Abstract
Genome-wide association (GWA) studies have identified several variants associated with brief self-report measures of smoking, predominately in European ancestry samples. GWA research has not applied intensive laboratory-based measures of smoking endophenotypes in African Americans. African American smokers have disproportionately low quit smoking rates and high tobacco-related disease risk. This study of non-Hispanic African American smokers tested associations of 93 candidate genetic variants identified in previous GWA smoking research and exploratory GWAs with laboratory-derived tobacco withdrawal phenotypes. African American daily cigarette smokers (N=528; ≥10 cig/day; 36.2% female) completed two counterbalanced visits following either 16-hours of tobacco deprivation or ad libitum smoking. At both visits, 22 self-report and 2 objective behavioral phenotypes were systematically assessed, collectively addressing 6 unique ‘sub-phenotype’ symptom domains within the tobacco withdrawal syndrome (Urge/Craving, Negative Affect, Low Positive Affect, Cognition, Hunger, and Motivation to Resume Smoking). Results of the candidate variant analysis found two significant small-magnitude associations. The alternate allele of rs11915747 in the CAD2M gene region was associated with .09 larger deprivation-induced changes in reported impulsivity (0-4 scale). The alternate allele of rs2471711 in the AC097480.1/AC097480.2 gene region was associated 0.26 lower deprivation-induced changes in confusion (0-4 scale). For both variants, associations were opposite in direction to previously published research. Individual genetic variants may exert only weak influences on tobacco withdrawal in African Americans. Larger sample sizes of non-European ancestry individuals might be needed to investigate both known and novel loci that may be ancestry-specific.
Keywords: genetics, tobacco withdrawal, smoking, African Americans, health disparities
Tobacco-related disease is the leading cause of preventable death (Control & Prevention, 2005) and disproportionately affects populations subject to health disparities, including African Americans (Haiman et al., 2006; Irvin Vidrine, Reitzel, & Wetter, 2009). Most smokers have difficulty quitting (Creamer et al., 2019; Fiore et al., 2008), and quit rates are low amongst African American smokers (Bacio, Guzman, Shapiro, & Ray, 2014; Kahende, Malarcher, Teplinskaya, & Asman, 2011), even when treated with front-line smoking cessation pharmacotherapies (Nollen et al., 2019). Consequently, elucidating the etiology of tobacco addiction amongst African Americans is important for informing future translational treatment research that can counteract the tobacco-related disease epidemic and reduce health disparities.
Genetic epidemiology research that agnostically scans the genome for variants associated with smoking phenotypes provides the promise of discovering biological pathways that underpin tobacco addiction. Primary features of modern smoking genome-wide association (GWA) research is the use of: (1) large samples, predominately of European ancestry, capable of detecting weak associations; and (2) low-resource, brief survey assessments of smoking phenotypes. In the largest smoking GWA study to date, the Genetic Sequencing Consortium of Alcohol and Nicotine (GSCAN) meta-analyzed data from 1.2 million individuals of European ancestry (Liu et al., 2019). This study found that 89 genetic variants across the genome were significantly, but weakly, associated with self-reported smoking phenotypes implicated in persistent smoking (e.g., smoking duration, heaviness, and cessation). We see two next steps in leveraging existing smoking GWA findings to further knowledge of smoking etiology and address tobacco-related health disparities.
It is critical to advance the sparse knowledge of genetic epidemiology of smoking in African Americans. If variants identified in European ancestry smoking GWA research are found to be associated with smoking phenotypes among African Americans—a population with genetic architecture distinct from European Americans—there is a greater likelihood that such variants are causal (or potentially further narrowing the putative list of variants within a locus). Additionally, studying GWA variants in African Americans allows for greater clinical translation to tobacco addiction treatment in this understudied population and progress towards addressing tobacco-related health disparities (Tate & Goldstein, 2004).
It is vital to disentangle the genetic bases of chronic, heavy, and persistent smoking patterns, which GWA research indicates are explained by the accumulation of hundreds of unique weak genetic influences. The genetic contribution to these distal (end-point) smoking phenotypes are likely attributed to an inherited vulnerability toward developing tobacco addiction, which is a highly heterogenous syndrome (Lerman, Perkins, & Gould, 2009). Addiction is a complex set of several narrow endophenotypes (e.g., withdrawal, tolerance). These endophenotypes loosely cluster together, and are more proximal to the underlying biological etiologies than the distal endpoints and may each have unique genetic causes (Cannon & Keller, 2006; Lerman et al., 2009; Ray, MacKillop, & Monti, 2010). As hundreds of variants across the genome are each weakly associated with distal smoking phenotypes, examining GWAs with one addiction endophenotype may reveal a subset of stronger genetic influences for biological pathways that directly underpin that endophenotype.
Tobacco withdrawal—psychobiological changes that emerge upon the cessation of chronic smoking—may be a promising endophenotype because it interferes with cessation and maintains smoking (Hughes, 2006), and can be isolated in the laboratory by studying response to a pharmacological tobacco deprivation challenge (Shiffman, West, & Gilbert). The tobacco withdrawal syndrome has a variety of manifestations (e.g., anxiety, anger, sadness, craving, hunger, anhedonia, concentration, motivation to resume smoking) that can be measured using quantitative phenotypic indexes (Hughes, 2007; A. M. Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010), each of which may be distinct sub-phenotypes with unique genetic influences. We know of only two previous GWA studies of tobacco withdrawal (Hällfors et al., 2019; Jensen et al., 2017), both of which utilized retrospective self-report measures of withdrawal that amalgamated numerous withdrawal sub-phenotypes into a single composite variable.
