Abstract
Purpose
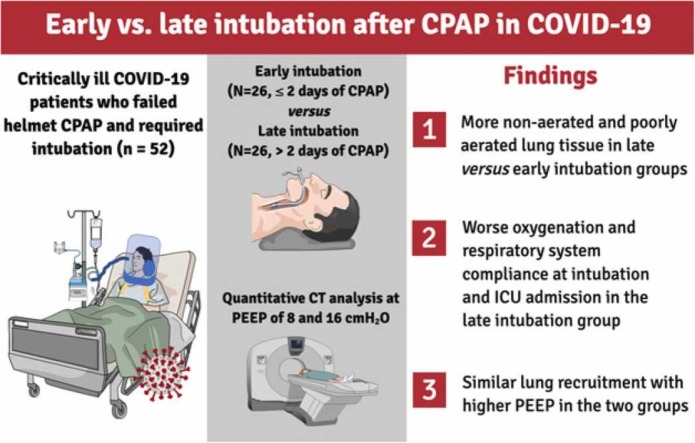
To describe the effects of timing of intubation in COVID-19 patients that fail helmet continuous positive airway pressure (h-CPAP) on progression and severity of disease.
Methods
COVID-19 patients that failed h-CPAP, required intubation, and underwent chest computed tomography (CT) at two levels of positive end-expiratory pressure (PEEP, 8 and 16 cmH2O) were included in this retrospective study. Patients were divided in two groups (early versus late) based on the duration of h-CPAP before intubation. Endpoints included percentage of non-aerated lung tissue at PEEP of 8 cmH2O, respiratory system compliance and oxygenation.
Results
Fifty-two patients were included and classified in early (h-CPAP for ≤2 days, N = 26) and late groups (h-CPAP for >2 days, N = 26). Patients in the late compared to early intubation group presented: 1) lower respiratory system compliance (median difference, MD −7 mL/cmH2O, p = 0.044) and PaO2/FiO2 (MD −29 mmHg, p = 0.047), 2) higher percentage of non-aerated lung tissue (MD 7.2%, p = 0.023) and 3) similar lung recruitment increasing PEEP from 8 to 16 cmH2O (MD 0.1%, p = 0.964).
Conclusions
In COVID-19 patients receiving h-CPAP, late intubation was associated with worse clinical presentation at ICU admission and more advanced disease. The possible detrimental effects of delaying intubation should be carefully considered in these patients.
Keywords: COVID-19, CPAP, Mechanical ventilation, Computed tomography, Intubation
Graphical Abstract
1. Introduction
The coronavirus disease 2019 (COVID-19) has posed unprecedented challenges to intensive care unit (ICU) physicians (Huang et al., 2020; Zhu et al., 2020). Clinical manifestations range from asymptomatic to acute hypoxemic respiratory failure requiring invasive mechanical ventilation and admission to the ICU (Huang et al., 2020; Ren et al., 2020). Early intubation has been recommended in patients with signs of respiratory distress to prevent progression from moderate to severe lung injury (Marini and Gattinoni, 2020) due to increased respiratory drive and risk of patient self-inflicted lung injury (P-SILI) (Battaglini et al., 2021). However, there are controversies regarding this approach (Marini and Gattinoni, 2020; Tobin et al., 2020) which might result in higher incidence of ventilator-associated pneumonia and ventilator-induced lung injury (Tobin et al., 2020), as reflected by an increased use of non-invasive respiratory support during the evolution of the pandemic (Doidge et al., 2021). Among the different types of non-invasive respiratory support, continuous positive airway pressure (CPAP) delivered through an helmet (h-CPAP) has been widely applied especially in the European countries, since it is easy to use and for its potential of reducing environmental dispersion of droplets (Amirfarzan et al., 2021). Because of the shortage of critical care resources and number of ICU beds, most centers extensively employed non-invasive respiratory support strategies even in patients with radiological and functional (respiratory mechanics and gas exchange parameter) parameters that indicate the need for invasive mechanical ventilation (Franco et al., 2020).
The effects of delaying intubation in COVID-19 patients on clinical outcome are matter of debate. In a recent meta-analysis, intubation within 24 h from ICU admission was not superior to intubation at any time after 24 h of ICU admission (Papoutsi et al., 2021). Nevertheless, only observational trials were included, and the time spent under non-invasive respiratory support prior to ICU admission was not reported. Therefore, in COVID-19 patients that fail h-CPAP, the effects of timing of intubation on physiological parameters and severity of disease at ICU admission are still unknown. In our ICU, a large proportion of intubated patients was systematically assessed with chest computed tomography (CT) performed at two fixed levels of positive end-expiratory pressure (PEEP) to assess extension of disease and alveolar recruitment (Ball et al., 2021b).
The present study was performed to describe the physiologic effects of early versus late intubation in COVID-19 patients previously receiving h-CPAP. We hypothesized that, in COVID-19 patients treated with h-CPAP, late compared to early intubation may be associated with higher amounts of non-aerated tissue, greater alveolar recruitment, as well as worse gas-exchange and lower respiratory system compliance.
2. Methods
This retrospective cohort study was conducted in a tertiary care hospital in Genoa, Northern Italy from March to December 2020, covering two pandemic surges. The protocol of the study was approved by the ethical review board (Comitato Etico Regione Liguria, protocol n. 163/2020) and the need for written informed consent was waived for retrospectively collected data; consent was delayed after discharge for prospectively collected data in unconscious patients. The study is reported in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) (von Elm et al., 2007) and REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) (Benchimol et al., 2015) recommendations.
2.1. Clinical context and indications for CT scan
At our institution, starting from March 20th 2020, a standardized protocol was introduced to acquire chest CT scans obtained at two fixed PEEP levels of 8 and 16 cmH2O (Ball et al., 2021b). During the pandemic surges, ICU admission was limited to intubated patients, while in low and intermediate-care settings patients were managed using conventional oxygen therapy or h-CPAP. The decision to intubate was performed by a dedicated team of intensivists and main criteria were inability to maintain oxygenation with h-CPAP, respiratory rate above 28 min−1, decline of consciousness and signs of respiratory distress (Robba et al., 2020). A trial of h-CPAP was used in patients receiving conventional oxygen therapy in case of clinical deterioration, before considering intubation. Unavailability of ICU beds was the leading reason for delaying intubation in the context of pandemic surges. Once intubated and admitted to the ICU, all patients with an indication for CT underwent two-PEEP CT scan to assess disease severity and response to PEEP.
