---|
| Study | Group | Hospital stay. Days‐ median (IQR/range ) | High dependency unit stay Days ‐ median (IQR) |
| Rahman 1995 | Atorvastatin group N=20 | 7 (5‐47) | 2 (1‐6) |
| Rahman 1995 | Placebo group N=20 | 8 (5‐25) | 1 (1‐6) |
| Rahman 1995 | Significance test (Mann‐Whitney U test) | p=0.869 | p=0.756 |
| STAR VaS study | AA group N = 15 | 6 (5‐8) (mean 9.07 SD= 9.34) | |
| STAR VaS study | PA group N=7 | 6 (4‐7) (mean 6.00 SD =1.53) | |
| STAR VaS study | PP group N=8 | 6 (4.5‐7) ( mean 6.75 SD = 4.50) | |
1.8. Analysis.
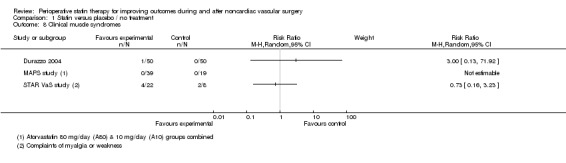
Comparison 1 Statin versus placebo / no treatment, Outcome 8 Clinical muscle syndromes.
Comparison of high‐dose with low‐dose statin
Only two studies compared outcomes in participants who had received high‐ and low‐dose statin. The MAPS study reported all outcomes, but only one MI was reported in the low‐dose (A10) group and one new atrial fibrillation in the high‐dose (A80) group. In preliminary reports from the APVS study, only combined cardiovascular endpoints (cardiac death, non‐fatal MI and stroke) were reported with 3/53 (5.7% incidence) in the A80 group and 7/53 (13.2%) in the A20 group. This gives a relative risk of 0.43 (95% CI 0.12 to 1.57). We do not have sufficient data to present effect estimates on any of our prespecified outcomes for this comparison.
Sensitivity analyses
We were unable to carry out planned sensitivity analyses on type of intervention, elective or emergency surgery or duration of statin use because of low numbers of studies. The STAR VaS study had a shorter duration of statin use than other studies and had a non‐significant increase in MI among statin users, but no significant heterogeneity was noted between study results.
We excluded the DECREASE III study because of concerns about validity, but we ran sensitivity analyses on its impact on our results for comparison 1-statin versus placebo/no treatment. This trial consisted of 497 participants who were scheduled for AAA repair, distal aorto‐iliac reconstruction, lower limb arterial reconstruction or carotid endarterectomy at Erasmus Medical Centre, Rotterdam, Netherlands. In these analyses, our achieved sample size was 356 in the statin group and 320 in the control group. Results from DECREASE were similar to those of included studies, and in these analyses, the pooled estimates were as follows: for all‐cause mortality, RR 0.61 (95% CI, 0.32 to 1.17); for death from cardiovascular causes, RR 0.56 (95% CI, 0.19 to 1.65); for MI, RR 0.47 (95% CI, 0.24 to 0.92); and for stroke, RR 0.34 (95% CI, 0.07 to 1.72). No clinical muscle syndrome events were reported in DECREASE III. Inclusion of these data would have led to a statistically significant pooled result for MI reduction in statin users but otherwise would not have altered results.
Discussion
Summary of main results
Our review has found no evidence that perioperative administration of statins has a beneficial effect on the outcome of patients undergoing vascular surgery. Our results for all outcomes are consistent with increased and decreased risk of cardiovascular events in the 30 days after surgery. For some of our primary outcomes (all‐cause mortality and non‐fatal MI), the included studies show a decrease in risk of approximately 50% in the group treated with statins, but because of low numbers of events, the difference in risk between participant groups did not reach statistical significance. For a control population with a 4% risk of MI, this means that participants treated with statins could have a risk of 0.6% to 6.1% (Table 1).
The risk of clinical muscle syndromes was low in these study populations with only 5/111 events in statin users and 2/77 in the control group across all studies. We were focusing on clinical symptoms rather than enzyme levels, and variations in the incidence of muscle effects indicate that there may have been differences in ascertainment across studies with 1/100 cases in Durazzo 2004 but 6/30 in the STAR VaS study, so we decided not to present a pooled estimate of effect. However overall, it would appear that adverse clinical muscle syndromes were unusual in the perioperative period. This is confirmed by observational data from vascular surgery participants with estimates of rhabdomyolysis incidence of between 0.1% and 0.5% and no clear evidence that statins increase the risk of postoperative muscle syndromes (Biccard 2008; Biccard 2009). In one observational study, statin use decreased the risk of elevated creatinine kinase after vascular surgery (Biccard 2009).
Only two studies-one with only preliminary data (APVS study; MAPS study)-have investigated the influence of dose of statin on outcomes, and we could not present an effect estimate for any of our outcomes. We found a non‐significantly decreased risk of a combined cardiovascular endpoint in the group receiving the higher statin dose.
Overall completeness and applicability of evidence
Despite a comprehensive search strategy and review of 5804 titles, evidence for our outcomes is sparse. Exclusion of the largest available study (DECREASE III) has severely restricted the power of the review. Power calculations indicate that to detect a 50% reduction in MI incidence from 4% to 2% with 80% power and 5% significance would require inclusion of 1141 participants in each group. Our sample size was less than 10% of this. Two additional studies included had small samples and few events (MAPS study; STAR VaS study). Six remaining studies appear to have the correct design and intervention but with no available outcome data, and it is possible that these studies may show different effects. However, all of these studies are small, and there are concerns about the quality of data on outcomes that were not prespecified for the trial.
It is striking that only one study reported on participant‐reported outcomes; this shows an important deficit in the literature.
Quality of the evidence
The included studies were well designed and executed. Based on the study publications, we had serious concerns about potential detection bias in only two studies, in which clinicians were not blinded (MAPS study; Ramo 1995). Including the MAPS study data is somewhat problematic as the outcome data collection was not planned and may not be of high quality. However, given the small numbers of events in this study, sensitivity analyses removing it had only a minor effect on effect size and confidence intervals.The MAPS study had received commercial funding but stated that this work was supported by an unrestricted educational grant.
The most serious concern about the quality of the evidence concerned DECREASE III, for which the authors were investigated about consent obtained and whether study results can be related to participant data. These papers have not been withdrawn, but we decided to exclude them from the review.
Potential biases in the review process
We were unable to undertake funnel plots or to assess publication bias because we identified fewer than 10 studies for any outcome.
Agreements and disagreements with other studies or reviews
Existing reviews of RCTs have considered all types of noncardiac surgery as a single group and were not restricted to vascular surgery participants. This increased the size and the number of trials. All reviews conclude that statins significantly reduce the incidence of CV events with relative risk for postoperative MI and all‐cause mortality reported as 0.52 (95% CI 0.36 to 0.74) and 0.59 (95% CI 0.31 to1.12), respectively, in Chopra 2012 and as 0.57 (95% CI 0.46 to 0.70) and 0.61 (95% CI 0.37 to 1.17) in Winchester 2010. There is considerable, but not complete, overlap between the studies included in this review and in previous reviews. DECREASE III is important in other published estimates.
Authors' conclusions
Implications for practice.
Evidence is insufficient to allow review authors to conclude that perioperative statin use in patients not already receiving statins reduces or increases the risk of complications following noncardiac vascular surgery. We are also unable to comment on the safety of statins around the time of surgery.
Implications for research.
The existing body of evidence leaves unanswered questions about the benefits of perioperative use of statins for vascular surgery. The study populations were statin‐naive, which led to large loss of eligible participants, as many patients were already receiving statins. With the more widespread use of statins, it may now be difficult to undertake the large RCTs needed to demonstrate any effect on rare events such as mortality in a statin‐naive population. Future trials could focus on the impact of different dosages in patients already taking statins. It is important to understand the patient's view and to include more patient‐reported outcomes.
Acknowledgements
We would like to thank Anna Lee (content editor); Cathal Walsh (statistical editor); Bita Mesgarpour, Gerry Stansby, Peter Kruger and Yannick Le Manach (peer reviewers); and Anne Lyddiatt (consumer reviewer) for their help and editorial advice during the preparation of this systematic review.
We would like to thank the following authors for the time and effort they took in responding to our queries: Dr Bruno Caramelli (Durazzo 2004); Professor Ian Chetter (Rahman 1995); Professor Paolo Pauletta and Dr Massimo Puato (MAPS study) and Professor David Neilipovitz (STAR VaS study).
