ABSTRACT
Acceptance of COVID-19 vaccine among health-care workers (HCWs) is crucial for controlling the pandemic and ensuring HCW and patient safety. Information on the acceptance of different COVID-19 vaccines is lacking. Despite the United Arab Emirates (UAE) having vaccinated most of its population, vaccine acceptance still raises concerns. This study explores COVID-19 vaccine acceptance, vaccine choice, and associated factors among HCWs in the UAE. An online national cross-sectional study was conducted among 517 HCWs. Acceptance and choice of COVID-19 vaccines were assessed, and logistic regression analysis identified predictors for vaccine acceptance. More than half (58%) of HCWs were willing to take the vaccine and give it to their family. Reasons for taking the vaccine were concerns for families contracting COVID-19 (67%) and social responsibility (64%). Reasons for refusals included concerns with side-effects (61%). Most HCWs knew of the Pfizer (79%) and Sinopharm (57%) vaccines; however, acceptance was higher for Pfizer (35%) and AstraZeneca (21%) vaccines. Being male and being influenza vaccinated predicted willingness to take the vaccine (aOR: 2.34; 95% CI:1.34–4.08; p ≤ 0.001) and (aOR: 2.13; 95% CI: 1.29–3.51; p ≤ 0.001), respectively. HCWs who expressed concerns with inadequate safety data were less likely to take the vaccine (aOR: 0.17; 95% CI: 0.10–0.30; p ≤ 0.001). Additionally, side effects, perception of risk, and level of trust of company and country of manufacture predicted acceptance and choice of vaccines. Effective vaccine policy campaigns to improve acceptance should target HCW’s knowledge and awareness of perceived risks of COVID-19, safety data, social responsibility, and individual preferences for vaccine choice.
KEYWORDS: COVID-19, healthcare workers, acceptance, hesitancy, vaccine, Pfizer, AstraZeneca
Introduction
The Coronavirus Disease-19 (COVID-19) pandemic became a global health crisis after being identified in Wuhan, China, in December 2019. The virus, responsible for COVID-19 disease, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not as virulent as other viruses in the same family; however, it has high transmissibility.
The preventative measures currently in place (such as social distancing and quarantine) may slow the spread of (SARS-CoV-2) and flatten the epidemic curve; however, this is not sufficient to curb the spread of the virus completely.1 Herd immunity can be acquired by infection or vaccination, though the latter is a more effective method for controlling the disease with new variants. The control of COVID-19 heavily relies on developing a vaccine and administering it to the majority of the population to achieve sufficient vaccine coverage.1
The rapid infection rate of COVID-19 worldwide initiated the development of multiple vaccines within a short and rapid timeframe and as of 31st, January 2021, 63 and 174 candidate vaccines were in clinical and pre-clinical evaluation.2 The vaccines that gained media attention and received emergency approval to begin manufacture and dissemination globally during this time were, Sinopharm (China), Pfizer (BNT162b2; USA): Moderna RNA (mRNA-1273: USA), AstraZeneca (ChAdOx1 nCoV-19: Oxford University), Sputnik V (Gam-Covid-Vac: Russia), and Johnson and Johnson (adenovirus type 26 vector; Ad26.COV2-S: USA). In the UAE, Sinopharm was approved by the Ministry of Health and Prevention (MoHaP) for use on the 9thDecember 20203 with mass vaccination campaigns starting mid-December 2020. On 22 December 2020, MoHaP announced the emergency registration of Pfizer-BioNTech’s COVID-19 vaccine and on the next day, Dubai Health Authority (DHA) started its free vaccination drive against COVID-19.4 Other Emirates received approval for the use of Pfizer in April 2021.
Although immunization has succeeded in reducing the burden of diseases and mortality rates worldwide, acceptance of vaccines by the public remains compromised. This is attributed to vaccine hesitancy, leading to delay and refusal of vaccination and disease outbreaks. Patients trust and rely on healthcare workers (HCWs) for information about vaccines and vaccine-preventable diseases, particularly in the MENA region. Consequently, attitudes of HCWs toward vaccines are of utmost importance, and those with negative attitudes tend to recommend vaccines less regularly and vice versa. Vaccine uptake can be influenced by attitudes and knowledge of HCWs.5,6
Since HCWs are constantly in contact with COVID-19 patients, vaccination against COVID-19 becomes a priority to curb the spread of disease within hospitals and health institutions and ensure HCW safety. However, HCWs attitudes toward COVID-19 vaccines have varied globally, with vaccination acceptance among HCWs ranging between 27.7%-77.3%.7 In recent months studies conducted to explore COVID-19 vaccine hesitancy and associated factors in the MENA region have also reported varying levels of vaccine acceptance.8–11 Higher levels of vaccine acceptance among HCWs were found in the Kingdom of Saudi Arabia (KSA) (60%),12 Iran (58%),10 and Israel (70%),13 while lower levels were reported for Egypt (21%),11 and Palestine (38%).14 However, evidence from the United Arab Emirates (UAE) remains unclear. Moreover, none of these studies have attempted to assess perceptions of HCWs on the different types of vaccines and the reasons for choosing specific vaccine brands. This study attempts to fill this knowledge gap by exploring the factors influencing vaccine acceptance amongst HCWs in the UAE. We aim to examine their knowledge of existing COVID-19 vaccines, their attitudes regarding the safety, efficacy, and acceptability of these vaccines, their choice of vaccine, and their willingness to be vaccinated.
Materials and methods
Study design and data collection
Since data for this study were collected at a single point in time, a cross-sectional study design was used to achieve the objectives of this study.
