Abstract
Objective
We aimed to characterize the evolution of humoral immune response up to 1 year after SARS-CoV-2 infection in healthcare workers (HCWs) during the first wave of COVID-19 in Paris.
Methods
Serum samples from 92 HCWs were tested at month 0 (M0), M6, and M12 after SARS-CoV-2 infection for IgG targeting the nucleocapsid (N), IgG targeting the receptor-binding domain (RBD) of spike (S) protein, IgA targeting S, and anti-RBD neutralizing antibodies. After M6, 46 HCWs received a single dose of COVID-19 vaccine.
Results
We observed a significant decrease in all SARS-CoV-2 immunologic markers at M6 post-infection: median decreases were 0.26 log binding antibody units/mL (M0: 1.9 (interquartile range (IQR) 1.47–2.27); M6: 1.64 (IQR 1.22–1.92)) for anti-RBD IgG; 4.10 (index) (M0: 4.94 (IQR 2.72–6.82); M6: 0.84 (IQR 0.25–1.55)) for anti-N IgG; 0.64 (index) (M0: 2.50 (IQR 1.18–4.62); M6: 1.86 (IQR 0.85–3.54)) for anti-S IgA; and 24.4% (M0: 66.4 (IQR 39.7–82.5); M6: 42.0 (IQR 16.8–68.8)) inhibition activity for the RBD neutralizing antibodies. Between M6 and M12, anti-RBD IgG level, anti-S IgA index, and anti-RBD neutralizing activity significantly increased among COVID-19 vaccinated HCWs, whereas they remained stable among unvaccinated HCWs. Anti-N IgG index significantly decreased between M6 and M12 among both vaccinated (median: 0.73 (IQR 0.23–1.11) at M6 and 0.52 (IQR 0.20–0.73) at M12) and unvaccinated HCWs (median: 0.79 (IQR 0.21–4.67) at M6 and 0.34 (IQR 0.24–2.78) at M12).
Discussion
A steady decline in the anti-N IgG response was observed during the first year after SARS-CoV-2 infection among HCWs, whereas the anti-RBD IgG and the anti-S IgA responses remained stable and could be enhanced by COVID-19 vaccination.
Keywords: IgA, IgG, SARS-CoV-2, Seroneutralization, Vaccines
Introduction
During the beginning of the COVID-19 pandemic, the contamination risk of healthcare workers (HCWs) by SARS-CoV-2 was of major concern. The SEROCOV multicentre cohort study conducted among 1062 frontline HCWs from five Parisian hospitals reported a rate of SARS-CoV-2 infection of 14.6% at the end of the first COVID-19 wave, by detection of anti-nucleocapsid protein (N) IgG in HCW sera [1]. Several studies have shown that anti-SARS-CoV-2 IgG levels decreased after infection over time and that COVID-19 vaccination led to a rise in antibodies levels [2,3]. The present retrospective study aimed to characterize the evolution of the humoral immune response among SARS-CoV-2–infected HCWs from the SEROCOV study during the first year post-infection.
Methods
For the SEROCOV study (registered on ClinicalTrials.gov: NCT04304690), first registered on March 11, 2020 and approved by the ethics committee (CPP Sud-Ouest et Outre-Mer I, approval no. 2-20-023 id7257), HCWs from Pitié-Salpêtrière, Bichat, Tenon, Trousseau and Saint-Antoine hospitals were included from March 16, 2020 to April 24, 2020 for a 3-month follow-up. HCWs with a positive detection of SARS-CoV-2 anti-N IgG in the serum at the end of the initial 3-month period were included in the present study for an additional 9-month follow-up. Humoral immune responses were evaluated at month zero (M0) (corresponding to the time of seroconversion), M6, and M12 (5–6 and 11–12 months after seroconversion, respectively). All participant signed an informed consent form [1].
Semi-quantification (index) of IgG against N and quantification (log binding antibody units (BAU)/mL) of Ig against the receptor-binding domain (RBD) of spike (S) protein were assessed by chemiluminescence assay (ALINITY i System, Abbott, Abbott Park, IL). Semi-quantitative (index) ELISA assay was performed for anti-S IgA (ELISA Anti-SARS-CoV-2 IgA kit, Euroimmun, Lübeck, Germany). Anti-RBD neutralizing activity of sera was measured with a semi-quantitative ELISA assay (SARS-CoV-2 Surrogate Virus Neutralization Test, GenScript, Piscataway, NJ) based on the binding inhibition of labelled RBD to angiotensin converting enzyme 2 (ACE2) by the anti-RBD neutralizing antibodies (results expressed in percentage).
