Abstract
Vaccination programs against SARS-CoV-2 constitute the mainstay of public health interventions against the global COVID-19 pandemic. Currently available vaccines have shown 90% or better rates of protection against severe disease and mortality. Barely a year after vaccines became available, the Omicron variant and its unprecedented speed of transmission has posed a new challenge. Overall, Omicron presents increased immune escape, transmissibility, and decreased pathogenicity. Vaccines do not offer a full protection against SARS-CoV-2 acquisition, since “breakthrough” infections may occur in fully vaccinated individuals, who may in turn spread the virus to others. Breakthrough infections may be causally related to the viral profile (viral variant and load, incubation period, transmissibility, pathogenicity, immune evasion), immunity characteristics (mucosal versus systemic immunity, duration of immunity, etc.), host determinants (age, comorbidities, immune status, immunosuppressive drugs) and vaccination properties (platform, antigen dose, dose number, dose interval, route of administration). Determining the rate of breakthrough infections may be challenging and necessitates the conduction of population-based studies regarding vaccine effectiveness as well as neutralizing antibody testing, a surrogate of immune protection. In this review, we analyze the causes of breakthrough infections, their clinical consequences (severity of infection and transmission), methods of determining their incidence as well as challenges and perspectives. Long COVID as well as multi-inflammatory syndrome in adolescents may be significantly reduced in breakthrough infections. The need for universal pancoranavirus vaccines that would aim at protecting against a plethora of SARS-CoV-2 variants as well as emerging variants is discussed. Finally, novel vaccine strategies, such as nasal vaccines, may confer robust mucosal and systemic protection, reducing efficiently transmission.
Keywords: Antibody, Breakthrough infection, COVID-19, Immunity, Neutralizing antibody, Omicron, SARS-CoV-2, Pancoronavirus, Vaccine, Variant of concern, Variant
1. Introduction
As the joint efforts towards the development of specific, effective, and safe anti-viral therapeutic agents against SARS-CoV-2 infection are in progress, vaccination strategies represent the spearhead of worldwide public health strategies against COVID-19 [[1], [2], [3]]. Among the current vaccination platforms, mRNA vaccines constitute the most used vaccines in Europe and the USA. Although all vaccines against SARS-CoV-2 present a remarkable efficacy and effectiveness in preventing particularly severe Covid-19 with an overall favorable adverse event profile [[4], [5], [6], [7]], they cannot efficiently prevent transmission. Almost a year after the rapid development of anti-SARS-CoV-2 vaccines and their spectacular success, the latest viral variant, Omicron, has posed a new challenge. Omicron has displaced the Delta variant due to its additional mutations, superior immune evasion and transmissibility.
The spike glycoprotein of SARS-CoV-2 presents two main antigenic domains; mutations in these locations may contribute to antigenic evasion and decreased immunity against infection [8]. The receptor binding domain (RBD) binds to the SARS-CoV-2 receptor, the angiotensin-converting enzyme 2 (ACE2). Amino acid alterations in RBD may influence the affinity of the spike glycoprotein for ACE2, and hence transmissibility and virulence of SARS-CoV-2 variants [8]. On November 24, 2021, the World Health Organization was first notified of the new SARS-CoV-2 B.1.1.529 Variant (Omicron). In the USA, by the end of January, over 90% of Covid cases were related to this variant, which has rapidly become the dominant circulating variant of concern [9]. The Omicron variant harbors a remarkable 59–amino acid substitution throughout its genome relative to the ancestral Wuhan-hu-1 SARS-CoV-2 virus, where almost 37 of these nonsynonymous mutations are located within the spike protein, the target of neutralizing antibodies against SARS-CoV-2 [10]. Overall, emerging data have suggested that the omicron variant presents increased transmissibility and ability to dodge immunity whether from vaccination or previous infection as well decreased pathogenicity [11,12]. In the meantime, the Omicron sub-lineage BA.2 has already been identified with an even faster growth rate. Of note, transmission within the household appears to be greater for the new omicron subvariant [13]. The BA.2 subvariant seems to be substantially more infectious than the BA.1 subvariant, reflecting greater viral load and/or protracted duration of infection, hence explaining its rapid expansion in Qatar [14]. Besides the importance of vaccines in preventing severe and fatal COVID-19, vaccines do not always prevent “breakthrough” infections, i.e. infections appearing in fully vaccinated individuals, thus permitting subsequent transmission to other subjects. In this review, we analyze the causes of breakthrough infections, their clinical consequences (severity of infection and transmission), methods of determining their incidence as well as challenges and perspectives (Fig. 1).
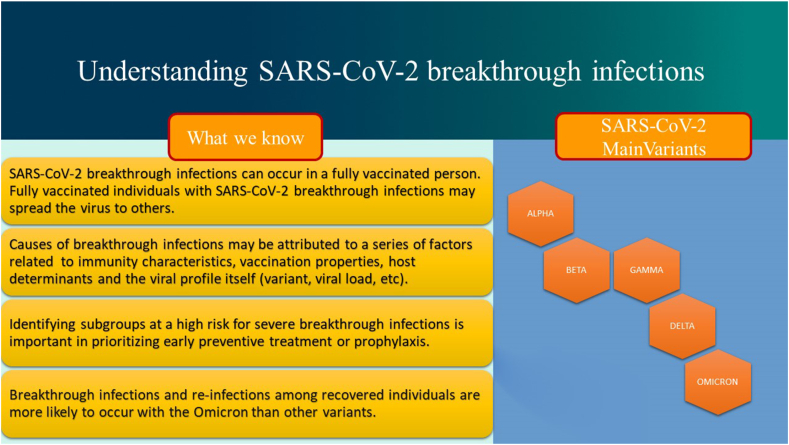
Fig. 1.
Understanding SARS-CoV-2 breakthrough infections.
