Abstract
Postoperative neurological disorders, including postoperative delirium (POD), postoperative cognitive dysfunction (POCD), postoperative covert ischemic stroke, and hemorrhagic stroke, are challenging clinical problems in the emerging aged surgical population. These disorders can deteriorate functional outcomes and long‐term quality of life after surgery, resulting in a substantial social and financial burden to the family and society. Understanding predisposing and precipitating factors may promote individualized preventive treatment for each disorder, as several risk factors are modifiable. Besides prevention, timely identification and treatment of etiologies and symptoms can contribute to better recovery from postoperative neurological disorders and lower risk of long‐term cognitive impairment, disability, and even death. Herein, we summarize the diagnosis, risk factors, prevention, and treatment of these postoperative complications, with emphasis on recent advances and perspectives.
Keywords: cognitive disorders, covert stroke, delirium, hemorrhagic stroke, postoperative neurological disorders, stroke
This review described general features and the latest evidence‐based knowledge of postoperative neurological disorders with emphasis on recent advances and perspectives. This review mapped the existing evidence regarding risk factors, prevention, and treatment for postoperative neurological disorders to help guide future research and clinical management.
1. INTRODUCTION
Postoperative neurological disorders have been attracting increasing attention in the world, with a vast amount of research conducted on these poorly understood disorders. Postoperative neurological disorders contain postoperative delirium (POD), postoperative cognitive dysfunction (POCD), postoperative covert ischemic stroke, and hemorrhagic stroke, causing cognition decline and poor long‐term functional outcome in the elderly. Postoperative neurological disorders increase mortality and cause substantial financial burden on family and society. With a rapid increase in the number of elderly patients undergoing elective surgical procedures, postoperative neurological disorders need more concern and further investigation. This review aims to describe general features and the latest evidence‐based knowledge of postoperative neurological disorders.
2. POSTOPERATIVE NEUROLOGICAL DISORDERS
Postoperative neurological disorders include neurological complications such as delirium, cognitive dysfunction, acute cerebral ischemic stroke, and hemorrhagic stroke that occur after surgery, especially in the elderly. As more elderly patients undergo surgery, the incidence of postoperative neurological disorders is rapidly increased. Although their exact etiology and pathogenesis remain elusive, several risk factors have been recognized.
2.1. POD
Postoperative delirium is defined as acute emergence of confusion, disorientation, perceptual disturbances, emotional dysregulation, or sleep disturbances, manifesting within a certain period of time. The prevalence of delirium ranged from 5% to 50% when assessed with Confusion Assessment Method for the Intensive Care Unit (CAM‐ICU). 1 POD can occur soon after general anesthesia and operation, for example, in the post‐anesthesia care unit (PACU). In many cases, POD is frequently linked to anesthesia. 2 Compared to dementia which chronically deteriorates brain function, POD is usually acute, transient, and presenting common causative factors. 3 It contributes to prolonged hospitalization, increased mortality rate, and reduced long‐term quality of life, which adds an additional burden to patients and families.
Besides DSM‐5 listed in Table 1 and ICD‐10 diagnostic criteria which supplements a disturbance in sleep‐awake cycle including insomnia, reverse of sleep‐awake cycle, various assessment tools have been developed to recognize POD. 4 The CAM‐ICU is a screening tool which consists of the assessment of four characteristics: 1) acute onset and fluctuating course of mental state, 2) inattention, 3) disorganized thinking, and 4) altered level of consciousness. 5 Delirium is diagnosed when both characteristics 1) and 2) are satisfied with 3) or 4) electively satisfied. 6
TABLE 1.
DSM‐5 Diagnostic criteria for delirium
| DSM−5 Diagnostic criteria for delirium |
|---|
|
Abbreviation: DSM‐5, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
2.2. POCD
Postoperative cognitive dysfunction, also defined as postoperative neurocognitive disorder (pNCD), 7 , 8 is characterized by cognitive decline persisting for more than 30 days but less than 12 months following surgery. Unlike POD (Table 2), consciousness, orientation, and attention are not obviously affected in POCD. 9 However, patients can still manifest impairment in memory, perceptual function, and language. 9 , 10 The incidence increases among the elderly, especially those over 60 years old. 11 For elderly patients, cognitive decline may result in prolonged hospitalization, reduced quality of life, even increased mortality, which has been neglected in the assessment of patient's prognosis, especially for those undergoing general anesthesia and surgery. 12 The diagnosis criteria for POCD are more complex than for POD, as POCD requires a subjective impression of postoperative cognitive decline in neuropsychological test. 13
TABLE 2.
Differential diagnosis of POD and POCD
| POD | POCD | |
|---|---|---|
| Epidemiology | In all ages but more common in older people over 60 | In all ages but more common in older people over 60 |
| Manifestation | Disturbance in attention and awareness, emotion, cognition, and fluctuating severity of consciousness. | Cognitive deficits (impairment of memory, perceptual function, language, ability to combine tasks) |
| Diagnostic tools | Various delirium scale, for example, CAM‐ICU | Pre‐ and postoperative psychometric testing |
| Timing | Days to weeks | Persisting for months |
| Prognosis | Reversible if underlying causative factors are treatable | Reversible but with long‐time impairment |
Abbreviations: POCD, postoperative cognitive dysfunction; POD, postoperative delirium.
2.3. Postoperative covert stroke
Cerebrovascular disease, a leading global cause of death and disability with approximately 6.2 million deaths due to stoke, is estimated to become the second leading cause of death by 2030. 14 According to a systemic analysis for the Global Burden of Disease Study, the mortality rates caused by stroke range from 30.6% to 48.3% 15 and are significantly related to operations. 16 Covert stroke has been increasingly recognized over the years. It represents brain infarcts with silent and subtle manifestations that can be detected on brain imaging. 17 , 18 Covert stroke may contribute more to poor outcomes and prognosis in elderly patients presenting cognitive decline, as its subtle manifestation can lead to ignorance of cognitive symptoms. 19
The incidence of postoperative covert stroke has gradually increased due to the aging population. So far, only a few studies have examined its mechanisms. 20 Little is known about perioperative covert stroke except its association with substantially increased mortality. 21 One multicenter prospective cohort study reported that postoperative covert stroke was found in 7% among 1114 participants over 65 years old who underwent inpatient, elective, noncardiac surgery, which were assessed with brain magnetic resonance imaging (MRI) after surgery and Montreal Cognitive Assessment (MoCA) on preoperative baseline and 1‐year follow‐up. 22 Among the patients with a complete 1‐year follow‐up, cognitive decline after surgery occurred in 42% of participants who had postoperative covert stroke and 29% of participants who did not have postoperative covert stroke. 22 In addition, another study suggested that covert stroke can increase the risk of POD, overt stroke, or transient ischemic attack (TIA) during one‐year follow‐up. 22
2.4. Hemorrhagic stroke
Although hemorrhagic stroke comprises only 20% of all strokes, the perioperative hemorrhagic stroke could detrimentally deteriorate patients’ recovery and prognosis. 23 According to the American Heart Association (AHA) and the American Stroke Association (ASA), 24 , 25 hemorrhagic stroke is divided into the following conditions: 1) focal bleeding in brain parenchyma and 2) in subarachnoid space or ventricular space due to rupture of blood vessel other than trauma. Perioperative hemorrhagic stroke may occur after cerebral hyper‐perfusion due to a sudden surge of blood pressure (BP) for a certain amount of time. 26 Hypertension is the most common risk factor for hemorrhagic stroke. 27 Most anesthetics induce hypotension, so they are unlikely to provoke hemorrhagic stroke while under anesthesia. 28 However, if postoperative hypertension persists for several hours, it can lead to certain condition linked to a sudden surge of cerebral perfusion. 29
What discussed above is a brief introduction of four major types of postoperative neurological disorders with current understanding, which present disorientation, memory deficit, changes in awareness and attention from the baseline. Cognitive decline and poor prognosis of elderly patients after surgery have been an increasing concern around the world. With few studies on postoperative neurological disorders, the mechanisms and pathophysiology remain unknown, 30 , 31 especially for POD, POCD, and covert stroke. Besides, various manifestations of postoperative neurological disorders add more difficulties for research. As postoperative complications become increasing concerns, the mechanisms, prevention, and management are focus points that still need further research.