For statistical analyses, Mann-Whitney U tests and nonparametric Wilcoxon paired tests were performed with the GraphPad Prism, version 8.0.2 software, and p < 0.05 was considered statistically significant.
Results
The study included 92 SARS-CoV-2–infected HCWs from the SEROCOV cohort: 22 males, 70 females, median age of 33 years (interquartile range (IQR) 28–41). A total of 91 and 55 serum samples were available at M6 and M12, respectively.
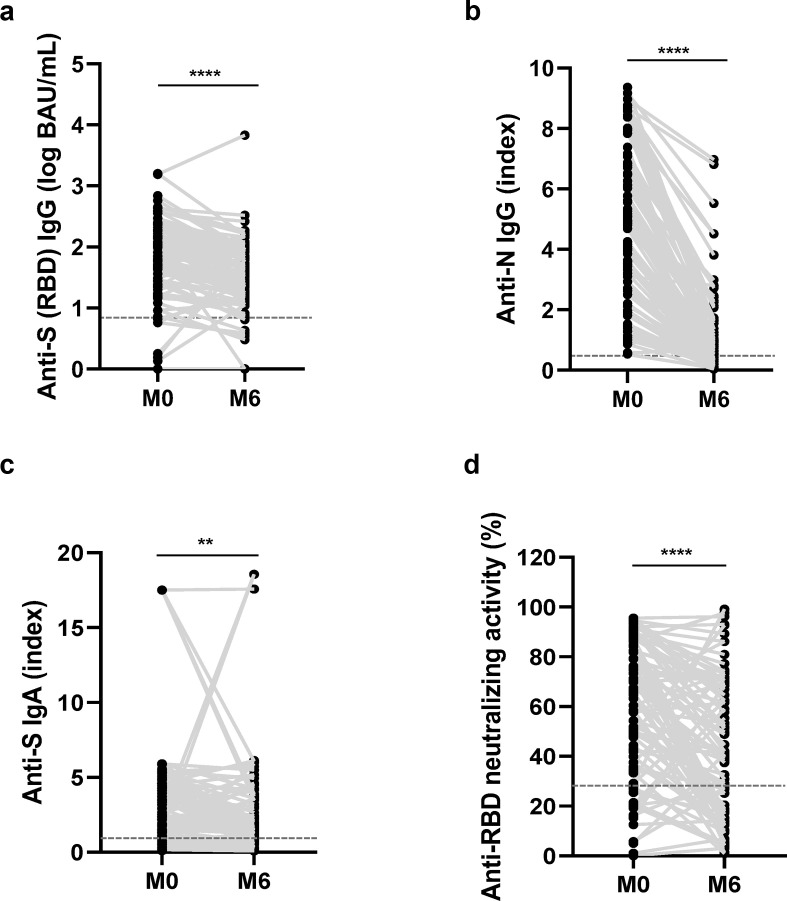
We first evaluated the natural evolution of humoral anti-SARS-CoV-2 immune response between M0 and M6. The anti-RBD IgG median level decreased significantly by 0.26 log BAU/mL between M0 (1.90 log BAU/mL (IQR 1.47–2.27)) and M6 (1.64 log BAU/mL (IQR 1.22–1.92)) (Fig. 1 (a)). The anti-N IgG median index also significantly decreased by 4.10 during this period: 4.94 (IQR 2.72–6.82) at M0 and 0.84 (IQR 0.25–1.55) at M6 (Fig. 1(b)). We also observed a significant 0.64 decline in the anti-S IgA median index between M0 (2.50 (IQR 1.18–4.62)) and M6 (1.86 (IQR 0.85–3.54)) (Fig. 1(c)). Considering the anti-RBD neutralizing activity, a median decay of 24.4% of inhibition was observed: 66.4% (IQR 39.7–82.5) at M0 and 42.0% (IQR 16.8–68.8) at M6 (Fig. 1(d)).
Fig. 1.
