Abstract
In a prospective, randomized clinical trial, the toxicity of 1 mg of amphotericin B (AmB) per kg of body weight per day infused in 5% dextrose was compared with that of AmB infused in lipid emulsion in children with malignant disease. In an analysis of 82 children who received a full course of 6 days or more of AmB (117 courses), it was shown that there were significant increases in plasma urea and creatinine concentrations and in potassium requirement after 6 days of therapy with both AmB infused in dextrose and AmB infused in lipid emulsion, with there being no difference between the two methods of AmB administration. An intent-to-treat comparison of the numbers of courses affected by acute toxicity (fever, rigors) and chronic toxicity (nephrotoxicity) also indicated that there was no significant difference between AmB infused in dextrose (78 courses) and AmB infused in lipid emulsion (84 courses). The pharmacokinetics of AmB were investigated in 20 children who received AmB in dextrose and 15 children who received AmB in lipid emulsion. Blood samples were collected up to 24 h after administration of the first dose, and the concentration of AmB in plasma was analyzed by a high-performance liquid chromatography assay. The clearance (CL) of AmB in dextrose (0.039 ± 0.016 liter · h−1 · kg−1) was significantly lower (P < 0.005) than the CL of AmB in lipid emulsion (0.062 ± 0.024 liter · h−1 · kg−1). The steady-state volume of distribution for AmB in dextrose (0.83 ± 0.33 liter · kg−1) was also significantly lower (P < 0.005) than that for AmB in lipid emulsion (1.47 ± 0.77 liter · kg−1). Although AmB in lipid emulsion is apparently cleared faster and distributes more widely than AmB in dextrose, this study did not reveal any significant advantage with respect to safety and tolerance in the administration of AmB in lipid emulsion compared to its administration in dextrose in children with malignant disease.
Amphotericin B (AmB) is an antifungal agent used as treatment for patients with established or suspected fungal infections and prophylaxis for immunosuppressed patients. Many children with malignant diseases who are admitted with fever receive empiric AmB therapy when the fever persists to prevent fungal superinfection and to control clinical or subclinical fungal infection. However, the side effects of AmB therapy are significant and include nephrotoxicity, electrolyte abnormalities (particularly hypokalemia), anemia, and infusion-related nausea, fever, and rigors (3, 7, 11, 12, 14, 31). Five clinical trials (9, 10, 18, 20, 24) with adults have compared the toxicity of AmB infused in lipid emulsion (AmB-IL) with that of the AmB infused in 5% dextrose (AmB-DX), which is conventionally used, and the results from three of these studies (9, 10, 20) have suggested that there is reduced nephrotoxicity and improved clinical tolerance when AmB is administered in lipid emulsion. Pharmacokinetic studies with adults (2, 15) have shown that the pharmacokinetics of AmB are different when it is administered in different infusion diluents. No studies have compared the toxicities and pharmacokinetics of AmB-IL and AmB-DX in children.
The aim of this study was to examine the toxicity and pharmacokinetics of AmB in children with malignant disease randomized to receive either conventional AmB-DX or AmB-IL (20% Intralipid).
MATERIALS AND METHODS
Study design.
This study was a prospective, single-center, randomized, open-label, controlled comparison of the clinical tolerance, toxicities, and pharmacokinetics of AmB-DX and AmB-IL. Restricted randomization was used in this study; the allocation arms were randomly assigned to consecutively numbered envelopes. The protocol was approved by the New Children’s Hospital Ethics Committee. The parents of all children in the study gave informed consent.
Patients.
All oncology patients who commenced AmB therapy, including those who had previous bone marrow transplants, were eligible for the study. Patients were allowed to be reentered into the study and rerandomized with subsequent courses of AmB. Patients were randomly assigned to one of the two AmB treatment groups (AmB-DX or AmB-IL).
Patients with suspected sepsis were treated according to the oncology unit’s standard antimicrobial policy. Blood cultures were performed for immunosuppressed patients with suspected sepsis (being usually febrile and neutropenic), and the patients were given antibiotics in an initial combination of gentamicin, cephalothin, and ticarcillin with clavulanate. Sodium chloride was also administered with ticarcillin. For children who were persistently febrile with negative bacterial cultures, empiric AmB was added after 3 or 4 days of fever. Antibiotics were continued until the fever resolved and there were early signs of myeloid recovery. Management also included supplementation of potassium to maintain the plasma potassium level at or above 3.0 mmol/liter.
A total of 130 children (the whole cohort) were randomized to receive 162 courses of AmB-DX (78 courses) or AmB-IL (84 courses). Of the whole cohort, 12 children were rerandomized to receive AmB-DX more than one time and 20 children were rerandomized to receive AmB-IL more than one time.
AmB was ceased in under 6 days in 45 courses. The reasons for cessation were the fact that (i) AmB was no longer indicated (n = 38), (ii) another drug was substituted (e.g., fluconazole for Candida parapsilosis infection; n = 3), (iii) the patient had deteriorating renal function (n = 3), or (iv) AmB was clinically toxic (n = 1).
For the remaining 117 courses (82 children), 6 days or more of AmB was administered (the full-course group). AmB-DX was administered in 50 courses and AmB-IL was administered in 67 courses. In this full-course group, 6 children who received AmB-DX and 10 children who received AmB-IL were rerandomized more than one time.
Table 1 presents the baseline characteristics of the whole cohort and full-course groups who received AmB-DX and AmB-IL. For the whole cohort, it was not possible to calculate the potassium requirement for 25 courses of AmB-DX and 15 courses of AmB-IL. Information about fever and rigors during the infusion was not obtained for 24 courses of AmB-DX and 18 courses of AmB-IL.