2.2. Patient inclusion, ventilatory management and collection of clinical data
This study included all consecutive patients that received at least 2 h of h-CPAP prior to intubation and ICU admission and that underwent a two-PEEP CT scan during their ICU stay. In patients with more than one two-PEEP CT available, the scan closest to intubation was chosen. Reasons for not performing two-PEEP scan and therefore exclusion criteria were: clinical instability hampering transport to the CT facility, absence of a clinical indication for chest CT, need for contrast-enhanced CT, contraindications to high PEEP (e.g., undrained pneumothorax). Patients were ventilated using a tidal volume of 6 mL per kg of predicted body weight with tolerance of higher values if the driving pressure was below 15 cmH2O. Part of the patients were included in another study (Ball et al., 2021b). However, in the present study the effects of timing of intubation in COVID-19 patients that fail h-CPAP treatment on the progression and severity of disease were investigated. The respiratory rate was set targeting pH above 7.25 tolerating moderate hypercapnia, the clinical PEEP level was set by the treating physician to maintain the PaO2 above 60 mmHg and limiting the plateau pressure below 27 cmH2O, if feasible. Clinical data were gathered from the electronic clinical record on the day of intubation and ICU admission and on the day of the two-PEEP CT scan, survival was assessed at ICU discharge. The ventilatory ratio (Sinha et al., 2009), an estimator of ventilation impairment correlated with dead-space in COVID-19 (Diehl et al., 2020), was computed as:
2.3. Protocol for the two-PEEP CT scan acquisition and analysis
All chest two-PEEP CT scans were performed using a Somatom Definition Flash scanner (Siemens, Erlangen, Germany), operated at 140 kVp with dose modulation. The first scan was acquired at PEEP 8 cmH2O during expiratory breath-hold, then PEEP was increased to 16 cmH2O and the scan was repeated after one minute. This resulted in a ventilation reaching plateau pressures from 25 to 35 cmH2O between the two scans. The range of pressures reached and the time spent between the two scan was sufficient to recruit most respiratory units susceptible to the PEEP effect (Crotti et al., 2001; Katz et al., 1981). Images were reconstructed with a slice thickness of 0.75 mm or 1.25 mm and a sharp convolution kernel (B80f). Lung parenchyma segmentation was performed using an automated multi-resolution convolutional neural network with automated airway exclusion (Gerard et al., 2020) followed by manual refinement using ITKSnap (http://www.itksnap.org). Images were analyzed with Matlab (Mathworks, Massachussetts, US) using custom-made scripts based on established quantitative analysis methods, assuming density proportional to the gas and tissue fraction contained within each voxel and approximating tissue density to 1 g per mL (Protti et al., 2014). We defined hyper-aerated, normal, poorly aerated, and non-aerated lung regions based on Hounsfield Units (HU) thresholds (below −900 HU, −900 HU to −500 HU, −500 HU to −100 HU and above −100 HU, respectively) (Ball et al., 2016). Three regions of interest (ROI) of equal lung weight (Güldner et al., 2016; Scaramuzzo et al., 2020) were defined along the ventral-dorsal and cranio-caudal axes. Lung recruitment was defined as the proportion of total lung weight accounted for non-aerated tissue at PEEP 8 cmH2O that was re-aerated at PEEP of 16 cmH2O, as previously described (Gattinoni et al., 2006):
The excess lung weight was defined as the percent difference between the lung weight measured with the CT at PEEP 8 cmH2O and the expected lung weight of a supine healthy patient of the same height:
where (Cressoni et al., 2013).
2.4. Definition of groups and sensitivity analysis
Patients were divided in two groups (early vs. late intubation) based on the number of days spent under h-CPAP support before intubation. The median time spent under h-CPAP before intubation in our population was 2 days, therefore this value was used as cut-off to divide into early and late intubation groups. To further investigate the effects of prolonged h-CPAP, a sensitivity analysis was performed dividing patients in very late versus early-intermediate intubation, with an arbitrary cut-off of 7 days of pre-ICU h-CPAP.
2.5. Statistical analysis
The primary endpoint of the study was the percent amount of non-aerated lung tissue. Among patients included in a previous study (Ball et al., 2021b), those that received less than 2 days of h-CPAP before intubation had 36% ± 8% non-aerated lung tissue mass. Accounting for the use of non-parametric statistics, we needed to analyze at least 50 patients divided in two equally sized groups to achieve 80% power (1-β) to detect a 20% relative increase (36–43%) of the non-aerated lung tissue in the late intubation group at an α level of 0.05. Data are reported as median [interquartile range], unless otherwise specified. We compared data between groups with the Mann–Whitney U, χ 2 or Fisher’s exact test, as appropriate. Parameters derived from the CT scan acquired at two PEEP levels were compared with the Wilcoxon signed-rank test. Correlations were sought using the Spearman’s rho. Median differences between groups with their 95% confidence intervals (CI) were computed with the Hodges–Lehmann estimator. All statistical analyses were performed in R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Significance was assumed at two-tailed p < 0.05.