Natural evolution of humoral immune response after SARS-CoV-2 infection among healthcare workers (HCWs). Evolution of antibody response during 6 months for (a) anti-RBD IgG, (b) anti-N IgG, (c) anti-S IgA, and (d) anti-RBD neutralizing activity (M0, n = 92; M6, n = 91). On each graph, the horizontal dotted line represents the positivity cut-off of the technique: (a) 50 BAU/mL, (b) 0.5 (index), (c) 1.1 (index), (d) 30%. BAU, binding antibody units; RBD, receptor-binding domain. ∗∗p < 0.005; ∗∗∗∗p < 0.0001.
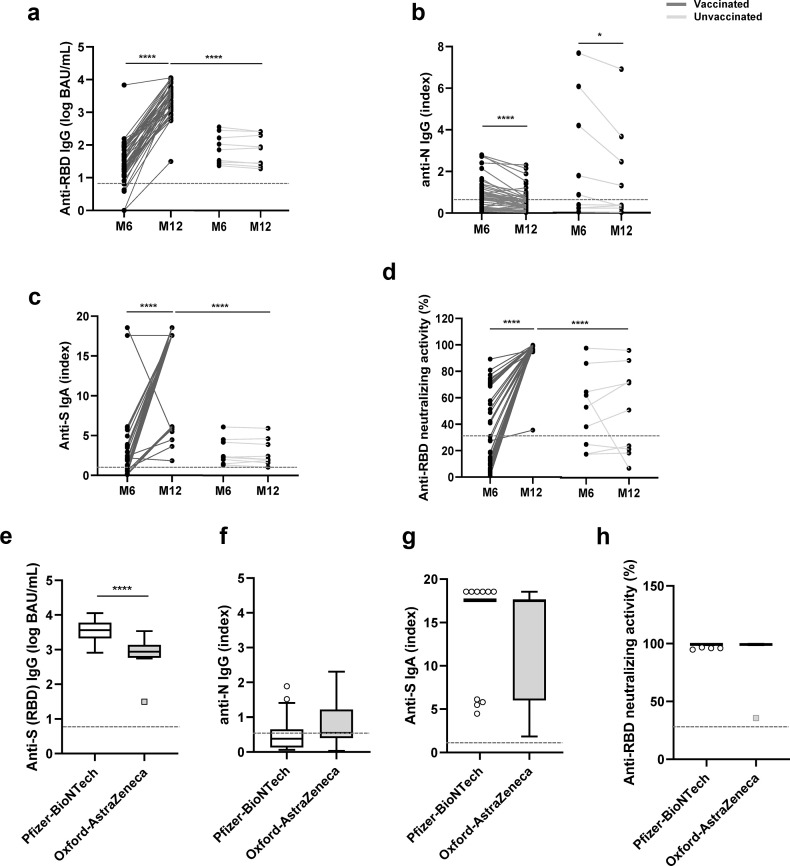
After M6, 46 (79%) HCWs received a single dose of COVID-19 vaccine: 35 (76%) Pfizer-BioNTech and 11 (24%) Oxford-AstraZeneca. The anti-SARS-CoV-2 humoral immune response was compared between vaccinated and unvaccinated HCWs. In the unvaccinated group of HCWs, the natural evolution of antibody responses could be analyzed. The levels of anti-RBD IgG, anti-S IgA, and the anti-RBD neutralizing activity were stable between M6 and M12 (Fig. 2 (a.c.d)), whereas we observed a significant decrease in the anti-N IgG median index during the same period: 0.79 (IQR 0.21–4.67) at M6 and 0.34 (IQR 0.24–2.78) at M12 (Fig. 2(b)). A significant decrease in the anti-N IgG median index was also observed in the vaccinated group of HCWs: 0.73 (IQR 0.23–1.11) at M6 and 0.52 (IQR 0.20–0.73) at M12 (Fig. 2(b)). However, the single-dose vaccination induced a strong increase in the anti-RBD IgG level (+1.95 log BAU/mL), anti-S IgA index (+16.30), and anti-RBD neutralizing activity (+71.4% of inhibition) (Fig. 2(a–d)).
Fig. 2.