3. RISK FACTORS OF ACUTE POSTOPERATIVE NEUROLOGICAL DISORDERS
The exact mechanisms and pathophysiology of POD, POCD, and postoperative stroke are unclear. In the following paragraphs, we discuss potential risk factors of postoperative neurological disorders.
3.1. Risk factors of POD and POCD
It is commonly accepted that interactions between predisposing factors and precipitating factors play an important role in the occurrence of postoperative neurological disorders. 32 The smaller vulnerability a patient has, the less occurrence of neurological disorders. 32 For example, as advanced age is a predisposing factor, patients over 65 years old may present POD or POCD when exposed to only a few precipitating factors. 33 On the contrary, younger patients exposed to the same precipitating factors may not experience POD or POCD. The recent evidence‐based risk factors including some novel candidates for POD as listed in Table 3. 32 POD and POCD share almost the same risk factors based on current limited researches. 34 Moreover, SARS‐CoV‐2 (COVID‐19) infection, a new uprising disease, has been found to be a potential novel risk factor of POD and POCD during the pandemic in the past two years, which is related to an accelerated onset with neurological manifestations 35 and deterioration of cognitive decline. 36
TABLE 3.
Risk factors of POD and POCD in perioperative patients
| Risk factors | Reference | |
|---|---|---|
| Preoperative factors | Advanced age | 32 |
| Comorbidities (eg, cerebrovascular including stroke, cardiovascular, peripheral vascular diseases, diabetes, anemia, Parkinson's disease, depression, chronic pain, anxiety disorders, renal failure, and alcohol use disorders) | 126, 127, 128, 129, 130, 131, 132, 133 | |
| Preoperative fluid fasting and dehydration | 32, 131 | |
| Preoperative blood transfusion | 130 | |
| Hyponatremia and hypernatremia | 128 | |
| Drugs with anticholinergic effects | 32 | |
| Quinolone | 130 | |
| Urine albumin to creatinine ratio (UACR) | 134 | |
| Intraoperative factors | Site of surgery (abdominal and cardiothoracic) | 126, 135 |
| Hybrid procedure | 130 | |
| Intraoperative bleeding | 136 | |
| Duration of surgery | 126 | |
| Bispectral index (too low or too high) | 127 | |
| Intraoperative electrolyte disturbance | 128 | |
| Hyperthermia or hypothermia | 137, 138 | |
| The rate of decline in intraoperative hemoglobin concentration | 136 | |
| Postoperative factors | Pain | 139 |
| Use of bed sensors | 140 | |
| Nursing home residency | 141 | |
| Delayed ambulation | 129 |
Abbreviations: POD, postoperative delirium; POCD, postoperative cognitive dysfunction
3.2. Risk factors of postoperative stroke
Postoperative stroke remains one of the most serious complications after surgery. As for both ischemic stroke and hemorrhagic stroke, risk factors include conventional vascular risk factors, the type of surgery, and other perioperative events (Table 4). Vascular factors and nutritional state are important preoperative risk factors for stroke and postoperative stroke. Postoperative stroke happens more often in cardiovascular, general thoracic, and neurosurgery. 37 , 38 Among factors below, the BP and coagulation state are specific modifiable risk factors for postoperative stroke according to large‐scale database studies, which will be further discussed.
TABLE 4.
Risk factors of perioperative stroke
| Risk factors | Reference | |
|---|---|---|
| Preoperative factors | Vascular factors (eg, age, sex, history of stoke or TIA, arrhythmia, coagulopathy) | 37, 41, 42, 142, 143, 144 |
| Anemia | 42 | |
| Malnutrition | ||
| Preoperative central nervous system malperfusion | 145 | |
| Cerebral diffusion‐weighted imaging lesions | 146 | |
| Renal dysfunction | 147, 148 | |
| Intraoperative factors | Type of surgery (cardiovascular, neurosurgery, left pneumonectomy other types of surgery) | 37, 38, 42 |
| Specific intraoperative events (arrhythmia, hypertension, hypotension) | 44 | |
| Postoperative factors | Adverse events (cardiac arrest, severe arrhythmia) | 149 |
| New‐onset atrial fibrillation | 150, 151 |
Abbreviation: TIA, transient ischemic attack
3.2.1. BP and stroke
Blood pressure fluctuation is an important risk factor for postoperative stroke. 39 Emergency surgeries raise the incidence of neurological disorders and even affect long‐term cognitive functions. 40 As mentioned before, older patients are usually complicated with underlying diseases including hypertension, coronary artery disease, and arrhythmia, and thus, they have a greater risk of suffering from postoperative neurological disorders. 37 , 41 , 42 It has been reported that patients developing postoperative stroke (ischemic or hemorrhagic) have higher average mean arterial blood pressure (MAP >80 mmHg). 37 Moreover, a previous study suggests a strong relationship between elevated pulse pressure and stroke, which increases cerebral vulnerability to ischemic stroke. 42 Additionally, evidence showed that intraoperative hypotension (IOH) is associated with the risk of major postoperative cardiac or cerebrovascular events. 43 The short exposure to MAP of 55–65 mmHg is significantly associated with postoperative adverse cerebrovascular events, while maintaining systolic blood pressure (SBP) within 10% of the reference value may prevent postoperative adverse events compared with standard care (only treating if SBP <80 mmHg or <40% of the reference value). 44 , 45
3.2.2. Coagulation and stroke
For elderly patients, oral anticoagulants, including vitamin K antagonist (VKA) and non‐vitamin K oral antagonists (NOACs), 46 are common and effective therapies for the prevention of thromboembolism and stroke. However, they may lead to an inherent risk of bleeding. For elective and urgent surgery, reversal of anticoagulants is a necessary process for perioperative management. 47 Traditional broader anticoagulants VKAs, such as warfarin, while effective, had multiple dietary and drug interactions and a great risk for intracranial hemorrhage, where 4‐factor (II, VII, IX, and X) prothrombin complex concentrate (4F‐PCC) should be used for VKAs reversal. 48 Recently new types of anticoagulants targeted specific clotting factors (factors IIa and Xa), including dabigatran and rivaroxaban, have been approved for anticoagulation use with less adverse effect and less risk of hemorrhage. 49 In addition, it is recommended to monitor the coagulation state of patients during the perioperative period. 50
Postoperative neurological disorders occur by the interaction of predisposing factors and precipitating factors. Risk factors of POD, POCD, and perioperative stroke are listed above, and there may be other underlying risk factors unknown. Therefore, more researches are needed to understand possible risk factors and pathophysiology related to postoperative neurological disorders. The preventive strategies and protocols need to be established for known risk factors as well.
4. PREVENTION
4.1. Perioperative prevention of POD
Prevention strategies should be designed based on predisposing factors and parts of precipitating factors, which are the most effective measures against delirium. 51 The Hospital Elder Life Program (HELP) released a multicomponent intervention guideline to prevent delirium 52 (Figure 1).
FIGURE 1.