We also thank Jenny Tancock for assistance with designing and running searches and Michael Lowe for assistance with title sifting.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Vascular Surgical Procedures, this term only #2 MeSH descriptor Peripheral Vascular Diseases explode all trees #3 MeSH descriptor Aortic Aneurysm, Abdominal explode all trees #4 MeSH descriptor Aortic Aneurysm, Thoracic explode all trees #5 MeSH descriptor Endarterectomy, Carotid explode all trees #6 MeSH descriptor Amputation, this term only #7 MeSH descriptor Carotid Stenosis explode all trees #8 MeSH descriptor Atherosclerosis explode all trees #9 MeSH descriptor Intermittent Claudication explode all trees #10 ((vascular or aort* or aneurysm or carotid) near (repair or procedur* or surg* or operat*)):ti,ab or ((abdominal or thoracic or thoracoabdominal or endovascular) near aneurysm*):ti,ab or (femoropopliteal near (bypass or graft)) or carotid endarterectomy:ti,ab or peripheral revascularisation:ti,ab or infrainguinal bypass or amputation:ti,ab or (aorta near (abdominal or thoracic)) near surgery:ti,ab #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Hydroxymethylglutaryl‐CoA Reductase Inhibitors explode all trees #13 (statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatinor lipitor or lescol or lipostat or crestor or zocor) #14 (#12 OR #13) #15 (#11 AND #14)
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp Vascular Surgical Procedures/ or Peripheral Vascular Diseases/ su, th or exp Aortic Aneurysm, Abdominal/ su, th or exp Aortic Aneurysm, Thoracic/ su, th or exp Endarterectomy, Carotid/ or Amputation/su, th or ((Aorta, Abdominal/ or Aorta, Thoracic/) and surgery.ti,ab.) or exp Carotid Stenosis/ su, th or exp Atherosclerosis/su, th or exp Intermittent Claudication/ su, th or ((vascular or aort* or aneurysm or carotid) adj3 (repair or procedur* or surg* or operat*)).mp. or ((abdominal or thoracic or thoracoabdominal or endovascular) adj3 aneurysm*).mp. or (femoropopliteal adj3 (bypass or graft)).mp. or carotid endarterectomy.mp. or peripheral revascularisation.mp. or infrainguinal bypass.mp. or amputation.ti,ab. 2. exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ or (statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatinor lipitor or lescol or lipostat or crestor or zocor).af. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 3. EMBASE (Ovid SP) search strategy
1. vascular surgery/ or peripheral vascular disease/su, th or abdominal aorta aneurysm/su, th or thoracic aorta aneurysm/su, th or carotid endarterectomy/ or amputation/su, th or ((abdominal aorta/ or thoracic aorta/) and surgery.ti,ab.) or carotid artery obstruction/su, th or atherosclerosis/su, th or intermittent claudication/su, th or ((vascular or aort* or aneurysm or carotid) adj3 (repair or procedur* or surg* or operat*)).ti,ab. or ((abdominal or thoracic or thoracoabdominal or endovascular) adj3 aneurysm*).ti,ab. or (femoropopliteal adj3 (bypass or graft)).mp. or carotid endarterectomy.mp. or peripheral revascularisation.ti,ab. or infrainguinal bypass.mp. or amputation.ti,ab. 2. hydroxymethylglutaryl coenzyme A reductase inhibitor/ or (statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatinor lipitor or lescol or lipostat or crestor or zocor).ti,ab. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. CINAHL (EBSCOhost) search strategy
S1. ((MH "Surgery, Cardiovascular") OR (MH "Peripheral Vascular Diseases") OR (MH "Aortic Aneurysm, Abdominal") OR (MH "Aortic Aneurysm, Thoracic") OR (MH "Endarterectomy, Carotid") OR (MH "Amputation") OR (MH "Carotid Stenosis") OR (MH "Atherosclerosis") OR (MH "Intermittent Claudication")) OR (((vascular or aort* or aneurysm or carotid) and (repair or procedur* or surg* or operat*))) OR (((abdominal or thoracic or thoracoabdominal or endovascular) and aneurysm*)) OR ( femoropopliteal and (bypass or graft)) OR carotid endarterectomy OR peripheral revascularisation OR infrainguinal bypass OR TI amputation S2. TI (statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatinor lipitor or lescol or lipostat or crestor or zocor ) OR AB ( statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatinor lipitor or lescol or lipostat or crestor or zocor) S3. S1 and S2
Appendix 5. ISI Web of Science search strategy
#1 TS=(aorta SAME (abdominal or thoracic) SAME surgery) or TS=carotid stenosis or TI=atherosclerosis or TS=intermittent claudication or TS=((vascular or aort* or aneurysm or carotid) SAME (repair or procedur* or surg* or operat*)) or TS=((abdominal or thoracic or thoracoabdominal or endovascular) SAME aneurysm*) or TS=(femoropopliteal SAME (bypass or graft)) or TS=carotid endarterectomy or TS=(peripheral revascularisation or infrainguinal bypass) or TI=amputation #2 TS=(statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatinor lipitor or lescol or lipostat or crestor or zocor) #3 TS=(random* or multicenter or prospective or placebo*) or TS=(trial* SAME (controlled or clinical)) or TS=((blind* or mask*) SAME (single or double or triple or treble)) #4 #1 and #2 and #3 and #4
Appendix 6. Additional searches
|
Searches – 16/4/12 Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present |
|
| 1 | exp Vascular Surgical Procedures/ or exp/amputation |
| 2 | exp Peripheral Vascular Diseases/su, th or exp aortic aneurysm, abdominal/su, th or exp aortic aneurysm, thoracic/su, th or exp aortic rupture/su, th or exp Carotid Stenosis/su, th or exp Atherosclerosis/su, th or exp Intermittent Claudication/su, th or ((exp Aorta, Abdominal/ or exp Aorta, Thoracic/) and surg*.ti,ab.) |
| 3 | ((abdominal or thoracic or thoracoabdominal) adj3 aneurysm*).mp. |
| 4 | (((iliac* adj3 arter*) or infrainguinal or (femoropop* or femoro‐pop*) or (vascular or AAA or aort* or aneurysm* or carotid)) adj3 (endovascular or bypass or graft* or surg* or revasc*or repair* or procedur* or operat*)).mp. |
| 5 | (endarterectomy or peripheral revasculari#ation or lower extremity revasculari#ation or critical limb ischaemia or critical limb ischemia).mp. |
| 6 | (interm* adj3 claud*).mp. |
| 7 | amputation*.ti,ab. |
| 8 | or/1‐7 |
| 9 | (statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatin).af. |
| 10 | (lipitor or lescol or lipostat or crestor or zocor).af. |
| 11 | exp Hydroxymethylglutaryl‐CoA‐Reductase Inhibitors/ |
| 12 | or/9‐11 |
| 13 | ((randomized controlled trial or controlled clinical trial).pt. or randomi#ed.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. |
| 14 | 8 and 12 and 13 |
|
Searches – 16/4/12 Embase 1974 to 2012 April 03++++++++ |
|
| 1 | placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. |
| 2 | ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. |
| 3 | 1 or 2 |
| 4 | (animals not (humans and animals)).sh. |
| 5 | 3 not 4 |
| 6 | exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ |
| 7 | (statin* or simvastatin or rosuvastatin or fluvastatin or cerivastatin or lovastatin or pravastatin or atorvastatin).af. |
| 8 | (lipitor or lescol or lipostat or crestor or zocor).af. |
| 9 | 6 or 7 or 8 |
| 10 | exp vascular surgery/ or exp limb amputation/ |
| 11 | exp peripheral vascular disease/su, th or exp carotid artery obstruction/su, th or exp carotid atherosclerosis/su, th or exp peripheral occlusive artery disease/su, th or exp atherosclerosis/su, th or ((exp abdominal aorta/ or exp thoracic aorta/) and surg*.ti,ab.) or exp aorta aneurysm/su, th or exp aorta atherosclerosis/su, th or exp aorta occlusion/su, th or exp aorta rupture/su, th |
| 12 | ((abdominal or thoracic or thoracoabdominal) adj3 aneurysm*).mp. |
| 13 | (((iliac* adj3 arter*) or infrainguinal or (femoropop* or femoro‐pop*) or (vascular or aort* or AAA or aneurysm* or carotid)) adj3 (endovascular or bypass or graft* or surg* or revasc*or repair* or procedur* or operat*)).mp. |
| 14 | (endarterectomy or peripheral revasculari#ation or lower extremity revasculari#ation or critical limb ischaemia or critical limb ischemia).mp. |
| 15 | (interm* adj3 claud*).mp. |
| 16 | amputation*.ti,ab. |
| 17 | or/10‐16 |
| 18 | 5 and 9 and 17 |
| 19 | exp in vitro study/ |
| 20 | exp animal experiment/ |
| 21 | exp animal model/ |
| 22 | exp nonhuman/ |
| 23 | exp animals/ |
| 24 | exp human/ |
| 25 | or/19‐23 |
| 26 | 25 not 24 |
| 27 | 18 and 26 |
| 28 | 18 not 26 |
|
CENTRAL 16/3/12 |
|
| ID | Search |
| #1 | MeSH descriptor Vascular Surgical Procedures explode all trees |
| #2 | MeSH descriptor Hydroxymethylglutaryl‐CoA Reductase Inhibitors explode all trees |
| #3 | statin* OR simvastatin OR rosuvastatin OR fluvastatin OR cerivastatin OR lovastatin OR pravastatin OR atorvastatin in Clinical Trials |