3. Results
3.1. Population description and clinical characteristics at ICU admission
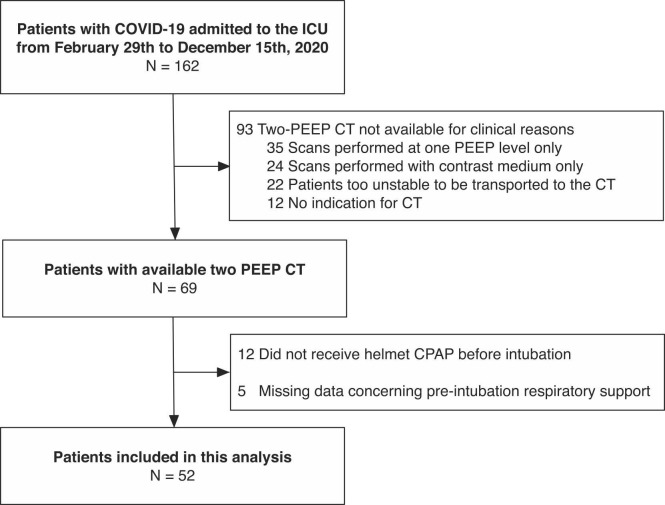
As illustrated in Fig. 1 , of 162 patients admitted to the ICU in the study period, 52 received at least 2 h of h-CPAP before intubation and underwent chest CT scan at two PEEP levels and were therefore analyzed. Of these, 26 patients were included in a previous unrelated study on alveolar recruitment in COVID-19 (Ball et al., 2021b). The median [interquartile range] duration of h-CPAP before intubation was 2 [1−7] days: 26 patients were classified in the early group (duration of h-CPAP ≤ 2 days) and 26 in the late group (h-CPAP for more than 2 days before intubation). The ICU mortality was 12/26 (46%) in the early group and 16/26 (62%) in the late group (p = 0.404). Characteristics of patients in the two groups at ICU admission are described in Table 1. Patients in the late group, compared to the early intubation group, had a longer time elapsed from symptoms onset and hospital admission to ICU admission, but similar comorbidities and sequential organ failure assessment (SOFA) score. At ICU admission, in the late compared to the early group, the respiratory system compliance was lower (median difference −7 mL/cmH2O, 95% CI from −14 to −1 mL/cmH2O, p = 0.044), the respiratory rate was higher (median difference 3 min−1, 95% CI from 1 to 6 min−1, p = 0.016) and the PaO2/FiO2 was lower (median difference −29 mmHg, 95% CI from −73 to −1 mmHg, p = 0.047). Plateau pressure was similar in the two groups, but patients in the late intubation group were ventilated at lower PEEP level, reflecting the need of limiting PEEP to maintain plateau pressure below a safety threshold value (Table 1).
Fig. 1.
Patient inclusion flow.
Table 1.
Patients’ characteristics after intubation. IQR: interquartile range; PBW predicted body weight; PEEP: Positive End-Expiratory Pressure. Gas exchange and ventilator settings measured at the clinical PEEP level. *Significant p < 0.05.
| Parameter | All (N = 52) | Early intubation (N = 26) | Late intubation (N = 26) | p |
|---|---|---|---|---|
| Duration of helmet CPAP before intubation, median [IQR], days | 2 [1–7] | 1 [1–2] | 7 [4–13] | < 0.001* |
| Age, median [IQR], years | 64 [59–67] | 62 [58–67] | 64 [60–67] | 0.783 |
| Body mass index, median [IQR], kg m−2 | 28 [25–31] | 27 [25–29] | 29 [25–31] | 0.197 |
| Male sex, N (%) | 40 (80) | 19 (73) | 21 (80) | 0.743 |
| Time from symptom onset, median [IQR], days | 12 [8–18] | 9 [7–12] | 17 [12–22] | < 0.001* |
| Time from hospital admission, median [IQR], days | 5 [2–10] | 2 [2–4] | 9 [6–15] | < 0.001* |
| SOFA score, median [IQR] | 4 [4–6] | 4 [4–6] | 4 [4–5] | 0.643 |
| Pandemic surge | ||||
| First (before June 2020), N (%) | 38 (73) | 21 (81) | 17 (65) | 0.349 |
| Second (after June 2020), N (%) | 14 (27) | 5 (19) | 9 (35) | |
| Comorbidities | ||||
| Hypertension, N (%) | 26 (50) | 11 (42) | 15 (58) | 0.406 |
| Cardiovascular disease, N (%) | 8 (15) | 3 (12) | 5 (19) | 0.703 |
| Smoker, N (%) | 4 (7.7) | 1 (3.8) | 3 (12) | 0.610 |
| Former smoker, N (%) | 5 (9.6) | 3 (12) | 2 (7.7) | 0.999 |
| Diabetes, N (%) | 6 (12) | 1 (3.8) | 5 (19) | 0.191 |
| Ventilator settings in invasive ventilation | ||||
| Tidal volume, median [IQR], mL kg−1 PBW | 7.7 [6.4–8.5] | 7.8 [6.4–8.5] | 7.4 [5.7–8.5] | 0.410 |
| Respiratory rate, median [IQR], min−1 | 20 [16–22] | 18 [15–20] | 22 [18–25] | 0.016* |
| PEEP, median [IQR], cmH2O | 12 [10–14] | 14 [12–15] | 10 [10–13] | 0.005* |
| Plateau pressure, median [IQR], cmH2O | 26 [24–29] | 28 [24–29] | 26 [24–28] | 0.639 |
| FiO2, median [IQR] | 0.80 [0.64–0.90] | 0.75 [0.52–0.90] | 0.80 [0.66–0.90] | 0.605 |
| Respiratory system compliance, median [IQR], mL cmH2O−1 | 37 [29–48] | 39 [33–51] | 33 [25–44] | 0.044* |
| Blood gas analysis | ||||
| pH, median [IQR] | 7.37 [7.33–7.43] | 7.35 [7.32–7.42] | 7.40 [7.33–7.43] | 0.295 |
| PaO2, median [IQR], mmHg | 86 [70–118] | 100 [72–136] | 83 [70–115] | 0.111 |
| PaCO2, median [IQR], mmHg | 47 [42–56] | 48 [44–52] | 45 [42–57] | 0.855 |
| PaO2/FiO2, median [IQR], mmHg | 129 [84–167] | 146 [105–189] | 117 [81–154] | 0.047* |
| Lactate, median [IQR], mmol L−1 | 1.0 [0.8–1.6] | 0.9 [0.8–1.1] | 1.4 [1.0–1.7] | 0.001* |
| Ventilatory ratio, median [IQR] | 1.8 [1.5–2.2] | 1.7 [1.4–2.1] | 1.9 [1.5–2.2] | 0.276 |
| Blood analyses | ||||
| D-dimer, median [IQR], ug L−1 | 1313 [812–4135] | 1263 [757–2514] | 1804 [989–5515] | 0.146 |
| C reactive protein, median [IQR], mg L−1 | 77 [24–128] | 98 [43–134] | 57 [19–120] | 0.170 |
| Procalcitonin, median [IQR], ug L−1 | 0.20 [0.06–0.46] | 0.27 [0.07–0.46] | 0.09 [0.04–0.32] | 0.099 |
| Creatinine, median [IQR], mg dL−1 | 0.8 [0.6–1.0] | 0.9 [0.7–1.0] | 0.7 [0.5–1.0] | 0.184 |
| Haemodynamics | ||||
| Heart rate, median [IQR], min−1 | 77 [67–89] | 77 [69–95] | 77 [66–88] | 0.451 |
| Mean arterial pressure, median [IQR], mmHg | 83 [78–94] | 84 [80–92] | 81 [77–98] | 0.799 |
3.2. Quantitative CT parameters and clinical characteristics on the day of CT scan
Clinical and quantitative chest CT parameters in the two groups are reported in Table 2 and Table 3, respectively. The median time from start of invasive mechanical ventilation to chest CT was 8 [4 − 13] days and did not differ between groups (Table 2). On the day of the CT scan, patients in the late compared to the early intubation group were ventilated with similar PEEP levels, but required higher FiO2 (median difference 0.15, 95% CI from 0.05 to 0.20, p = 0.004) and higher respiratory rate (median difference 3 min−1, 95% CI from 1 to 6 min−1, p = 0.038). Moreover, they had lower respiratory system compliance (median difference −8 mL/cmH2O, 95% CI from −15 to −2 mL/cmH2O, p = 0.010) and lower PaO2/FiO2 ratio (median difference −30 mmHg, 95% CI from −54 to −6 mmHg, p = 0.014) and a trend for higher PaCO2 (median difference 6 mmHg, 95% CI from 0 to 13 mmHg, p = 0.053).