A validated and structured self-administered questionnaire compromising 26 items covering aspects of HCW’s perceptions on COVID-19 vaccines was used. The questionnaire was adopted and slightly modified from a previously published study in KSA after obtaining the authors’ permission.12 Data were collected between 20th November 2020 and 3rd January 2021, just before and during the vaccine roll-out in the UAE. HCWs were divided into four groups: (1) Physicians (Consultant/specialist, general practitioner, and medical resident), (2) nurses, (3) pharmacy/laboratory staff and (4) others which comprised of paramedics, radiology technicians and ancillary staff. HCWs from across the UAE were invited to participate in the research via participating hospital e-mail lists and social media platform groups through an online survey hosted on SurveyMonkey (www.surveymonkey.com). Considering the need for a rapid data collection method to assess vaccine acceptance of HCWs during a critical and vulnerable period during the pandemic, we used convenience and snowball sampling to recruit HCWs from across the UAE to the study. HCWs were also asked to send the survey link to their colleagues. Before participation, the aim and objectives of the study were outlined, and HCWs’ confidentiality and anonymity were assured. Participants were able to ask questions via a dedicated e-mail address. Participants’ acceptance of continuing the survey indicated their willingness and consent for participation.
Sample size
We estimated a proportion of 60% of HCWs willing to take the vaccine based on a previous study investigating vaccine confidence and hesitancy among HCWs in Saudi Arabia.12 Using this proportion and a margin of error of 0.05, confidence level of 95%, and study power of 80%, the sample size required for this study was 363. To account for non-response, we increased the sample size by 20%, making the minimum sample size required 436 HCWs.
Data collection
The questionnaire was divided into six sections and collected sociodemographic data, history of chronic medical conditions, previous exposure or contact with COVID-19 patients or samples, previous influenza vaccination, willingness to take the COVID-19 vaccine, attitudes, and knowledge of COVID-19 vaccines, and factors contributing to acceptance and choice of vaccines. Questions were a combination of 5-item Likert scales, multiple-choice, and true/false responses and prepared in English as most HCWs have an excellent command of the English language. We piloted the final version of the survey among seven HCW colleagues to ensure clarity, consistency and face validity. We asked research and HCW expert colleagues to assess content validity of the questionnaire and to ensure that questions covered all aspects of the constructs being measured. The underlying constructs “attitude towards vaccines,” “Knowledge of vaccines” and “acceptance of vaccines” showed a high level of internal consistency with Cronbach’s alpha of 0.854, 0.793 and 0.82 respectively.
Variables
Demographic data comprised of age, gender, occupation, type of work setting, level of hospital/clinic and type of work unit which was categorized into 2 categories (1-high risk (isolation wards/ICU) and 2-low risk (general inpatient/outpatient/laboratory/Pharmacy/academics/and dental clinic)); health-related data (included previously influenza vaccinated, preexisting comorbidities, contact with COVID-19 patients, and previous infection with COVID); information about and reasons for participants willingness to take the vaccines, (worried about contracting COVID-19 themselves, worried about their children/family members contracting COVID-19, self-perception of being high risk for developing complications from COVID-19 if infected, sense of social responsibility toward taking the vaccine); reasons for not willing to take the vaccine; factors important for choosing type of COVID vaccine (including perceptions of reliability, availability, better vaccine strategy/technology used, manufacturing company’s side effects, media coverage, and personal preference).
Knowledge about the vaccine
HCWs knowledge of COVID-19 vaccines was assessed by whether they knew of or did not know any of the seven vaccines available. A score was generated by summing responses for all vaccines ranging between 7 and 14 (mean = 10.33 ± SD 2.04). Good knowledge of available vaccines was defined as HCWs who scored above the mean and poor knowledge below the mean.
Attitude toward vaccine
Attitude toward COVID-19 vaccines was measured on a 5-point Likert scale. Attitude questions covered HCWs responses to how likely they believed the vaccine would stop the pandemic, the safety of the COVID vaccine, and whether the vaccine could avoid complications. Attitude response scores were normally distributed and ranged between 3 and 15. HCWs who scored above the mean (mean = 10.65 ± SD 2.55) were classified as having a positive attitude toward COVID-19 vaccines. HCWs with scores below the mean were classified as having a negative attitude.
Data analysis
Data were analyzed using SPSS, version 27 (IBM Corp., Armonk, NY, USA). Continuous and categorical data were analyzed using descriptive statistics (frequency and percentages). Chi-square (χ2) test was used to determine the association between the two dependent variables (HCW’s willingness to take the vaccine this year and the likelihood of recommending it to their family members) and independent variables (medical and sociodemographic characteristics, occupation, contact cases, influenza vaccine, reasons for not taking the vaccines, reasons for taking the vaccine, attitude about vaccines, and knowledge attitude about vaccines).
Chi-square (χ2) test was also used to study the association between acceptance of the Pfizer and Sinopharm vaccines and medical and sociodemographic characteristics, occupation, contact cases, influenza vaccine, attitude about vaccines, and factors affecting the choice of a vaccine.
Statistically significant factors in the univariate analysis were included in the multivariable logistic regression to predict willingness of HCWs to take the vaccine and willingness to recommend it to their family members. The estimates of the strength of associations were demonstrated by the Odds ratio (OR) with a 95% confidence interval (CI). A two-tailed p < .05 was considered statistically significant.
Results
In total, we received 735 responses from HCWs across the UAE. Complete data from 517 responses were analyzed, giving this study a completion rate of (70%).
Among the 517 respondents, almost half of the HCWs were aged 25 to 34 years (44%), were predominantly females (64%), and from the emirate of Sharjah (36%) The majority of HCWs in our study were physicians (53%) followed by pharmacists/laboratory technicians (23%) and worked in governmental/public hospitals (47%). The majority were healthy and reported they did not suffer from any chronic medical condition (86%). Most participants (81%) reported they had been in contact with COVID-19 patients, and 11% reported being infected with COVID-19. Almost half (47%) indicated they were influenza vaccinated (Table 1).
Table 1.