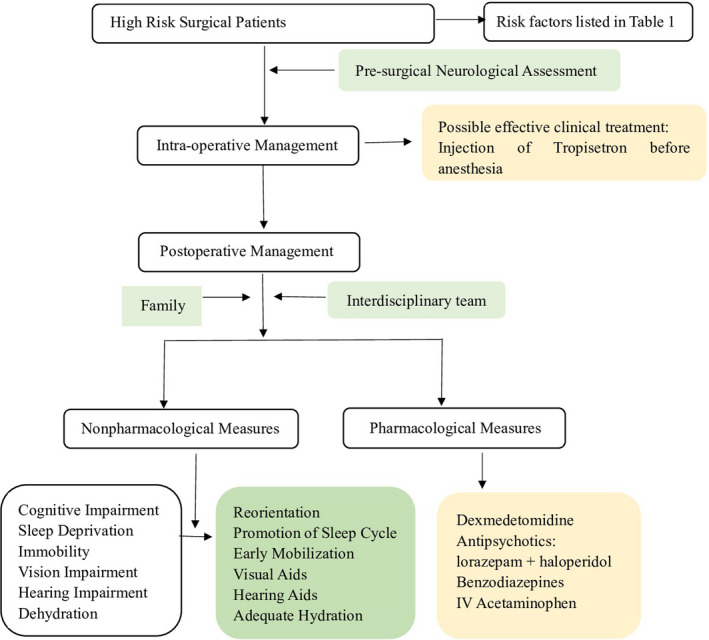
Nonpharmacological and pharmacological interventions during preventive process of POD recommended in HELP and latest evidence‐based advance research on prevention of POD, including interventions throughout the whole process of surgery and possible medications for treatment that needed further research. POD, postoperative delirium; HELP, Hospital Elder Life Program
4.1.1. Nonpharmacological interventions
Based on the studies of HELP, the multicomponent nonpharmacological intervention significantly reduces the incidence of delirium, including reorientation (using orientation calendar, clocks), early mobilization, promotion of sleep cycle, adequate hydration, visual and hearing aids, and increased supervision in hospital. 53 If implemented by a skilled interdisciplinary team, these measures are effective against POD. 54 Apart from common contents of HELP, a recent clinical trial has found that tailored, family‐involved HELP could be beneficial for reducing POD, maintaining, or improving cognitive function, which may increase the implementation of this program. 55 During the operation, however, the EEG (electroencephalography)‐guided anesthetic administration, compared with usual care, failed to decrease the incidence of POD. 56
4.1.2. Pharmacological interventions
Although several clinical trials have run various pharmacologic measures, there is a lack of strong evidence for effective prevention. One network meta‐analysis demonstrated that haloperidol plus lorazepam might be the best treatment, while ramelteon may be the best preventive medicine for POD. 57 Besides, a recent randomized clinical trial has evaluated the effectiveness of tropisetron and found that tropisetron could decrease the incidence of delirium after noncardiac procedures in adults. 58 Another trial has found that postoperative scheduled intravenous (IV) acetaminophen, combined with IV propofol or dexmedetomidine, could reduce the incidence of POD in hospital versus placebo group, while patients receiving one single intravenous anesthetic had no significant improvement for POD. 59 According to a Cochrane review that examined antipsychotic medications for preventing delirium in hospitalized, non‐ICU patients, medications such as cholinesterase inhibitors, melatonin, and melatonin‐receptor agonists have no clear effect in preventing delirium. 60
Besides, measures against precipitating factors are mainly adopted in the perioperative period, including limiting fasting time, reducing the use of preoperative medications such as benzodiazepines, opioids, and anticholinergic medications. For elderly patients with risk factors, preoperative neurological assessment is essential for predicting and preventing of POD. 61
4.2. Prevention of POCD
4.2.1. Preoperative interventions
The evaluation of patients’ baseline is important for the identification and prevention of POCD. Neuropsychological tests should be used before and after operations. 62 Also, cognitive training and exercise 63 have been proven to be beneficial for preventing POCD occurrence. 64 Besides, Lu et al. found that pretreatment of parecoxib sodium combined with dexmedetomidine can decrease the incidence of POCD in patients undergoing arthroscopy by over 10%. 65 These interventions should be particularly considered in high‐risk patients.
4.2.2. Intraoperative interventions
In addition to preoperative interventions, there are several intraoperative measures to consider, including minimal exposure to anesthetics with careful monitoring. Regarding the anesthetic choice, Chen et al. found that the use of inhaled anesthetics 66 in cardiac surgery generated higher postoperative scores in the Mini Mental State Exam (MMSE) compared with total intravenous anesthetics. 67 Also, propofol may have a significant advantage in reducing POCD incidence compared with dexmedetomidine and midazolam sedation in elderly patients, in which midazolam has the highest inhibitory effects on cognitive functions. 68
As for monitoring measures, one trial has shown a decreased incidence of short‐term POCD with bispectral index (BIS)‐guided deep anesthesia during the operation. 69 Lastly, it has been suggested that postoperative management, including early identification and treatment of postoperative complications, may decrease the risk of POCD, which will be discussed in the treatment section. All interventions discussed above are listed in Figure 2.
FIGURE 2.
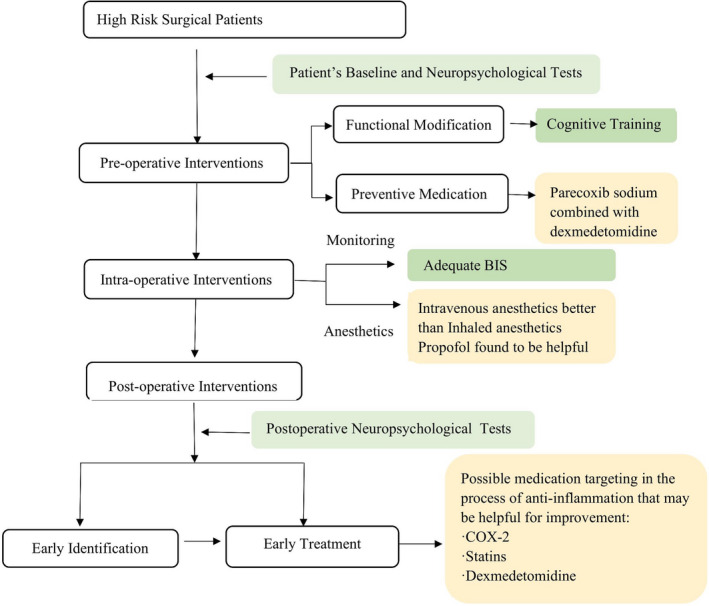
Preventive measures of POCD based on latest research on it during preoperative, intraoperative, and postoperative process and possible effective medications that may contribute to the prevention. POCD, postoperative cognitive dysfunction. BIS, bispectral index. COX‐2, cyclooxygenase‐2
4.3. Prevention of stroke
As for perioperative ischemic (covert) stroke and hemorrhagic stroke, the control of modifiable risk factors is the most effective preventive strategy. These identified modifiable risk factors include hypertension, diabetes mellitus, hyperlipidemia, obesity, and smoking. 70
4.3.1. Modifiable risk factor—hypertension
Hypertension is the most important modifiable factor for stroke, 71 attributing to more than half of all stroke events worldwide. 72 Antihypertensive medications are recommended for patients with BP over 140/90 mmHg. 73 The most common medications include β‐adrenergic agonists (β‐blockers), calcium channel blockers (CCB), diuretics, angiotensin‐converting enzyme inhibitors (ACEI), and angiotensin II receptors blockers (ARB), and the choice of therapy depends on individual comorbidities. 73 Adequate BP control is important, while the goal adjustment should also be considered for older patients to avoid complications such as hypotension and dizziness. Although perioperative use of β‐blockers might be beneficial in reducing heart rate and sympathetic activity and controlling BP, there is no association between β‐blockers and perioperative outcomes. 74 , 75
4.3.2. Modifiable risk factor—hyperlipidemia
Hyperlipidemia is another remarkable risk factor, as several clinical trials and meta‐analyses have reported decreased vascular events and mortality rates in patients with treatment for hyperlipidemia, especially lowering LDL‐C (low‐density lipoprotein‐cholesterol). 72 A randomized trial evaluated the benefits of statin as secondary prevention of stroke and found that atorvastatin can reduce the incidence of stroke in patients with recent stroke or TIA. 76
Treatments of hypertension and hyperlipidemia have been proven to prevent stroke. However, the effectiveness of other factors such as weight and blood glucose control, or smoking cessation may warrant further trials. Besides these control measures against risk factors, there are some other strategies proved to prevent postoperative stroke. Researchers have found that perioperative use of dexmedetomidine reduced the incidence of postoperative stroke and delirium in elderly patients following cardiac surgery. 77 And in another clinical trial, left atrial appendage (LAA) surgical exclusion is effective on prevention of stroke, particularly in patients with atrial fibrillation after mitral valve replacement (MVR). 55
5. CLINICAL MANAGEMENT AND PRECLINICAL STRATEGIES
Immediate treatment of etiologies and symptoms can contribute to a shorter duration of postoperative neurological disorders and lower risk of long‐term cognitive impairment, disability, and even death. 78 , 79 The clinical treatments and preclinical studies of POD, POCD, postoperative ischemic stroke, and postoperative hemorrhagic stroke are discussed below.