Table 2.
Patients’ characteristics the day of CT scan. IQR: interquartile range; PBW predicted body weight; PEEP: Positive End-Expiratory Pressure; ICU: intensive care unit. Gas exchange and ventilator settings measured at the clinical PEEP level. *Significant p < 0.05.
| Parameter | All (N = 52) | Early intubation (N = 26) | Late intubation (N = 26) | p |
|---|---|---|---|---|
| Time from start of invasive ventilation to CT, median [IQR], days | 8 [4–13] | 8 [3–12] | 8 [4–14] | 0.707 |
| Superimposed ventilator-associated pneumonia, N (%) | 17 (33) | 9 (35) | 8 (31) | 0.999 |
| Ventilator settings | ||||
| Tidal volume, median [IQR], mL kg−1 PBW | 7.3 [6.3–7.8] | 7.5 [7.1–8.1] | 7.0 [6.1–7.6] | 0.056 |
| Respiratory rate, median [IQR], min−1 | 20 [17–25] | 20 [16–22] | 24 [18–28] | 0.038* |
| PEEP, median [IQR], cmH2O | 10 [9–12] | 10 [10–12] | 10 [8–12] | 0.703 |
| Plateau pressure, median [IQR], cmH2O | 25 [22–27] | 24 [21–27] | 26 [24–29] | 0.102 |
| FiO2, median [IQR] | 0.70 [0.60–0.75] | 0.60 [0.50–0.70] | 0.70 [0.66–0.79] | 0.004* |
| Respiratory system compliance, median [IQR], mL cmH2O−1 | 35 [29–43] | 38 [34–45] | 29 [23–40] | 0.010* |
| Blood gas analysis | ||||
| pH, median [IQR] | 7.43 [7.37–7.48] | 7.44 [7.40–7.48] | 7.42 [7.35–7.46] | 0.153 |
| PaO2, median [IQR], mmHg | 72 [63–91] | 73 [64–86] | 68 [64–95] | 0.475 |
| PaCO2, median [IQR], mmHg | 50 [43–56] | 48 [42–52] | 52 [45–63] | 0.053 |
| PaO2/FiO2, median [IQR], mmHg | 111 [87–155] | 122 [105–184] | 100 [80–136] | 0.015* |
| Lactate, median [IQR], mmol L−1 | 1.1 [0.8–1.8] | 1.1 [0.8–1.6] | 1.4 [1.0–1.8] | 0.236 |
| Ventilatory ratio, median [IQR] | 1.8 [1.6–2.5] | 1.7 [1.5–2.2] | 2.1 [1.7–2.7] | 0.087 |
| Blood analyses | ||||
| D-dimer, median [IQR], ug L−1 | 2077 [1170–5095] | 1760 [1048–3853] | 2538 [1428–5106] | 0.173 |
| C reactive protein, median [IQR], mg L−1 | 49 [21–113] | 42 [17–85] | 52 [34–136] | 0.260 |
| Procalcitonin, median [IQR], ug L−1 | 0.30 [0.10–0.96] | 0.19 [0.08–0.60] | 0.33 [0.16–0.97] | 0.400 |
| Creatinine, median [IQR], mg dL−1 | 0.9 [0.7–1.1] | 1.0 [0.8–1.4] | 0.8 [0.7–0.9] | 0.062 |
| Haemodynamics | ||||
| Heart rate, median [IQR], min−1 | 84 [72–100] | 78 [68–90] | 88 [80–105] | 0.050 |
| Mean arterial pressure, median [IQR], mmHg | 83 [77–95] | 82 [73–95] | 84 [78–96] | 0.492 |
Table 3.
Quantitative CT analysis parameters. Data are presented as median [interquartile range]. PEEP: positive end-expiratory pressure; HU: Hounsfield Units; IQR: interquartile range; CI: confidence interval. *Significant p < 0.05.