Socio-demographic and medical characteristics of HCWs, UAE, January 2021
| Variable |
Frequency (N) |
Percent (%) |
| Age group (Years) | ||
| 45 and above | 70 | 13.54 |
| 35–44 | 121 | 23.40 |
| 25–34 | 227 | 43.91 |
| 18–24 | 99 | 19.15 |
| Gender | ||
| Female | 330 | 63.83 |
| Male | 187 | 36.17 |
| HCW profession | ||
| Physician | 273 | 52.80 |
| Nurse | 51 | 9.86 |
| Pharmacist/Laboratory HCWs | 121 | 23.40 |
| Others | 72 | 13.93 |
| Emirate | ||
| Abu Dhabi | 101 | 19.54 |
| Dubai | 157 | 30.36 |
| Sharjah | 184 | 35.59 |
| Other | 75 | 14.51 |
| Type of work setting | ||
| Private/Others | 160 | 30.91 |
| Public/ Government | 242 | 46.81 |
| University Hospital | 115 | 22.24 |
| Chronic medical conditions | ||
| No | 442 | 85.49 |
| Yes | 75 | 14.51 |
| Contact with COVID-19 patients | ||
| No | 100 | 19.34 |
| Yes | 417 | 80.66 |
| Had COVID-19 infection | ||
| No | 461 | 89.17 |
| Yes | 56 | 10.83 |
| Had Influenza shot | ||
| No | 272 | 52.61 |
| Yes | 245 | 47.39 |
| Total | 517 |
Willingness to take the vaccine/give it to family: Around half of participants indicated their willingness to take the COVID-19 vaccine and recommend it to their families (58% and 52%, respectively). Among those willing to take the vaccine, 57% indicated they were ready to take it as soon as possible, while 43% stated they would delay it for a few months (Figure 1).
Figure 1.
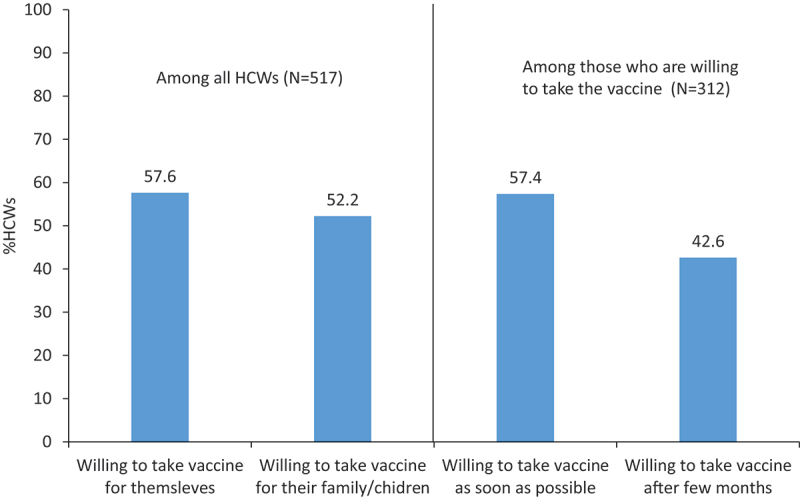
Willingness to take the vaccine/give it to family and preferred timing of vaccine UAE, January 2021.
The reasons HCWs were willing to take the COVID-19 vaccine or not once available are outlined in Table 2. Among those who wanted to take the vaccine, the most frequently reported reason was worry and concern for family members, including children, contracting the disease 67% (n = 208). The second most common reason was a sense of social responsibility 64% (n = 199). Among those who did not want to take the vaccine, the majority expressed concerns about side effects (61%), followed by worries of vaccine ineffectiveness (29%), low-risk perception (16%), and avoided vaccines/ medicines in general (15%) (Table 2).
Table 2.
Reasons cited for taking or not taking the COVID-19 vaccine among HCWs, UAE, January 2021
| |
Frequency |
Percent |
| Reasons cited among those who want to take vaccine (N = 312) | ||
| Worried about contracting COVID-19 myself | 146 | 46.79 |
| Worried about my children/family members contracting COVID-19 | 208 | 66.67 |
| Perceive myself as being high risk to develop complications from COVID-19 | 70 | 22.44 |
| Feels sense of social responsibility to take the vaccine | 199 | 63.78 |
| Reasons cited among those who do not want to take vaccine (N = 205) | ||
| Inadequate data about the safety of vaccine | 172 | 83.9 |
| Already infected with COVID-19 so don’t need | 17 | 8.29 |
| Avoid vaccines/ medicines in general | 31 | 15.12 |
| Concerned about side effects | 125 | 60.98 |
| Concerned about the vaccine being ineffective | 60 | 29.27 |
| Prior adverse reaction to vaccines | 16 | 7.8 |
| Don’t perceive myself at high risk | 32 | 15.61 |
| Others | 9 | 4.39 |
Knowledge of the type of vaccines
The vaccine most participants knew about was the Pfizer RNA vaccine (79%), followed by Sinopharm (China) (57%) and AstraZeneca (52%). The least known vaccine to HCWs was Novavax (USA) (23%), followed by Johnson and Johnson (28%) as shown in Figure 2. Although physicians reported better knowledge of the different vaccine brands (53.1%) compared to nurses (35.1%) and pharmacists/laboratory workers (49.6%), these differences were not significant χ2 (3, N = 517) = 5.48, p = .14. Similarly, no significant differences were found between type of clinic (private/governmental/public) and knowledge of different vaccine brands χ2 (3, N = 517) = 3.33, p = .189. The most accepted vaccine by participants was Pfizer RNA (35%), followed by AstraZeneca (21%). Only 4% of HCWs indicated they would accept the Sputnik (Russia) vaccine. (Figure 2).
Figure 2.