| #4 | lipitor OR lescol OR lipostat OR crestor OR zocor in Clinical Trials |
| #5 | (#2 OR #3 OR #4) |
| #6 | MeSH descriptor Peripheral Vascular Diseases explode all trees with qualifiers: SU,TH |
| #7 | MeSH descriptor Aortic Aneurysm, Abdominal explode all trees with qualifiers: SU,TH |
| #8 | MeSH descriptor Aortic Aneurysm, Thoracic explode all trees with qualifiers: TH,SU |
| #9 | MeSH descriptor Aortic Rupture explode all trees with qualifiers: SU,TH |
| #10 | MeSH descriptor Carotid Stenosis explode all trees with qualifiers: TH,SU |
| #11 | MeSH descriptor Atherosclerosis explode all trees with qualifiers: TH,SU |
| #12 | MeSH descriptor Intermittent Claudication explode all trees with qualifiers: TH,SU |
| #13 | MeSH descriptor Aorta, Abdominal explode all trees with qualifiers: TH,SU |
| #14 | MeSH descriptor Aorta, Thoracic explode all trees with qualifiers: TH,SU |
| #15 | surger*:ti,ab in Clinical Trials |
| #16 | (( #13 OR #14 ) AND #15) |
| #17 | (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #16) |
| #18 | MeSH descriptor Amputation explode all trees |
| #19 | (((vascular or aort* or aneurysm or carotid) near/3 (repair or procedur* or surg* or operat*)) or ((abdominal or thoracic or thoracoabdominal or endovascular) near/3 aneurysm*) or( (femoropop* or femoro‐pop*) near/3 (bypass or graft or surg*)) or (iliac* near/3 arter* near/3 (bypass or graft or surg*)) or (carotid endarterectomy or peripheral revascularisation or infrainguinal bypass) or (interm* near/3 claud*)) |
| #20 | (amputation):ti,ab,kw |
| #21 | (#1 OR #17 OR #18 OR #19 OR #20) |
| #22 | (#5 AND #21) |
Appendix 7. Draft data extraction form
1. General Information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report that data are extracted from) |
|
|
Report ID (ID for this paper/abstract/report) |
|
|
Study ID (surname of first author and year first full report of study was published (e.g. Smith 2001) |
|
|
Report IDs of other reports of this study (e.g. duplicate publications, follow‐up studies) |
|
|
Reference details |
|
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
|
Notes: | |
2. Study Eligibility
| Study Characteristics |
Eligibility criteria (insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Randomized controlled trial | |||||
| Controlled clinical trial (quasi‐randomized trial & cluster‐randomized) |
||||||
|
Participants |
Adults > 18 years scheduled for noncardiac vascular surgery |
|||||
| Types of intervention and comparison | Any statin in any dose compared with |
|||||
| Placebo | ||||||
| No treatment or standard care | ||||||
| Different dose of statin | ||||||
| Types of outcome measures | All‐cause mortality within 30 days |
|||||
| Non‐fatal cardiac events | ||||||
| Incident atrial fibrillation | ||||||
| Acute renal failure | ||||||
| Stroke/TIA | ||||||
| Participant‐reported outcome-QoL | ||||||
| Length of stay | ||||||
| Adverse muscle effects | ||||||
| INCLUDE | EXCLUDE | |||||
|
Reason for exclusion |
||||||
|
Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and Setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶/fig/table) |
||
|
Population description (types of surgical procedures included) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
|
Informed consent obtained |
Yes No Unclear |
||
|
Notes: | |||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Aim of study |
|||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, cluster/groups or body parts) |
|||
|
Start date |
|
||
|
End date |
|
||
|
Total study duration |
|||
| Ethical approval needed/ obtained for study |
Yes No Unclear |
||
|
Notes: | |||
5. Risk of Bias Assessment
| Domain |
Risk of bias |
Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: mortality |
||||
|
Outcome group: non‐fatal cardiac events/AF |
|||||
|
Outcome group: stroke/TIA |
|||||
|
Outcome group: renal failure |
|||||
|
Outcome group: participant‐reported outcomes incl time to return to work |
|||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: mortality |
||||
| (if required) |
Outcome group: non‐fatal cardiac events/AF |
||||
|
Outcome group: stroke/TIA |
|||||
|
Outcome group: renal failure |
|||||
|
Outcome group: participant‐reported outcomes |
|||||
|
Incomplete outcome data (attrition bias) |
Outcome group: mortality |
||||
|
Outcome group: non‐fatal cardiac events/AF |
|||||
|
Outcome group: stroke/TIA |
|||||
|
Outcome group: renal failure |
|||||
|
Outcome group: participant‐reported outcomes |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
|
Other bias |
|||||
|
Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Total no. randomly assigned (or total pop. at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Statin treatment before start of trial allowed? | ||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
| Severity of illness | ||
|
Comorbidities |
||
| Other treatment received(details of standard care such as beta‐blockers) | ||
|
Other relevant sociodemographics |
||
|
Subgroups measured |
||
|
Subgroups reported |
||
|
Notes: | ||
7. Intervention Groups
.7.1 Statin Group
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
Statin group | |
|
No. randomly assigned to group (specify whether no. people or clusters) |
||
| Description(type, dose ) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc., if relevant) |
||
|
Cointerventions |
||
| Number (%) on statins before randomization | ||
|
Notes: | ||
7.2 Comparison Group-Repeated as Required
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group type (placebo, no treatment or different dose) |
||
|
No. randomly assigned to group (specify whether no. people or clusters) |
||
| Description(format , contents) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc., if relevant) |
||
|
Cointerventions |
||
| Number (%) on statins before randomization | ||
|
Notes: | ||
8. Outcomes
8.1 Dichotomous Outcomes Such as Mortality, Non‐fatal Events
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Outcome name |
Mortality | |
| Time points measured | ||
| Time points reported | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
|
Notes: | ||
8.5 Continuous Outcomes Such as Length of Stay or Participant‐Reported Outcomes
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name (with diagnostic criteria if relevant) |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? |
Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
9. Results
9.1 Dichotomous Outcomes
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported-such as OR, HR, RRs | ||||||
|
Unit of analysis(by individuals, cluster/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) |
Yes No Unclear |
|||||
| Reanalysis possible? |
Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
9.2 Continuous Outcomes
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Postintervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, cluster/ groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) |
Yes No Unclear |
|||||||||
| Reanalysis possible? |
Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in the intervention effect) |
Yes No Unclear |
|
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) |
Yes No Unclear |
|
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
|
|
Notes: | ||
11. Other Information
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Key conclusions of study authors |
||
|
References to other relevant studies |
||
| Correspondence required for further study information(from whom, what and when) | ||
|
Notes: | ||
Data and analyses
Comparison 1. Statin versus placebo / no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.31, 1.75] |
| 2 Death from cardiovascular causes | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.07, 16.20] |
| 3 Myocardial infarction (non‐fatal) | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.15, 1.52] |
| 4 Stroke or TIA (non‐fatal) | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 2.25] |
| 5 New atrial fibrillation | 2 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.20, 14.84] |
| 6 Length of stay | Other data | No numeric data | ||
| 7 Graft patency | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Restenosis required | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.19, 1.45] |
| 7.2 Amputation required | 2 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.16, 2.53] |
| 8 Clinical muscle syndromes | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
1.3. Analysis.
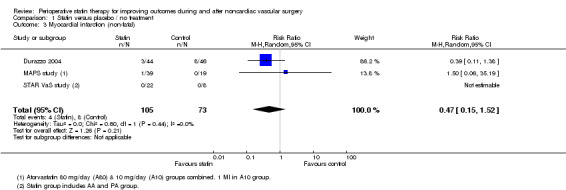
Comparison 1 Statin versus placebo / no treatment, Outcome 3 Myocardial infarction (non‐fatal).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
APVS study.