2. What are the causes of breakthrough infections?
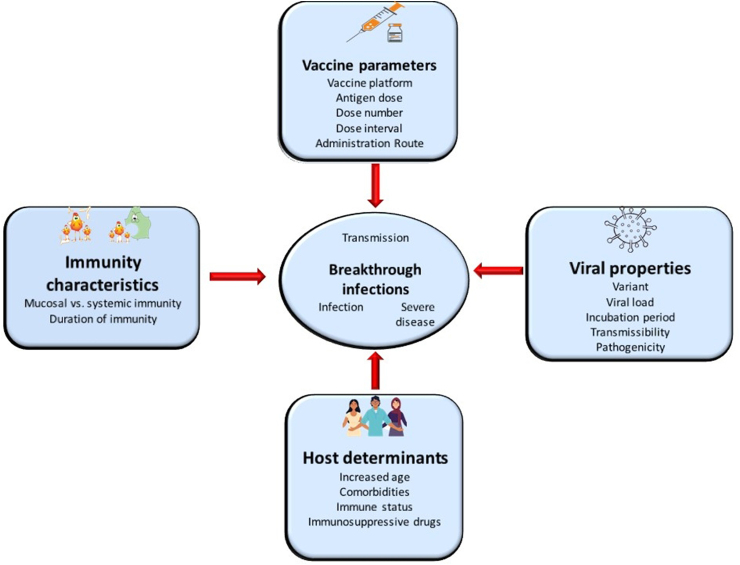
Vaccines can mitigate transmission via two main mechanisms: 1) prevention of infection and 2) less infectivity of breakthrough cases. Vaccinated subjects experiencing breakthrough infections may present decreased viral load and/or a rapid viral clearance with a lower potential for onward transmission. Causes of breakthrough infections may be attributed to a series of factors related to the viral profile itself, including variant, viral load, incubation period, transmissibility, pathogenicity and immune evasion; host determinants (age, comorbidities, immune status, immunosuppressive drugs); immunity characteristics (mucosal versus systemic immunity, duration of immunity, etc.); and vaccination properties (platform, antigen dose, dose number, dose interval, route of administration) [15]. Fig. 2 depicts the main causes of breakthrough infections.
Fig. 2.
Potential mechanisms implicated in breakthrough infections. Breakthrough infections may affect transmission of SARS-CoV-2.
Overall, mRNA vaccine platforms present high capacity at eliciting increased neutralizing antibody and T cell responses compared to other vaccine platforms. Also, neutralizing antibody levels after vaccination with mRNA platforms are higher than those after SARS-CoV-2 infection or after vaccination with other platforms. Although CD4 and CD8 T cell repertoires are broader against a plethora of peptides from SARS-CoV-2, compelling evidence has highlighted only for immunity to the spike protein as immunoprotective. The spike protein has already been established as the primary immunogen candidate for Sarbecoviruses, the family of Coronaviruses including SARS-CoV-2. In particular, the stabilized prefusion conformation of the spike protein has been the target of vaccines [[16], [17], [18], [19], [20], [21], [22]].
Vaccine-induced immune responses may be influenced by the genetic variant of SARS-CoV-2 and the viral load. Evidence from in vitro studies in sera from vaccinated subjects has shown a 3 to 15-fold, 1.4 to 3-fold and 25- to 40-fold reduction in neutralizing activity of antibodies for the Beta, Delta and Omicron variants respectively in comparison to earlier variants of SARS-CoV-2 [[16], [17], [18], [19], [20], [21], [22]]. These results are generally consistent with data from epidemiologic studies suggesting that the probability of breakthrough infections increases with the Omicron variant than Delta or Alpha variants [23]. Other virologic characteristics such as the increased viral load, the shorter incubation chronic periods, the reduction of the time of immune memory to respond effectively, and the increased fusogenicity of the SARS-CoV-2 spike protein observed in the Delta variant may ease fusion of the virus and increase its propagation, resulting in decreased effectiveness of humoral immunity [24]. Interestingly, time to response of the immune system may not effectively prevent infection given a short incubation period of the pathogen, but it may control disease progression as the antibody titer is increasing.
Based on previous data from other vaccines, increased viral inoculum may decrease vaccine effectiveness and augment the risk of breakthrough infections [25]. Therefore, theoretically, masking and vaccination may act synergistically in preventing breakthrough infections.
In comparison to respiratory viruses that cause systemic infections (such as rubella, measles, varicella-zoster virus infections), non-systemic respiratory viruses (RSV, influenza and parainfluenza viruses) and coronaviruses causing common cold, present limited contact with the systemic immune system, by infecting mainly epithelia of mucosal surfaces. Such non-systemic infections induce short and incomplete mucosal immunity, thus permitting reinfections and suboptimal responses to systemically administered vaccines leading to breakthrough infections [26]. Systematically injected vaccines elicit decreased and short-lived levels of IgG and monomeric IgA antibodies as well as secretory IgA antibodies at the mucosal surface of the upper respiratory tract, which represents the main entry of respiratory viruses [15].
Upon viral exposure, the immunity state of the individual plays an important role. It is not unexpected that older age, comorbidities, immunocompromised conditions such as hematologic malignancies, and immunosuppressive treatment may be linked to decreased neutralizing antibody activity and titers, and decreased T-cell immunity, leading to a higher risk of breakthrough infections with increasing severity [[27], [28], [29], [30], [31], [32], [33]].