Evolution of immune response after SARS-CoV-2 infection among COVID-19 vaccinated and unvaccinated healthcare workers (HCWs). Evolution of antibody response between M6 and M12 among vaccinated (dark grey, n = 46) and unvaccinated (light grey, n = 9) HCWs: (a) anti-RDB IgG, (b) anti-N IgG, (c) anti-S IgA, and (d) anti-RBD neutralizing activity. Comparison of antibody response at M12 among HCWs vaccinated with Pfizer-BioNTech vaccine (white, n = 35) and with Oxford-AstraZeneca vaccines (grey, n = 11): (e) anti-RBD IgG, (f) anti-N IgG, (g) anti-S IgA, and (h) anti-RBD neutralizing activity. On each graph, the horizontal dotted line represents the positivity cut-off of the technique: (a) and (e) 50 BAU/mL, (b) and (f) 0.5 (index), (c) and (g) 1.1 (index), and (d) and (h) 30%. BAU, binding antibody units; RBD, receptor-binding domain. ∗p < 0.05; ∗∗∗∗p < 0.001.
We also investigated the impact of the COVID-19 vaccine type (Pfizer-BioNTech, New York, NY or Oxford-AstraZeneca, Cambridge, UK) on the humoral immune response of HCWs. No difference was observed between vaccines at M12 for the anti-N IgG index, anti-S IgA index, or anti-RBD neutralizing activity (Figs. 2(f–h)). Conversely, a significantly higher level of anti-RBD IgG was observed in the Pfizer-BioNTech group of HCWs than among in the Oxford-AstraZeneca group of HCWs (median: 3.56 log BAU/mL (IQR 3.33–3.78) vs. 2.94 log BAU/mL (IQR 2.76–3.14)) (Fig. 2(e)).
Discussion
The evolution of the humoral immunity after SARS-CoV-2 infection is an important element in studying the dynamic of the COVID-19 pandemic. Our study reinforces and brings new evidence to support the fact that anti-S antibodies (IgG and IgA) decreased but remained detectable over time, in contrast to anti-N antibodies, and can be strongly enhanced after vaccination.
As previously shown [3,4], we observed a continuous decrease in anti-N IgG over 1 year. The anti-RBD IgG level also decreased until M6 but remained stable above the positive threshold over a year. These data were consistent with those observed in other European HCWs an in symptomatic/asymptomatic patients [2,3,[5], [6], [7]]. We observed the same pattern of evolution for the anti-S IgA antibodies. Previous works have shown that anti-S IgA levels decreased to a smaller degree compared to anti-RDB IgG levels over a time period of 6 to 9 months [2,8,9]. The present study confirmed this decrease at M6 but showed that, similar to anti-RBD IgG, it remained stable over a year. These patterns of antibody evolution are coherent with the kinetics of B-cell and T-cell expansion after SARS-CoV-2 infection [10] and suggest that active and young adult HCWs could exhibit an efficient immune response in case of virus re-exposure after 1 year.
Moreover, a strong increase in antibody titres was observed between M6 and M12 after one dose of vaccine. Consistent with previous studies [11,12], only one dose of vaccine after SARS-CoV-2 infection was enough to increase strongly immune response makers.
One of the limitations of our study is the low number of available serum samples from SARS-CoV-2–infected HCWs, particularly for the unvaccinated group and the Oxford-AstraZeneca group. Indeed, we only observed a lower level of anti-RBD IgG with the Oxford-AstraZeneca group, which is consistent with the literature [12]. No significant differences were observed for anti-S IgA level and anti-RDB neutralizing activity. Those results could be explained by the fact that the last two immunologic markers were assessed by semi-quantitative assays, which do not allow precise quantification.
Conclusion
Anti-RBD IgG and anti-S IgA levels decreased until 6 months and then stabilized until 12 months after SARS-CoV-2 infection in HCWs. Anti-N IgG levels showed a continuous decline throughout the study period. COVID-19 vaccination (Pfizer-BioNTech and Oxford-AstraZeneca) led to a strong increase in all anti-SARS-CoV-2 immunologic markers, except for the anti-N IgG response.
Transparency declaration
Authors declare that they have no conflict of interest.
ET was supported by the ANRS-MIE (Agence Nationale de Recherches sur le SIDA et les hépatites virales-Maladies Infectieuses Emergentes) (AC43, Medical Virology). The SEROCOV study was funded by the French Ministry of Health (Programme Hospitalier de Recherche Clinique) and the French Agency for Research (Fond d'amorçage de l’Agence National pour la Recherche) and sponsored by the Assistance Publique-Hôpitaux de Paris (AP-HP) and the URC Pitié-Salpêtrière.