In the current study, non-Hispanic African American daily smokers completed a laboratory-based overnight tobacco deprivation challenge protocol, which provided phenotype data for a variety of symptomatic and behavioral components of the acute tobacco withdrawal syndrome. We estimated associations of the laboratory-derived withdrawal phenotypes with 89 variants across the genome identified in the GSCAN GWA study of 1.2 million European Americans (Liu et al., 2019). Secondarily, we report the results of GWAs with each tobacco withdrawal phenotype.
METHODS
Participants
528 non-treatment seeking African American smokers were recruited from the greater Los Angeles area. Eligible participants were regular daily cigarette smokers for at least 2+ years and aged 18 years or older, currently smoking ≥ 10 cigarettes per day, fluent in English, and self-reported racial/ethnic identification as Non-Hispanic African American (see Table 1 for descriptive statistics of race/ethnicity of grandparents’ ancestry). Exclusion criteria included: 1) current Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) non-nicotine substance dependence (including cannabis dependence) in order to minimize alcohol and drug withdrawal symptoms during experimental conditions (DSM-IV substance abuse [including cannabis abuse] was allowed); 2) breath carbon monoxide (CO) levels < 10 parts per million (ppm) at intake; 3) daily use of any other non-cigarette forms of tobacco products (e.g., cigars/cigarillos) or cannabis products (non-daily use permitted); 4) current use of nicotine replacement therapy, varenicline, or bupropion; 5) currently pregnant or breastfeeding or intent to get pregnant in the next 30 days; and 6) planning to cut down or quit smoking in the next 30 days. This study was approved by the University of Southern California Institutional Review Board (HS-13-00225) and participants were compensated approximately $200 for completing the study.
Table 1.
Descriptive Statistics of Sample Characteristics
| Variables | N (%) / M (SD) | Available N |
|---|---|---|
| Demographics | ||
| Age, M (SD) | 49.96 (10.79) | 527 |
| Gender (Female), N (%) | 190 (36.26) | 524 |
| Race/Ethnicity of Grandparents’ Ancestry | ||
| Grandmother (on mother’s side) | 527 | |
| Hispanic or Latino | 19 (3.6) | |
| Not Hispanic or Latino | 453 (86.0) | |
| Don’t Know | 55 (10.4) | |
| Grandfather (on mother’s side) | 527 | |
| Hispanic or Latino | 16 (3.0) | |
| Not Hispanic or Latino | 452 (85.8) | |
| Don’t Know | 59 (11.2) | |
| Grandmother (on father’s side) | 527 | |
| Hispanic or Latino | 16 (3.0) | |
| Not Hispanic or Latino | 449 (85.2) | |
| Don’t Know | 62 (11.8) | |
| Grandfather (on father’s side) | 527 | |
| Hispanic or Latino | 18 (3.4) | |
| Not Hispanic or Latino | 441 (83.7) | |
| Don’t Know | 68 (12.9) | |
| Grandmother (on mother’s side) | 525 | |
| American Indian or Alaskan Native | 25 (4.8) | |
| Asian | 2 (0.4) | |
| Black or African American | 434 (82.7) | |
| Middle Eastern | 1 (0.2) | |
| Pacific Islander | 0 (0.0) | |
| White | 11 (2.1) | |
| Other | 17 (3.2) | |
| Multiracial | 35 (6.7) | |
| Grandfather (on mother’s side) | 523 | |
| American Indian or Alaskan Native | 12 (2.3) | |
| Asian | 3 (0.6) | |
| Black or African American | 445 (85.0) | |
| Middle Eastern | 1 (0.2) | |
| Pacific Islander | 2 (0.4) | |
| White | 9 (1.7) | |
| Other | 26 (5.0) | |
| Multiracial | 25 (4.8) | |
| Grandmother (on father’s side) | 524 | |
| American Indian or Alaskan Native | 25 (4.8) | |
| Asian | 2 (0.4) | |
| Black or African American | 440 (84.0) | |
| Middle Eastern | 2 (0.4) | |
| Pacific Islander | 0 (0.0) | |
| White | 3 (0.6) | |
| Other | 31 (5.9) | |
| Multiracial | 21 (4.0) | |
| Grandfather (on father’s side) | 520 | |
| American Indian or Alaskan Native | 9 (1.7) | |
| Asian | 2 (0.4) | |
| Black or African American | 446 (85.8) | |
| Middle Eastern | 2 (0.4) | |
| Pacific Islander | 0 (0.0) | |
| White | 11 (2.1) | |
| Other | 38 (7.3) | |
| Multiracial | 12 (2.3) | |
| Smoking Characteristics | ||
| Age started smoking, M (SD) | 19.59 (5.73) | 526 |
| Number of cigarettes per day, M (SD)a | 15.12 (7.33) | 522 |
| Menthol cigarette use, N (%) | 323 (61.52) | 525 |
| FTND, M (SD)b | 5.55 (1.93) | 526 |
Note. N= 528. Race/Ethnicity of Grandparents’ Ancestry (“Ethnicity of Your Relatives” [fill in one bubble for each grandparent]; “Race of Your Relatives” [fill in every bubble that applies for each grandparent]).
Response range 2-60.
FTND = Fagerström Test for Nicotine Dependence (range 0-10).
Procedure
After a phone screen, participants were scheduled to attend a baseline session involving informed consent, in-person eligibility screening involving CO breath assessment and psychiatric interviews, and completion of questionnaires assessing demographics and smoking history and characteristics. After a 30-minute period involving no eating, drinking, smoking, or chewing gum, participants provided 2 mL of saliva into a Genotek Oragene DNA self-collection kit with stabilizing liquid.