However, waning immunity is inevitable and might only be reinforced by a booster dose or infection. The modeling of declining kinetics of antibody responsiveness after vaccination or infection, has predicted a half-life response that would protect most individuals beyond a year. However, antibody waning has been more rapid than predicted. From functional neutralization assays and antibody binding essays, antibody titers decline from approximately 4 to 5 months corresponding to the increase of breakthrough infections after the second vaccine dose. This correlates with the assumed inverse relationship between the level of immunity and infection/disease severity. Nevertheless, there is lasting protection from severe infection, hospitalization, admission to the Intensive Care Unit (ICU) and death, probably due to the stimulation of memory B and T cell immunity. Indeed, during the early chronic period after vaccination, mRNA vaccines presented a remarkable efficacy and effectiveness above 90% from symptomatic infection to mortality, as observed in randomized clinical trials and in population-based observational studies respectively [34]. In the case of BNT162b2, the initial robust humoral immune response which peaks after 4–30 days after a complete vaccination course, is followed by a gradual decay of antibody titers, with a virtually linear decrease of anti-Spike IgG-titers over time, reaching levels after 6 months that are roughly 18-fold lower compared to their peak [35]. The drop of anti-Spike IgG levels is apparent as early as 70 days after the completion of a full vaccination scheme, with a two- and five-fold reduction compared with the levels at 20–40 days for BNT162b2 and ChAdOx1 recipients, respectively [36]. Given that there is a parallel trend regarding neutralizing antibody titers [35], which may serve as a surrogate of immunity against SARS-CoV-2 [37], this humoral phenomenon coincides with a gradually increasing susceptibility of fully vaccinated individuals for breakthrough infections in the months following immunization [[38], [39], [40], [41]]. As immunity diminishes over time, the most notable decreases in vaccine effectiveness have occurred in milder forms and asymptomatic infections, in those vaccinated earliest, in older subjects and in those infected with the Omicron variant [15,23]. Therefore, there is an urgent need to examine the effectiveness of both prior SARS-CoV-2 infection and vaccination against the Omicron variant. Table 1 presents data on the vaccine effectiveness against the Omicron variants and subvariants related with the 2nd and 3rd booster doses. Table 2 depicts data on the effectiveness of a 3rd booster dose of mRNA vaccines against the Omicron variant by country.
Table 1.
Vaccine effectiveness against the Omicron variant.
| Principal Defense | Outcomes | Dose 2 |
Dose 3 |
Dose 4 | ||||
|---|---|---|---|---|---|---|---|---|
| 0–3 months | 4–6 months | >6 months | 0–3 months | 4–6 months | >6 months | |||
| Neutralizing antibodies | Infection | Not sufficient data | Not sufficient data | “Small effect” | ||||
| Symptomatic infection | 25–70% | 5–30% | 0–10% | 50–75% | 40–50% | Not sufficient data | ||
| Memory B and T cells | Hospitalization | 65–85% | 55–65% | 30–35% | 80–95% | 75–85% | Not sufficient data | Not sufficient data |
| Mortality | Not available yet | 40–70% | 85–99% | Not sufficient data | ||||
| Symptomatic infection from BA.1/BA.2 subvariant | BA.1 | BA.2 | BA.1 | BA.2 | Not sufficient data | |||
| 9–11% | 5–29% | 48–69% | 37–77% | |||||
Table 2.
Effectiveness of a 3rd booster dose of mRNA vaccines against the Omicron variant by country.
| Study, year and Reference | Country and study design | Effectiveness |
|---|---|---|
| Tseng et al., 2022 [77] | USA Test-negative case-control study including 26,683 SARS-CoV-2 cases |
Effectiveness of booster vaccination 71.6% (after 14–60 days), 47.4% (>60 days) |
| Monge et al., 2022 [78] | Spain Case-control study among 3,111,159 individuals receiving a booster vaccine and equal number of matched controls |
Booster effectiveness (after 7–34 days): mRNA-1273 booster: 52.5% (95% CI: 51.3–53.7) BNT162b2 booster: 46.2% (95% CI: 43.5–48.7) |
| Abu-Raddad et al., 2022 [79] | Qatar Retrospective cohort study including 230.526 receivers of a BNT162b2 booster vaccination |
Booster effectiveness: mRNA-1273 booster (after 35 days): 50.8% (95% CI: 43.4–57.3) BNT162b2 booster (after 49 days): 50.1% (95% CI: 47.3–52.8%) |
| Accorsi E et al., 2022 [80] | USA Test-negative case-control analysis among 70,155 tests from 4,666 sites |
Effectiveness 66.3% (95% CI: 64.3–68.1) against Omicron after BNT162b2 or mRNA-1273 booster |
| Thomson et al., 2022 [81] | USA Test-negative case-control analysis among 222722 emergency department and urgent care Encounters |
Effectiveness 94% (95% CI: 93–94) against Delta and 82% (95% CI: 79–84) against Omicron after BNT162b2 or mRNA-1273 booster |
| Kislaya I et al., 2022 [61] | Portugal Case-control study of 4,898 omicron variant cases among 13,134 SARS-CoV-2 infections |
Booster effectiveness against omicron variant 68.8% (95% CI: 46.4–81.7%), against delta 94.0% (95% CI: 93.4–94.6) Odds ratio for omicron breakthrough infection after booster: 5.2 (95% CI: 3.1–8.8) vs. delta |
| UK Health Security Agency [82] | United Kingdom UK Health Security Agency COVID-19 vaccine surveillance report |
Effectiveness against mild infection 2–4 weeks after mRNA booster 60–75%, after >15 weeks 25–40% Effectiveness against hospitalization initially ∼90%, dropping to ∼75% after 10–14 weeks for BNT162b2 booster 90–95% up to 9 weeks after mRNA-1273 booster. |
| Andeweg SP et al., 2022 [49] | the Netherlands Test-negative case-control analysis among 528,488 tests |
Effectiveness against Omicron BA.1: 76% (95% CI: 72–79) among those with previous infection and 68% (95% CI: 67–69) without previous infection |
A recent report from the Centers for Disease Control and Prevention has registered the decreasing vaccine effectiveness on outcomes of visits to the emergency department and hospitalization at 4 months after the 3rd dose as compared to a 2-month interval. The UK Health Security Agency (UKHSA) reports as well as other studies have also confirmed the descending vaccine effectiveness [42,43]. While further studies are needed, results from laboratory studies have suggested that the immunity resulting from a combination of vaccination and prior infection (hybrid immunity) or combined vaccination with different vaccine types (heterologous immunity) may offer some protection against the variant, which may be superior to vaccination with a single platform alone [44,45]. The Omicron variant, characterized by great immune evasion, escapes the majority of existing SARS-CoV-2 neutralizing antibodies [46]. However, recent data have shown that a third-dose mRNA vaccine booster, or double vaccination followed by Delta breakthrough infection, or prior infection followed by mRNA vaccine double vaccination, all appear to restore neutralizing activity against Omicron [[47], [48], [49]].