| Parameter | All (N = 52) | Early intubation (N = 26) | Late intubation (N = 26) | p |
|---|---|---|---|---|
| Scan at PEEP 8 cmH2O | ||||
| Total lung volume, median [IQR], (mL) | 2965 [2517–3810] | 3245 [2734–3811] | 2693 [2338–3419] | 0.219 |
| Total lung weight (g) | 1560 [1292–1973] | 1440 [1146–1880] | 1623 [1436–2019] | 0.168 |
| Excess lung weight (%) | 58 [28–82] | 50 [22–62] | 66 [46–90] | 0.048* |
| Gas volume, median [IQR], (mL) | 1277 [889–2032] | 1603 [1210–2126] | 1086 [744–1646] | 0.029* |
| As proportion of the total lung volume, median [IQR], (%) | 46.7 [33.7–56.1] | 54.3 [43.7–62.8] | 39.5 [32.6–50.5] | 0.006* |
| Mean attenuation, median [IQR], (HU) | -451 [− 561 to − 324] | -543 [− 628 to − 436] | -369 [− 498 to − 316] | 0.005* |
| Hyper-aerated mass, median [IQR], (g) | 12 [5–24] | 16 [9–27] | 8 [5–21] | 0.157 |
| Normally aerated mass, median [IQR], (g) | 350 [245–458] | 388 [322–465] | 320 [203–380] | 0.031* |
| Poorly aerated mass, median [IQR], (g) | 525 [394–688] | 398 [341–633] | 577 [486–694] | 0.014* |
| Non-aerated mass, median [IQR], (g) | 716 [426–841] | 527 [375–811] | 755 [586–876] | 0.049* |
| Changes from PEEP 8 cmH2O to PEEP 16 cmH2O | ||||
| Lung recruitment (changes in non-aerated), median [IQR], (%) | 3.1 [1.4–4.7] | 3.2 [1.4–4.6] | 2.9 [1.5–4.8] | 0.964 |
| Changes in poorly aerated, median [IQR], (%) | 1.4 [0.0–3.1] | 3.1 [0.7–3.6] | 1.1 [− 0.2–1.5] | 0.001* |
| Changes in gas volume, median [IQR], (mL) | 397 [280–484] | 448 [373–609] | 277 [230–397] | < 0.001* |
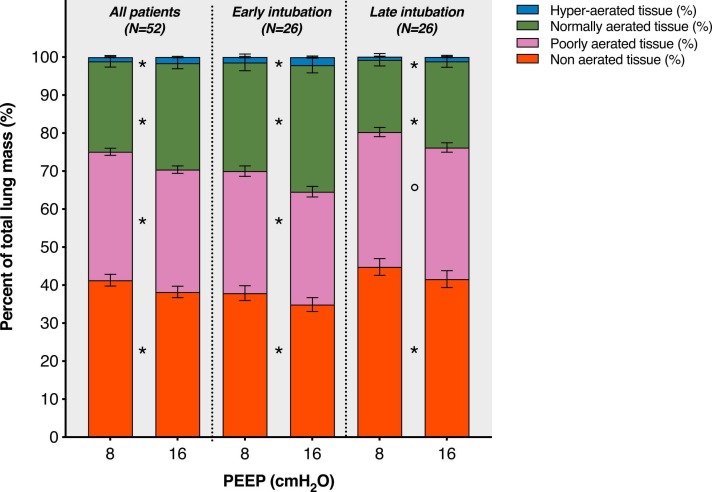
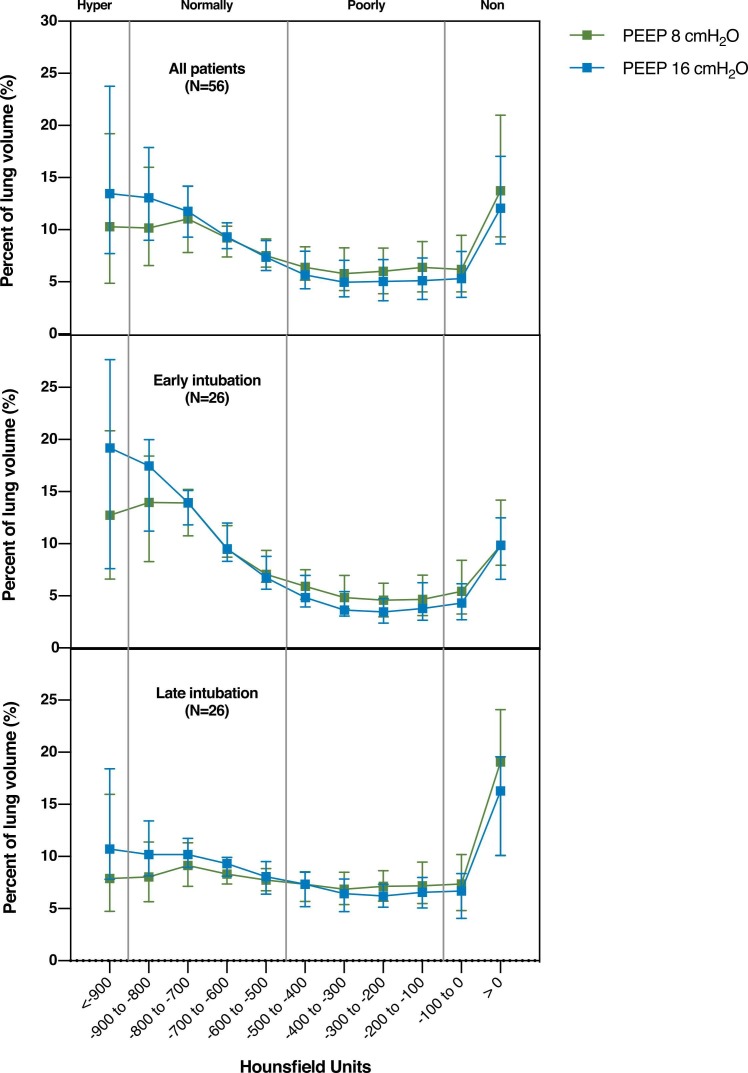
Patients in the late versus early intubation group had higher percentage of non-aerated lung tissue at PEEP of 8 cmH2O (median difference 7.2%, 95% CI from 1.3% to 12.9%, p = 0.023, Fig. 2), less normally aerated tissue (median difference −11.2%, 95% CI from −15.8% to 3.2%, p = 0.004, Fig. 2) and higher percentage of excess lung tissue mass (median difference 22%, 95% CI from 1% to 42%, p = 0.048, Table 3). In both groups, the amount of non-aerated and poorly aerated tissue was modestly reduced increasing PEEP from 8 to 16 cmH2O (Fig. 2 and Table 3). Lung recruitment was similar in the two groups (median difference 0.1%, 95% CI from −1.8% to 2.0%, p = 0.964, Table 3). The effects of PEEP increase according to lung density are depicted in Fig. 3. Loss of aeration was distributed along a ventral to dorsal and a cranial to caudal gradients (eFig. 1 and eFig. 2).
Fig. 2.
Distribution of aeration compartments, expressed as percent of the total lung tissue, at PEEP of 8 and 16 cmH2O. Data are reported overall and stratified in the early intubation and late intubation groups. Bars represent means, error bars the standard error of mean. Significant difference between the two PEEP levels: *p < 0.001, °p < 0.01. PEEP: positive end-expiratory pressure.
Fig. 3.