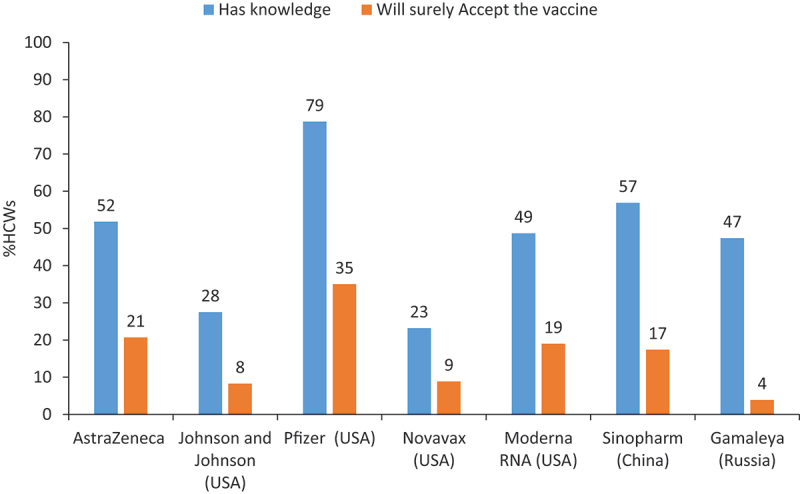
Knowledge and acceptance of type of vaccines among HCWs, UAE, January 2021.
Univariate analysis
Chi-square analysis examined the association between participants’ willingness to take COVID-19 vaccine and choice of vaccine type. Detailed results are provided in the appendix (Appendix Tables A1 and A2). Results suggest a significant association between willingness to take COVID-19 vaccine and age χ2 (3, N = 517) = 21.25, p < .01; gender χ2 (1, N = 517) = 20.11, p < .01; influenza vaccinated χ2 (1, N = 517) = 30.11, p < .01; knowledge about vaccines χ2 (1, N = 517) = 9.94, p < .01; attitude toward vaccine χ2 (1, N = 517) = 124.11, p < .01; inadequate safety data χ2 (1, N = 517) = 111.06, p < .01; previous COVID-19 infection χ2 (1, N = 517) = 7.70, p < .01; overall vaccine avoidance χ2 (1, N = 517) = 33.73, p < .01; concerns about side effects χ2 (1, N = 517) = 72.44, p < .01; concern about vaccine ineffectiveness χ2 (1, N = 517) = 32.65, p < .01; and low risk perception χ2 (1, N = 517) = 33.24, p < .01. No significant associations were found between willingness to take a vaccine and HCW profession, emirate or work setting (Appendix Table A1).
Similarly, willingness for their family to be vaccinated were associated with, age χ2 (3, N = 517) = 12.63, p < .01, gender χ2 (1, N = 517) = 10.10, p < .01, influenza vaccinated χ2 (1, N = 517) = 15.11, p < .01; knowledge about vaccines χ2 (1, N = 517) = 6.73, p < .01; attitude toward vaccine χ2 (1, N = 517) = 131.87, p < .01; inadequate safety data χ2 (1, N = 517) = 95.21, p < .01; previous COVID-19 infection χ2 (1, N = 517) = 4.55, p < .05; avoid vaccines/ medicines in general χ2 (1, N = 517) = 22.05, p < .01; concerns about side effects χ2 (1, N = 517) = 58.58, p < .01; concerns about vaccine ineffectiveness χ2 (1, N = 517) = 24.76, p < .01; and low risk perception χ2 (1, N = 517) = 25.09, p < .01. For HCWs preference for taking the Pfizer or Sinopharm vaccines, significant associations were found between age, gender, work setting, influenza vaccinated, attitude about vaccines, and choice of vaccine. HCWs profession, and emirate were not associated with preference for either the Pfizer or Sinopharm vaccines. (Appendix Table A2).
Multiple logistic regression
All independent variables that showed significant associations with the dependent variables in the univariate analysis were included in the logistic regression model. Predictors for HCWs’ willingness to take the COVID-19 vaccine and their willingness to recommend it to family members are shown in Table 3. Male HCWs (aOR:2.34;95% CI: 1.34–4.08), being influenza vaccinated (aOR: 2.13; 95% CI: 1.29–3.51), and positive attitude toward vaccines (aOR:6.36; 95% CI: 3.67–11.02) had higher odds of willingness to take the vaccine. HCWs who indicated they were worried about the inadequate safety data available (aOR: 0.17; 95% CI: 0.10–0.30), those who avoided vaccines/medicines (aOR: 0.30; 95% CI: 0.10–0.88), those who were concerned about side effects (aOR:0.50; 95% CI: 0.29–0.88), and those who don’t perceive themselves at higher risk (aOR:0.11; 95% CI: 0.03–0.35) had lower odds of willing to take the vaccine. As for willingness to give the vaccine to their families, those with a positive attitude toward vaccines (aOR:6.72; 95% CI: 4.13–10.91) had higher odds of potentially giving the vaccine to family/children, whereas those who indicated inadequate data about the safety of vaccine (aOR:0.25; 95% CI: 0.16–0.42) had lower odds of giving the vaccine to family/children. (Table 3).
Table 3.