Apart from time since vaccination for some anti-SARS-CoV-2 vaccines, recent data have highlighted that increasing the interval time between the first and the second doses as well as the increased antigen presented in the vaccine (such as in the mRNA-1273-Moderna vaccine: 100 μg of mRNA versus 30 μg of mRNA in BNT162b2-Pfizer) may augment immune response and protection. The latter feature may in part explain the slightly more pronounced effectiveness of mRNA-1273 versus BNT162b2 in the prevention of infection, hospitalization, intensive care admission and death [50]. With the emergence of future variants of SARS-CoV-2 presenting increased immune escape, boosting with the original Wuhan-hu-1 SARS-CoV-2 spike protein may be ineffective. For example, influenza vaccines are updated twice a year based on the prevalent and projected circulating strains of influenza virus, and have also the capacity of an updated vaccine to elicit an antibody response in a small group of volunteers, avoiding the requirement for larger, time-consuming and expensive clinical trials. mRNA vaccines may be rapidly updated and produced in large scale compared to other vaccine platforms. However, an Omicron booster dose has not shown to provide greater immunity or protection compared to a booster dose with the current mRNA-1273 vaccine in macaques [51].
3. How can we determine breakthrough infections?
Recording breakthrough infections is challenging due to the fact that many unvaccinated individuals are likely to have some immunity probably due to previous infections along with the wide spectrum of symptoms and outcomes of COVID-19. Comparing the incidence rate of infections amongst vaccinated and unvaccinated subjects offers a rough but crucial estimate. Despite the fact that randomized control trials (RCTs) can provide this information, they lack insight into the waning of vaccine protection over time as well as the role of viral variants. Efficacy is generally assessed mainly against one variant, and significant RCTs presented the comparison of vaccines against placebo without a head-to-head comparison [15].
The use of observational studies could overcome the limitations of RCTs which can compare either infection incidence amongst vaccinated and unvaccinated persons during the outbreak of a new viral variant or the effectiveness of vaccines among different variants. Additionally, prospective observational studies can be used to count the weekly incidence rate of breakthrough infections compared to that of the unvaccinated individuals. In particular, cohort studies in healthcare workers may be a useful investigational tool for the study of breakthrough infections. Retrospective case-control studies can be equally helpful as they are easily conducted, being used in the same way as the test negative design employed by the WHO to estimate the efficacy of influenza vaccines. In this approach, which is used for the evaluation of effectiveness of a plethora of vaccines, the vaccination status of confirmed COVID-19 patients (cases) is compared to that of patients who had similar symptoms but tested negative for Covid-19 (controls) [52,53].
Furthermore, in vitro measurements, such as neutralization assays, may help assessing the risk of breakthrough infections. These assays provide quantifiable results of the ability and activity of anti-SARS-CoV-2 specific antibodies to protect cells against infection. However, in order to conduct live virus neutralization assays, which is the gold standard method, a biosafety level (BSL) −3 or higher facility is required, in comparison to the more frequent use assays employing SARS-CoV-2 pseudotyped viral particles like the spike protein, which can be conducted in a BSL-2 facility [15]. These safer approaches have to be validated employing live virus neutralization assays. Although evidence has shown that assays performed with pseudotyped viruses correlate with those performed with wild-type viruses, some pseudotyped viruses may be more prone to neutralization [54].
Overall, the functional titer of neutralizing anti-Spike antibodies and, to a lesser extent, the concentration of anti-IgG antibodies against the spike protein and its RBD have been considered as a proxy of immune protection. Of note, not all anti-spike or RBD antibodies are capable of neutralizing SARS-CoV-2. Nonetheless, some non-neutralizing antibody activities, such as antibody-dependent cellular cytotoxicity may play an important role in immune protection. Also, animal studies have shown that nucleocapsid may be highly immunogenic for antibodies and T cells, and may be incorporated in multi-antigen vaccines. Although neutralizing antibody titers are an important determinant of protective immunity against COVID-19, they are not the only correlate of immunity. First, neutralizing antibody titers in the serum do not translate into protection. Serum neutralizing antibodies do not normally diffuse into mucosal fluids. Therefore, immune protection against superficial respiratory infection resides in mucosal antibodies, such as IgA, and mucosal T cells of the respiratory tract [55]. Second, non-neutralizing antibody responses, T cell responses, and innate immune responses could also affect the outcome of SARS-CoV-2 infections, and these factors are not easily determined [56,57]. T cell immune responses could be important in buttressing neutralizing antibody–mediated protection while maintaining long-term protection [55]. However, more granularity in T cell memory studies is required to examine causal associations between specific T cell repertoires and phenotypes conferring protection. Cell mediated immunity presents added benefits providing protection against a plethora of viral strains and emerging variants [58]. More importantly, the protective role of mucosal T cells, comprising resident memory T cells, highlights the significance of studying immune responses in relevant tissue locations, not just in the serum [59].
Neutralizing antibody testing is not widely used by many laboratories to detect protection against COVID-19 for many reasons. Firstly, some laboratory tests do not detect antibodies produced after vaccination as well as neutralizing antibodies, and use different units of measurement. In addition, a specific serum antibody threshold that predicts protection has not been defined yet. Finally, the tests need to be standardized and calibrated, as it has been done for other tests used in vaccines in the past. FDA has highlighted that antibody testing cannot prove vaccination effectiveness and subsequent protection [60].
While clearer and more specific determinations for immune protection are yet to come, the combining vaccination, wearing masks, avoiding crowded indoor spaces and washing hands remain a good protective measure against breakthrough infections.