TABLE 1.
Comparison of patient groups receiving AmB-DX or AmB-IL
| Characteristic | Whole cohort
|
Full-course group
|
||||
|---|---|---|---|---|---|---|
| AmB-DX | AmB-IL | Significance | AmB-DX | AmB-IL | Significance | |
| No. of courses | 78 | 84 | 50 | 67 | ||
| No. of patients rerandomized | 12 | 20 | NSa,b | 6 | 10 | NSa |
| Time to rerandomization (days [mean ± SD]) | 132 ± 177 | 138 ± 108 | NSc | 37 ± 27 | 119 ± 113 | P < 0.05c |
| AmB dose (mg [mean ± SD]) | 23.7 ± 10.8 | 20.6 ± 9.9 | NSc | 23.6 ± 11.4 | 20.5 ± 10.2 | NSc |
| No. of days of daily AmB (mean ± SD) | 6.3 ± 2.9 | 6.9 ± 3.5 | NSc | 7.8 ± 2.2 | 7.8 ± 3.0 | NSc |
| Median (range) age (mo) | 80 (9–2,190) | 70 (4–191) | NSc | 81.5 (9–219) | 71 (4–191) | NSc |
| Wt (kg [mean ± SD]) | 23.9 ± 10.9 | 20.8 ± 9.8 | NSc | 23.9 ± 11.6 | 20.7 ± 10.2 | NSc |
| Ht (cm [mean ± SD]) | 119 ± 24 | 112 ± 25 | NSc | 118 ± 25 | 111 ± 27 | NSc |
| Surface area (m2 [mean ± SD]) | 0.90 ± 0.30 | 0.82 ± 0.29 | NSc | 0.89 ± 0.32 | 0.81 ± 0.29 | NSc |
| Baseline plasma creatinine level (μM [mean ± SD]) | 48.3 ± 13.3 | 45.2 ± 12.0 | NSc | 47.8 ± 14.4 | 45.1 ± 12.6 | NSc |
| Gender (no. of males/no. of females) | 49/29 | 54/30 | NSc | 29/21 | 45/22 | NSa |
| Diagnosis (no. of patients) | ||||||
| Acute myeloid leukemia | 9 | 11 | NSa | 9 | 13 | NSa |
| Acute lympoblastic leukemia | 22 | 25 | NSa | 11 | 20 | NSa |
| Neuroblastoma | 9 | 8 | NSa | 8 | 7 | NSa |
| Rhabdomyosarcoma | 13 | 7 | NSa | 10 | 6 | NSa |
| Non Hodgkins Lymphoma | 3 | 7 | NSa | 2 | 5 | NSa |
| Other | 16 | 22 | NSa | 10 | 16 | NSa |
| Recent BMTd | ||||||
| Yes/No | 20/58 | 22/62 | NSa | 19/31 | 21/46 | NSa |
| TPNe with AmB (no. yes/no. no/no. unknown) | 29/44/5 | 40/35/9 | NSa | 25/22/3 | 37/24/6 | NSa |
| Cyclosporin administration (no. yes/no. no/no. unknown) | 12/62/4 | 9/69/6 | NSa | 11/39 | 9/58 | NSa |
| Diuretic administration (no. yes/no. no/no. unknown) | 15/59/4 | 25/53/6 | NSa | 15/35 | 22/45 | NSa |
Chi-square test.
NS, not significant.
Mann-Whitney U test.
BMT, bone marrow transplantation.
TPN, total parenteral nutrition.
For a subgroup of the full-course group, AmB pharmacokinetic analyses were also performed on the first day of treatment. Pharmacokinetic analyses were performed with 20 children who were administered AmB-DX and 15 children who were administered AmB-IL. Blood samples for the first-dose pharmacokinetic analysis were taken when convenient, that is, on weekdays when nursing staff levels were adequate, and hence, pharmacokinetic analyses could not be performed for all patients. Blood for determination of AmB trough concentrations was collected prior to the administration of each dose for the majority of patients in the study.
Monitoring of side effects.
Assessment of the extent of renal dysfunction was based on changes in plasma creatinine, urea, and potassium concentrations and in potassium requirement.
Potassium requirement was calculated for a 24-h period on the 1st and 7th days of AmB therapy as the sum of the potassium content in the parenteral nutrition and that in the intravenous and oral potassium supplements. Dietary potassium intake was not taken into account. Most patients had mucositis and poor oral intake.
The percent change in plasma creatinine and plasma urea concentrations and in potassium requirements for the full-course group was calculated by the following equation: percent change = (C7 − C1)/C1 × 100, where C1 is the concentration of or requirement for the analyte on day 1 and C7 is the concentration or requirement on day 7. For the whole cohort, the following equation was used: percent change = (Cz − C1)/C1 × 100, where Cz was either the value on day 7 or the value following administration of the last AmB dose when AmB treatment was ceased in under 7 days.
Clinical toxicity, determined by observation, was defined as the occurrence of fever and/or rigors during the AmB infusion.
Formulation and administration of AmB.
The AmB desoxycholate (Fungizone; Brystol-Myers-Squibb) dose, calculated as 1 mg/kg of body weight, was reconstituted with distilled water and was diluted with 5% dextrose solution (AmB-DX) or parenteral fat emulsion (20% Intralipid; Kabi Pharmacia) (AmB-IL). Doses of 40 mg of AmB or less were given in 100 ml of solution (i.e., maximum concentration of 0.4 mg/ml). Doses of over 40 mg (with a maximum of 50 mg) were given in 250 ml of solution. The AmB dose was administered as a single daily 2-h intravenous infusion. All patients were premedicated routinely with hydrocortisone (3 mg/kg). All patients had a double-lumen central line into the right atrium, so that one lumen could be used for drug administration and one could be used for sampling.