Predictors for HCWs taking the vaccine or giving it to family/children, UAE, January 2021
| Yes, I will take it |
Yes, I will give it to my family/children |
||||
| |
|
aOR |
95%CI |
aOR |
95%CI |
| Age | 45 and above | 1 | 1 | ||
| 35–44 | 1.81 | 0.75,4.38 | 0.76 | 0.35,1.68 | |
| 25–34 | 0.92 | 0.41,2.05 | 0.69 | 0.33,1.45 | |
| 18–24 | 1.68 | 0.69,4.12 | 1.05 | 0.45,2.42 | |
| Gender | Female | 1 | 1 | ||
| Male | 2.34* | 1.34,4.08 | 1.39 | 0.85,2.28 | |
| Had influenza shot | No | 1 | 1 | ||
| Yes | 2.13* | 1.29,3.51 | 1.23 | 0.78,1.95 | |
| Knowledge about vaccines | Poor knowledge | 1 | 1 | ||
| Good knowledge | 1.55 | 0.95,2.53 | 1.31 | 0.83,2.06 | |
| Attitude about vaccine | Negative attitude | 1 | 1 | ||
| Positive attitude | 6.36* | 3.67,11.02 | 6.72* | 4.13,10.91 | |
| Reasons cited for taking COVID-19 vaccine | |||||
| Inadequate data about the safety of vaccine | No | 1 | 1 | ||
| Yes | 0.17* | 0.10,0.30 | 0.25* | 0.16,0.42 | |
| Already infected with covid-19 | No | 1 | 1 | ||
| Yes | 0.34 | 0.12,1.01 | 0.58 | 0.21,1.56 | |
| Avoid vaccines/ medicines in general | No | 1 | 1 | ||
| Yes | 0.30* | 0.10,0.88 | 0.53 | 0.20,1.39 | |
| Concerned about side effects | No | 1 | 1 | ||
| Yes | 0.50* | 0.29,0.88 | 0.65 | 0.38,1.10 | |
| Concerned about the vaccine being ineffective | No | 1 | 1 | ||
| Yes | 0.79 | 0.41,1.53 | 0.78 | 0.41,1.47 | |
| Prior adverse reaction to vaccines | No | 1 | 1 | ||
| Yes | 2.5 | 0.92,6.74 | 2.15 | 0.84,5.50 | |
| Don’t perceive myself at high risk | No | 1 | 1 | ||
| Yes | 0.11* | 0.03,0.35 | 0.18* | 0.06,0.54 | |
| Others | No | 1 | 1 | ||
| Yes | 0.19* | 0.04,0.85 | 1.05 | 0.32,3.44 | |
| Pseudo R-squared | 0.40 | 0.31 | |||
*indicates p-value <.05.
The male gender (aOR: 1.85; 95% CI: 1.16–2.97), those working in public/government settings (aOR:2.02; 95% CI: 1.17–3.48), positive attitude (aOR:3.70; 95% CI: 2.28–6.02), believing the vaccine was more reliable (aOR:2.95; 95% CI: 1.76–4.95), and overall trust of the company’s reputation/manufacturing country (aOR:6.83; 95% CI: 4.18–11.14) had higher odds of choosing the Pfizer vaccine. Those worried about the vaccine’s side effects were less likely to choose Pfizer (aOR: 0.43; 95% CI: 0.24–0.75).
As for choosing the Sinopharm vaccine, the male gender (aOR: 2.06; 95% CI: 1.21–3.52), a positive attitude toward vaccines (aOR:2.75; 95% CI: 1.55–4.88), and the vaccines’ availability (aOR:3.45; 95% CI: 2.00–5.95) were significant predictors. Participants were less likely to choose Sinopharm due to the company’s reputation/manufacturing country (aOR: 0.54; 95% CI: 0.31–0.92) (Table 4).
Table 4.
Predictors for choice of Pfizer and Sinopharm among HCWs, UAE, January 2021
| Pfizer |
Sinopharm |
||||
| |
|
aOR |
95%CI |
aOR |
95%CI |
| Age | 45 and above | 1 | 1 | ||
| 35–44 | 1.38 | 0.63,3.01 | 1.49 | 0.68,3.30 | |
| 25–34 | 0.94 | 0.45,2.00 | 0.61 | 0.27,1.37 | |
| 18–24 | 1.09 | 0.46,2.60 | 0.97 | 0.37,2.54 | |
| Gender | Female | 1 | 1 | ||
| Male | 1.85* | 1.16,2.97 | 2.06* | 1.21,3.52 | |
| Type of work setting | Private + other | 1 | 1 | ||
| Public/ Government | 2.02* | 1.17,3.48 | 1.18 | 0.64,2.18 | |
| University Hospital | 1.03 | 0.54,1.95 | 1.08 | 0.52,2.24 | |
| Had influenza shot | No | 1 | 1 | ||
| Yes | 0.88 | 0.55,1.42 | 1.3 | 0.75,2.24 | |
| Has Chronic morbidity | No | 1 | 1 | ||
| Yes | 0.59 | 0.30,1.18 | 1.64 | 0.81,3.31 | |
| Attitude about vaccine | Negative attitude | 1 | 1 | ||
| Positive attitude | 3.70* | 2.28,6.02 | 2.75* | 1.55,4.88 | |
| Important factors for choosing COVID-19 vaccine type | |||||
| This COVID-19 vaccine seems more reliable | No | 1 | 1 | ||
| Yes | 2.95* | 1.76,4.95 | 1.04 | 0.59,1.85 | |
| This vaccine is available | No | 1 | 1 | ||
| Yes | 1.24 | 0.74,2.09 | 3.45* | 2.00,5.95 | |
| Like the vaccine strategy/technology used | No | 1 | 1 | ||
| Yes | 0.75 | 0.46,1.22 | 0.93 | 0.53,1.63 | |
| Due to company’s reputation/Manufacturing country | No | 1 | 1 | ||
| Yes | 6.83* | 4.18,11.14 | 0.54* | 0.31,0.92 | |
| Less side effects from this vaccine | No | 1 | 1 | ||
| Yes | 0.43* | 0.24,0.75 | 0.72 | 0.38,1.37 | |
| Media Coverage | No | 1 | 1 | ||
| Yes | 0.62 | 0.34,1.10 | 0.94 | 0.47,1.85 | |
| Personal preference | No | 1 | 1 | ||
| Yes | 0.98 | 0.58,1.66 | 0.74 | 0.39,1.43 | |
| Others | No | 1 | 1 | ||
| Yes | 0.77 | 0.32,1.83 | 1.16 | 0.47,2.86 | |
| Pseudo R-squared | 0.27 | 0.18 | |||
*indicates p-value <.05.