Distribution of aeration along the Hounsfield units scale at PEEP 8 and 16 cmH2O in the overall population (top panel) and in the early (middle panel) versus late (lower panel) groups. PEEP: positive end-expiratory pressure.
3.3. Sensitivity analysis
Clinical characteristics of patients receiving very late (≥ 7 days, N = 16) versus early-intermediate (< 7 days, N = 36) intubation after h-CPAP at ICU admission and on the day of CT scan are reported in eTable 1 and eTable 2, respectively. Patients in the very late, compared to the early-intermediate intubation group, had more compromised respiratory mechanics both at ICU admission and on the day of CT scan (eTable 1 and eTable 2). Quantitative CT parameters showed more non-aerated and less normally aerated lung tissue. Gas-exchange parameters were similar at ICU admission but were worse in the very late intubation group on the day of the CT scan, reflecting more severe respiratory function deterioration compared to the early-intermediate intubation group. The ICU mortality in the very late compared to the early-intermediate intubation group was 12/16 (75%) versus 16/36 (44%), p = 0.070.
4. Discussion
In patients with severe COVID-19 pneumonia receiving h-CPAP prior to intubation, late versus early intubation resulted in: 1) higher amount of non-aerated lung tissue; 2) comparable lung recruitment after PEEP increase from 8 cmH2O to 16 cmH2O; and 3) worse respiratory mechanics and gas exchange at ICU admission and during ICU stay.
We performed standardized acquisition of chest CT images at two fixed levels of PEEP, allowing a precise comparison between groups, independent of the ventilatory strategy adopted during the ICU stay. Moreover, the analysis of the effects of 16 versus 8 cmH2O of PEEP provided detailed information on the nature of lung lesions. Our cohort was characterized by a wide range of exposure time to non-invasive respiratory support prior to intubation, well representative of different clinical management strategies adopted during the pandemic. Since all the patients included ultimately failed h-CPAP and required intubation, the time spent under h-CPAP was considered as an objective marker of timeliness of intubation. The pre-intubation and ICU management of patients was standardized at our institution (Robba et al., 2021, Robba et al., 2020). We included only patients that received h-CPAP, which was the most commonly used non-invasive respiratory support in our center, reducing the possible confounding effect of different devices. Furthermore, the two groups were homogeneous according to comorbidities and non-respiratory disease severity at ICU admission and timing from intubation to chest CT scan.
Non-invasive respiratory support has been considered a bridge therapy to overcome gas exchange impairment. Patients with late intubation presented, at PEEP of 8 cmH2O, lower gas volume and normally aerated tissue, as well as higher poorly- and non-aerated tissue; this may be attributed to the duration of h-CPAP thus increasing the risk of P-SILI. In this line, during h-CPAP, high respiratory drive and transpulmonary pressure can promote progression of lung injury (Cruces et al., 2020) through increased trans-alveolar and trans-capillary pressure gradients, especially in juxta-diaphragmatic regions (Battaglini et al., 2021). Another possible mechanism explaining the worse lung injury observed in the CT analysis in the late intubation group is viral disease progression per se. Both disease progression and superimposed P-SILI could result in h-CPAP failure and need for intubation. Additionally, late intubation was associated with worse oxygenation and respiratory mechanics parameters. The reduction in respiratory system compliance may be associated with the relevant loss of lung gas volume and normally aerated tissue, while oxygenation impairment may be explained by the higher proportion of non-aerated and poorly aerated tissue in the late intubation group. Other studies suggested a relevant role of perfusion abnormalities in the non-aerated (Ball et al., 2021a) and poorly aerated (Busana et al., 2021) regions in determining the severity of gas exchange impairment.
The non-aerated tissue may be caused by several mechanisms: 1) increased vascular permeability resulting in higher alveolar and interstitial edema; 2) consolidation and/or 3) fibrosis. Increasing PEEP from 8 to 16 cmH2O resulted in minimal variations in the non-aerated tissue, showing a modest role of edema in determining such alterations. Differently from conventional acute respiratory distress syndrome (ARDS), characterized by higher edema and lung recruitability (Coppola et al., 2021; Gattinoni et al., 2006) at late phase of lung injury, COVID-19 patients in the late intubation group had a severe lung disease which was not associated with increased recruitability (increased response to PEEP). These findings suggest that the mechanisms leading to worsening of respiratory function ultimately resulting in h-CPAP failure might be related to more consolidation and fibrosis rather than atelectasis and interstitial edema (Barisione et al., 2021; Grillo et al., 2020; Tonelli et al., 2021). Another determinant of gas-exchange impairment in COVID-19 is the extension of ground glass opacities, corresponding to poorly aerated lung tissue (Busana et al., 2021). The pathophysiological meaning of these lesions is under debate. Studies on lung perfusion showed that these regions might act both as areas of high or low ventilation/perfusion ratio (Ball et al., 2021a), depending on the complex interaction of vasodilation, hypoxic or mechanical vasoconstriction and microthrombosis (Busana et al., 2021; Marini and Gattinoni, 2020). Increasing PEEP led to greater reduction in poorly aerated areas in the early intubation group. This might be explained by less advanced consolidative and fibrotic processes in the initial phase of the disease. However, the ability of PEEP of reducing poorly aerated areas might have negative effects. In fact, COVID-19 is characterized by a pro-coagulant condition especially in the pulmonary circulation (Xiong et al., 2020), thus mechanical compression of blood vessels induced by higher PEEP might increase pulmonary coagulopathy, while only transiently improving oxygenation through diversion of flow from poorly aerated areas. Both non-aerated and poorly aerated regions were distributed across a gradient in the ventral-dorsal and apical-caudal directions. However, in the late compared to early intubation group, non- and poorly aerated regions were larger but distributed in a similar way. Self-inflicted lung injury should affect predominantly the caudal and dorsal regions. However, our findings did not confirm this hypothesis. The sensitivity analysis that was performed using 7 days instead of 2 days as cut-off to define late intubation, confirmed these results and identified a subgroup of patients with particularly severe chest CT findings and respiratory mechanics parameters.
This study has limitations that should be acknowledged. First, the observational nature of the study does not allow to infer a causal link between timing of intubation and physiologic and CT parameters. Therefore, these findings should be interpreted cautiously regarding clinical recommendations. Second, only patients that failed h-CPAP and were intubated were included, not exploring the characteristics of patients that were successfully managed with h-CPAP only. Third, severe patients too unable to be transported to CT scan were excluded from the study. Fourth, the time elapsed between chest CT scans was short, therefore lung recruitment could have been numerically underestimated.