Blood samples for pharmacokinetic analysis.
Heparinized blood samples (1 to 2 ml) for the measurement of the AmB concentration for the pharmacokinetic analysis were collected before the infusion and at 2 (infusion end), 3, 4, 6, 12, 18, and 24 h after the start of the infusion on the first day of therapy. For subsequent doses given once daily, blood samples for trough AmB level determinations were collected immediately prior to administration of the next AmB dose. Blood was centrifuged in a Beckman GS-6R centrifuge at 3,000 rpm for 10 min at 4°C, and plasma was stored at −40°C and analyzed within 1 week of collection. AmB has previously been shown to be stable in human plasma at 4 and −20°C for 24 days (13).
Assay of AmB.
The AmB concentration in plasma was determined by a modified version of a previously reported method (13). To an aliquot of plasma (0.1 ml) in a microcentrifuge tube was added internal standard solution (20 μl of a 4-mg/liter solution of p-nitroaniline) and acetonitrile (0.25 ml). The tube was vortexed for 10 s and was allowed to stand for 5 min before centrifugation at 1,200 × g for 2 min, and a 50-μl sample of the acetonitrile extract was injected into the high-performance liquid chromatography (HPLC) system. The HPLC system consisted of an Altex model 100 pump and a Waters model 480 UV detector set at 405 nm with a Phenomenex Spherex (15 cm by 4.3 mm by 5 μm) C18 column fitted with a Brownlee (1.5 cm by 3.2 mm by 7 μm) RP-18 precolumn. AmB eluted with a retention time of 9 min at room temperature when 1 ml (per min) of 5.6 mM sodium acetate buffer (pH 7.4) with 43.3% acetonitrile and 0.9% EDTA was used. The internal standard had a retention time of 4.5 min. The peak height ratio (ratio of the AmB peak height to the internal standard peak height) of the unknown sample was compared with the peak height ratios of spiked plasma standards containing 0, 0.5, 1.0, 1.5, and 2.0 μg of AmB per ml. The standard curve was demonstrated to be linear for between 0.1 and 6 μg of AmB per ml on several occasions. The between-day coefficient of variation of the assay was 15% for a concentration of 0.5 μg of AmB per ml (n = 83) and 11% for a concentration of 1.5 μg/ml (n = 83), and the limit of detection was 0.1 μg/ml.
Pharmacokinetic analysis.
First-dose pharmacokinetic analysis was performed with AmB concentration-time data for 20 children who received AmB-DX and 15 children who received AmB-IL (see Table 2). Mean residence time (MRT) and the area under the plasma concentration-time curve (AUC) were estimated by the linear trapezoidal rule with extrapolation to infinity (23). Clearance (CL) was calculated by dividing the dose (in milligrams per kilogram) by the AUC extrapolated to infinity (AUC0–∞). The steady-state volume of distribution (VSS) was calculated as the product of CL and MRT. The terminal elimination rate constant (kel) was determined from the slope of the terminal portion of the log concentration-time curve. There were at least three and up to five datum observations in the log-linear terminal phase. The slope of the terminal decline in the log-linear concentrations was determined by unweighted linear regression. The elimination half-life (t1/2) (harmonic mean) was calculated by dividing the natural logarithm of 2 (0.693) by the mean kel value. The initial volume of distribution (V) was calculated by dividing CL by kel.
TABLE 2.
Comparison of AmB pharmacokinetics after administration in either dextrose or lipid emulsion
| Parameter | AmB-DX (n = 20) | AmB-IL (n = 15) | P valuea |
|---|---|---|---|
| Median (range) age (mo) | 112 (27–172) | 71 (10–190.5) | NS |
| Wt (kg [mean ± SD]) | 25.8 ± 10.0 | 22.5 ± 12.2 | NS |
| Dose (mg/kg [mean ± SD]) | 0.989 ± 0.065 | 1.010 ± 0.054 | NS |
| Cmax (μM [mean ± SD]) | 2.43 ± 1.27 | 1.65 ± 1.01 | <0.005 |
| C24 h,trough (μM [mean ± SD]) | 0.41 ± 0.19 | 0.26 ± 0.08 | NS |
| CSS,trough (μM [mean ± SD]) | 0.84 ± 0.37 (n = 26) | 0.63 ± 0.39 (n = 40) | <0.01 |
| AUC0–24 (μM · h [mean ± SD]) | 22.0 ± 11.1 | 13.1 ± 6.0 | <0.01 |
| CL (liter · h−1 · kg−1 [mean ± SD]) | 0.039 ± 0.016 | 0.062 ± 0.024 | <0.005 |
| VSS (liter · kg−1 [mean ± SD]) | 0.83 ± 0.33 | 1.47 ± 0.77 | <0.005 |
| V (liter · kg−1) Mean ± SD | 0.92 ± 0.43 | 1.64 ± 0.83 | <0.005 |
| MRT (h [mean ± SD]) | 25.5 ± 15.3 | 23.8 ± 8.0 | NS |
| kel (h−1 [mean ± SD]) | 0.046 ± 0.022 | 0.042 ± 0.015 | NS |
| t1/2 (h [harmonic mean]) | 15.1 | 16.5 |
Mann-Whitney U test. NS, not significant.
The trough AmB concentration (Ctrough) was defined as the AmB concentration in any plasma sample taken between 20 and 24 h after administration of the dose. First-dose trough AmB concentration (C24 h,trough) was defined as the AmB concentration in a plasma sample taken between 20 and 24 h after administration of the first dose. Steady-state trough AmB concentration (CSS,trough) was the Ctrough on the 7th day after the start of daily therapy with AmB or, if this value was not available, on the 5th, 6th, or 8th day after the start of therapy.