4. What are the consequences of breakthrough infections?
The clinical impact of SARS-CoV-2 breakthrough infections generally pertains to the severity of the infection and the transmission of the virus. Recent studies about the infectiousness of Alpha and Delta variants, have reported that vaccinated individuals, especially with a third (booster) dose, seem to present reduced viral loads in breakthrough infections, but also smaller contact-transmission rates, compared with the unvaccinated population [61]. Available evidence has pointed towards that transmissibility of the Omicron variant is considerably higher compared with other dominant variants [62]. Accordingly, modelling estimates have suggested that the rates of high-, very high and super-emitters are particularly increased among those infected with the Omicron, compared with previous variants (1 every 10–20 infected persons) [63]. Nevertheless, this does not seem to emerge as a result of a higher viral load, since patients infected with the Omicron variant present lower infectious titers and a shorter clearance phase [64,65]. Vaccines may alter the kinetics of viral shedding diminishing transmission as well as the association between viral load and symptomatology [66,67]. In the chronic period of the Alpha variant, vaccination decreased transmission to unvaccinated subjects [68]. Unvaccinated household members were 23% more likely to become infected with Omicron than with Delta. Household members who had received three doses of a SARS-CoV-2 vaccine were more than twice as likely to get infected with Omicron than with Delta. An individual who had received three vaccine doses is also about twice as likely to transmit Omicron on to another household member than Delta. Evidence has highlighted that a third vaccine dose presented a marginal beneficial effect on the transmission of Omicron or Delta variants compared to only two doses, although this effect was more pronounced for Delta than it was for Omicron [69,70].
Overall, breakthrough infections are milder than infections in unvaccinated individuals and may fuel a relative decrease of future surges, boosting individual immune responses and strengthening collective immunity. Also, since immunity wanes over time, the third booster dose decreases drastically the incidence of severe disease across all age groups [71] as well as the rate of symptomatic infections, albeit to a lesser extent [13,43]. Currently, there are not sufficient data regarding the effectiveness against symptomatic infection after a 4th booster dose. Based on a small clinical trial in Israel, the fourth dose of a COVID-19 vaccine may restore antibody titers to levels observed after the third dose but confers only a modest boost in protection against infection [72].
With regards to severe manifestations, it has been noted that post-SARS-CoV-2 infection (i.e. long COVID) seems to be significantly reduced in breakthrough infections [61]. However, even if vaccine effectiveness is high, severe breakthrough infections may still occur in older individuals, patients with comorbidities and immunosuppression [15]. According to a review of the UKHSA, subjects who had been fully vaccinated using all current vaccine platforms against COVID-19 presented a 50% reduction of developing long COVID-19 symptoms compared to individuals who had received only one vaccine dose or were unvaccinated [73]. Also, adolescents receiving 2 doses of the Pfizer-BioNTech vaccine present a high level of protection against Multisystem Inflammatory Syndrome, highlighting the importance of the vaccination among all eligible children [74].
Further studies are awaited to examine the severity of breakthrough infections and their dependance on the level of an individual's immunity at any moment. Identifying high risk populations for severe breakthrough infections could lead to earlier prevention strategies such as the administration of specific anti-viral drugs (e.g. molnupiravir or nirmatrelvir and ritonavir) and/or monoclonal antibodies.
5. Challenges and perspectives
Several important questions remain unanswered. Firstly, it remains controversial whether it is possible to achieve high and sustained levels of herd immunity against SARS-COV-2 infection, as it is a mucosal one without an obligate stage of dissemination through lymph or blood. A viable future approach in vaccinal strategy would include the production of nasal vaccines that mimic the natural entry of the virus and may confer important mucosal immunity with rapid and local protection. In a recent animal study in mice from the Yale School of Medicine, nasal vaccines have been shown to provide robust mucosal and systemic immunity, including IgA, memory B and T cell responses against SARS-CoV-2, and other similar respiratory viruses [75]. Nasal vaccines may be effective at preventing both infection and transmission.
Moreover, it is unclear whether determining the level of individual immune responses can predict how protected the individual is against breakthrough infections. As mentioned in the 2nd section, although the level of neutralizing antibodies correlates with overall immune protection, an analysis of the large Moderna mRNA-1273 vaccine efficacy trial has shown that neutralizing antibodies accounted for only 68% of the protection [72]. Also, will we continue to develop boosters with the prototypic Wuhan Hu-1 spike sequence or focus on future predominant variants of concern (VOCs)? Until now, no specific vaccine against the Omicron variant has yet entered clinical trials. An important issue is whether regulatory agencies will accept evidence of immune responses as surrogate of immune protection rather than efficacy clinical trials.
It is worth investigating if a fourth dose of vaccination is qualitatively different from the third or whether the protection levels will return to the pre-boost level afterwards [76]. A practical issue that needs to be addressed is whether a longer interval in booster doses may effectively increase immunogenicity and protection from symptomatic breakthrough infections. Besides, the idea of reboosting every 4–6 months is not a practical and viable public health strategy. Therefore, there is need for universal pancoranavirus vaccines that would aim at protecting against a plethora of SARS-CoV-2 variants as well as emerging variants. The vaccine from the Walter Reed Army Institute of Research (SARS-COV-2-Spike-Ferritin-Nanoparticle Vaccine with Army Liposomal Formulation QS21 Adjuvant), which leads in the development of a pancoronavirus vaccine candidate, has just completed its phase 1 trial (NCT04784767). This offered increased neutralizing antibody titers against VOCs and other Sarbecoviruses, Other pancoronavirus candidate vaccines may commence first trials during 2022.
Also, there is an increasing need to monitor duration of protection and vaccine effectiveness against different variants [[73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88]]. Such a system will be efficient in promptly adapting vaccine antigens and dosing intervals, as required. In the future, VOCs will gain a lot of their transmission potential from immune evasion resembling seasonal influenza viruses. Besides pathogenicity of future VOCs which cannot be predicted, the infection fatality ratio is expected to decline mainly due to the increasing herd immunity [88]. As the SARS-COV-2 infection is gradually transitioning from pandemic to endemic, the high breakthrough infection rate requires applicable rigorous and goal directed research to enforce the implementation of guidelines by public health services throughout the world, thereby preventing possible future outbreaks.
Financial support
None.
Declaration of competing interest
No conflict of interest to disclose.
Contributor Information
Evropi Amanatidou, Email: a.evropi@gmail.com.