| Methods | Atorvastatin in the Perioperative of Vascular Surgery (APVS) study. Single‐centre randomized controlled trial. | |
| Participants | 106 participants undergoing vascular surgery (aortic aneurysm, carotid endarterectomy and lower limb arterial surgery) in University Hospital, Brazil. Statin naive. Mean age, 69.6 years in A20 group and 65.8 years in A80 group. |
|
| Interventions | High‐dose (80 mg/day) atorvastatin (A80) compared with 20 mg/day atorvastatin (A20). 53 participants in each group, received drug for 60 days. | |
| Outcomes | Follow‐up for 30 days after surgery. Primary endpoint: composite of cardiac death, non‐fatal myocardial infarction and stroke. 3 events in A80 group and 7 events in A20 group. Secondary endpoint: reduction in total and low‐density cholesterol CRP. Safety monitored via hepatic enzymes and creatinine kinase. |
|
| Funding sources | No statement about funding sources. | |
| Duration of statin use before surgery | 33 days average between randomization and surgery in both groups. | |
| Notes | Study reported in two conference abstracts only. Emailed for further information 20/8/12 and 27/9/12. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) Mortality /complications | Low risk | Study described as "double‐blind". |
| Blinding of outcome assessment (detection bias) Mortality/complications | Unclear risk | Criteria and assessment process not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Premiminary reports only. Combined endpoint only reported. |
| Other bias | Unclear risk | Insufficient information to assess other potential risks of bias. |
Durazzo 2004.
| Methods | Single‐centre randomized controlled trial. | |
| Participants | 100 participants scheduled for elective non‐cardiac vascular surgery-aortic,femoropopliteal and carotid procedures. University Hospital, Sao Paulo, Brazil. Statin naive. Atorvastatin: 66% > 65 years, 20% female. Placebo: 64% > 65 years, 22% female. |
|
| Interventions | 50 participants randomly assigned to intervention group 20 mg/day atorvastatin. 44 participants underwent planned surgery. 50 participants randomly assigned to control group, placebo. 46 participants underwent planned surgery. 45‐Day course. Surgery not earlier than 2 weeks after start of therapy. Beta‐blockers if indicated by current guidelines. 56% in atorvastatin group, 64% in placebo group. |
|
| Outcomes | All‐cause mortality at 30 days: 7 in atorvastatin group, including 1 stroke; 10 in control group, including 1 cardiac death. Non‐fatal MI at 30 days: 3 in atorvastatin group and 8 in control group. Non‐fatal stroke at 30 days: 1 in control group. Adverse muscle effects: 1 participant with rhabdomyolysis and elevated aminotransferase levels in atorvastatin group. Study primary outcome: composite of death from cardiac causes, non‐fatal MI, ischaemic stroke and unstable angina. Hepatic transaminase and creatine kinase (CK) monitored during hospital stay. Lipid profile after discharge. |
|
| Funding sources | Supported by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo, Brazil. Statement that no conflicts of interest present. | |
| Duration of statin use before surgery | Average, 30 days before surgery. | |
| Notes | Authors supplied additional data by email October 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer algorithm used. |
| Allocation concealment (selection bias) | Low risk | Additional information from authors. "Adequate allocation concealment was obtained using sequentially numbered and sealed opaque envelopes and randomization was performed by the pharmacy of the hospital". |
| Blinding of participants and personnel (performance bias) Mortality /complications | Low risk | "All clinical and study investigators were blinded to study group assignments throughout all phases of the trial". Described as double‐blind. Authors confirmed that participants and clinical staff were blinded to study allocation. |
| Blinding of outcome assessment (detection bias) Mortality/complications | Low risk | Data collected by study investigators analysed by endpoint committee unaware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT: ll participants included in analysis, including 6 participants in atorvastatin group and 4 in placebo group who did not undergo surgery. These participants are not eligible and were excluded from analyses of cardiovascular outcomes. . |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section reported. |
| Other bias | Low risk | Baseline characteristics, β‐blocker use and type of operation similar in the 2 groups. |
MAPS study.
| Methods | Multicenter Atorvastatin Plaque Stabilization (MAPS) study. Multicentre randomized controlled trial. | |
| Participants | 60 hypercholesteraemic participants with symptomatic carotid stenosis undergoing CEA. Italy. Statin naive. Mean age (SD) years: A80, 79.0 (5.0); A10, 78.4 (5.1); control, 78.7 (5.3). % female: A80, 45%; A10, 65%; control, 55%. |
|
| Interventions | 20 participants randomly assigned to intervention groups: 80 mg atorvastatin/ day (A80) 20 underwent surgery. 20 participants randomly assigned to 10 mg atorvastatin/day (A10); 19 underwent surgery. 20 participants randomly assigned to control group received sitosterol and cholestyramine; 19 underwent surgery. Treatment for 12 weeks before surgery. Participants continued statins for at least 1 year after surgery. |
|
| Outcomes | Blood lipid levels reported and endarterectomy specimens analysed for macrophage cell content. Clinical outcomes obtained in personal communication from authors. I MI in A10 group, I incident AF with heart failure in A80 group and 1 TIA in control group. |
|
| Funding sources | Biomedical Foundation for Cardiovascular Research and Gene Therapy of Padova and Pfizer (by an unrestricted educational grant). | |
| Duration of statin use before surgery | 12 weeks. | |
| Notes | Additional information obtained from authors October 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) Mortality /complications | High risk | Described as single‐blind (user) on clinicaltrials.gov. No details of blinding in published papers. Clinicians probably not blinded. |
| Blinding of outcome assessment (detection bias) Mortality/complications | High risk | Described as single‐blind (user) on clinicaltrials.gov. No details of blinding in published papers. Clinical outcomes not predefined for trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up minimal: 1/20 in A10 group and 1/20 in control group. |
| Selective reporting (reporting bias) | Low risk | Authors supplied clinical outcome data on request. |
| Other bias | Low risk | Baseline characteristics similar in both groups. |
Rahman 1995.
| Methods | Single‐centre randomized controlled trial. | |
| Participants | 40 participants undergoing open repair of > 5.5 cm. Academic vascular surgery unit, Hull, UK. Statin naive. Mean age (years)/% female: atorvastatin group, ‐ 77.5 years/14%. Placebo: 72.0 years/15%. |
|
| Interventions | Intervention (N = 20) 80 mg/day atorvastatin. Placebo (N = 20). Therapy given for 4 weeks before surgery. After surgery, statin prescription dealt with by primary care (communication from authors), so duration unclear but likely to have lasted at least 48 hours. | |
| Outcomes | Primary outcome: level of MMP‐9 in aortic wall. Secondary outcome: levels of MMP‐2, MMP‐8 and other enzymes in arterial wall. Length of stay reported in Table. |
|
| Funding sources | No statement about funding sources. | |
| Duration of statin use before surgery | Four weeks before surgery. | |
| Notes | Additional information obtained from authors 28/9/12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated sequence, with a subgroup size of 4". |
| Allocation concealment (selection bias) | Low risk | Carried out by pharmacy. |
| Blinding of participants and personnel (performance bias) Length of stay | Low risk | Paper and personal communication report that study was double‐blind; thus neither participants nor staff members were aware of allocation. Placebo tablets were identical in shape and colour (but were possibly missing numbers and letters). |
| Blinding of outcome assessment (detection bias) Length of stay | Low risk | Personal communication from authors. The clinical team (including physiotherapists) assessed fitness for discharge. No formal criteria but that participant was "generally independent and safe". Clinical team unaware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analyses. No details of loss to follow‐up but all outcomes during hospital stay. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Baseline characteristics and type of operation similar in both groups. |
Ramo 1995.
| Methods | Single‐centre randomized controlled trial. | |
| Participants | 37 participants undergoing percutaneous transluminal angioplasty (PTA) below inguinal ligament for critical ischaemia or severe claudication (< 20 m). University Hospital, Helsinki, Finland. Not stated that statin naive. Mean age, 68 years 45% female. |
|
| Interventions | Intervention (N = 18): 20 mg/day lovastatin plus 250 mg aspirin/day. Commenced on referral and angiogram usually within 2 days. Assume continued for 1 year. Control (N = 19) had aspirin only. |
|
| Outcomes | Participants followed up for 1 year. Outcomes reported repeated PTA (4/18 statin and 8/19 control), time to repeat PTA, amputation (2/18 statin users and 4/19 control). |
|
| Funding sources | No statement about funding sources. | |
| Duration of statin use before surgery | 2 days on average. | |
| Notes | Authors emailed for further information 20/8/12 and 27/9/12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All patients were randomized into two groups". No further details given. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) Mortality /complications | High risk | No mention of blinding and no placebo given. |
| Blinding of outcome assessment (detection bias) Mortality/complications | High risk | "Angiograms were evaluated by two experienced interventional radiologists independently and without knowing the treatment patients were receiving". But angiograms during 1‐year follow‐up initiated by clinical staff who were aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed follow‐up. |
| Selective reporting (reporting bias) | Low risk | Study objective was to study restenosis only. |
| Other bias | Low risk | Statin group had worse clinical condition and fewer run‐off vessels at baseline. Therefore, reduced restenosis rate may be under‐estimate. |
STAR VaS study.