Statistical analysis.
The analysis of the adverse effects of AmB was performed for two groups of patients. The first group included all patients who were enrolled in the study, according to the intent-to-treat principle (the whole cohort). The second group included all patients who received a full course of AmB for 6 days or more (the full-course group).
Descriptive statistics (means, standard deviations [SDs], and 95% confidence intervals) and correlations were performed with Microsoft Excel (version 5.0) software. Comparisons between groups were performed by either the nonparametric Mann-Whitney test, the Wilcoxon matched-pairs signed-rank test, or the chi-square test with SPSS version 6.1.4 (SPSS Inc., Chicago, Ill.). t1/2 data were presented by using the harmonic mean.
RESULTS
Patients.
Table 1 compares the baseline characteristics of the groups of patients in the whole cohort and in the full-course group randomized to receive AmB-DX or AmB-IL. There was no significant difference in age, weight, height, surface area, gender, underlying disease, total parenteral nutrition administration, baseline creatinine concentration, days of daily AmB therapy, or AmB dose between the two treatment groups for both study populations (the whole cohort and the full-course group). Exposures to other potentially nephrotoxic agents were also similar. For the AmB-DX and AmB-IL treatment groups there were no significant differences in the numbers of courses after bone marrow transplantation or the numbers of courses in which cyclosporin or diuretics were used.
In both study populations the numbers of children who were rerandomized into the study were not significantly different for the AmB-DX and AmB-IL treatment groups. There was no significant difference between AmB-DX and AmB-IL in the time to rerandomization for the whole cohort. There was a significant difference for the full-course group, but the numbers were very small.
There was no significant difference in age, weight, or dose for the patients who received AmB-DX compared with those who received AmB-IL in the pharmacokinetic analysis subgroup (Table 2).
Clinical outcome.
Of the whole cohort of 162 courses of AmB, positive fungal cultures were observed for 20 courses: there were 10 documented fungal infections in the AmB-DX treatment group (6 Candida spp., 1 Fusarium sp., 1 Aspergillus sp., 1 Scedosporium sp., and one unknown) and 10 documented fungal infections in the AmB-IL treatment group (all Candida spp.). One child died (AmB-DX arm) from a disseminated fungal infection (a 0.9% mortality rate). AmB appeared to be successful in all other instances in preventing or controlling fungal infection.
Pharmacokinetics.
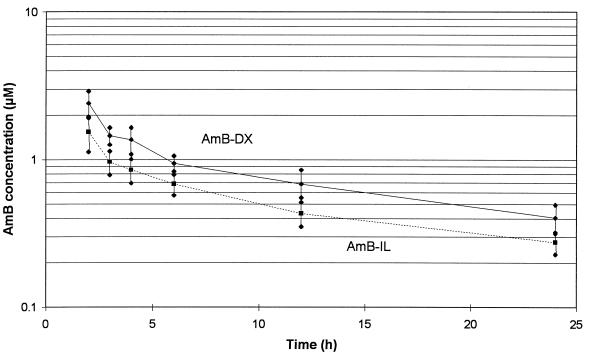
A semilogarithmic plot of the disposition of AmB in 20 children who received AmB-DX and in 15 children who received AmB-IL is shown in Fig. 1. Mean plasma AmB concentrations and 95% confidence intervals for the two groups of patients are shown for the first 24 h. Plasma AmB concentrations were generally lower for the AmB-IL treatment group than for the AmB-DX treatment group.
FIG. 1.
Semilogarithmic plot of mean AmB concentrations and 95% confidence intervals after administration of the first dose in dextrose or lipid emulsion in children with malignant disease.
The pharmacokinetic data for AmB administered as AmB-DX and AmB-IL are compared in Table 2. The maximum concentration of AmB in serum (Cmax) for AmB-IL (1.65 ± 1.01 μM) was significantly lower (32%) than the Cmax for AmB-DX (2.43 ± 1.27 μM), and the AUC from 0 to 24 h (AUC0–24) for AmB-IL (13.1 ± 6.0 μM · h) was significantly lower (40%) than the AUC0–24 for AmB-DX (22.0 ± 11.1 μM · h). The CL of AmB after administration in lipid (0.062 ± 0.024 liter · h−1 · kg−1) was significantly higher (159%) than the CL of AmB after administration in dextrose (0.039 ± 0.016 liter · h−1 · kg−1). The VSS for AmB-IL (1.47 ± 0.77 liters · kg−1) was significantly higher (177%) than the VSS for AmB-DX (0.83 ± 0.33 liter · kg−1), and V was also significantly higher (178%) for AmB-IL (1.64 ± 0.83 liter · kg−1) than for AmB-DX (0.92 ± 0.43 liter · kg−1).
The C24 h,trough of AmB-IL was not significantly different from the C24 h,trough of AmB-DX, but the CSS,trough of AmB-IL (0.63 ± 0.39 μM) was significantly lower (25%) than the CSS,trough of AmB-DX (0.84 ± 0.37 μM) (Table 2).
There was no significant difference between AmB-DX and AmB-IL in the kel for AmB. AmB-DX had a mean kel of 0.046 ± 0.022 h−1, corresponding to a t1/2 (harmonic mean) of 15.1 h and AmB-IL had a kel of 0.042 ± 0.015 h−1, corresponding to a t1/2 (harmonic mean) of 16.5 h.
Effects on markers of renal function.