5. Conclusions
In COVID-19 patients with severe pneumonia that fail h-CPAP and require invasive mechanical ventilation, late intubation was associated with worse CT findings and clinical presentation at ICU admission. In the management of COVID-19 patients receiving prolonged h-CPAP, the possible detrimental effects of delaying intubation should be carefully considered.
Funding
This research was partly funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (401700/2020-8), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/210.181/ 2020). The funders had no role in the study design nor manuscript preparation.
Authors contributions
LB had full access to all data in this study and takes responsibility for the integrity of the data and the accuracy of data analysis, designed the study, analyzed images, performed statistical analysis, interpreted the results and drafted the manuscript PP, NP, PRMR, MC designed the study, analyzed data, interpreted the results and drafted the manuscript. CR, JH, SG, YX, MP, AB, FI, DB, IB, GM, SS, AV, DRG, MB, LC collected and analyzed images, collected clinical data, revised critically the manuscript for important intellectual content All authors revised and approved the final version of the manuscript.
Competing interests
Dr. Bassetti reports personal fees and other from Angelini, personal fees and other from AstraZeneca, other from Bayer, personal fees and other from Cubist, personal fees and other from Pfizer, personal fees and other from Menarini, personal fees and other from MSD, other from Nabriva, other from Paratek, other from Roche, other from Shionogi, other from Tetraphase, other from The Medicine Company, personal fees and other from Astellas Pharma Inc., personal fees from Gilead Sciences, personal fees from Teva, personal fees from Novartis, grants from Ranbaxy, personal fees from Correvio, personal fees from Molteni, personal fees from Thermo Fisher, outside the submitted work. Dr. Herrmann is cofounder and shareholder in OscillaVent, Inc, and consultant for ZOLL Medical Corporation, both outside the submitted work. Dr. Giacobbe reports personal fees from Stepstone Pharma GmbH, personal fees from MSD Italia, personal fees from Correvio Italia, outside the submitted work. Dr. Rocco reports personal fees from SANOFI as a DSMB member. All other authors declared no conflict of interest.
Acknowledgments
We are grateful for the efforts of the collaborators of the GECOVID (GEnoa COVID-19) group: Angelo Gratarola, Paolo Frisoni, Francesco Nicosia, Giordano Casalini, Maurizio Loconte, Alexandre Molin, Federico Costantino, Marco Micali, Dario Battioni, Giulio Bovio, Gerolama Buconte, Alessandro Casaleggio, Giuseppe Cittadini, Luca Dogliotti, Federica Briano, Veronica Giasotto, Elena Santacroce, Chiara Dentone, Lucia Taramasso, Laura Magnasco, Federica Briano.
Edited by Mathias Dutschmann
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.resp.2022.103889.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- Amirfarzan H., Cereda M., Gaulton T.G., Leissner K.B., Cortegiani A., Schumann R., Gregoretti C. Use of Helmet CPAP in COVID-19 - a practical review. Pulmonology. 2021;0437(21) doi: 10.1016/j.pulmoe.2021.01.008. Jul. 9: S2531, 00135-00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L., Brusasco C., Corradi F., Paparo F., Garlaschi A., Herrmann P., Quintel M., Pelosi P. Lung hyperaeration assessment by computed tomography: correction of reconstruction-induced bias. BMC Anesth. 2016;16:67. doi: 10.1186/s12871-016-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L., Robba C., Herrmann J., Gerard S.E., Xin Y., Mandelli M., Battaglini D., Brunetti I., Minetti G., Seitun S., Bovio G., Vena A., Giacobbe D.R., Bassetti M., Rocco P.R.M., Cereda M., Rizi R.R., Castellan L., Patroniti N., Pelosi P. Lung distribution of gas and blood volume in critically ill COVID-19 patients: a quantitative dual-energy computed tomography study. Crit. Care. 2021;21(25):214. doi: 10.1186/s13054-021-03610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L., Robba C., Maiello L., Herrmann J., Gerard S.E., Xin Y., Battaglini D., Brunetti I., Minetti G., Seitun S., Vena A., Giacobbe D.R., Bassetti M., Rocco P.R.M., Cereda M., Castellan L., Patroniti N., Pelosi P., GECOVID (GEnoa COVID-19) group Computed tomography assessment of PEEP-induced alveolar recruitment in patients with severe COVID-19 pneumonia. Crit. Care Lond. Engl. 2021;25:81. doi: 10.1186/s13054-021-03477-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisione E., Grillo F., Ball L., Bianchi R., Grosso M., Morbini P., Pelosi P., Patroniti N.A., De Lucia A., Orengo G., Gratarola A., Verda M., Cittadini G., Mastracci L., Fiocca R. Fibrotic progression and radiologic correlation in matched lung samples from COVID-19 post-mortems. Virchows Arch. Int. J. Pathol. 2021;478:471–485. doi: 10.1007/s00428-020-02934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglini D., Robba C., Ball L., Leme Silva P., Ferreira Cruz F., Pelosi P., Rieken Macedo Rocco P. Mechanisms of patient self-inflicted lung injury (P-SILI) in COVID-19: a narrative review. Br. J. Anaesth. 2021;127(3):353–364. doi: 10.1016/j.bja.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchimol E.I., Smeeth L., Guttmann A., Harron K., Moher D., Petersen I., Sørensen H.T., von Elm E., Langan S.M., RECORD Working Committee The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busana M., Giosa L., Cressoni M., Gasperetti A., Di Girolamo L., Martinelli A., Sonzogni A., Lorini L., Palumbo M.M., Romitti F., Gattarello S., Steinberg I., Herrmann P., Meissner K., Quintel M., Gattinoni L. The impact of ventilation - perfusion inequality in COVID-19: a computational model. J. Appl. Physiol. 2021;00871:2020. doi: 10.1152/japplphysiol.00871.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola S., Pozzi T., Gurgitano M., Liguori A., Duka E., Bichi F., Ciabattoni A., Chiumello D. Radiological pattern in ARDS patients: partitioned respiratory mechanics, gas exchange and lung recruitability. Ann. Intensive Care. 2021;11:78. doi: 10.1186/s13613-021-00870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressoni M., Gallazzi E., Chiurazzi C., Marino A., Brioni M., Menga F., Cigada I., Amini M., Lemos A., Lazzerini M., Carlesso E., Cadringher P., Chiumello D., Gattinoni L. Limits of normality of quantitative thoracic CT analysis. Crit. Care Lond. Engl. 2013;17:R93. doi: 10.1186/cc12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti S., Mascheroni D., Caironi P., Pelosi P., Ronzoni G., Mondino M., Marini J.J., Gattinoni L. Recruitment and derecruitment during acute respiratory failure: a clinical study. Am. J. Respir. Crit. Care Med. 2001;164:131–140. doi: 10.1164/ajrccm.164.1.2007011. [DOI] [PubMed] [Google Scholar]