| Methods | Short Term Atorvastatin Regime for Vasculopathic Subjects (STAR_VaS) study. Single‐centre randomized controlled trial with 3 study arms. Randomization to 2 groups depending on how much time available before surgery. |
|
| Participants | 56 participants undergoing non‐cardiac surgery (aortic surgery and infrainguinal revascularization and including 16 with non‐vascular surgery). University Hospital, Ottawa, Canada. Statin naive. Mean age/% female: AA, 71 years/15%; PA, 67 years/25%; PP, 69 years/18%. Baseline results for all participants, not just vascular surgery. |
|
| Interventions | Intervention group AA: (N = 26, of which 15 vascular surgery with outcome data (8 aortic and 7 PVD)); atorvastatin 80 mg/day for 7 days preoperatively, on day of surgery and for 7 days after surgery. Intervention group PA: (N = 16, of which 7 vascular surgery with outcome data (5 aortic and 2 PVD)); placebo for 7 days preoperatively, atorvastatin 80 mg/day on day of surgery and for 7 days after surgery. Control group PP: (N = 17, of which 8 vascular surgery with outcome data (5 aortic and 3 PVD)); placebo for 7 days preoperatively, on day of surgery and for 7 days after surgery. Anaesthesia, surgery and postoperative care at discretion of study team. Beta‐blocker prescribed: AA 35%, PA 50%, PP 12%. Baseline results for all participants, not just vascular surgery. |
|
| Outcomes | Primary outcome: CRP at 48 hours postoperatively. Secondary outcomes: lipids, creatinine kinase and liver transaminases. Also documented occurrence within 7 days of surgery of myocardial ischaemia, myocardial infarction, cardiac arrest, new‐onset cardiac arrhythmias, cerebral vascular accident and death from all causes. Outcomes in participants who had vascular surgery obtained from authors: no deaths, MI or strokes. 1 incident AF in AA and 1 in PA group. I amputation required in AA group. Duration of hospital stay recorded and obtained from authors. Participant‐reported nausea obtained from authors. |
|
| Funding sources | Funded by grants from Heart and Stroke Foundation of Ontario, Canadian Anaesthesiologists' Society, Departement of Anaesthesiology, University of Ottawa and Ottawa Anaesthesia Alternate Funds Association. Statement that no commercial or non‐commercial affiliations present that are perceived to be a conflict of interest. | |
| Duration of statin use before surgery | AA: 7 days before surgery. PA: on day of surgery. |
|
| Notes | AA & PA combined in comparisons. Additional information received from authors on 30/10/12. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐ generated random number table created by investigator not involved with bedside care or outcome assessment. Randomized in blocks of 6, stratified by type of surgery. |
| Allocation concealment (selection bias) | Low risk | "A pharmacist who was not involved with the study prepared and dispensed all study medications as identical capsules in similar sequentially numbered containers". |
| Blinding of participants and personnel (performance bias) Mortality /complications | Low risk | "Participants, care providers, investigators and research personnel remained blinded to intervention throughout the study". |
| Blinding of participants and personnel (performance bias) Length of stay | Low risk | As above. |
| Blinding of participants and personnel (performance bias) Patient‐reported | Low risk | As above |
| Blinding of outcome assessment (detection bias) Mortality/complications | Low risk | Participants contacted by telephone on day 30 to determine occurrence of complications or death. |
| Blinding of outcome assessment (detection bias) Length of stay | Low risk | "Duration of hospital stay was also noted". No details of who decided fitness for discharge and whether any criteria were used, but given extensive blinding of staff, bias is unlikely. |
| Blinding of outcome assessment (detection bias) Patient reported | Low risk | Participants blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Numbers reported in study flow chart, text and tables not consistent. Outcomes obtained from authors on 31 of total 43 participants who underwent vascular surgery. |
| Selective reporting (reporting bias) | Low risk | Length of stay not reported, but otherwise full data. |
| Other bias | Unclear risk | Baseline characteristics and distribution of operations similar between groups but based on all participants, not those with outcome data. |
AA= intervention group in STAR VaS study receiving atorvastatin 7 days preoperatively and from day of surgery ; AAA = Abdominal aortic aneurysm; AF = Atrial fibrillation; CEA = Carotid endarterectomy; CRP = C‐reactive protein; ITT = Intention‐to‐treat; MI = Myocardial infarct; MMP = Matrix metalloproteinase; PA=intervention group in STAR VaS study receiving placebo 7 days preoperatively and atorvastatin from day of surgery ; PP = intervention group in STAR VaS study receiving placebo 7 days preoperatively and from day of surgery; PTA = Percutaneous transluminal angioplasty; PVD = Peripheral vascular disease; TIA = Transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbruzzese 2004 | Observational study of participants undergoing infrainguinal bypass. |
| Blann 2001 | RCT. Study population consists of participants with peripheral arterial diease but not undergoing surgery. |
| Brili 2012 | Postop administration of statins. Design unclear. |
| DECREASE III | Eligible trial excluded because of concerns about scientific integrity. |
| DECREASE IV | RCT. No vascular surgical participants included in trial. Concerns about scientific integrity. |
| ELIMIT 2007 | RCT. Intervention and control both receive statins. |
| Font Padros 2008 | Participants scheduled for CEA divided into those who received statins and those who did not. Not clear from abstract report, but probably observational study.Unable to find contact details for author. |
| Hagenaars 2001 | RCT. Study population consists of participants with peripheral arterial diease but not undergoing surgery. |
| Hong 2010 | RCT. Study population receiving coronary artery stents. |
| HPS 2007 | RCT. Long‐term statin use in subgroup of peripheral arterial disease population who underwent surgery. |
| Kennedy 2005 | Obsevational study of participants undergoing CEA. |
| Kertai 2004 | Observational study of participants undergoing AAA repair. |
| Luijendijk 2012 | RCT. Participants undergoing coarctation repair. Statins given postoperatively only. |
| Martin‐Ventura 2008 | RCT. Participants undergoing CEA. Both intervention and control receive statins. |
| O'Neil‐Callahan 2005 | Observational study of participants undergoing noncardiac vascular surgery. |
| Poldermans 2003 | Observational study of participants undergoing noncardiac vascular surgery. |
| Schouten 2005a | Observational study of participants undergoing noncardiac vascular surgery. |
| van Gestel 2008 | Observational study of participants undergoing noncardiac vascular surgery. |
| Ward 2005 | Observational study of participants undergoing infrainguinal bypass. |
AAA = Abdominal aortic aneurysm; CEA = Carotid endarterectomy; RCT = Randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
ATROCAP study.
| Methods | Atorvastatin and Thrombogenicity of the Carotid Atherosclerotic Plaque (ATROCAP) study. Six–centre randomized controlled trial. |
| Participants | 59 participants undergoing bilateral CEA. Italy. Statin naive. |
| Interventions | Intervention group (N = 29) received 20 mg atorvastatin/day from day after 1st CEA to after 2nd CEA. Intervention group (N = 30) received placebo. Mean duration, 133 (SD 63) days. Not clear when treatment was stopped. |
| Outcomes | No clinical outcomes reported. Endarterectomy specimens analysed for tissue factor and tissue factor pathway inhibitor antigens. |
| Notes | Unable to contact authors; emailed 20/8/12 and 27/9/12. |
Crisby 2001.
| Methods | Single‐centre randomized controlled trial. |
| Participants | 24 participants undergoing CEA. University Hospital, Stockholm, Sweden. Statin naive. |
| Interventions | Intervention group (N = 11) 40 mg pravastatin/day for 3 months before surgery. Control group (N = 13) received no treatment. |
| Outcomes | No clinical outcomes. Endarterectomy specimens analysed for lipoproteins and inflammatory markers. |
| Notes | Not clear how participants were allocated. States not randomized but appears to be an intervention study. No relevant outcome data. Unable to contact authors; emailed 20/8/12 and 27/9/12. |
Cuccurollo 2006.
| Methods | Single‐centre randomized controlled trial. |
| Participants | 70 participants with type 2 diabetes undergoing CEA. Italy. Statin naive. |
| Interventions | Intervention group (N = 35) received 40 mg simvastatin/day plus diet and aspirin 100 mg/day for 4 months. Control group (N =35) received aspirin and diet advice. |
| Outcomes | No clinical outcomes reported. Endarterectomy specimens analysed for RAGE expression. |
| Notes | Unable to contact authors; emailed 20/8/12 and 27/9/12. |
Evans 2007.
| Methods | Single‐centre randomized controlled trial. |
| Participants | 21 participants undergoing elective repair of large AAA. UK. Statin naive. |
| Interventions | Intervention group (N = 10) received 40 mg simvastatin/day for three weeks. Control group (N = 11) received identical placebo. |
| Outcomes | No clinical outcomes reported. Aneurysm wall specimens analysed for MMP activity. |
| Notes | Unable to contact authors; emailed 20/8/12 and 27/9/12. |
Kajimoto 2009.
| Methods | Single‐centre randomized controlled trial. |
| Participants | 20 participants undergoing open repair of AAA. University Hospital, Tokyo, Japan. Statin naive. |
| Interventions | Intervention group (N = 10) received 20 mg atorvastatin/day for 4 weeks. Control group (N = 10) received no treatment. |
| Outcomes | No clinical outcomes reported. Aneurysm wall specimens analysed for MMP activity. |
| Notes | Unable to contact authors; emailed 20/8/12 and 27/9/12. |
Martin Ventura 2005.
| Methods | Single‐centre randomized controlled trial. |
| Participants | 20 participants undergoing CEA. Madrid, Spain. Statin naive. |
| Interventions | Intervention group (N = 11) received 80 mg atorvastatin/day for 1 month. Control group (N = 19) received no treatment. |
| Outcomes | No clinical outcomes reported. Aneurysm wall specimens analysed for inflammatory markers. |
| Notes | Unable to contact authors; emailed 20/8/12 and 27/9/12. |
AAA = Abdominal aortic aneurysm; CEA = Carotid endarterectomy; MMP = Matrix metalloproteinase; RAGE = The Receptor for Advanced Glycation Endproducts.