In Table 3, the mean plasma creatinine, urea, and potassium concentrations, as well as the potassium requirement, are shown for both the 1st and 7th days of therapy for the children who received the full course of AmB-DX and AmB-IL. There was wide interpatient variation in both plasma creatinine and urea levels on day 7, and most were within the normal reference interval of 30 to 75 μM for the plasma creatinine concentration in children ages 5 to 15 years. Only 2 (of 162) courses of AmB were associated with nephrotoxicity, which was defined previously (13) as a greater than 100% increase in the plasma creatinine level from the baseline to a level above normal (see Table 5). Despite the low incidence of AmB-induced nephrotoxicity, the results in Table 3 do provide evidence that renal function was affected in patients receiving both AmB-DX and AmB-IL. It was observed that there were significantly higher plasma creatinine and plasma urea concentrations and significantly higher potassium requirements on the 7th day of AmB therapy compared with those on the 1st day.
TABLE 3.
Changes in renal function markers after 6 days of therapy with AmB in dextrose or lipid emulsion (full-course group)
| Group and day | Creatinine concn (μM [mean ± SD]) | Urea concn (mM [mean ± SD]) | Potassium concn (mM [mean ± SD]) | Potassium requirement (mmol/kg/day [mean ± SD]) |
|---|---|---|---|---|
| AmB-DX (n = 50) | ||||
| 1 | 48 ± 14 | 3.4 ± 1.7 | 3.6 ± 0.6 | 2.4 ± 2.1 |
| 7 | 54 ± 20 | 7.0 ± 3.5 | 3.5 ± 0.8 | 5.6 ± 2.4 |
| P valuea | <0.01 | <0.0005 | NS | <0.0005 |
| AmB-IL (n = 67) | ||||
| 1 | 45 ± 13 | 3.7 ± 2.2 | 3.5 ± 0.6 | 2.3 ± 1.9 |
| 7 | 49 ± 16 | 6.5 ± 3.6 | 3.5 ± 0.7 | 5.0 ± 2.2 |
| P valuea | <0.05 | <0.0005 | NS | <0.0005 |
Wilcoxon matched-pairs signed-rank test for significant differences between day 1 and day 7 values. NS, not significant.
TABLE 5.
Comparison of numbers of courses adversely affected by therapy with AmB-DX and AmB-IL (whole cohort)
| Group | No. of courses
|
||||
|---|---|---|---|---|---|
| Fever and/or rigors during infusiona | Nephro-toxicityb | Change in plasma creatinine concn >24% from baselinec | Change in plasma urea concn >148% from baselinec | Change in potassium requirement >313% from baselinec | |
| AmB-DX (n = 78) | 19 | 1 | 20 | 21 | 14 |
| AmB-IL (n = 84) | 21 | 1 | 16 | 13 | 16 |
| Significanced | NS | NS | NS | NS | NS |
Information about whether fever and/or rigors occurred during AmB infusion is missing for 24 courses of AmB-DX and 18 courses of AmB-IL.
Nephrotoxicity is defined as a change in plasma creatinine concentration greater than 100% above the baseline to a level above normal.
The cutoff points of 24, 148, and 313% for change in plasma creatinine and plasma urea concentrations and potassium requirement, respectively, were determined from the 75th percentiles.
Test for significance: chi-square test. NS, not significant.
There was no significant difference between the AmB-DX and AmB-IL methods of AmB administration in the changes in plasma creatinine or plasma urea concentration or potassium requirement that occurred after a full course of AmB (Table 4).
TABLE 4.
Comparison of changes in renal function markers that occurred after 6 days of therapy with AmB administered in either dextrose or lipid emulsion (full-course group)
| Group | % Change (mean ± SD)
|
||
|---|---|---|---|
| Plasma creatinine concn | Plasma urea concn | Potassium requirement | |
| AmB-DX | 5 ± 33 | 121 ± 93 | 231 ± 251 |
| AmB-IL | 10 ± 26 | 97 ± 116 | 179 ± 193 |
| Significancea | NS | NS | NS |
Test for significance: Mann-Whitney U test. NS, not significant.
Table 5 presents an intent-to-treat comparison of the numbers of courses associated with fever and rigors, nephrotoxicity, or increased changes in plasma creatinine and plasma urea concentrations and potassium requirements (changes above the 75th percentile). No significant difference between AmB-DX and AmB-IL was observed.
Bone marrow transplantation 1 to 2 weeks prior to AmB therapy did not significantly affect the percent change in the plasma creatinine or plasma urea concentration or the potassium requirement over 6 days of therapy (data not shown).
DISCUSSION
This study compares the pharmacokinetics and side effects of AmB-DX with AmB-IL (20% Intralipid) in children with malignant disease. Pharmacokinetic studies were performed only after administration of the first dose of AmB, and model-independent pharmacokinetic analyses were used.
The pharmacokinetics of AmB-DX in children has been reported previously (4, 19, 25), and comparisons of previously published data with the results from this study are shown in Table 6. CL, volume of distribution (V), and elimination t1/2 for AmB-DX for this study closely agreed with those of Benson and Nahata (4), who investigated AmB-DX infusion in a group of oncology patients with an age range similar to that of the patients in this study and who also used model-independent pharmacokinetic analysis. There were methodological differences between the various studies, with Starke et al. (25) and Koren et al. (19) using a one-compartment pharmacokinetic modeling approach and with Starke et al. (25) including 7 (of 10) patients under 1 year of age. The variation in AmB pharmacokinetics reported from these different studies may be due to these methodological differences.
TABLE 6.