Participants were scheduled for two counterbalanced experimental sessions starting around noon: 1) 16-hour tobacco deprived condition where participants were instructed to withhold from smoking after 8 pm the night before their session; and 2) non-deprived condition after ad libitum smoking. Both conditions were identical with the exception that participants were administered a cigarette of their preferred brand at the beginning of the non-deprived condition to standardize smoking recency across the sample. Participants were also instructed to avoid alcohol and use of any other tobacco products or cannabis prior to their visits. We then confirmed compliance with instructions by testing for breath alcohol (BrAC = 0.000 required) and CO levels (CO < 10 ppm (SRNT Subcommitte on Biochemical Verification, 2002) at the beginning of each session. Participants with CO level readings ≥ 10 ppm were considered non-abstinent and rescheduled for a second attempt; those not meeting CO criteria twice were discontinued (n = 11). Next, participants completed self-report tobacco withdrawal measures described below at a single time-point. Participants subsequently completed a behavioral smoking task that evaluated the motivational value of initiating smoking.
Phenotype Measures
Smoking Urges.
The 10-item Brief Questionnaire of Smoking Urges (QSU; Cox, Tiffany, & Christen, 2001) consists of statements that evaluate desire to smoke for pleasure and intention to smoke (Factor 1; e.g., “A cigarette would taste good.”; 5 items) and desire to smoke to relieve negative affect (Factor 2; e.g., “Smoking would make me less depressed.”; 5 items). Participants rated the extent to which they agreed with the 10 statements based on how they felt “right now” on 6-point Likert scales (0 = Strongly disagree to 5 = Strongly agree). In addition to the total scale that averages all 10 items, each subscale (Factor 1 and Factor 2) is computed based on mean rating per item within each respective subscale, with Factors 1 and 2 reflecting appetitive and aversive urges, respectively.
Nicotine Withdrawal Symptoms.
The 28-item Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) captures 5 major symptom components of nicotine withdrawal in separate subscales (i.e., anger, anxiety, sadness, concentration, hunger, craving) plus an overall total scale. Participants rated their level of agreement with self-statements reflecting withdrawal symptoms on 5-point Likert scales based on how they have felt “so far today” (0 = Strongly disagree to 4 = Strongly agree), which yielded a composite index based on mean response within items in a subscale or all items for the total composite.
Affect.
The 72-item Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971) asked participants to rate adjectives of affect states (e.g., friendly, unhappy, confused, panicky, annoyed) based on how they were feeling “right now” on a 5-point Likert scale (0 = Not at all to 4 = Extremely). We computed specific subscales for those in both negative (Anger [12 items], Anxiety [8 items], Confusion [7 items], Depression [15 items]) and positive (Elation [6 items], Friendliness [8 items], and Vigor [8 items]) affect domains on mean rating per item within respective subscale. Overall scores for negative affect valence (NM; mean of 4 negative affect subscale scores) and positive affect valence (PM; mean of 3 positive affect subscales) were also computed.
Anhedonia.
The 14-item Snaith-Hamilton Pleasure Scale (SHAPS; Snaith, 1993) instructs participants to agree or disagree with statements of hedonic response in pleasurable situations (e.g., “I would be able to enjoy my favorite meal.”) experienced “right now, in the current moment” on 4-point Likert scales (1 = Strongly disagree to 4 = Strongly agree). A composite index was computed based on mean response across all items and reverse-coded , with higher SHAPS scores indicating greater levels of diminished hedonic pleasure capacity (i.e., anhedonia).
Current Impulsivity.
The 19-item Current Impulsivity Scale (CIS) assesses subjective impulsive states. Participants rated statements reflecting state impulsive tendencies (e.g., “Feeling like doing or saying things without thinking.”) based on how they felt “right now” on 5-point Likert scales (0 = Not at all to 4 = Extremely), which yielded a composite index based on mean response across all items.
Behavioral Smoking Task.
This behavioral economics-based objective task assesses the motivational reward value of smoking relative to money (McKee, 2009). Participants received a tray containing 8 cigarettes of their preferred brand, a lighter, and ashtray. During the delay portion of the task, participants were instructed that they could smoke at any point within the next 50 minutes, but for each 5 minutes that they delayed smoking, they would earn $0.20 for a maximum of $2.00. The delay period ended when the participant decided that they would like to smoke once or after all 50 minutes had elapsed for those who chose not to smoke. After the delay period, participants began the self-administration portion of the task where they were instructed that they could smoke as little or as many cigarettes as they wished for the next 60 minutes. Participants were instructed that they had a $1.60 credit and each cigarette they lit would cost $0.20. The primary outcomes were time to smoking initiation during the delay period (0-50 min, participants who choose not to smoke receive the maximum value of 50) and the number of cigarettes purchased during the self-administration period (possible range: 0-8 cigarettes), which reflect the reward value of initiating smoking and continuing to smoke once given the opportunity, respectively. Following the self-administration period, participants began a rest period during which they were not allowed to smoke (rest time range: 60-110 minutes).
Genotyping, Quality Control, and Imputation
DNA samples were genome-wide genotyped on the Smokescreen Genotyping Array, which includes 646,247 additional variants to provide comprehensive coverage of loci known to be related to smoking phenotypes (Baurley, Edlund, Pardamean, Conti, & Bergen, 2016). Using 200 ng of genomic DNA, array plates were prepared using the Axiom 2.0 Reagent Kits and then processed on the GeneTitan MC instrument (Thermo Fisher Scientific, Wilmington, DE, USA). Analysis of the raw data was performed using Affymetrix Power tools (APT) v-1.16 according to the Affymetrix best practices workflow. Additional steps were performed using SNPolisher to identify and select the best performing probe sets and high-quality variants for downstream analysis. 563,065 genetic variants remained after QC filtering. Additional quality control steps were performed with PLINK v1.90p 64-bit (25 Mar 2016) including observed versus expected heterozygosity. Before imputation, genotypes were reported on the forward strand and removed if their alleles did not match the reference genome or were out of Hardy-Weinberg equilibrium (p < 1E-10), leaving 548,820 variants. 1000 Genomes Phase 3 v5 was selected as a reference panel, haplotype phasing was performed using Eagle v2.4.1, and genotype imputation was performed using Minimac4 v1.0.1.