Characteristics of ongoing studies [ordered by study ID]
LOAD study.
| Trial name or title | Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD). |
| Methods | Multicenter Randomized Controlled Trial. |
| Participants | Participants older than 45 years of age undergoing noncardiac surgery with an expected hospital stay of at least 24 hours and any 1 of the following criteria:
|
| Interventions | 80 mg atorvastatin 3 to 6 hours before surgery; then 40 mg atorvastatin 12 hours after loading dose; then 40 mg atorvastatin every night for 7 days. Control group receives placebo. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | August 2012. Hope to complete recruitment by November 2014. |
| Contact information | Otavio Berwanger, MD, PhD; 3053‐6611, ext 8237; oberwanger@hcor.com.br |
| Notes | NCT01543555. |
Vita 2012.
| Trial name or title | Clinical Utility of Endothelial Dysfunction in PAD. |
| Methods | Randomized controlled trial. |
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | May 2004. Estimated study completion date June 2012. |
| Contact information | Joseph A. Vita, Professor of Medicine, Boston University. |
| Notes | NCT00491751. |
MI = Myocardial infarction; PAD = Peripheral arterial disease; VTE = Venous thromboembolism.
Differences between protocol and review
Robert Sanders' affiliation has changed.
The search of electronic databases included CINHAL and Web of Science, which were not listed in the protocol.
We have made the following changes to outcomes because stroke outcomes are important for carotid surgery:
Death from cardiovascular causes (including death from cerebrovascular events and stroke) rather than just cardiac causes; and
We have put stroke/TIA at the top of secondary outcomes.
Adverse muscle effects and participant‐reported outcomes added to Table 1.
Contributions of authors
Conceiving of the review: Robert D Sanders (RDS).
Co‐ordinating the review: Amanda Nicholson (AN).
Undertaking manual searches: AN and RDS.
Screening search results: AN and Sharon R Lewis (SRL).
Organizing retrieval of papers: SRL and AN.
Screening retrieved papers against inclusion criteria: AN, SRL and RDS.
Appraising quality of papers: SRL, AN and RDS.
Resolving disagreements: Andrew F Smith (AFS) and Phil Alderson (PA).
Abstracting data from papers: SRL, AN and RDS.
Resolving disagreements: AFS and PA.
Writing to authors of papers for additional information: SRL and AN.
Providing additional data about papers: AN, SRL and RDS.
Obtaining and screening data on unpublished studies: AN, SRL and RDS.
Providing data management for the review: AN.
Entering data into Review Manager (RevMan 5.1): SRL and AN.
Analysing RevMan statistical data: AN
Performing other statistical analysis not using RevMan: AN
Interpreting data: AN, RDS, PA and AFS.
Making statistical inferences: AN, RDS, PA and AFS.
Writing the review: all authors.
Securing funding for the review: AFS and PA.
Performing previous work that served as the foundation of the present study: N/A.
Serving as guarantor for the review (one author): AFS.
Taking responsibility for reading and checking review before submission: AN.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Collaboration Programme Grant. Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04 ‐, UK.
This grant funds the work of AN, AS, PA & SL on this review.
Declarations of interest
Robert D Sanders has acted as a consultant for Air Liquide on the development of medical gases and has received an honorarium from Hospira for speaking at the Canadian Society of Anesthesiologists meeting.
Amanda Nicholson worked for the Cardiff Research Consortium, which provides research and consultancy services to the pharmaceutical industry. This included work on a project assessing cardiovascular risk in patients with high lipid levels and different treatment options. Cardiff Research Consortium has no connection with AN’s work with The Cochrane Collaboration. AN’s husband has small direct holdings in several drug and biotech companies as part of a wider balanced share portfolio.
Sharon R Lewis, Andrew F Smith, Phil Alderson: none known (see Sources of support).
Edited (no change to conclusions)
References
References to studies included in this review
APVS study {published data only (unpublished sought but not used)}
- Almeida R, Coelho OR, Meneses FH, Oliveira G, Molinari G, Franca R, et al. The APVS (atorvastatin in the perioperative of vascular surgery) study. Abstract from World Congress of Cardiology Scientific Sessions 2010, Beijing China. Published in Circulation 2010. 2010; Vol. 122 (2):e345. [EMBASE: 70233157]
- Almeida RC, Coelho OR, Menezes FH, Oliveira GP, Molinari GJPD, Franca RP, et al. Comparison of effects of high dose (80 mg) atorvastatin versus 20 mg atorvastatin on C‐reactive protein and lipoproteins in patients undergoing major vascular surgery. Abstract from European Society of Cardiolog Congress 2010 Stockholm, Sweden. Published in European Heart Journal. 2010; Vol. 31:64. [EMBASE: 70279734]
Durazzo 2004 {published and unpublished data}
- Caramelli B, Calderaro D, Yu PC, Gualandro D. Preoperative lipid‐lowering therapy on postoperative outcome. American Journal of Cardiology 2007; Vol. 100, issue 7:1185. [PUBMED: 17884389] [DOI] [PubMed]
- Caramelli B, Durazzo A, Ikeoka D, Bernoche C, Monachini M, Puech‐Leao P, et al. Short‐term treatment with atorvastatin for prevention of cardiac complications after vascular surgery [abstract no:086]. Atherosclerosis Supplements 2002, issue 2:83. [CENTRAL: CN‐00784215]
- Durazzo AEDS, Machado FS, Ikeoka DT, Bernoche C, Monachini MC, Puech‐Leao P, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. Journal of Vascular Surgery 2004; Vol. 39, issue 5:967‐76. [EMBASE: 2004198566] [DOI] [PubMed]
- Durazzo AES, Machado FS, Ikeoka D, Puech‐Leao P, Caramelli B. Effect of atorvastatin on cardiovascular events after vascular surgery. Circulation 2002, issue 19 Suppl:II‐343. [CENTRAL: CN‐00520506]
MAPS study {published and unpublished data}
- Puato M, Elisabetta F, Rattazzi M, Cipollone F, Zambon A, Grego F, et al. Atorvastatin reduces macrophage accumulation in the atherosclerotic plaque in patients undergoing carotid endarterectomy: a comparison versus non‐statin based regimen. Abstract from 78th European Atherosclerosis Society Congress, Hamburg Germany. Published in Atherosclerosis Supplements. 2010; Vol. 11 (2):23. [EMBASE: 70201591]
- Puato M, Faggin E, Rattazzi M, Zambon A, Cipollone F, Grego F, et al. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin‐based regimen in patients undergoing carotid endarterectomy. Stroke 2010; Vol. 41, issue 6:1163‐8. [EMBASE: 2010315441] [DOI] [PubMed]
Rahman 1995 {published and unpublished data}
- Rahman MA, Khan JA, Mazari FAK, Mockford K, McCollum PT, Chetter IC. A randomized placebo controlled trial of the effect of preoperative statin use on matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in areas of low and peak wall stress in patients undergoing elective open repair of abdominal aortic aneurysm. Annals of Vascular Surgery 2011; Vol. 25, issue 1:32‐8. [EMBASE: 2010702366] [DOI] [PubMed]
Ramo 1995 {published data only (unpublished sought but not used)}
- Ramo OJ, Juha L, Martti S, Hannu H, Pauliina RM, Risto M. Effects of lovastatin in prevention of restenosis after percutaneous transluminal angioplasty in lower limbs. International Journal of Angiology 1995; Vol. 4, issue 4:173‐6. [EMBASE: 1995373334]
STAR VaS study {published and unpublished data}
- Neilipovitz DT, Bryson GL, Taljaard M. STAR VaS-Short Term Atorvastatin Regime for Vasculopathic Subjects: a randomized placebo‐controlled trial evaluating perioperative atorvastatin therapy in noncardiac surgery. Canadian Journal of Anaesthesia 2012; Vol. 59, issue 6:527‐37. [PUBMED: 22528165] [DOI] [PubMed]