Comparison of pharmacokinetic results for AmB obtained in this study with those obtained in previous studies with children receiving the conventional formulation
| Investigators | No. of children | Age range (mo) | Dose range (mg/kg) | CL (ml · min−1 · kg−1 [mean ± SD]) | V (liters · kg−1 [mean ± SD]) | t1/2 (h [mean ± SD]) |
|---|---|---|---|---|---|---|
| This study | ||||||
| AmB-DX | 20 | 27–172 | 1.0 | 0.64 ± 0.27 | 0.92 ± 0.43 | 15.1 |
| AmB-IL | 15 | 10–190.5 | 1.0 | 1.04 ± 0.41 | 1.64 ± 0.83 | 16.5 |
| Starke et al. (25) | 9 | 0.5–180 | 0.25–1.0 | 3.72 ± 3.91 | 3.10 ± 2.32 | 17.7 ± 17.6 |
| Koren et al. (19) | 13 | 1–216 | 0.5–1.0 | 0.43 ± 0.08 | 0.38 ± 0.02 | 9.9 ± 1.5 |
| Benson and Nahata (4) | 9 | 4–168 | 0.25–1.5 | 0.46 ± 0.20 | 0.76 ± 0.52 | 18.1 ± 6.65 |
The pharmacokinetics of AmB-IL differed considerably from those of AmB-DX. When AmB-IL was administered, the values for CL, V, and VSS were 1.6-, 1.8-, and 1.8-fold higher, respectively, than those obtained when AmB-DX was administered. The Cmax, CSS,trough, and AUC0–24 h values for AmB-IL were 0.68, 0.75, and 0.60 the values for AmB-DX, respectively. We found no significant difference between AmB-DX and AmB-IL C24 h,troughs, but the numbers were smaller than those for the comparison of CSS,troughs. The terminal t1/2 and the MRT were also unaffected by the method of AmB administration. A previous study performed with adults (2) also showed that AmB-IL was associated with a 1.9-fold higher VSS compared with that for AmB-DX, with the t1/2 and MRT remaining constant.
It is possible that differences in AmB aggregate formation could contribute to the observed differences in the pharmacokinetics of AmB-IL and AmB-DX. It has been reported previously (26) that AmB does not mix well with lipid emulsion, with there being a greater degree of self-association of the AmB molecules in the lipid emulsion. Intralipid has a particle size of less than 1 μm (27), but Trissel (26) found that there were substantially more particles, many of them greater than 10 μm in diameter, in AmB-IL than in AmB-DX. Ranchere and colleagues (22) found that the particle size in an infusion containing AmB and Intralipid was up to fourfold greater than that in an infusion with dextrose and Intralipid. The possibility therefore exists that, as discussed previously (15, 30), AmB administered in lipid emulsion leads to an increased uptake of the drug by the organs of the reticuloendothelial system (liver, spleen, and lung) due to the larger particle size. This could account for the increased CL of the drug (especially uptake into the liver and subsequent hepatic elimination) and an increase in VSS due to more extensive tissue uptake. In support of this, there appears to be increased uptake of AmB-IL into the lungs of mice compared with that of AmB-DX (17).
Another possible explanation for the increased V, VSS, and CL associated with AmB-IL is that there may be a difference in the concentration of unbound AmB in AmB-IL compared with that in AmB-DX. It has previously been postulated (16) that the unbound concentration of AmB may differ widely between dose forms (dextrose versus lipid emulsion versus liposomal form), although this has not been investigated. The unbound fraction is more available for hepatic elimination and distribution into the tissues. AmB is highly bound in plasma (96.5% [21] and 91 to 95% [5]), mainly to plasma lipoproteins (6, 28, 29). The unbound AmB concentration is therefore likely to be very small, and a change could significantly affect the pharmacokinetics of AmB. Intralipid may compete with lipoproteins for the binding of AmB molecules, effectively increasing the fraction of AmB that is unbound. An increase in the unbound fraction could thus explain the observed increases in V, VSS and CL that occurs when AmB is administered in lipid emulsion.
We observed that therapy with either AmB-DX or AmB-IL over 6 days was associated with significant increases in plasma urea and creatinine concentrations, increases in potassium requirement, and infusion-related fever and rigors. The magnitude of the change in creatinine was generally not very large, and nephrotoxicity was evident for only 2 (of 162) courses of AmB. Altered renal function is well known to be a consequence of AmB therapy (31), and the changes in creatinine and urea concentrations and potassium requirements observed after 6 days of AmB therapy appeared to be very useful markers of this side effect of AmB in children.
The low incidence of nephrotoxicity observed in this study may be due to a number of reasons. Patient age and underlying disease state may affect the pharmacokinetics of AmB in such a way that fewer side effects are manifested. The fact that patients were premedicated with hydrocortisone and were not sodium depleted at the initiation of AmB therapy may also have contributed to the low incidence of side effects.
The changes in potassium requirement and plasma urea or plasma creatinine concentration after 6 days of AmB therapy were not significantly different for AmB-DX and AmB-IL, indicating that there was no difference in the way that they affected renal function, despite the lower AUC for AmB-IL.
In an intent-to-treat analysis there was also no detectable difference between AmB-DX and AmB-IL in the numbers of children experiencing nephrotoxicity or infusion-related fever and rigors (although not all patients had a full follow-up with respect to this end point). Previous studies with adults have shown that AmB-IL is better tolerated than AmB-DX, with fewer patients experiencing adverse reactions (9, 10, 20), but we found no such difference in children. Joly and colleagues (18) also found that AmB-IL did not have substantially reduced renal toxicity compared with AmB-DX in AIDS patients, although Intralipid infusion did reduce the infusion-related toxicity of AmB without altering the antifungal efficacy. In addition, a recent investigation comparing the safety of AmB-DX and AmB-IL in adult neutropenic patients found that administration of AmB-IL provided no benefits and was associated with increased occurrences of acute pulmonary side effects (24).