Analytic Plan
Descriptive analyses of phenotypes.
We first calculated descriptive statistics for demographics, race/ethnicity of grandparents’ ancestry, and smoking characteristics (see Table 1). We then utilized paired sample t-tests to assess whether tobacco deprivation affected each tobacco withdrawal phenotype outcome and reported internal consistency estimates by deprivation condition (Cronbach’s α; Table 2). Deprivation-induced change scores were calculated for each phenotype outcome (i.e., difference score in tobacco deprivation condition – score in nicotine satiated condition). To facilitate interpretation, we grouped presentation of the tobacco withdrawal phenotypes into 6 phenomenologically distinct domains: 1) Urge/Craving, 2) Negative Affect, 3) Low Positive Affect, 4) Cognition, 5) Other Withdrawal Symptoms, and 6) Behavioral Smoking Task.
Table 2.
Descriptive Statistics of Tobacco Withdrawal Phenotypes by Tobacco Deprivation Status
| Withdrawal Outcomes | Deprived | Non-Deprived | Deprivation-Induced Change Score | Deprivation Effect | Available Data | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| M (SD) | α | M (SD) | α | M (SD) | t | d | N | |
| Smoking Motivation | ||||||||
| WSWS-Craving | 2.57 (0.98) | .85 | 1.65 (0.99) | .82 | 0.92 (1.02) | 20.56† | 0.90 | 517 |
| QSU-Total | 3.04(1.29) | .92 | 1.20 (1.28) | .95 | 1.84 (1.37) | 30.62† | 1.34 | 520 |
| QSU-Factor 1 (Appetitive Urge) |
3.83 (1.29) | .91 | 1.51 (1.50) | .94 | 2.32 (1.66) | 31.73† | 1.40 | 517 |
| QSU Factor 2 (Aversive Urge) | 2.26 (1.52) | .88 | 0.89 (1.19) | .90 | 1.36 (1.38) | 22.54† | 0.99 | 520 |
| Negative Affect | ||||||||
| POMS-Negative Affect | 0.69 (0.63) | .97 | 0.54 (0.53) | .96 | 0.16 (0.52) | 6.80† | 0.30 | 516 |
| WSWS-Anger | 1.64 (1.15) | .88 | 0.93 (0.99) | .85 | 0.71 (1.14) | 14.12† | 0.62 | 517 |
| POMS-Anger | 0.53 (0.67) | .91 | 0.34 (0.52) | .87 | 0.19 (0.60) | 7.17† | 0.32 | 516 |
| WSWS-Anxiety | 1.94 (0.88) | .69 | 1.46 (0.89) | .72 | 0.48 (0.93) | 11.88† | 0.52 | 516 |
| POMS-Anxiety | 0.94 (0.77) | .87 | 0.65 (0.61) | .85 | 0.29 (0.70) | 9.59† | 0.42 | 516 |
| WSWS-Sadness | 1.54 (0.74) | .64 | 1.20 (0.74) | .57 | 0.34 (0.77) | 10.06† | 0.44 | 517 |
| POMS-Depression | 0.46 (0.64) | .93 | 0.37 (0.58) | .93 | 0.09 (0.53) | 3.71† | 0.16 | 516 |
| Low Positive Affect | ||||||||
| POMS-Positive Affect | 1.70 (0.88) | .96 | 2.15 (0.84) | .95 | −0.44 (0.76) | −13.32† | −0.59 | 515 |
| POMS-Elation | 1.46 (0.92) | .86 | 1.90 (0.90) | .85 | −0.44 (0.84) | −11.99† | −0.53 | 516 |
| POMS-Friendliness | 1.98 (0.96) | .90 | 2.50 (0.88) | .89 | −0.51 (0.84) | −13.88† | −0.61 | 515 |
| POMS-Vigor | 1.66 (0.92) | .89 | 2.04 (0.91) | .88 | −0.38 (0.82) | −10.47† | −0.46 | 516 |
| SHAPS-Anhedonia | 1.90 (0.54) | .93 | 1.73 (0.47) | .91 | 0.17 (0.52) | 7.33† | 0.32 | 521 |
| Cognition | ||||||||
| POMS-Confusion | 0.86 (0.64) | .75 | 0.71 (0.57) | .71 | 0.15 (0.57) | 6.00† | 0.26 | 516 |
| WSWS-Concentration | 1.54 (0.94) | .77 | 1.04 (0.78) | .62 | 0.50 (0.89) | 12.75† | 0.56 | 517 |
| CIS-Impulsivity | 1.49 (0.48) | .71 | 1.29 (0.43) | .66 | 0.21 (0.42) | 11.12† | 0.49 | 519 |
| Other Withdrawal Symptoms | ||||||||
| WSWS-Hunger | 2.21 (0.85) | .76 | 1.86 (0.82) | .71 | 0.35 (0.88) | 8.97† | 0.40 | 516 |
| WSWS-Total | 1.95 (0.63) | .81 | 1.50 (0.58) | .82 | 0.46 (0.56) | 18.47† | 0.82 | 518 |
| Behavioral Smoking Task | ||||||||
| Time Delayed (min) | 18.37 (22.37) | - | 36.07 (19.85) | - | −17.70 (23.43) | −17.26† | −0.76 | 522 |
| Cigarettes Smoked | 1.50 (0.96) | - | 1.25 (1.04) | - | 0.25 (1.02) | 5.40† | 0.24 | 504 |
Note: WSWS = Wisconsin Severity of Withdrawal Scale (range 0-4); QSU = Questionnaire of Smoking Urges (range 0-5); POMS = Profile of Mood States (range 0-4); CIS = Current Impulsivity Scale (range 0-4); Time Delay (range 0-50 minutes); Cigarettes smoked (range 0-8). Deprivation-Induced Change Score = Score in Deprivation Condition – Score in Non-Deprived Condition. Two composite
p<.001
Genetic association analyses.