References to studies excluded from this review
Abbruzzese 2004 {published data only}
- Abbruzzese TA, Havens J, Belkin M, Donaldson MC, Whittemore AD, Liao JK, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. Journal of Vascular Surgery 2004; Vol. 39, issue 6:1178‐85. [PUBMED: 15192555] [DOI] [PMC free article] [PubMed]
Blann 2001 {published data only}
- Blann AD, Gurney D, Hughes E, Buggins P, Silverman SH, Lip GY. Influence of pravastatin on lipoproteins, and on endothelial, platelet, and inflammatory markers in subjects with peripheral artery disease. The American Journal of Cardiology 2001, issue 1:A7‐8, 89‐92. [CENTRAL: CN‐00348899] [DOI] [PubMed]
Brili 2012 {published data only}
- Brili S, Tousoulis D, Antonopoulos AS, Antoniades C, Hatzis G, Bakogiannis C, et al. Effects of atorvastatin on endothelial function and the expression of proinflammatory cytokines and adhesion molecules in young subjects with successfully repaired coarctation of aorta. Heart 2012; Vol. 98, issue 4:325‐9. [PUBMED: 22076019] [DOI] [PubMed]
DECREASE III {published data only}
- Poldermans D. Fluvastatin XL use is associated with improved cardiac outcome after major vascular surgery: results from a randomized placebo controlled trial: DECREASE III. European Society of Cardiology Congress; August 2008; Munich, Germany. [CN‐00713534]
- Poldermans D. Statins and noncardiac surgery: current evidence and practical considerations. Cleveland Clinic Journal of Medicine 2009; Vol. (76 Suppl 4):S79‐83. [PUBMED: 19880840] [DOI] [PubMed]
- Poldermans D, Schouten O, Bax J, Winkel TA. Reducing cardiac risk in non‐cardiac surgery: evidence from the DECREASE studies. European Heart Journal Supplements 2009; Vol. 11, issue A:A9‐A14. [WOS:000265835900003]
- Schouten O, Boersma E, Hoeks SE, Benner R, Urk H, Sambeek MRHM, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. New England Journal of Medicine 2009; Vol. 361, issue 10:980‐9. [EMBASE: 2009479335] [DOI] [PubMed]
- Schouten O, Hoeks SE, Boersma H, Voute MT, Ravensbergen N, Bastos Goncalves F, et al. Long‐term outcome of the DECREASE III trial; the impact of initiation of statin therapy prior to surgery. Abstract from European Society of Cardiology Congress, 2011, Paris France. Published in European Heart Journal. 2011; Vol. 32:332‐3. [EMBASE: 70534292]
- Schouten O, Hoeks SE, Poldermans D. Fluvastatin in patients undergoing vascular surgery (REPLY). New England Journal of Medicine 2009; Vol. 361, issue 22:2187‐8. [WOS:000272117800018] [DOI] [PubMed]
- Schouten O, Hoeks SE, Voute MT, Boersma E, Verhagen HJ, Poldermans D. Long‐term benefit of perioperative statin use in patients undergoing vascular surgery: Results from the DECREASE III trial. Abstract from Vascular Annual Meeting of the Society for Vascular Surgery, 2011, Chicago, IL United States. Published in Journal of Vascular Surgery. 2011; Vol. 53 (6 SUPPL. 1):20S‐1S. [EMBASE: 70435717]
- Schouten O, Hoeks SE, Voute MT, Ravensbergen N, Luijtgaarden K, Bastos Goncalves F, et al. Perioperative myocardial ischemia: A preventable cause of poor long‐term outcome after vascular surgery?. Abstract from European Society of Cardiology Congress, 2011, Paris, France. Published in European Heart Journal. 2011; Vol. 32:556. [EMBASE: 70535145]
- Schouten O, Poldermans D, Visser L, Kertai MD, Klein J, Urk H, et al. Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study. American Heart Journal 2004; Vol. 148, issue 6:1047‐52. [WOS:000225970400021] [DOI] [PubMed]
- Schouten O, Verhagen HJ, Poldermans D. Endovascular repair of abdominal aortic aneurysm. New England Journal of Medicine 2010; Vol. 363, issue 15:1480‐1; author reply 1481‐2. [EMBASE: 20931722] [DOI] [PubMed]
DECREASE IV {published data only}
- Dunkelgrun M, Boersma E, Schouten O, Koopman‐Van Gemert AWMM, Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing noncardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Annals of Surgery 2009; Vol. 249, issue 6:921‐6. [EMBASE: 2009367606] [DOI] [PubMed]
ELIMIT 2007 {published data only}
Font Padros 2008 {published data only}
- Font Padros MA, Fernandez A, Rico I, Turu MM, Luque A, Gamez C, et al. Statin treatment prior to carotid endarterectomy (CEA) reduces inflammation as seen on consecutive 18FDG‐PET imaging. Cerebrovascular Diseases 2008, issue Suppl 2:41. [CENTRAL: CN‐00660930]
Hagenaars 2001 {published data only}
Hong 2010 {published data only}
- Hong YJ, Jeong MH, Choi YH, Ma EH, Ko JS, Lee MG, et al. Usual dose of simvastatin does not inhibit plaque progression and lumen loss at the peri‐stent reference segments after bare‐metal stent implantation: a serial intravascular ultrasound analysis. Korean Journal of Internal Medicine 2010; Vol. 25, issue 4:356‐63. [PUBMED: 21179272] [DOI] [PMC free article] [PubMed]
HPS 2007 {published data only}
- Heart Protection Study Collaborative G. Randomized trial of the effects of cholesterol‐lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high‐risk conditions. Journal of Vascular Surgery 2007, issue 4:645‐54; discussion 653‐4. [CENTRAL: CN‐00579613] [DOI] [PubMed]
Kennedy 2005 {published data only}
Kertai 2004 {published data only}
- Kertai MD, Boersma E, Westerhout CM, Klein J, Urk H, Bax JJ, et al. A combination of statins and beta‐blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. European Journal of Vascular & Endovascular Surgery 2004; Vol. 28, issue 4:343‐52. [PUBMED: 15350554] [DOI] [PubMed]
Luijendijk 2012 {published data only}
- Luijendijk P, Bouma BJ, Vriend JWJ, Groenink M, Vliegen HW, Groot E, et al. Rationale and design of a trial on the effect of high dose statins on cardiovascular risk in adults after successful coarctation repair. Contemporary Clinical Trials 2012; Vol. 33, issue 2:410‐6. [EMBASE: 2012055919] [DOI] [PubMed]
Martin‐Ventura 2008 {published data only}
- Martin‐Ventura JL, Muoz‐Garcia B, Blanco‐Colio LM, Martin‐Conejero A, Madrigal‐Matute J, Vega M, et al. Treatment with amlodipine and atorvastatin has additive effect on blood and plaque inflammation in hypertensive patients with carotid atherosclerosis. Kidney International 2008; Vol. 74(Suppl 111):S71‐4. [EMBASE: 2008568017] [DOI] [PubMed]
O'Neil‐Callahan 2005 {published data only}
- O'Neil‐Callahan K, Katsimaglis G, Tepper MR, Ryan J, Mosby C, Ioannidis JPA, et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: The Statins for Risk Reduction in Surgery (StaRRS) study. Journal of the American College of Cardiology 2005; Vol. 45, issue 3:336‐42. [EMBASE: 2005060716] [DOI] [PubMed]
Poldermans 2003 {published data only}
Schouten 2005a {published data only}
- Schouten O, Waning VH, Kertai MD, Feringa HHH, Bax JJ, Boersma E, et al. Perioperative and long‐term cardiovascular outcomes in patients undergoing endovascular treatment compared with open vascular surgery for abdominal aortic aneurysm or iliaco‐femoro‐popliteal bypass. American Journal of Cardiology 2005; Vol. 96, issue 6:861‐6. [WOS:000232096700029] [DOI] [PubMed]
van Gestel 2008 {published data only}
- Gestel YR, Hoeks SE, Sin DD, Simsek C, Welten GM, Schouten O, et al. Effect of statin therapy on mortality in patients with peripheral arterial disease and comparison of those with versus without associated chronic obstructive pulmonary disease. American Journal of Cardiology 2008; Vol. 102, issue 2:192‐6. [EMBASE: 2008311443] [DOI] [PubMed]
Ward 2005 {published data only}
- Ward RP, Leeper NJ, Kirkpatrick JN, Lang RM, Sorrentino MJ, Williams KA. The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery. International Journal of Cardiology 2005; Vol. 104, issue 3:264‐8. [WOS:000232752700004] [DOI] [PubMed]
References to studies awaiting assessment
ATROCAP study {published data only (unpublished sought but not used)}