5.1. Treatment of POD
5.1.1. Nonpharmacological measures
Nonpharmacological measures are beneficial for both preventing and treating POD, 80 including reorientation, early mobilization, promotion of sleep cycle, adequate hydration, and visual and hearing aids. They can modify and create a safe and calm environment for patients.
5.1.2. Pharmacological measures
There is no strong evidence for pharmacological management of POD, although the use of dexmedetomidine resulted in more ventilator‐free time at 7 days among patients with agitated delirium in the intensive care unit. 81 Medications are generally applied for delirium‐associated behaviors. Two types of medications are frequently used, that is, antipsychotics and benzodiazepines. 82
For agitation with perceptual disturbance or sleep‐wake cycle abnormalities, antipsychotics can be useful. 83 Nevertheless, patients with POD, which is more common in advanced age, may respond poorly to antipsychotic medications. 84 Benzodiazepines are historically used to sedate patients with delirium for decades. However, evidence suggests that benzodiazepines may increase the risk and duration of delirium, especially in elderly patients. 85 Thus, benzodiazepines should be mainly used in the treatment of agitation associated with sedative withdrawal. 82 Another randomized trial showed that the addition of lorazepam to haloperidol resulted in a significantly reduction in agitation at 8 hours in hospitalized cancer patients with agitated delirium. 86 Given the above evidence, pharmacologic treatment is not strongly recommended but can be used to treat severe agitation and life‐threatening POD complication.
5.2. Treatment of POCD
Preventive interventions discussed above can also be used for the treatment of POCD. Several preclinical and clinical studies suggested that targeting postoperative neuro‐inflammation 87 , 88 , 89 , 90 , 91 may be a potential way to treat POCD. There are some medications used in the clinical trials attempted to block the process of neuroinflammation.
The cyclooxygenase‐2 (COX‐2), which is responsible for catalyzing the conversion of arachidonic acid to pro‐inflammatory prostaglandins 92 and increasing blood‐brain barrier (BBB) permeability, 93 , 94 is considered to be an important mediator of neuroinflammation and thus a potential target for POCD treatment. Moreover, a meta‐analysis suggested that the administration of parecoxib was effective in treating early POCD within 7 days and reducing interleukin‐6 (IL‐6) and S100 calcium‐binding protein B protein (S100β) concentrations within 2 days after operations. 95 Other anti‐inflammatory medications, such as minocycline 96 and dexamethasone, 97 may also provide possible treatments for POCD. However, no prospective clinical trials investigated the promising effect of antioxidative agents for the prevention of POCD.
Statins are reversible competitive inhibitors of the rate‐limiting enzyme in cholesterol synthesis. 98 They have been widely proven to be beneficial for neurological disorders, including dementia 99 and delirium. 100 In POCD, a clinical trial showed a significant reduction in memory dysfunction comparing statin to placebo in patients undergoing off‐pump coronary artery bypass grafting (CABG). 101
Dexmedetomidine has downstream effects on reducing serum pro‐inflammatory cytokines in POCD. 102 Dexmedetomidine treatment has been shown to ameliorate neurological dysfunction and decrease the incidence of cognitive impairment following surgical trauma in a hyperlipidemia rat model. 103
5.3. Treatment of postoperative ischemic stroke
Postoperative covert stroke, as a type of postoperative ischemic stroke, has been studied little so far, and thus, there is no recommended treatment. Focusing on underlying treatable causes may be helpful but it is still in need of further investigation. Further research developed, treatment protocol may be clearer with more information. There are several clinical treatments for stroke, including intravenous thrombolysis, mechanical thrombectomy, intravenous infusion of unfractionated heparin, 104 which are major treatments for stroke. As for postoperative stroke, some of the treatments may not be suitable for patients undergoing surgeries. For example, intravenous unfractionated heparin is not recommended for postoperative stroke due to the high incidence of bleeding. However, the prolonged therapeutic window and new thrombectomy devices will give more opportunities to treat postoperative ischemic stroke in the future. Several effective treatments of postoperative ischemic stroke are listed below, which may be possible complications of covert stroke.
5.3.1. Intravenous thrombolysis, injecting intravenous medications to dissolve thrombus
For patients with ischemic stroke up to 4.5 hours after symptom onset, 105 intravenous thrombolysis using tissue‐type plasminogen activator (t‐PA) can be considered as the first‐line treatment. However, undergoing major surgery within 14 days is a contraindication for t‐PA administration because of bleeding of surgical sites. Therefore, it is challenging and controversial to use intravenous t‐PA even with clear symptoms for patients with postoperative stroke. 106 The use of t‐PA should be individualized based on the risk and benefit of the treatment.
5.3.2. Mechanical thrombectomy, using a guidewire to remove thrombus
Multiple clinical trials have demonstrated that endovascular therapy (EVT) has effective recanalization 107 and better clinical outcomes without the additional complication of hemorrhage in anterior circulation ischemia with large vessel occlusion (mainly of the intracranial internal carotid artery [ICA], middle cerebral artery [MCA] main trunk, or the M2 major branch of the MCA), which is considered as a treatment option for postoperative stroke. 108 Besides, studies have expanded the therapeutic window up to 24 hours after symptom onset for patients with a small core and large penumbra. 109 New devices continue to be developed for reaching distal branches, 110 which may provide standard treatment for perioperative stroke.
5.4. Treatment of postoperative hemorrhagic stroke
Some clinical trials suggested various effective treatment methods for hemorrhagic stroke, including the control of high BP and intracranial pressure (ICP), and treatment of complications and intracranial hemorrhage, 111 which are important treatment methods for hemorrhagic stroke. We will discuss the most effective methods below.
5.4.1. Controlled BP
Patients with hemorrhage usually present high BP. High SBP has been associated with neurological deterioration and death, 112 which should be gradually reduced by using antihypertensive drugs such as CCB and ACEI. The ASA recommended that for patients presenting with SBP between 150 and 220 mmHg, an acute and aggressive reduction of SBP is safe and beneficial for functional outcomes. 112 However, some clinical trials found no significant relationship between SBP reduction and hematoma expansion or outcome. 113 The goal and effect of BP control in postoperative hemorrhagic stroke need to be elucidated in future studies.
5.4.2. Interventions to control intracranial pressure (ICP)
The initial treatment should include elevating the head of the bed to 30° and the patients’ head facing midline to avoid excessive flexion or rotation of the neck. 114 Then, osmotic agents (mannitol and hypertonic saline) are given; for example, 20% mannitol is given at 1.0–1.5 g/kg. 115 Next, early intubation and mechanical ventilation should be applied, especially for patients in coma. ASA also recommends ICP monitoring with a ventricular catheter to ensure the cerebral perfusion pressure (CPP) between 50 and 60 mmHg to prevent cerebral ischemia. 112
5.4.3. Treatment of complications
Researches showed that 3% to 17% of patients have seizures 116 in the first two weeks after operation, which should be treated with antiepileptic drugs. 112 First‐generation antiepileptic drugs negatively affect cognitive function, which should be avoided in patients with postoperative stroke. 117 Drugs developed after 2000 are known as third‐generation antiepileptic drugs, such as eslicarbazepine acetate, lacosamide, perampanel, brivaracetam, rufinamide, and stiripentol. 118 These drugs, characterized with new mechanisms of action and favorable pharmacokinetics, can decrease the occurrence of side effects and drug‐drug or hormonal interactions compared with first‐generation antiepileptic drugs. These new drugs are also useful therapies in patients with intractable epilepsy. 119 However, currently there are no instructions on the use of these drugs for postoperative stroke patients.