Genome-wide scans were performed for each of the 24 smoking phenotypes. The models were fit using linear regression including the phenotype’s deprivation-induced change score (deprived – non-deprived) as the outcome and variant genotype dosage (i.e., the estimated number of alternate alleles for the variant from genotyping or imputation; range: 0-2) as the predictor. Each model additionally adjusted for sex and the respective non-deprived phenotype value as covariates. The results from these scans were inserted into a sqlite3 database and queried for each phenotype by: (1) 89 candidate genetic variants reported by the GSCAN consortium meta-analysis of age of smoking initiation, cigarettes per day, and smoking cessation (primary analysis; see Table S1 for depiction of all 89 variants) (Liu et al., 2019), and (2) genome-wide (secondary exploratory analysis). Variants were considered for reporting with good imputation quality (imputation r2 > 0.8) and with a minor allele frequency ≥ 1%. The threshold for statistical significance in the primary candidate gene analysis was 0.05 after PACT correction over the set of correlated phenotypes (Conneely & Boehnke, 2007). For the genome-wide scans of all variants on the entire Smokescreen array, we used the historic genome-wide significance value corrected for multiple phenotype testing (5E-8 / 24 = 2.08E-9) and interpreted GWA results for genetic variants only with minor allele frequencies ≥ 5%. Population stratification was evaluated by principal component analysis for significant associations (Price et al., 2006).
RESULTS
Descriptive Results
Depicted in Table 1, participants were, on average, 50 years old (SD = 10.8) and 63.7% male. 61.5% of participants smoked menthol-flavored cigarettes and smoked, on average, 15 cigarettes per day (SD = 7.33) and had medium levels of nicotine dependence (M = 5.55 [SD = 1.93]).
Table 2 reports the results of paired sample t-tests conducted to determine the effects of tobacco deprivation on tobacco withdrawal phenotype outcomes. Tobacco deprivation significantly impacted all phenotype outcomes in the expected direction and effect magnitudes ranged from small to large as indicated by Cohen’s d statistics (see Table 2).
Genetic Analyses
Analysis of candidate variants.
Results of tests associations of the 89 candidate genetic variants with each of the 24 withdrawal phenotypes found two significant associations after correction for multiple phenotype testing (Table 3). Both variants were previously associated with age of smoking onset in GSCAN. rs11915747 in the CAD2M gene region was associated with deprivation-induced changes in reported impulsivity (0-4 scale; B = 0.092, SE = 0.028, Uncorrected P = .0010, Figure, Panel A) and rs2471711 in the AC097480.1/AC097480.2 region with deprivation-induced changes in confusion (0-4 scale; B = −0.264, SE = 0.088, Uncorrected P =.0028, Figure, Panel B). These associations were unchanged after adjustment for population stratification by principal component analysis (rs11915747-impulsivity: Adjusted B = 0.092 Uncorrected P = .0015; rs2471711-confusion: Adjusted B= −0.289. Uncorrected P = .0010).
Table 3.
Significant Associations of Candidate Genetic Variants with Tobacco Withdrawal Phenotypes
| Genetic Variant Information | Association | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Phenotype | RS ID | Chrom | Ref Allele | Alt Allele | MAF | Nearest Gene(s) | Annotation | Genotyped or Imputed | B | SE | P |
|
| |||||||||||
| Confusiona | rs2471711 | 4 | C | T | 0.037 | AC097480.1/ AC097480.2 | NC Int | Imputed | −0.264 | 0.088 | .0028* |
|
| |||||||||||
| Impulsivityb | rs11915747 | 3 | C | G | 0.269 | CADM2 | Int | Imputed | 0.092 | 0.028 | .0010* |
Note. Chrom: Chromosome number location. Ref: Reference, Alt: alternate, MAF: Minor Allele Frequency; NC: Non-Coding; Int: Intronic. N/A: Not Applicable. B: unstandardized regression coefficient. SE: standard error of coefficient.
Deprivation-induced changes in Profile of Mood States confusion subscale (range 0-4)
Current Impulsivity Scale (range 0-4)
P < 0.05 after P_ACT correction over the set of correlated phenotypes.
Genome wide analysis.
The genome-wide scan produced no genetic associations with any of the withdrawal phenotypes that surpassed the corrected significance threshold of P < 2.08E-9 with minor allele frequencies ≥ 5%.
DISCUSSION
In this genetic epidemiology study of laboratory-based tobacco deprivation phenotype in non-Hispanic African American smokers, we did not replicate prior published findings or identify new loci. While it was anticipated that the current approach of using a rigorously characterized experimental endophenotype might reveal some strong genetic signals, this did not come to fruition in this study.
The candidate gene analysis of the 89 variants associated with relevant smoking phenotypes identified in a previous GWA in European Ancestry smokers found that an intronic variant in the CADM2 gene region was associated with deprivation-induced increases in self-reported impulsivity. CADM2, alternatively named SYNCAM2, is a cell-adhesion molecule implicated in brain development (Fogel et al., 2007; Frei, Andermatt, Gesemann, & Stoeckli, 2014). In addition to being associated with smoking, CADM2 variation has been associated with a wide range of behavioral phenotypes, including impulsive personality traits and behaviors (Sanchez-Roige et al., 2019). While the CADM2 gene is consistently implicated in behavior, this is the only study besides GSCAN to find this particular variant (rs11915747) to be associated with any behavioral phenotype. The statistically significant effect size for rs11915747 was small in GSCAN (association with age of smoking onset; Standardized Beta = .02) and in this study. Each additional alternate allele was associated with 0.09 points higher deprivation-induced increase on a 5-point scale.