Discussion
Vaccination is one of the most significant advances in public health; it is responsible for eradicating and controlling various infectious diseases.15 Since the announcement of the COVID-19 pandemic, scientists have been competing to develop a vaccine. Yet, there remains widespread public hesitancy and concerns regarding vaccine safety and concerns around adverse effects associated with vaccines. Healthcare workers are at the forefront and highly regarded by the population in eliminating some of these concerns. Therefore, it is necessary to understand HCW’s acceptance of COVID-19 vaccines and the predictors associated with vaccine acceptance since they can provide essential information about vaccines to the general population.16 To the best of our knowledge, this is one of the first studies exploring HCWs acceptance of COVID-19 vaccinations in the UAE and assessing determinants and predictors for choice of vaccine brands.
In this study, although more than half of HCWs were willing to take the vaccine, only half were ready to take it as soon as possible. A considerable proportion were hesitant in taking the vaccine immediately which is consistent with recent literature from the MENA region.11–14 Recent studies have reported that HCWs are uncertain about the safety of vaccines and would prefer to wait until they can review safety data before being vaccinated.17
Gaining reliable knowledge on COVID-19 vaccines can be challenging, especially with the rapid development of these vaccines and diverse types and different technology platforms. More than half of the HCWs in our study were aware of the different vaccines, namely, Pfizer, Sinopharm, AstraZeneca, Moderna RNA, and Sputnik V. Interestingly; most participants were aware of the Pfizer vaccine. These results are interesting considering that SinoPharm was the first vaccine approved by the UAE government. This could be because at the time of data collection for this research; the UAE had also offered the Pfizer vaccine in addition to the Chinese Sinopharm vaccine to its residents free of charge.18 Less than a third were aware of the Johnson and Johnson and Novavax vaccines. These results are consistent with research from Saudi Arabia where HCWs knowledge of the different types of vaccines were found to be low.12 Only forty percent of Saudi HCWs were aware of the AstraZeneca (ChAdOx1 nCoV-19), and only one-third were aware of the Pfizer (BNT162b2), Sputnik V (Gam-COVID-Vac), and Johnson and Johnson (Ad26.COV2-S) vaccines.19 The low percentage in the knowledge and understanding of different vaccines highlights the importance of conducting further studies to explore variables contributing to the acceptance or refusal of each type of vaccine. This understanding would aid policymakers in developing appropriate educational materials to boost confidence in various vaccine platforms.
Previous studies have reported that men are more likely to accept various vaccines than women.20,21 This was also the case in our study, where males had a higher COVID-19 vaccine acceptance than females. The higher acceptance among men might be because men had higher knowledge scores i.e., more men knew about types of vaccines than women. This is consistent with studies conducted in the Congo, KSA, France, and Indonesia, where men reported higher acceptance of COVID-19 vaccines than women.19,20,22,23 Many gender-based health diseases such as cardiovascular disease, chronic respiratory disease, and cancer have been broadly reviewed.24 Moreover, several reports have revealed higher risks for COVID-19 complications and death among males.25 The gender-based difference in COVID-19 mortality, as well as other diseases, may explain why males are more likely to indicate a willingness to take the vaccine, in addition to their perceived role as the paternal figure in their family and sense of social responsibility.
Exploring attitudes toward the COVID-19 vaccine is crucial since it is considered a pivotal construct to adapt and maintain behaviors. It is often regarded as a precursor to behavior change.26 HCWs with positive attitudes, those who believed that the COVID-19 vaccine was a scientific achievement, and those who believed the vaccine to be a reliable tool for preventing the infection were more likely to accept taking the vaccine. These positive attitudes, beliefs, and perceptions toward COVID-19 vaccines have also been reported to influence vaccine acceptance.17,23 Additionally, those who had taken the influenza vaccine were also more willing to take the COVID-19 vaccine. This highlights the previously reported association found between acceptance of the COVID-19 vaccine and overall vaccine acceptance and hesitancy.27,28 The WHO views vaccine hesitancy as one of the top ten global health threats.27 Despite scientific evidence for the success of large-scale vaccination campaigns being well established, some individuals remain hesitant to accepting vaccines making this a challenge for achieving the desired herd immunity within a community.
Perception of risk is also another factor which plays a significant role in the acceptance of vaccines. In this study, we found that those with higher perceived risk of contracting the virus were more likely to accept the vaccine than those with lower perceived risk. These results are consistent with previous studies conducted in Saudi Arabia and Indonesia.9,22 This highlights the importance of educating HCWs of the risks involved in their profession and their contribution in enhancing social responsibility among fellow HCWs and acceptance of the vaccines.
A significant proportion of HCWs reported frequent concerns about adverse safety, side-effects and vaccine ineffectiveness of the new vaccines. HCWs with these concerns were less willing to take the COVID-19 vaccine. Similar concerns were also reported in an Australian28 and European study29 where concerns and doubts for the safety and adverse effects from the vaccines were outlined. Doubts about vaccine safety have often been reported as the main obstacle to vaccination decision-making, particularly for newly introduced vaccines which have not been completely tested in the real world.30 These doubts can consistently affect the vaccine uptake27 which may cause challenges in achieving the vaccination coverage required for population immunity.
The choice of vaccine was significantly associated with gender and positive attitude. Males and having a positive attitude were both factors for choosing either the Pfizer or Sinopharm vaccines. HCWs who considered reliability and company/country’s reputation to be important factors were more likely to choose Pfizer. Whereas those who considered availability as an important factor were more likely to choose Sinopharm. Our study also found that HCWs who were to choose a vaccine based on the company/country’s reputation were less likely to choose Sinopharm. At the time of conducting this study, Sinopharm was not yet approved by the WHO which may have influenced HCWs opinion of the Sinopharm and their choice of vaccine.