Author contributions
DB, AGM, and ET planned the study; ET, KZ, SS, SM, MD, CS, and BA conducted the experiments; ET and DB analysed the data; ET, DB, and AGM wrote the manuscript; ET, DB, AGM, PH, and FT reviewed the manuscript; all authors approved the final version.
SEROCOV study group
Pierre Hausfater, Florence Tubach, Karine Lacombe, David Hajage, Alexandra Beurton, Margaux Dumont, Jean-Michel Constantin, Jade Ghosn, Alain Combes, Nicolas Cury, Romain Guedj, Michel Djibré, Rudy Bompard, Sandie Mazerand, Valérie Pourcher, Linda Gimeno, Ilaria Cherubini, Enfel Houas, Guillaume Payan, Sylvie Le Gac, Odile Fleurot, Elisabeth Bouvet, Christine Jestin, Clemence Marois, Justine Dorchies, Nathanelle Yeni, Olivia Da Conceicao, Lucie Touchar, Nicolas Mediamolle, Aida Zarhrate, Fatiha Bouchama, Marina Vaz, Mariem Ben Cheikh Souguir, Vincent Calvez.
Acknowledgements
The authors acknowledged all the HCWs of the Pitié-Salpêtrière, Bichat, Saint-Antoine/Trousseau, and Tenon for their participation in the SEROCOV study.
Editor: E. Bottieau
References
- 1.Hausfater P., Boutolleau D., Lacombe K., Beurton A., Dumont M., Constantin J.-M., et al. Cumulative incidence of SARS-CoV-2 infection and associated risk factors among frontline health care workers in Paris, France: the SEROCOV Prospective Cohort Study. Scientific reports. 2022 doi: 10.21203/rs.3.rs-960512/v1. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaebler C., Wang Z., Lorenzi J.C., Muecksch F., Finkin S., Tokuyama M., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallais F., Gantner P., Bruel T., Velay A., Planas D., Wendling M.-J., et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine. 2021;71:103561. doi: 10.1016/j.ebiom.2021.103561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumley S.F., Wei J., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) antibody responses in individual healthcare workers. Clin Infect Dis. 2021;73:e699–e709. doi: 10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe P.G., Kang C.K., Kim K.-H., Yi J., Kim E.S., Park S.W., et al. Persistence of neutralizing antibody response up to 1 year after asymptomatic or symptomatic SARS-CoV-2 infection. J Infect Dis. 2021;224:1097–1099. doi: 10.1093/infdis/jiab339. [DOI] [PubMed] [Google Scholar]
- 6.Masiá M., Fernández-González M., Telenti G., Agulló V., García J.A., Padilla S., et al. Durable antibody response one year after hospitalization for COVID-19: a longitudinal cohort study. J Autoimmun. 2021;123:102703. doi: 10.1016/j.jaut.2021.102703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capetti A.F., Borgonovo F., Mileto D., Gagliardi G., Mariani C., Lupo A., et al. One-year durability of anti-spike IgG to SARS-CoV-2: preliminary data from the anticrown prospective observational study one year durability of COVID-19 anti-spike IgG. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortega N., Ribes M., Vidal M., Rubio R., Aguilar R., Williams S., et al. Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses. Nat Commun. 2021;12:4740. doi: 10.1038/s41467-021-24979-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanov A., Semenova E. Long-term monitoring of the development and extinction of IgA and IgG responses to SARS-CoV-2 infection. J Med Virol. 2021;93:5953–5960. doi: 10.1002/jmv.27166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens D.S., McElrath M.J. COVID-19 and the path to immunity. JAMA. 2020;324:1279. doi: 10.1001/jama.2020.16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong D., Xiao S., Debes A.K., Egbert E.R., Caturegli P., Colantuoni E., et al. Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA. 2021;326:2524. doi: 10.1001/jama.2021.19996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyre D.W., Lumley S.F., Wei J., Cox S., James T., Justice A., et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin Microbiol Infect. 2021;27:1516.e7–1516.e14. doi: 10.1016/j.cmi.2021.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]