5.4.4. Surgical treatments of hemorrhage
There are different types of surgical treatments of hemorrhagic stroke, including craniotomy for hematoma drainage, decompressive craniectomy with or without hematoma drainage, minimally invasive endoscopic aspiration and catheter aspiration. 120
Open surgery
Although the approach of open surgery to treat patients with cerebral hemorrhage remains controversial, it is still one of the most common approaches applied for hematoma drainage. 121 The Surgical Trial in Intracerebral Hemorrhage (STICH), which was the first multicenter, multinational, randomized clinical trial that compared the benefits of hematoma drainage with conservative management, found that there was no overall benefit from early hematoma drainage compared with conservative treatment. 122 Subsequently, another international, multicenter, prospective, randomized trial, which included only patients with superficial hematomas within 1 cm from the brain's cortical surface of the brain, indicated that patients with superficial hematomas could benefit from early intervention of hematoma removal. 123
Minimally invasive approach
The practice of open craniotomy is complicated with brain tissue damage and related complications because it requires a large bone flap and exposure of the brain tissue. On the contrary, a minimally invasive approach with thrombolysis is safer, more feasible, and more efficacious. 124 However, it still showed no significant benefit of long‐term functional outcome than conservative treatment based on clinical trials. 124
In summary, decompressive craniectomy and hematoma evacuation are performed more frequently for hemorrhagic stroke in patients with Glasgow Coma Scale (GCS) scores of 8 or less and large hematomas. These procedures reduce mortality and may improve functional outcomes. 125
6. CONCLUSION AND FUTURE REMARKS
Postoperative neurological disorders are common complications without effective treatment that exert an enormous burden on patients, their families, hospitals, public resources, and society. The existing acknowledge of risk factors provides certain information to guide possible effective interventions that may need more repeating trials, but further interventional research is urgently needed to improve the outcomes or prognosis for hospitalized patients who experience the condition. Early prevention is likely to be more effective than treatment for prognosis. Thus, further prospective trials should gain deeper insight into uncertain mechanisms and contributing factors underlying these neurological disorders, avoiding simple risk factor evaluation, and validating possible prognostic models for interventional research.
Significantly, dexmedetomidine may be an effective medication for the prevention of POD and POCD as mentioned in multiple trials, which should be considered in future research. As for covert stroke, early identification may be more effective, likely given priority to in further research.
CONFLICT OF INTEREST
The authors declare to have no potential conflicts of interest. All data included in this study are available upon request by contact with the corresponding author.
ACKNOWLEDGEMENTS
This work is supported by National Natural Science Foundation of China (NSFC, 91957111, 81971096, 82061130224 to PL, 81971223 to WY), New Frontier Technology Joint Research Program (SHDC12019102 to PL), Shanghai Municipal Education Commission‐Gaofeng Clinical Medical Grant Support (20181805 to PL), Shuguang Program (20SG17 to PL), Shanghai Outstanding Academic Leaders Program (20XD1422400 to PL), Newton Advanced Fellowship grant provided by the UK Academy of Medical Sciences (NAF\R11\1010 to PL), Shanghai Municipal Key Clinical Specialty (shslczdzk03601 to WY), Innovation Program of Shanghai Municipal Education Commission (2019‐01‐07‐00‐01‐E00074 to WY), General Research Program of Shanghai Health and Family Planning Commission (201840241 to HZ). This work is also supported by the Shanghai Engineering Research Center of Peri‐operative Organ Support and Function Preservation (20DZ2254200).
Liu B, Huang D, Guo Y, et al. Recent advances and perspectives of postoperative neurological disorders in the elderly surgical patients. CNS Neurosci Ther. 2022;28:470–483. doi: 10.1111/cns.13763
Biying Liu and Dan Huang contributed equally to this work.
Contributor Information
Peiying Li, Email: peiyingli.md@gmail.com.
Weifeng Yu, Email: ywf808@yeah.net.
REFERENCES
- 1. Haenggi M, Blum S, Brechbuehl R, Brunello A, Jakob SM, Takala J. Effect of sedation level on the prevalence of delirium when assessed with CAM‐ICU and ICDSC. Intensive Care Med. 2013;39:2171‐2179. [DOI] [PubMed] [Google Scholar]
- 2. Sharma PT, Sieber FE, Zakriya KJ, et al. Recovery room delirium predicts postoperative delirium after hip‐fracture repair. Anest Analg. 2005;101:1215‐1220. [DOI] [PubMed] [Google Scholar]
- 3. Wang P, Velagapudi R, Kong C, et al. Neurovascular and immune mechanisms that regulate postoperative delirium superimposed on dementia. Alzheimer's & Dementia. 2020;16:734‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM‐5 approach. Nat Rev Neurol. 2014;10:634‐642. [DOI] [PubMed] [Google Scholar]
- 5. Shenkin SD, Fox C, Godfrey M, et al. Delirium detection in older acute medical inpatients: a multicentre prospective comparative diagnostic test accuracy study of the 4AT and the confusion assessment method. BMC Med. 2019;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotfis K, Marra A, Ely EW. ICU delirium — A diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiology Intensive. Therapy. 2018;50. [DOI] [PubMed] [Google Scholar]
- 7. Borchers F, Knaak C, Piper SK, Spies C. Empfehlungen zur Erfassung und Beschreibung perioperativer kognitiver Störungen in Wissenschaft und Praxis. Anästhesiol Intensivmed Notfallmed Schmerzther. 2019;54:652‐667. [DOI] [PubMed] [Google Scholar]
- 8. Le Y, Liu S, Peng M, et al. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery. PLoS One. 2014;9:e106837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP. Neurocognitive Function after Cardiac Surgery: From Phenotypes to Mechanisms. Anesthesiology. 2018;129:829‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131:477‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54:951‐956. [DOI] [PubMed] [Google Scholar]
- 12. Moller JT, Cluitmans P, Rasmussen LS, et al. Long‐term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351:857‐861. [DOI] [PubMed] [Google Scholar]
- 13. Olotu C. Postoperative neurocognitive disorders. Curr Opin Anaesthesiol. 2020;33:101‐108. [DOI] [PubMed] [Google Scholar]
- 14. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the global burden of disease study 2017. The Lancet. 2019;394:1145‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong Y, Cao W, Cheng X, et al. Risk factors and stroke characteristic in patients with postoperative strokes. J Stroke Cerebrovasc Dis. 2017;26:1635‐1640. [DOI] [PubMed] [Google Scholar]
- 17. Vlisides PE, Avidan MS, Mashour GA. Uncovering covert stroke in surgical patients. The Lancet. 2019;394:982‐984. [DOI] [PubMed] [Google Scholar]
- 18. Chen H‐F, Huang L‐L, Li H‐Y, et al. Microstructural disruption of the right inferior fronto‐occipital and inferior longitudinal fasciculus contributes to WMH‐related cognitive impairment. CNS Neurosci Ther. 2020;26:576‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu S, Li P. Cognitive declines after perioperative covert stroke: Recent advances and perspectives. Curr Opin Anaesthesiol. 2020;33:651‐654. [DOI] [PubMed] [Google Scholar]
- 20. Lukaszewicz AC, Bouchier B, Bruckert V. Perioperative covert stroke: An overlooked but sneaky event. Anaesth Crit Care Pain Med. 2020;39:19‐20. [DOI] [PubMed] [Google Scholar]
- 21. Mrkobrada M, Hill MD, Chan MTV, et al. Covert stroke after non‐cardiac surgery: A prospective cohort study. Br J Anaesth. 2016;117:191‐197. [DOI] [PubMed] [Google Scholar]