Due to the urgency of the COVID-19 pandemic, the development of vaccines has been different. To date, the development of COVID-19 vaccines has been rapid with several vaccines on the market. These vaccines are different in their technological mechanism, number of required doses, safety and efficacy rates. In the absence of uniformity in the efficacy rates or side-effects due to these vaccines, people are voicing their preferences depending on the factors they deem important. In our study, HCWs have cited different reasons for preferring one vaccine over another: HCWs chose Pfizer due to company/country’s reputation and Sinopharm due to availability. It can be assumed that having more choices and allowing HCWs and the general public to choose their vaccine based on their preferences might increase the uptake of vaccines. To date, COVID-19 vaccinations remain optional and have not been mandated for the general population nor HCWs, however; health care institutions highly recommend and encourage HCWs to be vaccinated.31 Although changes in vaccine policies may influence vaccine acceptance, it is essential that HCWs are well informed about their decision to vaccinate and their choice of vaccines. Incorporating and acknowledging individual preferences into vaccination drives will allow individuals to make informed choices hence reducing vaccine hesitancy and ensuring the complete roll-out of vaccination drives.
Strengths and limitations
This is one of the first studies in the UAE studying HCWs acceptance of COVID-19 vaccinations. Results from this study can be used as baseline data for future studies exploring vaccine hesitancy. A major strength of this study is the large number of independent variables that were included. This resulted in many significant associations that could aid in understanding HCW hesitancy toward COVID-19 vaccines. Moreover, our population was diverse with representation from both genders, different age groups, area of residence, marital status, occupation, and practice type.
However, the study also had several limitations. Firstly, the convenience sampling method limits the generalizability of the findings which may not be representative of all HCWs in the UAE. However, with the need for a rapid method to assess vaccine acceptability within a vulnerable population during a rapidly evolving pandemic, convenience sampling is a promising method for quick results. Furthermore, the UAE is a multi-ethnic population and data on ethnicity would have added valuable information on the role of ethnic and cultural backgrounds on vaccine acceptance and should be included in future studies. Most importantly, our study was conducted when information about COVID-19 vaccines was limited and had not been made public. As such, it is possible that the results from this study are time sensitive and may change as new information becomes publicly available. Further research exploring the long-term acceptance of vaccines with new information is required.
Conclusion
This study is among the first to document HCWs acceptance of vaccines in the UAE as well as provide an insight into HCWs choice of COVID-19 vaccine among a sub-group of the UAE population. More than half of HCWs were willing to take the vaccine and give it to their families, however, a substantial proportion expressed high levels of worry and concern associated with vaccine safety and adverse effects. Additionally, this study found a low percentage in knowledge and understanding of different vaccine brands highlighting the need for follow-up studies to explore factors contributing to acceptance or refusal of specific vaccine types. Acceptance of COVID-19 vaccination among HCWs is essential given the critical role HCWs can play in future vaccination drives. Furthermore, with recent reports suggesting the need for booster shots and the potential for COVID-19 vaccines becoming annual vaccines, adherence and compliance with routine vaccinations will be necessary. Future vaccination campaign strategies should be tailored to address HCWs concerns, and worries, as well as compliment public health and educational interventions to boost vaccine knowledge. Additionally, acknowledging individual preferences for choice of vaccine and the provision of reliable and scientific resources on different types of vaccines may tackle vaccine hesitancy and increase vaccine uptake.
Supplementary Material
Funding Statement
The research is supported by grants from, Clinical Epidemiology Research group operational grant [Grant code: 150389]; COVID-19 research grant [CoV19-0301] to BS, University of Sharjah, UAE; COVID-19 research grant [CoV19-0307], and collaborative research grant [Grant code: 2001090278] to RH, University of Sharjah, UAE; and by Prince Abdullah Ben Khalid Celiac Disease Research Chair, under the Vice Deanship of Research Chairs, King Saud University, Riyadh, Kingdom of Saudi Arabia.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
BS: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Funding acquisition, Supervision. NA: Methodology, Formal analysis, Writing – review & editing. AS: Methodology, Formal analysis, Writing – review & editing. HB: Methodology, Writing – original draft, Writing – review & editing. HHA: Writing – review & editing. NSA: Writing – review & editing. MHT: Conceptualization, Methodology, Writing – review & editing, Funding acquisition. RB: Resources, Writing – review & editing. QH: Resources, Writing – review & editing. RH: Conceptualization, Methodology, Resources, Writing – review & editing, Funding acquisition, Supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethical statement
The study was approved by the Research Ethics Committee at the University of Sharjah on November 12, 2020 (reference no. REC 20-03-03-02) and the Ethics and Research Committee at the University Hospital of Sharjah (UHS-HERC- 032-26032020). HCWs consented prior to participation.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2021.1994300
References
- 1.Gadoth A, Halbrook M, Martin-Blais R, Gray A, Tobin NH, Ferbas KG, Aldrovandi GM, Rimoin AW.. Cross-sectional assessment of COVID-19 vaccine acceptance among health care workers in Los Angeles. Ann Intern Med. 2021;174(6):882–85. doi: 10.7326/M20-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . COVID-19 vaccine tracker and landscape; 2021. [accessed 2021 Jan 1]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 3.Cyranoski D. Arab nations first to approve Chinese COVID vaccine - despite lack of public data. Nature. 2020;588(7839):548. doi: 10.1038/d41586-020-03563-z. [DOI] [PubMed] [Google Scholar]
- 4.MOHAP Ministry of Health and Prevention . Vaccines against COVID-19 in the UAE; 2021. [accessed 2021 July 7]. https://u.ae/en/information-and-services/justice-safety-and-the-law/handling-the-covid-19-outbreak/vaccines-against-covid-19-in-the-uae.