The candidate variant analysis also found an association of rs2471711 (an intronic variant in the AC097480.1/AC097480.2 region) with deprivation-induced changes in confusion. Again, the association magnitude was small (alternate allele associated with 0.26 lower score on a 5-point scale). While this is the only other investigation besides GSCAN to show rs2471711 was associated with a behavioral phenotype, other variants in this gene region have been associated with risk taking (Linnér et al., 2019), depression and negative affect traits (Nagel et al., 2018a; Nagel et al., 2018b) as well as cognition-relevant characteristics, including educational attainment (Lee et al., 2018). This gene region produces a novel lncRNA, which may act as an enhancer or be involved in other aspects of gene regulation, but the exact function is unknown.
For both of these significant associations, the directions of relations were opposite to expectations based on GSCAN’s findings. GSCAN found that that the alternate allele of rs11915747 was associated with older age of smoking onset and lower smoking chronicity, which presumably would reduce risk for withdrawal; yet, the rs11915747 alternate allele increased deprivation-related impulsivity here. The same discordance is observed for the rs2471711 reference allele. One explanation is that the associations here may be spurious. It is also possible that the results are valid and somehow these genes have pleiotropic effects that override any direct effect of smoking chronicity on withdrawal-related impulsivity and confusion; this explanation is less plausible. Also, differences between this sample and the GSCAN GWA meta-analysis might affect genetic associations, including different LD patterns due to ancestral differences in genetic architecture.
The exploratory GWA found no associations with an allele rate >5% with p-values that exceeded the multiple-phenotype corrected significance threshold of P < 2.08E-9. Because this study was underpowered for GWA, we did a post-hoc exploration of the 93 variants (not shown) with p-values that exceeded the non-corrected traditional GWA significance threshold (5E-8). We cross-referenced these 93 variants with hits in the most recent, largest GWA of tobacco withdrawal symptoms in African Americans that used a survey retrospective report phenotype (Gelernter et al., 2015). There were no common polymorphisms.
We would be remiss if we did not mention the role of environment in smoking among African Americans. While smoking was historically less common in African Americans than other races, over time the race gap in smoking prevalence has closed, with the recent 2018 data showing higher prevalence of smoking in U.S. non-Hispanic African Americans than the general population (Jamal et al., 2018). The reason for temporal trends in disparities is obviously non-genetic and due to environmental changes that disproportionately reduce smoking in groups other than African Americans. Racism and other forms of societal disadvantage have been implicated in tobacco withdrawal in African Americans, as has been shown previously (Bello et al., 2020; Calixte-Civil, 2020).
This study has limitations. First, the study utilized a smaller sample that may not be powered to identify weaker signals. Second, we studied associations with previously identified variants not based on known associated loci studied within African Americans specifically; the candidate variants were from European Ancestry smokers. Third, we did not have a replication sample with the same phenotype data, leaving unclear whether the two significant associations were spurious. Fourth, we tested univariate linear regression analyses to examine associations with individual tobacco withdrawal outcomes one at a time because we aimed for detailed characterization of various subphenotypes within the withdrawal syndrome, each of which could potentially have non-overlapping genetic etiologies that could be obscured if the phenotypes were collapsed. Future research characterizing meta-phenotypes that take into account variable-centered or person-centered analyses to reduce phenotypic multidimensionality warrant consideration. Another limitation is that differing degrees of genetic population admixture has been shown to be a complicating factor in previous genetic association studies of alcohol and substance dependence (Zuo et al., 2009) and thus, may have impacted our findings in the present study.
In sum, the behavioral pharmacology endophenotype approach to characterizing tobacco withdrawal provided suggestive evidence that different tobacco sub-phenotypes might be modestly associated with some genetic loci in African Americans. However, the endophenotype approach was not robust enough to generate noticeably strong signals or clear replications of prior studies, with the caveats of the small sample and the lack of a priori candidate loci specific to African Americans.
Supplementary Material
Figure 1. Estimated phenotype means, by genotype, for significant associations with candidate genetic variants.
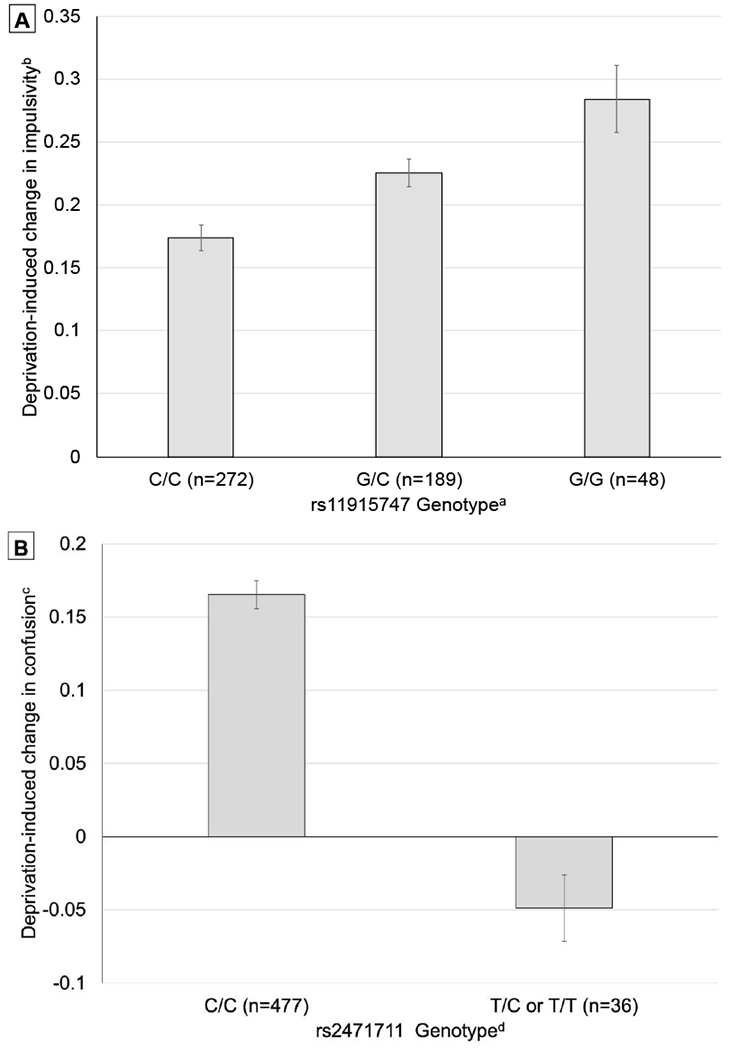
Panel A. Estimated Mean ± SE of deprivation-induced change in current impulsivity scale, by rs11915747 genotype in the CAD2M gene region, from linear regression adjusted for sex, and non-deprived current impulsivity scale score (corrected P-value = .0190).