This study, in agreement with previous clinical trials (8, 10, 18), showed that AmB-IL and AmB-DX are equally effective in preventing or controlling fungal infections. However, only 20 episodes of cultures positive for fungal infection were identified, and there were insufficient numbers for a good comparison of efficacy. It is possible that the efficacy of AmB-IL could be compromised by the observed lower plasma AmB concentrations because it has previously been suggested that maintenance of serum AmB concentrations above the MIC for the invading organism is a minimum requirement for successful therapy (1).
The low incidence of AmB-induced nephrotoxicity in this study was a limitation in the detection of differences in toxicity between AmB-DX and AmB-IL. However, this study has demonstrated that there is no advantage to administering AmB in lipid emulsion in children with malignant diseases, a patient group that tolerates AmB therapy well.
There are now reports that suggest that the lipid preparation of AmB is not suitable for routine use: the product is inconsistent and undesirable (22, 26, 30). Until it becomes clear why administration of AmB in lipid emulsion has such a marked effect on the pharmacokinetic parameters, including AUC, CSS,trough, CL, and VSS, and yet does not alter the pharmacodynamic parameters, including the changes in the plasma creatinine and plasma urea concentrations and the potassium requirement, the use of lipid emulsion for the administration of AmB must remain suspect. Further work comparing the unbound concentrations of AmB, the tissue distribution of AmB, and the distribution of AmB to the lipoprotein fractions of blood for AmB-DX and AmB-IL is needed to explain these effects, but in view of the possible safety concerns with AmB-IL, these may not be warranted.
In conclusion, this investigation found no significant advantage in safety or tolerance by administering AmB in lipid emulsion compared to administering it in dextrose in the treatment and prevention of fungal infection in children with malignant disease. In view of this finding and the reported incompatibility of AmB with lipid emulsion (26), we recommend the use of only conventional AmB-DX.
We thank the patients and their families for taking part in the study, the nursing staff in the oncology unit for their care of the patients including the taking of samples for pharmacokinetic analysis, and the staff in the Department of Biochemistry for measuring the plasma creatinine, urea, and potassium concentrations.
ACKNOWLEDGMENTS
C.E. Nath is supported by the Leukaemia Research and Support Fund.
REFERENCES
- 1.Atkinson A J, Jr, Bennett J E. Amphotericin pharmacokinetics in humans. Antimicrob Agents Chemother. 1978;13:271–276. doi: 10.1128/aac.13.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayestaran A, Lopez R M, Montoro J B, Estibalez A, Pou L, Julia A, Lopez A, Pascual B. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob Agents Chemother. 1996;40:609–612. doi: 10.1128/aac.40.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell N H, Andriole V T, Sabesin S M, Utz J P. On the nephrotoxicity of amphotericin B in man. Am J Med. 1962;33:64–69. doi: 10.1016/0002-9343(62)90277-2. [DOI] [PubMed] [Google Scholar]
- 4.Benson J M, Nahata M C. Pharmacokinetics of amphotericin B in children. Antimicrob Agents Chemother. 1989;33:1989–1993. doi: 10.1128/aac.33.11.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block E R, Bennett J E, Livoti L G, Klein W J, MacGregor R R, Henderson L. Flucytosine and amphotericin B: hemodialysis effects on the plasma concentration and clearance. Studies in man. Ann Intern Med. 1974;80:613–617. doi: 10.7326/0003-4819-80-5-613. [DOI] [PubMed] [Google Scholar]
- 6.Brajtburg J, Elberg S, Bolard J, Kobayashi G S, Levy R A, Ostlund R E, Jr, Schlessinger D, Medhoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984;149:986–997. doi: 10.1093/infdis/149.6.986. [DOI] [PubMed] [Google Scholar]
- 7.Burgess J L, Birchall R. Nephrotoxicity of amphotericin B, with emphasis on changes in tubular function. Am J Med. 1972;53:77–84. [PubMed] [Google Scholar]
- 8.Caillot D, Casasnovas O, Solary E, Chavanet P, Bonnotte B, Reny G, Entezam F, Lopez J, Bonnin A, Guy H. Efficacy and tolerance of an amphotericin B lipid (intralipid) emulsion in the treatment of candidaemia in neutropenic patients. J Antimicrob Chemother. 1993;31:161–169. doi: 10.1093/jac/31.1.161. [DOI] [PubMed] [Google Scholar]
- 9.Caillot D, Reny G, Solary E, Casasnovas O, Chavanet P, Bonnotte B, Perello L, Dumas M, Entezam F, Guy H. A controlled trial of the tolerance of amphotericin B infused in dextrose or in Intralipid in patients with haematological malignancies. J Antimicrob Chemother. 1994;33:603–613. doi: 10.1093/jac/33.3.603. [DOI] [PubMed] [Google Scholar]