- Cruces P., Retamal J., Hurtado D.E., Erranz B., Iturrieta P., González C., Díaz F. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit. Care. 2020;24:494. doi: 10.1186/s13054-020-03197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J.-L., Peron N., Chocron R., Debuc B., Guerot E., Hauw-Berlemont C., Hermann B., Augy J.L., Younan R., Novara A., Langlais J., Khider L., Gendron N., Goudot G., Fagon J.-F., Mirault T., Smadja D.M. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: a hypothesis-generating study. Ann. Intensive Care. 2020;10:95. doi: 10.1186/s13613-020-00716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doidge J.C., Gould D.W., Ferrando-Vivas P., Mouncey P.R., Thomas K., Shankar-Hari M., Harrison D.A., Rowan K.M. Trends in intensive care for patients with COVID-19 in England, Wales, and Northern Ireland. Am. J. Respir. Crit. Care Med. 2021;203:565–574. doi: 10.1164/rccm.202008-3212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco C., Facciolongo N., Tonelli R., Dongilli R., Vianello A., Pisani L., Scala R., Malerba M., Carlucci A., Negri E.A., Spoladore G., Arcaro G., Tillio P.A., Lastoria C., Schifino G., Tabbì L., Guidelli L., Guaraldi G., Ranieri V.M., Clini E., Nava S. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur. Respir. J. 2020;56:2002130. doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Caironi P., Cressoni M., Chiumello D., Ranieri V.M., Quintel M., Russo S., Patroniti N., Cornejo R., Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- Gerard S.E., Herrmann J., Kaczka D.W., Musch G., Fernandez-Bustamante A., Reinhardt J.M. Multi-resolution convolutional neural networks for fully automated segmentation of acutely injured lungs in multiple species. Med. Image Anal. 2020;60 doi: 10.1016/j.media.2019.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo F., Barisione E., Ball L., Mastracci L., Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect. Dis. 2020;21(4) doi: 10.1016/S1473-3099(20)30582-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldner A., Braune A., Ball L., Silva P.L., Samary C., Insorsi A., Huhle R., Rentzsch I., Becker C., Oehme L., Andreeff M., Vidal Melo M.F., Winkler T., Pelosi P., Rocco P.R.M., Kotzerke J., Gama de Abreu M. Comparative effects of volutrauma and atelectrauma on lung inflammation in experimental acute respiratory distress syndrome. Crit. Care Med. 2016;44:e854–e865. doi: 10.1097/CCM.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond. Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J.A., Ozanne G.M., Zinn S.E., Fairley H.B. Time course and mechanisms of lung-volume increase with PEEP in acute pulmonary failure. Anesthesiology. 1981;54:9–16. doi: 10.1097/00000542-198101000-00003. [DOI] [PubMed] [Google Scholar]
- Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. J. Am. Med. Assoc. 2020;323:2329. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- Papoutsi E., Giannakoulis V.G., Xourgia E., Routsi C., Kotanidou A., Siempos I.I. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit. Care. 2021;25:121. doi: 10.1186/s13054-021-03540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti A., Iapichino G.E., Milesi M., Melis V., Pugni P., Comini B., Cressoni M., Gattinoni L. Validation of computed tomography for measuring lung weight. Intensive Care Med. Exp. 2014;2:31. doi: 10.1186/s40635-014-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L.-L., Wang Y.-M., Wu Z.-Q., Xiang Z.-C., Guo L., Xu T., Jiang Y.-Z., Xiong Y., Li Y.-J., Li X.-W., Li H., Fan G.-H., Gu X.-Y., Xiao Y., Gao H., Xu J.-Y., Yang F., Wang X.-M., Wu C., Chen L., Liu Y.-W., Liu B., Yang J., Wang X.-R., Dong J., Li L., Huang C.-L., Zhao J.-P., Hu Y., Cheng Z.-S., Liu L.-L., Qian Z.-H., Qin C., Jin Q., Cao B., Wang J.-W. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Ball L., Pelosi P., Rocco P.R.M. Ten things you need to know about intensive care unit management of mechanically ventilated patients with COVID-19. Expert Rev. Respir. Med. 2021:1–10. doi: 10.1080/17476348.2021.1906226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Robba C., Battaglini D., Ball L., Patroniti N., Loconte M., Brunetti I., Vena A., Giacobbe D., Bassetti M., Rocco P.R.M., Pelosi P. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramuzzo G., Ball L., Pino F., Ricci L., Larsson A., Guérin C., Pelosi P., Perchiazzi G. Influence of positive end-expiratory pressure titration on the effects of pronation in acute respiratory distress syndrome: a comprehensive experimental study. Front. Physiol. 2020;11:179. doi: 10.3389/fphys.2020.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P., Fauvel N.J., Singh S., Soni N. Ventilatory ratio: a simple bedside measure of ventilation. Br. J. Anaesth. 2009;102:692–697. doi: 10.1093/bja/aep054. [DOI] [PubMed] [Google Scholar]
- Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli R., Marchioni A., Tabbì L., Fantini R., Busani S., Castaniere I., Andrisani D., Gozzi F., Bruzzi G., Manicardi L., Demurtas J., Andreani A., Cappiello G.F., Samarelli A.V., Clini E. Spontaneous breathing and evolving phenotypes of lung damage in patients with COVID-19: review of current evidence and forecast of a new scenario. J. Clin. Med. 2021;10:975. doi: 10.3390/jcm10050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet Lond. Engl. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Xiong M., Liang X., Wei Y.-D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br. J. Haematol. 2020;189:1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.