Anna Gkiouliava, Email: annagkio@gmail.com.
Eva Pella, Email: evap14@yahoo.gr.
Maria Serafidi, Email: marizaseraf@gmail.com.
Dimitrios Tsilingiris, Email: tsilingirisd@gmail.com.
Natalia G. Vallianou, Email: natalia.vallianou@hotmail.com.
Irene Karampela, Email: eikaras1@gmail.com.
Maria Dalamaga, Email: madalamaga@med.uoa.gr.
References
- 1.Dalamaga M., Karampela I., Mantzoros C.S. Commentary: phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metab, Clin Exp. 2020;109:154282. doi: 10.1016/j.metabol.2020.154282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karampela I., Vallianou N.G., Tsilingiris D., Christodoulatos G.S., Muscogiuri G., Barrea L., et al. Panminerva medica; 2021. Could inhaled corticosteroids be the game changers in the prevention of severe COVID-19? A review of current evidence. [DOI] [PubMed] [Google Scholar]
- 3.Vallianou N.G., Tsilingiris D., Christodoulatos G.S., Karampela I., Dalamaga M. Anti-viral treatment for SARS-CoV-2 infection: a race against time amidst the ongoing pandemic. Metabolism open. 2021;10:100096. doi: 10.1016/j.metop.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsilingiris D., Vallianou N.G., Karampela I., Liu J., Dalamaga M. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metabolism open. 2022;13:100159. doi: 10.1016/j.metop.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsilingiris D., Vallianou N.G., Karampela I., Dalamaga M. Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story. Metabolism open. 2021;11:100101. doi: 10.1016/j.metop.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallianou N.G., Tsilingiris D., Karampela I., Liu J., Dalamaga M. Herpes zoster following COVID-19 vaccination in an immunocompetent and vaccinated for herpes zoster adult: a two-vaccine related event? Metabolism open. 2022;13:100171. doi: 10.1016/j.metop.2022.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastie K.M., Li H., Bedinger D., Schendel S.L., Dennison S.M., Li K., et al. Defining variant-resistant epitopes targeted by SARS-CoV-2 antibodies: a global consortium study. Science. 2021;374:472–478. doi: 10.1126/science.abh2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambrou A.S., Shirk P., Steele M.K., Paul P., Paden C.R., Cadwell B., et al. Genomic surveillance for SARS-CoV-2 variants: predominance of the Delta (B. 1.617. 2) and omicron (B. 1.1. 529) variants—United States, June 2021–January 2022. MMWR (Morb Mortal Wkly Rep) 2022;71:206. doi: 10.15585/mmwr.mm7106a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sievers B.L., Chakraborty S., Xue Y., Gelbart T., Gonzalez J.C., Cassidy A.G., et al. Antibodies elicited by SARS-CoV-2 infection or mRNA vaccines have reduced neutralizing activity against Beta and Omicron pseudoviruses. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abn7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCallum M., Czudnochowski N., Rosen L.E., Zepeda S.K., Bowen J.E., Walls A.C., et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022;375:864–868. doi: 10.1126/science.abn8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiecco G., Storti S., Degli Antoni M., Focà E., Castelli F., Quiros-Roldan E. Omicron genetic and clinical peculiarities that may overturn SARS-CoV-2 pandemic: a literature review. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23041987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UK Health Security Agency . UK Health Security Agency; 2022. COVID-19 vaccine surveillance report.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1054071/vaccine-surveillance-report-week-6.pdf [Google Scholar]
- 14.Qassim S.H., Chemaitelly H., Ayoub H.H., AlMukdad S., Tang P., Hasan M.R., et al. Effects of BA.1/BA.2 subvariant, vaccination, and prior infection on infectiousness of SARS-CoV-2 Omicron infections. medRxiv. 2022 doi: 10.1101/2022.03.02.22271771. 2022.03.02.22271771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsitch M., Krammer F., Regev-Yochay G., Lustig Y., Balicer R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22:57–65. doi: 10.1038/s41577-021-00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 17.Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021;27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- 18.Edara V.V., Manning K.E., Ellis M., Lai L., Moore K.M., Foster S.L., et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep. Med. 2022;3:100529. doi: 10.1016/j.xcrm.2022.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Stewart-Jones G.B.E., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med. 2021;384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameroni E., Saliba C., Bowen J.E., Rosen L.E., Culap K., Pinto D., et al. 2021. Broadly Neutralizing Antibodies Overcome SARS-CoV-2 Omicron Antigenic Shift. bioRxiv : the preprint server for biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejnirattisai W., Shaw R.H., Supasa P., Liu C., Stuart A.S., Pollard A.J., et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399:234–236. doi: 10.1016/s0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021 doi: 10.1101/2021.12.08.21267417. [DOI] [Google Scholar]
- 23.Christensen P.A., Olsen R.J., Long S.W., Snehal R., Davis J.J., Saavedra M.O., et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in houston, Texas. Am J Pathol. 2022 doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langwig K.E., Gomes M.G.M., Clark M.D., Kwitny M., Yamada S., Wargo A.R., et al. Limited available evidence supports theoretical predictions of reduced vaccine efficacy at higher exposure dose. Sci Rep. 2019;9:3203. doi: 10.1038/s41598-019-39698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morens D.M., Taubenberger J.K., Fauci A.S. Universal coronavirus vaccines - an urgent need. N Engl J Med. 2022;386:297–299. doi: 10.1056/NEJMp2118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brockman M.A., Mwimanzi F., Lapointe H.R., Sang Y., Agafitei O., Cheung P., et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J Infect Dis. 2021 doi: 10.1093/infdis/jiab592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalamaga M., Christodoulatos G.S., Karampela I., Vallianou N., Apovian C.M. Understanding the Co-epidemic of obesity and COVID-19: current evidence, comparison with previous epidemics, mechanisms, and preventive and therapeutic perspectives. Curr Obes Rep. 2021;10:214–243. doi: 10.1007/s13679-021-00436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallianou N.G., Evangelopoulos A., Kounatidis D., Stratigou T., Christodoulatos G.S., Karampela I., et al. Diabetes mellitus and SARS-CoV-2 infection: pathophysiologic mechanisms and implications in management. Curr Diabetes Rev. 2021;17 doi: 10.2174/1573399817666210101110253. [DOI] [PubMed] [Google Scholar]