- 22. Mrkobrada M, Chan MTV, Cowan D, et al. Perioperative covert stroke in patients undergoing non‐cardiac surgery (NeuroVISION): A prospective cohort study. The Lancet. 2019;394:1022‐1029. [DOI] [PubMed] [Google Scholar]
- 23. Montaño A, Hanley DF, Hemphill JC. Chapter 13 ‐ Hemorrhagic stroke. In: Hetts SW, Cooke DL, eds. Handbook of clinical neurology. Elsevier; 2021:229‐248. [DOI] [PubMed] [Google Scholar]
- 24. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: A report from the american heart association. Circulation. 2020;141:e139‐e596. [DOI] [PubMed] [Google Scholar]
- 25. Selim M, Hanley D, Steiner T, et al. Recommendations for clinical trials in ICH. Stroke. 2020;51:1333‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kameyama M, Fujimura M, Tashiro R, et al. Significance of quantitative cerebral blood flow measurement in the acute stage after revascularization surgery for adult moyamoya disease: Implication for the pathological threshold of local cerebral hyperperfusion. Cerebrovasc Dis. 2019;48:217‐225. [DOI] [PubMed] [Google Scholar]
- 27. Dong H, Liu S, Jing L, et al. Hypertension among hemorrhagic stroke patients in northeast China: A population‐based study 2017–2019. Med Sci Monit. 2020;26:e926581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ranasinghe JS, Kafi S, Oppenheimer J, Birnbach DJ. Hemorrhagic stroke following elective cesarean delivery. Int J Obstet Anesth. 2008;17:271‐274. [DOI] [PubMed] [Google Scholar]
- 29. van Mook WNKA, Rennenberg RJMW, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4:877‐888. [DOI] [PubMed] [Google Scholar]
- 30. Lin X, Chen Y, Zhang P, Chen G, Zhou Y, Yu X. The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol. 2020;130: 110791. [DOI] [PubMed] [Google Scholar]
- 31. Abad‐Gurumeta A, Casans‐Frances R, Gomez‐Rios MA. Postoperative neurocognitive disorders: unknowns to solve and work to do. Minerva Anestesiol. 2020;86:908‐909. [DOI] [PubMed] [Google Scholar]
- 32. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence‐based and consensus‐based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192‐214. [DOI] [PubMed] [Google Scholar]
- 33. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21‐26. [DOI] [PubMed] [Google Scholar]
- 34. Kapoor I, Prabhakar H, Mahajan C. Postoperative cognitive dysfunction. Indian J Crit Care Med. 2019;23:S162‐S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baig AM. Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neurosci Ther. 2020;26:499‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei P, Lyu W, Wan T, et al. COVID‐19: a novel risk factor for perioperative neurocognitive disorders. Br J Anaesth. 2021;127:e113‐e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsiouris A, Heliopoulos I, Mikroulis D, Mitsias PD. Factors defining occurrence of ischemic and hemorrhagic strokes during continuous flow left ventricular assist device support. Gen Thorac Cardiovasc Surg. 2020;68:319‐327. [DOI] [PubMed] [Google Scholar]
- 38. Xie N, Meng X, Wu C, et al. Both left upper lobectomy and left pneumonectomy are risk factors for postoperative stroke. Sci Rep 2019;9:10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin M‐H, Kamel H, Singer DE, Wu Y‐L, Lee M, Ovbiagele B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. 2019;50:1364‐1371. [DOI] [PubMed] [Google Scholar]
- 40. Shin YS. Postoperative delirium in geriatric patients after emergency general surgery. J Am Coll Surg. 2020;231:188‐189. [DOI] [PubMed] [Google Scholar]
- 41. Ko S‐B. Perioperative stroke: pathophysiology and management. Korean J Anesthesiol. 2018;71:3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Al‐Qamari A, Adeleke I, Kretzer A, Hogue CW. Pulse pressure and perioperative stroke. Curr Opin Anaesthesiol. 2019;32:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khanna AK, Shaw AD, Stapelfeldt WH, et al. Postoperative hypotension and adverse clinical outcomes in patients without intraoperative hypotension, after noncardiac surgery. Anesth Analg. 2021;132:1410‐1420. [DOI] [PubMed] [Google Scholar]
- 44. Gregory A, Stapelfeldt WH, Khanna AK, et al. Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesth Analg. 2021;132:1654‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu Q, Qi J, Wang Y. Intraoperative hypotension and neurological outcomes. Curr Opin Anaesthesiol. 2020;33:646‐650. [DOI] [PubMed] [Google Scholar]
- 46. Bo M, Marchionni N. Practical use of direct oral anti coagulants (DOACs) in the older persons with atrial fibrillation. Eur J Intern Med. 2020;71:32‐38. [DOI] [PubMed] [Google Scholar]
- 47. Leonidas P, Jeremy M, Damianos GK, et al. Reversal of novel anticoagulants in emergent surgery and trauma: A comprehensive review and proposed management algorithm. Curr Pharm Des. 2018;24:4540‐4553. [DOI] [PubMed] [Google Scholar]
- 48. Kuramatsu JB, Sembill JA, Huttner HB. Reversal of oral anticoagulation in patients with acute intracerebral hemorrhage. Crit Care. 2019;23:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adeboyeje G, Sylwestrzak G, Barron JJ, et al. Major bleeding risk during anticoagulation with warfarin, dabigatran, apixaban, or rivaroxaban in patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23:968‐978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Milling TJ Jr, Ziebell CM. A review of oral anticoagulants, old and new, in major bleeding and the need for urgent surgery. Trends Cardiovasc Med. 2020;30:86‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lovell N, Maddocks M, Etkind SN, et al. Characteristics, symptom management, and outcomes of 101 patients With COVID‐19 referred for hospital palliative care. J Pain Symptom Manage. 2020;60:e77‐e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singler K, Thomas C. HELP – Hospital Elder Life Program – ein multimodales Interventionsprogramm zur Delirprävention bei älteren Patienten. Der Internist. 2017;58:125‐131. [DOI] [PubMed] [Google Scholar]
- 53. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non‐pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP series. PLoS One. 2015;10:e0123090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deng LX, Cao L, Zhang LN, Peng XB, Zhang L. Non‐pharmacological interventions to reduce the incidence and duration of delirium in critically ill patients: A systematic review and network meta‐analysis. J Crit Care. 2020;60:241‐248. [DOI] [PubMed] [Google Scholar]
- 55. Jiang S, Zhang H, Wei S, et al. Left atrial appendage exclusion is effective in reducing postoperative stroke after mitral valve replacement. J Card Surg. 2020;35:3395‐3402. [DOI] [PubMed] [Google Scholar]
- 56. Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of electroencephalography‐guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: The ENGAGES randomized clinical trial. JAMA. 2019;321:473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu Y‐C, Tseng P‐T, Tu Y‐K, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: A network meta‐analysis. JAMA Psychiatry. 2019;76:526‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun Y, Lin D, Wang J, et al. Effect of tropisetron on prevention of emergence delirium in patients after noncardiac surgery: A trial protocol. JAMA Netw Open 2020;3:e2013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: The DEXACET randomized clinical trial. JAMA. 2019;321:686‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non‐ICU patients. Cochrane Database of Systematic Reviews. 2016;3:CD005563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mistraletti G, Umbrello M, Anania S, et al. Neurological assessment with validated tools in general ICU: multicenter, randomized, before and after, pragmatic study to evaluate the effectiveness of an e‐learning platform for continuous medical education. Minerva Anestesiol. 2017;83:145‐154. [DOI] [PubMed] [Google Scholar]
- 62. Rasmussen LS, Larsen K, Houx P, et al. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45:275‐289. [DOI] [PubMed] [Google Scholar]