- 5.Connors CM, Miller NC, Fau - Krause VL, Krause VL. Universal hepatitis B vaccination: hospital factors influencing first-dose uptake for neonates in Darwin. Aust N Z J Public Health. 1998;22(1):143–45. doi: 10.1111/j.1467-842X.1998.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 6.Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9(8):1763–73. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Luo Y, Watson R, Zheng Y, Ren J, Tang J, Chen Y. Healthcare workers’ (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Medical Journal. 2021.. doi: 10.1136/postgradmedj-2021-140195. Epub ahead of print. PMID: 34193545. [DOI] [PubMed] [Google Scholar]
- 8.Qattan AMN, Alshareef N, Alsharqi O, Al Rahahleh N, Chirwa GC, Al-Hanawi MK. Acceptability of a COVID-19 vaccine among healthcare workers in the Kingdom of Saudi Arabia. 2021;8:644300. doi: 10.3389/fmed.2021.644300. PMID: 33732723; PMCID: PMC7959705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Mohaithef M, Padhi BK. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. J Multidiscip Healthc. 2020;13:1657–63. doi: 10.2147/JMDH.S276771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Askarian M, Fu L, Taghrir MH, Borazjani R, Shayan Z, Taherifard E, Taherifard E, Abarialiabad H, Longtin Y, Askarian A, et al. Factors affecting COVID-19 vaccination intent among Iranians: COVID-19 vaccination acceptance. [available 2020 December 3]. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3741968 [Google Scholar]
- 11.Fares S, Elmnyer MM, Mohamed SS, Elsayed R. COVID-19 vaccination perception and attitude among healthcare workers in Egypt. J Prim Care Community Health. 2021;12:21501327211013303. doi: 10.1177/21501327211013303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barry MA, Temsah M-H, Alhuzaimi AN, Alamro N, Al-Eyadhy A, Aljamaan F, Saddik B, Alhaboob AA, Alsohime F, Alhasan KA, et al. COVID-19 vaccine confidence and hesitancy among healthcare workers: a cross-sectional survey from a MERS-CoV experienced nation. medRxiv. 2020. 2020.2012.2009.20246447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dror AA, Eisenbach N, Taiber S, Morozov NG, Mizrachi M, Zigron A, Srouji S, Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–79. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maraqa B, Nazzal Z, Rabi R, Sarhan N, Al-Shakhra K, Al-Kaila M. COVID-19 vaccine hesitancy among health care workers in Palestine: a call for action. Prev Med. 2021;149:106618–106618. doi: 10.1016/j.ypmed.2021.106618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubé E. Addressing vaccine hesitancy: the crucial role of healthcare providers. Clin Microbiol Infect. 2017;23(5):279–80. doi: 10.1016/j.cmi.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360(19):1981–88. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 17.Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, Barrett E, Pal S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines. 2021;9(2):2. doi: 10.3390/vaccines9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turak N. Dubai is offering the Pfizer vaccine to residents for free in addition to China’s Sinopharm shot. HEALTH AND SCIENCE Web site; 2020. updated 2020 Dec 23 [accessed 2021 Mar 17]. https://www.cnbc.com/2020/12/23/dubai-offering-pfizer-sinopharm-covid-vaccines-to-residents-for-free.html.
- 19.Temsah M-H, Barry M, Aljamaan F, Alhuzaimi A, Al-Eyadhy A, Saddik B, Alrabiaah A, Alsohime F, Alhaboob A, Alhasan K, et al. Adenovirus and RNA-based COVID-19 vaccines’ perceptions and acceptance among healthcare workers in Saudi Arabia: a national survey. BMJ Open. 2021;11(6):e048586. doi: 10.1136/bmjopen-2020-048586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pulcini C, Massin S, Launay O, Verger P. Factors associated with vaccination for hepatitis B, pertussis, seasonal and pandemic influenza among French general practitioners: a 2010 survey. Vaccine. 2013;31(37):3943–49. doi: 10.1016/j.vaccine.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 21.Jones M, Cook R. Intent to receive an HPV vaccine among university men and women and implications for vaccine administration. J ACH. 2008;57(1):23–32. doi: 10.3200/JACH.57.1.23-32. [DOI] [PubMed] [Google Scholar]
- 22.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, Setiawan AM, Rajamoorthy Y, Sofyan H, Mudatsir M, et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Front Public Health. 2020;8:381. doi: 10.3389/fpubh.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabamba Nzaji M, Kabamba Ngombe L, Ngoie Mwamba G, Banza Ndala DB, Mbidi Miema J, Luhata Lungoyo C, Lora Mwimba B, Cikomola Mwana Bene A, Mukamba Musenga E. Acceptability of vaccination against COVID-19 among healthcare workers in the Democratic Republic of the Congo. Pragmatic Obs Res. 2020;11:103–09. doi: 10.2147/POR.S271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ (Clinical Research Ed). 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- 25.Galbadage T, Peterson BM, Awada J, Buck AS, Ramirez DA, Wilson J, Gunasekera RS. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. Front Med. 2020;7:348. doi: 10.3389/fmed.2020.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glasman LR, Albarracín D. Forming attitudes that predict future behavior: a meta-analysis of the attitude-behavior relation. Psychol Bull. 2006;132(5):778–822. doi: 10.1037/0033-2909.132.5.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet (London, England). 2020;396(10255):898–908. doi: 10.1016/S0140-6736(20)31558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes A, Hoq M, Measey M-A, Danchin M. Intention to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2021;21(5):e110. doi: 10.1016/S1473-3099(20)30724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson H, deFigueiredo A, Karafillakis E, Rawal M. State of vaccine confidence in the EU 2018; 2018. [accessed 2021 Apr 15]. https://ec.europa.eu/health/sites/default/files/vaccination/docs/2018_vaccine_confidence_en.pdf.
- 30.Nguyen T, Henningsen KH, Brehaut JC, Hoe E, Wilson K. Acceptance of a pandemic influenza vaccine: a systematic review of surveys of the general public. Infect Drug Resist. 2011;4:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NCEMA . UAE national crisis and disasters management authority - UAE Coronavirus (COVID19) updates; 2021. [accessed 2021 July 7]. https://covid19.ncema.gov.ae/en/page/about-the-vaccine#:~:text=Is%20the%20vaccination%20mandatory%3F,Vaccination%20is%20optional.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