- 30.Karampelas M., Dalamaga M., Karampela I. Does COVID-19 involve the retina? Ophthalmol Ther. 2020;9:693–695. doi: 10.1007/s40123-020-00299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belanger M.J., Hill M.A., Angelidi A.M., Dalamaga M., Sowers J.R., Mantzoros C.S. Covid-19 and disparities in nutrition and obesity. N Engl J Med. 2020;383:e69. doi: 10.1056/NEJMp2021264. [DOI] [PubMed] [Google Scholar]
- 32.Chung D.J., Shah G.L., Devlin S.M., Ramanathan L.V., Doddi S., Pessin M.S., et al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Canc Discov. 2021;2:568–576. doi: 10.1158/2643-3230.bcd-21-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peeters M., Verbruggen L., Teuwen L., Vanhoutte G., Vande Kerckhove S., Peeters B., et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO open. 2021;6:100274. doi: 10.1016/j.esmoop.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kow C.S., Hasan S.S. Real-world effectiveness of BNT162b2 mRNA vaccine: a meta-analysis of large observational studies. Inflammopharmacology. 2021;29:1075–1090. doi: 10.1007/s10787-021-00839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/s0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Raddad L.J., Chemaitelly H., Bertollini R. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med. 2022;386:799–800. doi: 10.1056/NEJMc2117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/s0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin D.Y., Gu Y., Wheeler B., Young H., Holloway S., Sunny S.K., et al. Effectiveness of covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022 doi: 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UK Health Security Agency . UK Health Security Agency; 2022. SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 36.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1056487/Technical-Briefing-36-22.02.22.pdf [Google Scholar]
- 43.Ferdinands J.M., Rao S., Dixon B.E., Mitchell P.K., DeSilva M.B., Irving S.A., et al. 2022. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance—VISION Network, 10 states. August 2021–January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Zhao X., Song J., Wu J., Zhu Y., Li M., et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microb Infect. 2022;11:477–481. doi: 10.1080/22221751.2022.2030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuo F., Abolhassani H., Du L., Piralla A., Bertoglio F., de Campos-Mata L., et al. Heterologous immunization with inactivated vaccine followed by mRNA booster elicits strong humoral and cellular immune responses against the SARS-CoV-2 Omicron variant. medRxiv. 2022 doi: 10.1101/2022.01.04.22268755. 2022.01.04.22268755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Servellita V., Syed A.M., Brazer N., Saldhi P., Garcia-Knight M., Sreekumar B., et al. Neutralizing immunity in vaccine breakthrough infections from the SARS-CoV-2 Omicron and Delta variants. medRxiv. 2022 doi: 10.1101/2022.01.25.22269794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann M., Krüger N., Schulz S., Cossmann A., Rocha C., Kempf A., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456. doi: 10.1016/j.cell.2021.12.032. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gruell H., Vanshylla K., Tober-Lau P., Hillus D., Schommers P., Lehmann C., et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022:1–4. doi: 10.1038/s41591-021-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andeweg S.P., de Gier B., Eggink D., van den Ende C., van Maarseveen N., Ali L., et al. Protection of COVID-19 vaccination and previous infection against omicron BA.1 and Delta SARS-CoV-2 infections, The Netherlands, 22 november 2021- 19 January 2022. medRxiv. 2022 doi: 10.1101/2022.02.06.22270457. 2022.02.06.22270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickerman B.A., Gerlovin H., Madenci A.L., Kurgansky K.E., Ferolito B.R., Figueroa Muñiz M.J., et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. Veterans. N Engl J Med. 2022;386:105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gagne M., Moliva J.I., Foulds K.E., Andrew S.F., Flynn B.J., Werner A.P., et al. 2022. mRNA-1273 or mRNA-Omicron Boost in Vaccinated Macaques Elicits Comparable B Cell Expansion, Neutralizing Antibodies and Protection against Omicron. bioRxiv : the preprint server for biology. 2022.02.03.479037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan S.G., Tchetgen Tchetgen E.J., Cowling B.J. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184:345–353. doi: 10.1093/aje/kww064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dean N.E., Hogan J.W., Schnitzer M.E. Covid-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385:1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentley E.M., Mather S.T., Temperton N.J. The use of pseudotypes to study viruses, virus sero-epidemiology and vaccination. Vaccine. 2015;33:2955–2962. doi: 10.1016/j.vaccine.2015.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Openshaw P.J.M. Using correlates to accelerate vaccinology. Science. 2022;375:22–23. doi: 10.1126/science.abn0007. [DOI] [PubMed] [Google Scholar]
- 56.Mallajosyula V., Ganjavi C., Chakraborty S., McSween A.M., Pavlovitch-Bedzyk A.J., Wilhelmy J., et al. CD8(+) T cells specific for conserved coronavirus epitopes correlate with milder disease in COVID-19 patients. Science Immunol. 2021;6 doi: 10.1126/sciimmunol.abg5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farber D.L. Tissues, not blood, are where immune cells function. Nature. 2021;593:506–509. doi: 10.1038/d41586-021-01396-y. [DOI] [PubMed] [Google Scholar]
- 60.Abbasi J. The flawed science of antibody testing for SARS-COV-2 immunity. JAMA. 2021;326:1781–1782. doi: 10.1001/jama.2021.18919. [DOI] [PubMed] [Google Scholar]