- 63. Bliss ES, Wong RH, Howe PR, Mills DE. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J Cereb Blood Flow Metab. 2021;41:447‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Gara BP, Mueller A, Gasangwa DVI, et al. Prevention of early postoperative decline: A randomized, controlled feasibility trial of perioperative cognitive training. Anesth Analg. 2020;130:586‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lu J, Chen G, Zhou H, Zhou Q, Zhu Z, Wu C. Effect of parecoxib sodium pretreatment combined with dexmedetomidine on early postoperative cognitive dysfunction in elderly patients after shoulder arthroscopy: A randomized double blinded controlled trial. J Clin Anesth. 2017;41:30‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Berndt N, Kovács R, Schoknecht K, et al. Low neuronal metabolism during isoflurane‐induced burst suppression is related to synaptic inhibition while neurovascular coupling and mitochondrial function remain intact. J Cereb Blood Flow Metab. 0:0271678X211010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen F, Duan G, Wu Z, Zuo Z, Li H. Comparison of the cerebroprotective effect of inhalation anaesthesia and total intravenous anaesthesia in patients undergoing cardiac surgery with cardiopulmonary bypass: a systematic review and meta‐analysis. BMJ Open. 2017;7:e014629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Li W‐X, Luo R‐Y, Chen C, et al. Effects of propofol, dexmedetomidine, and midazolam on postoperative cognitive dysfunction in elderly patients: a randomized controlled preliminary trial. Chin Med J. 2019;132:437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Quan C, Chen J, Luo Y, et al. BIS‐guided deep anesthesia decreases short‐term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain and Behavior. 2019;9:e01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Udesh R, Mehta A, Gleason TG, Wechsler L, Thirumala PD. Perioperative strokes and early outcomes in mitral valve surgery: A nationwide analysis. J Cardiothorac Vasc Anesth. 2017;31:529‐536. [DOI] [PubMed] [Google Scholar]
- 71. Cipolla MJ, Liebeskind DS, Chan S‐L. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. 2018;38:2129‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case‐control study. The Lancet. 2016;388:761‐775. [DOI] [PubMed] [Google Scholar]
- 73. Malhotra K, Goyal N, Katsanos AH, et al. Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension. 2020;75:730‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. van Lier F, Schouten O, Hoeks SE, et al. Impact of prophylactic beta‐blocker therapy to prevent stroke after noncardiac surgery. Am J Cardiol. 2010;105:43‐47. [DOI] [PubMed] [Google Scholar]
- 75. Hajibandeh S, Hajibandeh S, Antoniou SA, Torella F, Antoniou GA. Effect of beta‐blockers on perioperative outcomes in vascular and endovascular surgery: A systematic review and meta‐analysis. Br J Anaesth. 2017;118:11‐21. [DOI] [PubMed] [Google Scholar]
- 76. Szarek M, Amarenco P, Callahan A, et al. Atorvastatin reduces first and subsequent vascular events across vascular territories: The SPARCL trial. J Am Coll Cardiol. 2020;75:2110‐2118. [DOI] [PubMed] [Google Scholar]
- 77. Cheng H, Li Z, Young N, et al. The effect of dexmedetomidine on outcomes of cardiac surgery in elderly patients. J Cardiothorac Vasc Anesth. 2016;30:1502‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Adults TAGSEPoPDiO . American geriatrics society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63:142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Young J, Murthy L, Westby M, Akunne A, O’Mahony R. Diagnosis, prevention, and management of delirium: Summary of NICE guidance. BMJ. 2010;341:c3704. [DOI] [PubMed] [Google Scholar]
- 80. Yürek F, Olbert M, Müller‐Werdan U, et al. Wie können postoperativ ein Delir und eine neurokognitive Störung verhindert werden? Anästhesiol Intensivmed Notfallmed Schmerzther. 2019;54:669‐683. [DOI] [PubMed] [Google Scholar]
- 81. Reade MC, Eastwood GM, Bellomo R, et al. Effect of dexmedetomidine added to standard care on ventilator‐free time in patients with agitated delirium: A randomized clinical trial. JAMA. 2016;315:1460‐1468. [DOI] [PubMed] [Google Scholar]
- 82. Lauretani F, Bellelli G, Pelà G, Morganti S, Tagliaferri S, Maggio M. Treatment of delirium in older persons: What we should not do! Int J Mol Sci. 2020;21:2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Aringhieri S, Carli M, Kolachalam S, et al. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol Ther. 2018;192:20‐41. [DOI] [PubMed] [Google Scholar]
- 84. Janssen TL, Alberts AR, Hooft L, Mattace‐Raso F, Mosk CA, van der Laan L. Prevention of postoperative delirium in elderly patients planned for elective surgery: Systematic review and meta‐analysis. Clin Interv Aging. 2019;14:1095‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Weinstein SM, Poultsides L, Baaklini LR, et al. Postoperative delirium in total knee and hip arthroplasty patients: a study of perioperative modifiable risk factors. Br J Anaesth. 2018;120:999‐1008. [DOI] [PubMed] [Google Scholar]
- 86. Hui D, Frisbee‐Hume S, Wilson A, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: A randomized clinical trial. JAMA. 2017;318:1047‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gong M, Wang G, Li G, et al. Dysfunction of inflammation‐resolving pathways is associated with postoperative cognitive decline in elderly mice. Behav Brain Res. 2020;386:112538. [DOI] [PubMed] [Google Scholar]
- 88. VanDusen KW, Eleswarpu S, Moretti EW, et al. The MARBLE study protocol: Modulating ApoE signaling to reduce brain inflammation, DeLirium, and PostopErative cognitive dysfunction. J Alzheimers Dis. 2020;75:1319‐1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ye J‐S, Chen L, Lu Y‐Y, Lei S‐Q, Peng M, Xia Z‐Y. SIRT3 activator honokiol ameliorates surgery/anesthesia‐induced cognitive decline in mice through anti‐oxidative stress and anti‐inflammatory in hippocampus. CNS Neurosci Ther. 2019;25:355‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Du S‐Q, Wang X‐R, Zhu W, et al. Acupuncture inhibits TXNIP‐associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci Ther. 2018;24:39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36:172‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Engblom D, Ek M, Saha S, Ericsson‐Dahlstrand A, Jakobsson P‐J, Blomqvist A. Prostaglandins as inflammatory messengers across the blood‐brain barrier. J Mol Med. 2002;80:5‐15. [DOI] [PubMed] [Google Scholar]
- 93. Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Huang X, Hussain B, Chang J. Peripheral inflammation and blood‐brain barrier disruption: Effects and mechanisms. CNS Neurosci Ther. 2021;27:36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Huang S, Hu H, Cai Y‐H, Hua F. Effect of parecoxib in the treatment of postoperative cognitive dysfunction: A systematic review and meta‐analysis. Medicine (Baltimore) 2019;98:e13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fan L, Wang TL, Xu YC, Ma YH, Ye WG. Minocycline may be useful to prevent/treat postoperative cognitive decline in elderly patients. Med Hypotheses. 2011;76:733‐736. [DOI] [PubMed] [Google Scholar]
- 97. Karaman T, Karaman S, Doğru S, Tapar H, Şahin A, Süren M. Short‐term and long‐term effects of dexamethasone on cognitive dysfunction induced by sevoflurane in adult rats. Turk J Anaesthesiol Reanim. 2017;45:158‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cerqueira N, Oliveira E, Gesto D, et al. Cholesterol biosynthesis: A mechanistic overview. Biochemistry. 2016;55:5483‐5506. [DOI] [PubMed] [Google Scholar]
- 99. Lee J‐W, Choi E‐A, Kim Y‐S, et al. Statin exposure and the risk of dementia in individuals with hypercholesterolaemia. J Intern Med. 2020;288:689‐698. [DOI] [PubMed] [Google Scholar]
- 100. Junhui C, Yuhai W, Ximin H, et al. The Role of Statins in the Management of Delirium: Recent Advances. CNS Neurol Disord Drug Targets. 2021;20:203‐215. [DOI] [PubMed] [Google Scholar]