aImputed genotype score, 0-0.49 = C/C, 0.5-1.49 = C/G, 1.5-2.0 = G/G.
bMean response to 13 statements reflecting state impulsive tendencies (e.g., “Feeling like doing or saying things without thinking.”) based feeling “right now” on 5-point Likert scales (0 = Not at all to 4 = Extremely, range: 0-4).
Panel B. Estimated Mean ± SE of deprivation-induced change in confusion score, by rs2471711 genotype in the AC097480.1/AC097480.2 region, from linear regression adjusted for sex, and non-deprived confusion score corrected P-value = .0486).
bMean response to 7 affective adjectives (e.g., “confusion” and “unable to concentrate”) from the Profile of Mood States based feeling “right now” on 5-point Likert scale (0 = Not at all to 4 = Extremely, range: 0-4).
dImputed genotype score, 0-0.49 = C/C, 0.5-1.49 = C/T, 1.5-2.0 = T/T. C/T (n=36) and C/C (n=1) were combined.
PUBLIC SIGNIFICANCE STATEMENTS.
This study is the first known to date to combine behavioral pharmacology with Genome-wide association (GWA) analysis in a large sample of African American smokers. Findings of this study demonstrated no statistically robust, biologically plausible, or historically consistent associations between any genetic variants and any laboratory-derived tobacco withdrawal phenotypes studied. Our study suggests it may be more effective to directly address social determinants of health in African Americans.
References
- Aguirre CG, Madrid J, & Leventhal AM (2015). Tobacco withdrawal symptoms mediate motivation to reinstate smoking during abstinence. Journal of Abnormal Psychology, 124(3), 623–634. doi: 10.1037/abn0000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameringer KJ, & Leventhal AM (2015). Psychological symptoms, smoking lapse behavior, and the mediating effects of nicotine withdrawal symptoms: A laboratory study. Psychology of Addictive Behaviors, 29(1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacio GA, Guzman IY, Shapiro JR, & Ray LA (2014). Differences in quit attempts between non-Hispanic Black and White daily smokers: The role of smoking motives. Addictive Behaviors, 39(12), 1769–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurley JW, Edlund CK, Pardamean CI, Conti DV, & Bergen AW (2016). Smokescreen: a targeted genotyping array for addiction research. BMC Genomics, 17, 145. doi: 10.1186/s12864-016-2495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello MS, Liautaud MM, De La Cerda JT, Pang RD, Ray LA, Ahluwalia JA, & Leventhal AM (2020). Association of frequency of perceived exposure to discrimination with tobacco withdrawal symptoms and smoking lapse behavior in African Americans. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrington H, … Williams B. (2013). Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry, 70(5), 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, & Munafò MR (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376. [DOI] [PubMed] [Google Scholar]
- Calixte-Civil PF (2020). The Effect of Acute Interpersonal Racial Discrimination on Smoking Motivation and Behavior among Black Smokers. [DOI] [PubMed]
- Cannon TD, & Keller MC (2006). Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology, 2, 267–290. [DOI] [PubMed] [Google Scholar]
- Conneely KN, & Boehnke M (2007). So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. The American Journal of Human Genetics, 81(6), 1158–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Control, C. f. D., & Prevention. (2005). Annual smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 1997-2001. MMWR. Morbidity and mortality weekly report, 54(25), 625. [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3(1), 7–16. [DOI] [PubMed] [Google Scholar]
- Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, … Neff L. (2019). Tobacco product use and cessation indicators among adults—United States, 2018. Morbidity and Mortality Weekly Report, 68(45), 1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DM, Visscher PM, & Wray NR (2009). Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Human Molecular Genetics, 18(18), 3525–3531. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, … Healton CG. (2008). Treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services. [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, & Biederer T (2007). SynCAMs organize synapses through heterophilic adhesion. Journal of Neuroscience, 27(46), 12516–12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei JA, Andermatt I, Gesemann M, & Stoeckli ET (2014). The SynCAM synaptic cell adhesion molecules are involved in sensory axon pathfinding by regulating axon–axon contacts. Journal of Cell Science, 127(24), 5288–5302. [DOI] [PubMed] [Google Scholar]
- Furberg H, Kim Y, Dackor J, Boerwinkle E, Franceschini N, Ardissino D, … Merlini PA (2010). Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature Genetics, 42(5), 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler HR, Sherva R, Almasy L, Herman AI, Koesterer R, … & Farrer LA. (2015). Genome-wide association study of nicotine dependence in American populations: identification of novel risk loci in both African-Americans and European-Americans. Biological psychiatry, 77(5), 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, & Le Marchand L (2006). Ethnic and racial differences in the smoking-related risk of lung cancer. New England Journal of Medicine, 354(4), 333–342. doi: 10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- Hughes JR (2006). Clinical significance of tobacco withdrawal. Nicotine & Tobacco Research, 8(2), 153–156. [DOI] [PubMed] [Google Scholar]
- Hughes JR (2007). Measurement of the effects of abstinence from tobacco: A qualitative review. Psychology of Addictive Behaviors, 21(2), 127. [DOI] [PubMed] [Google Scholar]