- 61.Kislaya I., Rodrigues E.F., Borges V., Gomes J.P., Sousa C., Almeida J.P., et al. Comparative effectiveness of coronavirus vaccine in preventing breakthrough infections among vaccinated persons infected with Delta and Alpha variants. Emerg Infect Dis. 2022;28:331–337. doi: 10.3201/eid2802.211789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022 doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riediker M., Briceno-Ayala L., Ichihara G., Albani D., Poffet D., Tsai D.H., et al. Higher viral load and infectivity increase risk of aerosol transmission for Delta and Omicron variants of SARS-CoV-2. Swiss Med Wkly. 2022;152:w30133. doi: 10.4414/smw.2022.w30133. [DOI] [PubMed] [Google Scholar]
- 64.Hay J.A., Kissler S.M., Fauver J.R., Mack C., Tai C.G., Samant R.M., et al. Viral dynamics and duration of PCR positivity of the SARS-CoV-2 Omicron variant. medRxiv. 2022 doi: 10.1101/2022.01.13.22269257. 2022.01.13.22269257. [DOI] [Google Scholar]
- 65.Puhach O., Adea K., Hulo N., Sattonnet P., Genecand C., Iten A., et al. Infectious viral load in unvaccinated and vaccinated patients infected with SARS-CoV-2 WT, Delta and Omicron. medRxiv. 2022 doi: 10.1101/2022.01.10.22269010. 2022.01.10.22269010. [DOI] [PubMed] [Google Scholar]
- 66.Levine-Tiefenbrun M., Yelin I., Katz R., Herzel E., Golan Z., Schreiber L., et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27:790–792. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- 67.Ke R., Martinez P.P., Smith R.L., Gibson L.L., Achenbach C.J., McFall S., et al. Longitudinal analysis of SARS-CoV-2 vaccine breakthrough infections reveal limited infectious virus shedding and restricted tissue distribution. medRxiv. 2021 doi: 10.1101/2021.08.30.21262701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Layan M., Gilboa M., Gonen T., Goldenfeld M., Meltzer L., Andronico A., et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. medRxiv. 2021 doi: 10.1101/2021.07.12.21260377. 2021.07.12.21260377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mallapaty S. COVID-19: how Omicron overtook Delta in three charts. 10.1038/d41586-022-00632-3, https://www.nature.com/articles/d41586-022-00632-3 Date Accessed:04/03/2022. [DOI] [PubMed]
- 70.Allen H., Tessier E., Turner C., Anderson C., Blomquist P., Simons D., et al. 2022. Comparative Transmission of SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants and the Impact of Vaccination: National Cohort Study, England. medRxiv. 2022.02.15.22271001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/s0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Regev-Yochay G., Gonen T., Gilboa M., Mandelboim M., Indenbaum V., Amit S., et al. 2022. 4th dose COVID mRNA vaccines' immunogenicity & efficacy against omicron VOC. medRxiv. 2022.02.15.22270948. [DOI] [Google Scholar]
- 73.Mahase E. Covid-19: vaccinated people are less likely to get long covid, review finds. BMJ. 2022;376:o407. doi: 10.1136/bmj.o407. [DOI] [PubMed] [Google Scholar]
- 74.Zambrano L.D., Newhams M.M., Olson S.M., Halasa N.B., Price A.M., Boom J.A., et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against Multisystem inflammatory syndrome in children among persons aged 12-18 Years - United States, July-december 2021. MMWR (Morb Mortal Wkly Rep) 2022;71:52–58. doi: 10.15585/mmwr.mm7102e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mao T., Israelow B., Suberi A., Zhou L., Reschke M., Peña-Hernández M.A., et al. 2022. Unadjuvanted Intranasal Spike Vaccine Booster Elicits Robust Protective Mucosal Immunity against Sarbecoviruses. bioRxiv : the preprint server for biology. 2022.01.24.477597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jalali N., Brustad H.K., Frigessi A., MacDonald E., Meijerink H., Feruglio S., et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron variant compared to the Delta variant: evidence from Norwegian contact tracing and vaccination data. medRxiv. 2022 doi: 10.1101/2022.02.07.22270437. 2022.02.07.22270437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and Delta variants. Nat Med. 2022 doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monge S, Rojas-Benedicto A, Olmedo C, Mazagatos C, Sierra MJ, Limia A, et al. The effectiveness of mRNA vaccine boosters for laboratory-confirmed COVID-19 during a period of predominance of the omicron variant of SARS-CoV-2. Available at SSRN 4035396.
- 79.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., AlMukdad S., Tang P., Hasan M.R., et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 boosters against SARS-CoV-2 Omicron (B.1.1.529) infection in Qatar. medRxiv. 2022 doi: 10.1101/2022.01.18.22269452. 2022.01.18.22269452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and Delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson M.G., Natarajan K., Irving S.A., Rowley E.A., Griggs E.P., Gaglani M., et al. 2022. Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 States. August 2021–January 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.UK Health Security Agency COVID-19 vaccine surveillance report week 4 UK health security agency. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf
- 83.Karampela I., Dalamaga M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch Med Res. 2020;51:741–742. doi: 10.1016/j.arcmed.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsilingiris D., Dalamaga M., Liu J. SARS-CoV-2 adipose tissue infection and hyperglycemia: a further step towards the understanding of severe COVID-19. Metabol Open. 2022;13:100163. doi: 10.1016/j.metop.2022.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Syriga M., Karampela I., Dalamaga M., Karampelas M. The effect of COVID-19 pandemic on the attendance and clinical outcomes of patients with ophthalmic disease: a mini-review. Metabol Open. 2021;12:100131. doi: 10.1016/j.metop.2021.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Altmann D.M., Boyton R.J. COVID-19 vaccination: the road ahead. Science. 2022;375(6585):1127–1132. doi: 10.1126/science.abn1755. [DOI] [PubMed] [Google Scholar]
- 87.Edwards A.M., Baric R.S., Saphire E.O., Ulmer J.B. Stopping pandemics before they start: lessons learned from SARS-CoV-2. Science. 2022;375(6585):1133–1139. doi: 10.1126/science.abn1900. [DOI] [PubMed] [Google Scholar]
- 88.Koelle K., Martin M.A., Antia R., Lopman B., Dean N.E. The changing epidemiology of SARS-CoV-2. Science. 2022;375(6585):1116–1121. doi: 10.1126/science.abm4915. [DOI] [PMC free article] [PubMed] [Google Scholar]