- 10.Chavanet P Y, Garry I, Charlier N, Caillot D, Kisterman J P, D’Athis M, Portier H. Trial of glucose versus fat emulsion in preparation of amphotericin for use in HIV infected patients with candidiasis. BMJ. 1992;305:921–925. doi: 10.1136/bmj.305.6859.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas J B, Healy J K. Nephrotoxic effects of amphotericin B, including renal tubular acidosis. Am J Med. 1969;46:154–162. doi: 10.1016/0002-9343(69)90067-9. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M A, Talbot G H, Maislin G, McKeon B P, Tynan K P, Strom B L. Risk factors for amphotericin B-associated nephrotoxicity. Am J Med. 1989;87:547–552. doi: 10.1016/s0002-9343(89)80612-6. [DOI] [PubMed] [Google Scholar]
- 13.Golas C L, Prober C G, Macleod S M, Soldin S J. Measurement of amphotericin B in serum or plasma by high performance liquid chromatography. J Chromatogr. 1983;278:387–395. doi: 10.1016/s0378-4347(00)84798-2. [DOI] [PubMed] [Google Scholar]
- 14.Heidemann H T, Gerkens J F, Spickard W A, Jackson E K, Branch R A. Amphotericin B nephrotoxicity in humans decreased by salt repletion. Am J Med. 1983;75:476–481. doi: 10.1016/0002-9343(83)90353-4. [DOI] [PubMed] [Google Scholar]
- 15.Heinemann V, Kahny B, Debus A, Wachholz K, Jehn U. Pharmacokinetics of liposomal amphotericin B (AmBisome) versus other lipid-based formulations. Bone Marrow Transplant. 1994;14(Suppl. 5):S8–S9. [PubMed] [Google Scholar]
- 16.Janknegt R, de Mare S, Bakker-Woudenberg I A J M, Crommelin D J A. Liposomal and lipid formulations of amphotericin B. Clin Pharmacokinet. 1992;23:279–291. doi: 10.2165/00003088-199223040-00004. [DOI] [PubMed] [Google Scholar]
- 17.Joly V, Farinotti R, Saint-Julien L, Cheron M, Carbon C, Yeni P. In vitro renal toxicity and in vivo therapeutic efficacy in experimental murine cryptococcosis of amphotericin B (Fungizone) associated with Intralipid. Antimicrob Agents Chemother. 1994;38:177–183. doi: 10.1128/aac.38.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joly V, Aubry P, Ndayiragide A, Carriere I, Kawa E, Mlika-Cabanne N, Couland J P, Larouze B, Yeni P. Randomized comparison of amphotericin B desoxycholate dissolved in dextrose or Intralipid for the treatment of AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1996;23:556–562. doi: 10.1093/clinids/23.3.556. [DOI] [PubMed] [Google Scholar]
- 19.Koren G, Lau A, Klein J, Golas C, Bologa-Campeanu M, Soldin S, Macleod S, Prober C. Pharmacokinetics and adverse effects of amphotericin B in infants and children. J Pediatr. 1988;113:559–563. doi: 10.1016/s0022-3476(88)80653-x. [DOI] [PubMed] [Google Scholar]
- 20.Moreau P, Milpied N, Fayette N, Ramee J F, Harousseau J L. Reduced renal toxicity and improved clinical tolerance of amphotericin B mixed with Intralipid compared with conventional amphotericin B in neutropenic patients. J Antimicrob Chemother. 1992;30:535–541. doi: 10.1093/jac/30.4.535. [DOI] [PubMed] [Google Scholar]
- 21.Morgan D J, Ching M S, Raymond K, Bury R W, Mashford L, Kong B, Sabto J, Gurr W, Somogyi A A. Elimination of amphotericin B in impaired renal function. Clin Pharmacol Ther. 1983;34:248–253. doi: 10.1038/clpt.1983.161. [DOI] [PubMed] [Google Scholar]
- 22.Ranchere J Y, Latour J F, Fuhrmann C, Lagallarde C, Loreuil F. Amphotericin B intralipid formulation: stability and particle size. J Antimicrob Chemother. 1996;37:1165–1169. doi: 10.1093/jac/37.6.1165. [DOI] [PubMed] [Google Scholar]
- 23.Rowland M, Tozer T N. Clinical pharmacokinetics: concepts and applications. 3rd ed. Baltimore, Md: The Williams & Wilkins; 1995. [Google Scholar]
- 24.Schoffski P, Freund M, Wunder R, Peterson D, Kohne C H, Hecker H, Schubert U, Ganser A. Safety and toxicity of amphotericin B in glucose 5% or Intralipid 20% in neutropenic patients with pneumonia or fever of unknown origin: randomised study. BMJ. 1998;317:379–384. doi: 10.1136/bmj.317.7155.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starke J R, Mason E O, Jr, Kramer W G, Kaplan S L. Pharmacokinetics of amphotericin B in infants and children J. Infect Dis. 1987;155:766–774. doi: 10.1093/infdis/155.4.766. [DOI] [PubMed] [Google Scholar]
- 26.Trissel L A. Amphotericin B does not mix with fat emulsion. Am J Health-Syst Pharm. 1995;52:1463–1464. doi: 10.1093/ajhp/52.13.1463. [DOI] [PubMed] [Google Scholar]
- 27.Tuckwell K R, editor. MIMS Annual. Sydney, Australia: MIMS; 1997. Intralipid. Pharmacia and UpJohn product information; pp. 19-1110–19-1111. [Google Scholar]
- 28.Wasan K M, Brazeau G A, Keyhani A, Hayman A C, Lopez-Berestein G. Roles of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993;37:246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasan K M, Cassidy S M. Role of plasma lipoproteins in modifying the biological activity of hydrophobic drugs. J Pharm Sci. 1998;87:411–424. doi: 10.1021/js970407a. [DOI] [PubMed] [Google Scholar]
- 30.Washington C, Lance M, Davis S S. Toxicity of amphotericin B emulsion formulations. J Antimicrob Chemother. 1993;31:806–808. doi: 10.1093/jac/31.5.806. [DOI] [PubMed] [Google Scholar]
- 31.Wilson R, Feldman S. Toxicity of amphotericin B in children with cancer. Am J Dis Child. 1979;133:731–734. doi: 10.1001/archpedi.1979.02130070067014. [DOI] [PubMed] [Google Scholar]