- Cortellaro M, Confrancesco E, Arbustini E, Rossi F, Negri A, Tremoli E, et al. Atorvastatin and thrombogenicity of the carotid atherosclerotic plaque: The ATROCAP Study. Thrombosis and Haemostasis 2002; Vol. 88, issue 1:41‐7. [EMBASE: 2002247459] [PubMed]
Crisby 2001 {published data only (unpublished sought but not used)}
- Crisby M, Nordin‐Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation 2001; Vol. 103, issue 7:926‐33. [PUBMED: 11181465] [DOI] [PubMed]
Cuccurollo 2006 {published data only (unpublished sought but not used)}
- Cipollone F, Fazia M, Iezzi A, Zucchelli M, Pini B, Cesare D, et al. Suppression of the functionally coupled cyclooxygenase‐2/prostaglandin E synthase as a basis of simvastatin‐dependent plaque stabilization in humans. Circulation 2003; Vol. 107, issue 11:1479‐85. [PUBMED: 12654603] [DOI] [PubMed]
- Cuccurullo C, Iezzi A, Fazia ML, Cesare D, Francesco A, Muraro R, et al. Suppression of rage as a basis of simvastatin‐dependent plaque stabilization in type 2 diabetes. Arteriosclerosis, Thrombosis, and Vascular Biology 2006; Vol. 26, issue 12:2716‐23. [EMBASE: 2006569544] [DOI] [PubMed]
Evans 2007 {published data only (unpublished sought but not used)}
- Evans J. Pre‐operative simvastatin reduces MMP‐9 in the wall of abdominal aortic aneurysms: a randomized trial. British Journal of Surgery 2007, issue Suppl 1:8‐9. [CENTRAL: CN‐00653659]
- Evans J, Powell JT, Schwalbe E, Loftus IM, Thompson MM. Simvastatin attenuates the activity of matrix metalloprotease‐9 in aneurysmal aortic tissue. European Journal of Vascular and Endovascular Surgery 2007; Vol. 34, issue 3:302‐3. [EMBASE: 2007378477] [DOI] [PubMed]
- Evans J, Schwalbe E, Loftus IM, Powell JT, Thompson MM. Pre‐operative simvastatin reduces MMP‐9 in the wall of abdominal aortic aneurysms: a randomised trial. The Vascular Society of Great Britain & Ireland Yearbook 2006:79. [CENTRAL: CN‐00691124]
Kajimoto 2009 {published data only (unpublished sought but not used)}
- Kajimoto K, Miyauchi K, Kasai T, Shimada K, Kojima Y, Shimada A, et al. Short‐term 20‐mg atorvastatin therapy reduces key inflammatory factors including c‐Jun N‐terminal kinase and dendritic cells and matrix metalloproteinase expression in human abdominal aortic aneurysmal wall. Atherosclerosis 2009; Vol. 206, issue 2:505‐11. [EMBASE: 2009510035] [DOI] [PubMed]
Martin Ventura 2005 {published data only (unpublished sought but not used)}
- Martin‐Ventura JL, Blanco‐Colio LM, Gomez‐Hernandez A, Munoz‐Garcia B, Vega M, Serrano J, et al. Intensive treatment with atorvastatin reduces inflammation in mononuclear cells and human atherosclerotic lesions in one month. Stroke 2005; Vol. 36, issue 8:1796‐800. [EMBASE: 2005353457] [DOI] [PubMed]
References to ongoing studies
LOAD study {unpublished data only}
- Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD).. Ongoing study August 2012. Hope to complete recruitment by November 2014..
Vita 2012 {unpublished data only}
- Clinical Utility of Endothelial Dysfunction in PAD.. Ongoing study May 2004. Estimated study completion date June 2012..
Additional references
Al‐Omran 2008
Biccard 2005
Biccard 2008
- Biccard BM. A peri‐operative statin update for non‐cardiac surgery. Part II: Statin therapy for vascular surgery and peri‐operative statin trial design. Anaesthesia 2008;63(2):162‐71. [PUBMED: 18211448] [DOI] [PubMed] [Google Scholar]
Biccard 2009
- Biccard BM. Investigation of predictors of increased creatine kinase levels following vascular surgery and the association with peri‐operative statin therapy. Cardiovascular Journal of Africa 2009;20(3):187‐91. [PUBMED: 19575084] [PMC free article] [PubMed] [Google Scholar]
Chen 2010
Chopra 2012
- Chopra V, Wesorick DH, Sussman JB, Greene T, Rogers M, Froehlich JB, et al. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta‐analysis. Archives of Surgery 2012; Vol. 147, issue 2:181‐9. [PUBMED: 22351917] [DOI] [PubMed]
Coveney 2011
- Coveney AP, O'Brien GC, Fulton GJ. ACE up the sleeve-are vascular patients medically optimized?. Journal of Vascular Health and Risk Management 2011; Vol. 7:15‐21. [PUBMED: 21339909] [DOI] [PMC free article] [PubMed]
Dagher 2007
Erasmus 2011
- Committee for the Investigation of Scientific Integrity. Erasmus MC, University Medical Center Rotterdam. Investigation into possible violation of scientific integrity. http://www.erasmusmc.nl/5663/135857/3664573/3397899/report_summary_investigation_integrity. November 2011.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008; Vol. 336, issue 7651:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; Vol. 343:d5928. [PUBMED: 22008217] [DOI] [PMC free article] [PubMed]
Hindler 2006
Hoeks 2008
Kapoor 2006
- Kapoor AS, Kanji H, Buckingham J, Devereaux PJ, McAlister FA. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006; Vol. 333, issue 7579:1149. [PUBMED: 17088313] [DOI] [PMC free article] [PubMed]
Katznelson 2009
Landesberg 2003
- Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, et al. Association of cardiac troponin, CK‐MB, and postoperative myocardial ischemia with long‐term survival after major vascular surgery. Journal of the American College of Cardiology 2003; Vol. 42, issue 9:1547‐54. [PUBMED: 14607436] [DOI] [PubMed]
Law 2003
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta‐analysis. BMJ 2003; Vol. 326, issue 7404:1423. [PUBMED: 12829554] [DOI] [PMC free article] [PubMed]
Le Manach 2005
Le Manach 2007
Le Manach 2011
Lee 1999
Liakopoulos 2010
- Liakopoulos OJ, Kuhn EW, Slottosch I, Wassmer G, Wahlers T. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008493.pub2] [DOI] [PubMed] [Google Scholar]
Marshall 2009
Mills 2011
Molnar 2011
- Molnar AO, Coca SG, Devereaux PJ, Jain AK, Kitchlu A, Luo J, et al. Statin use associates with a lower incidence of acute kidney injury after major elective surgery. Journal of the American Society of Nephrology 2011; Vol. 22, issue 5:939‐46. [PUBMED: 21493769] [DOI] [PMC free article] [PubMed]
NICE 2006
- NICE. Statins for the prevention of cardiovascular events. National Institute for Health and Clinical Excellence. Technology Appraisal 2006; Vol. 94.
Noordzij 2007
- Noordzij PG, Poldermans D, Schouten O, Schreiner F, Feringa HH, Dunkelgrun M, et al. Beta‐blockers and statins are individually associated with reduced mortality in patients undergoing noncardiac, nonvascular surgery. Coronary Artery Disease 2007;18(1):67‐72. [PUBMED: 17172933] [DOI] [PubMed] [Google Scholar]
Nowygrod 2006
Paraskevas 2011
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Sanders 2011
Sanders 2012
- Sanders RD, Bottle A, Jameson SS, Mozid A, Aylin P, Edger L, et al. Independent preoperative predictors of outcomes in orthopedic and vascular surgery: the influence of time interval between an acute coronary syndrome or stroke and the operation. Annals of Surgery 2012; Vol. 255, issue 5:901‐7. [PUBMED: 22504189] [DOI] [PubMed]
Singh 1000
- Singh N, Augoustides JG. Perioperative protective effects of statins. F1000 Medicine Reports 1000; Vol. 2. [PUBMED: 20948850] [DOI] [PMC free article] [PubMed]
Stalenhoef 2009
Taylor 2011
- Taylor F, Ward K, Moore THM, Burke M, Davey Smith G, Casas JP, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD004816.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thygesen 2007
Twine 2011
- Twine CP, Williams IM. Systematic review and meta‐analysis of the effects of statin therapy on abdominal aortic aneurysms. The British Journal of Surgery 2011;98(3):346‐53. [PUBMED: 21254006] [DOI] [PubMed] [Google Scholar]
VASGBI 2009
- VASGBI. The Vascular Society of Great Britain and Ireland. Lees T, Stansby G (editors).The National Vascular Database Report 2009. http://www.vasgbi.com/.
Wang 2008
- Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends in Molecular Medicine 2008; Vol. 14, issue 1:37‐44. [PUBMED: 18068482] [DOI] [PMC free article] [PubMed]