- Hällfors J, Palviainen T, Surakka I, Gupta R, Buchwald J, Raevuori A, … Madden PA. (2019). Genome-wide association study in Finnish twins highlights the connection between nicotine addiction and neurotrophin signaling pathway. Addiction Biology, 24(3), 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin Vidrine J, Reitzel LR, & Wetter DW (2009). The role of tobacco in cancer health disparities. Current Oncology Reports, 11(6), 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, & Neff LJ (2018). Current cigarette smoking among adults—United States, 2016. Morbidity and Mortality Weekly Report, 67(2), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, Smith AH, Herman AI, Farrer LA, Kranzler HR, Sofuoglu M, & Gelernter J (2017). A protocadherin gene cluster regulatory variant is associated with nicotine withdrawal and the urge to smoke. Molecular Psychiatry, 22(2), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahende JW, Malarcher AM, Teplinskaya A, & Asman KJ (2011). Quit attempt correlates among smokers by race/ethnicity. International Journal of Environmental Research and Public Health, 8(10), 3871–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Wedow R, Okbay A et al. (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50, 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Perkins KA, & Gould T (2009). Nicotine dependence endophenotypes in chronic smokers. Phenotypes, endophenotypes, and genetic studies of nicotine dependence. National Cancer Institute Monograph, 22. [Google Scholar]
- Leventhal AM (2017). Sociopharmacological Predictors of Tobacco Withdrawal Amongst African Americans. Paper presented at the 23rd annual scientific meeting of the Society for Research on Nicotine and Tobacco, Florence, Italy. [Google Scholar]
- Leventhal AM, Goldenson NI, Aguirre CG, Huh J, & Kirkpatrick MG (2019). Initial application of a human laboratory model for estimating the motivational substitutability of e-cigarettes for combustible cigarettes. Experimental and Clinical Psychopharmacology, 27(2), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, & Pickworth WB (2010). A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors, 35(12), 1120–1130. doi: 10.1016/j.addbeh.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnér RK, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, … & Beauchamp JP. (2019). Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nature genetics, 51(2), 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, … Twian C. (2019). Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nature Genetics, 51(2), 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA (2009). Developing human laboratory models of smoking lapse behavior for medication screening. Addiction Biology, 14(1), 99–107. doi: 10.1111/j.1369-1600.2008.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, & Droppleman L (1971). Manual for the profile of mood states (POMS). San Diego: Educational and Industrial Testing Service. [Google Scholar]
- Nagel M, Watanabe K, Stringer S, Posthuma D, & Van Der Sluis S (2018a). Item-level analyses reveal genetic heterogeneity in neuroticism. Nature communications, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Jansen PR, Stringer S, Watanabe K, De Leeuw CA, Bryois J, … & Posthuma D. (2018b). Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nature genetics, 50(7), 920–927. [DOI] [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Cox LS, Benowitz NL, Tyndale RF, Ellerbeck EF, … Ahluwalia JS. (2019). Factors that Explain Differences in Abstinence between Black and White Smokers: A Prospective Intervention Study. Journal of the National Cancer Institute. doi: 10.1093/jnci/djz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RD, Bello MS, Liautaud MM, Weinberger AH, & Leventhal AM (2019). Gender differences in negative affect during acute tobacco abstinence differ between African American and White adult cigarette smokers. Nicotine and Tobacco Research, 21(8), 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang RD, & Leventhal AM (2013). Sex differences in negative affect and lapse behavior during acute tobacco abstinence: a laboratory study. Experimental and Clinical Psychopharmacology, 21(4), 269–276. doi: 10.1037/a0033429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, & Reich D (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics, 38(8), 904–909. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, & Monti PM (2010). Subjective responses to alcohol consumption as endophenotypes: advancing behavioral genetics in etiological and treatment models of alcoholism. Substance use & misuse, 45(11), 1742–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, de Wit H, MacKillop J, & Palmer AA (2019). Genome-wide association studies of impulsive personality traits (BIS-11 and UPPS-P) and drug experimentation in up to 22,861 adult research participants identify loci in the CACNA1I and CADM2 genes. Journal of Neuroscience, 39(13), 2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, West R, & Gilbert D SRNT Work Group on the Assessment of Craving and Withdrawal in Clinical Trials.(2004). Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research, 6(4), 599–614. [DOI] [PubMed] [Google Scholar]
- Snaith P (1993). Anhedonia: a neglected symptom of psychopathology. Psychological Medicine, 23(4), 957–966. [DOI] [PubMed] [Google Scholar]
- Tate SK, & Goldstein DB (2004). Will tomorrow’s medicines work for everyone?. Nature Genetics, 36(11), S34–S42. [DOI] [PubMed] [Google Scholar]
- Verification, S. S. o. B. (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4(2), 149–159. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, & Baker TB (1999). Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology, 7(4), 354. [DOI] [PubMed] [Google Scholar]
- Zuo L, Luo X, Listman JB, Kranzler HR, Wang S, Anton RF, … & Gelernter J. (2009). Population admixture modulates risk for alcohol dependence. Human genetics, 125(5-6), 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