- 101. Das S, Nanda SK, Bisoi AK, Wadhawan AN. Effect of preoperative statin therapy on early postoperative memory impairment after off‐pump coronary artery bypass surgery. Ann Card Anaesth. 2016;19:38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Y, He R, Chen S, Qu Y. Effect of dexmedetomidine on early postoperative cognitive dysfunction and peri‐operative inflammation in elderly patients undergoing laparoscopic cholecystectomy. Exp Ther Med. 2015;10:1635‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang XP, Liu YR, Chai M, et al. High‐fat treatment prevents postoperative cognitive dysfunction in a hyperlipidemia model by protecting the blood‐brain barrier via Mfsd2a‐related signaling. Mol Med Rep. 2019;20:4226‐4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rabinstein A. Update on treatment of acute ischemic stroke. Continuum (Minneapolis, Minn). 2020;26:268‐286. [DOI] [PubMed] [Google Scholar]
- 105. Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta‐analysis of individual patient data from randomised trials. Lancet. 2014;384:1929‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tekle WG, Hassan AE, Jadhav AP, et al. Impact of periprocedural and technical factors and patient characteristics on revascularization and outcome in the DAWN trial. Stroke. 2020;51:247‐253. [DOI] [PubMed] [Google Scholar]
- 107. Laredo C, Renú A, Tudela R, et al. The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: A comprehensive whole‐brain computed tomography perfusion study. J Cereb Blood Flow Metab. 2020;40:966‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Smith WS. Endovascular stroke therapy. Neurotherapeutics. 2019;16:360‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11‐21. [DOI] [PubMed] [Google Scholar]
- 110. Kara B, Selcuk HH, Erbahceci Salik A, et al. Single‐center experience with the Tigertriever device for the recanalization of large vessel occlusions in acute ischemic stroke. J NeuroIntervent Surg. 2019;11:455‐459. [DOI] [PubMed] [Google Scholar]
- 111. Lapchak PA, Araujo DM. Advances in hemorrhagic stroke therapy: Conventional and novel approaches. Expert Opin Emerg Drugs. 2007;12:389‐406. [DOI] [PubMed] [Google Scholar]
- 112. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015;46:2032‐2060. [DOI] [PubMed] [Google Scholar]
- 113. Qureshi AI, Palesch YY, Martin R, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3‐month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67:570‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schizodimos T, Soulountsi V, Iasonidou C, Kapravelos N. An overview of management of intracranial hypertension in the intensive care unit. J Anesth. 2020;34:741‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Witherspoon B, Ashby NE. The use of mannitol and hypertonic saline therapies in patients with elevated intracranial pressure: A review of the evidence. Nurs Clin North Am. 2017;52:249‐260. [DOI] [PubMed] [Google Scholar]
- 116. Fukuma K, Kajimoto K, Tanaka T, et al. Visualizing prolonged hyperperfusion in post‐stroke epilepsy using postictal subtraction SPECT. J Cereb Blood Flow Metab. 2021;41:146‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Beghi E, Beghi M. Epilepsy, antiepileptic drugs and dementia. Curr Opin Neurol. 2020;33:191‐197. [DOI] [PubMed] [Google Scholar]
- 118. Stefanovic S, Jankovic SM, Novakovic M, Milosavljevic M, Folic M. Pharmacodynamics and common drug‐drug interactions of the third‐generation antiepileptic drugs. Expert Opin Drug Metab Toxicol. 2018;14:153‐159. [DOI] [PubMed] [Google Scholar]
- 119. Demir M, Akarsu EO, Dede HO, et al. Investigation of the roles of new antiepileptic drugs and serum BDNF levels in efficacy and safety monitoring and quality of life: A clinical research. Curr Clin Pharmacol. 2020;15:49‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen S, Zeng L, Hu Z. Progressing haemorrhagic stroke: categories, causes, mechanisms and managements. J Neurol. 2014;261:2061‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Babi M‐A, James ML. Spontaneous intracerebral hemorrhage: Should we operate? Front Neurol. 2017;8:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet. 2005;365:387‐397. [DOI] [PubMed] [Google Scholar]
- 123. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Fam MD, Hanley D, Stadnik A, et al. Surgical performance in minimally invasive surgery plus recombinant tissue plasminogen activator for intracerebral hemorrhage evacuation phase III clinical trial. Neurosurgery. 2017;81:860‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Takeuchi S, Wada K, Nagatani K, Otani N, Mori K. Decompressive hemicraniectomy for spontaneous intracerebral hemorrhage. Neurosurg Focus. 2013;34:E5. [DOI] [PubMed] [Google Scholar]
- 126. Wu J, Yin Y, Jin M, Li B. The risk factors for postoperative delirium in adult patients after hip fracture surgery: a systematic review and meta‐analysis. Int J Geriatr Psychiatry. 2021;36:3‐14. [DOI] [PubMed] [Google Scholar]
- 127. Chan MTV, Cheng BCP, Lee TMC, Gin T. Group tCT. BIS‐guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33‐42. [DOI] [PubMed] [Google Scholar]
- 128. Wang LH, Xu DJ, Wei XJ, Chang HT, Xu GH. Electrolyte disorders and aging: risk factors for delirium in patients undergoing orthopedic surgeries. BMC Psychiatry. 2016;16:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Robinson TN, Kovar A, Carmichael H, Overbey DM, Goode CM, Jones TS. Postoperative delirium is associated with decreased recovery of ambulation one‐month after surgery. Am J Surg. 2021;221:856‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liu J, Yang F, Luo S, et al. Incidence, predictors and outcomes of delirium in complicated type B aortic dissection patients after thoracic endovascular aortic repair. Clin Interv Aging. 2021;16:1581‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang Y, Bao H‐G, Lv Y‐L, et al. Risk factors for early postoperative cognitive dysfunction after colorectal surgery. BMC Anesthesiol 2019;19:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Baek W, Kim YM, Lee H. Risk factors of postoperative delirium in older adult spine surgery patients: A meta‐analysis. AORN J. 2020;112:650‐661. [DOI] [PubMed] [Google Scholar]
- 133. Yang Y, Zhao X, Dong T, Yang Z, Zhang Q, Zhang Y. Risk factors for postoperative delirium following hip fracture repair in elderly patients: A systematic review and meta‐analysis. Aging Clin Exp Res. 2017;29:115‐126. [DOI] [PubMed] [Google Scholar]
- 134. Guan H‐L, Liu H, Hu X‐Y, et al. Urinary albumin creatinine ratio associated with postoperative delirium in elderly patients undergoing elective non‐cardiac surgery: A prospective observational study. CNS Neurosci Ther;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhang J, Bi J‐J, Guo G‐J, et al. Abnormal composition of gut microbiota contributes to delirium‐like behaviors after abdominal surgery in mice. CNS Neurosci Ther. 2019;25:685‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sun Y, Feng H, Zou T, et al. Assessment of risk factors for postoperative cognitive dysfunction after coronary artery bypass surgery: A single‐center retrospective cohort study. Biosci Rep 2021;41:BSR20190719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Grocott HP, Mackensen GB, Grigore AM, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33:537‐541. [DOI] [PubMed] [Google Scholar]
- 138. Xiao H, Run X, Cao X, et al. Temperature control can abolish anesthesia‐induced tau hyperphosphorylation and partly reverse anesthesia‐induced cognitive impairment in old mice. Psychiatry Clin Neurosci. 2013;67:493‐500. [DOI] [PubMed] [Google Scholar]
- 139. Uysal Aİ, Altıparmak B, Yaşar E, et al. The effects of early femoral nerve block intervention on preoperative pain management and incidence of postoperative delirium geriatric patients undergoing trochanteric femur fracture surgery: A randomized controlled trial. Ulus Travma Acil Cerrahi Derg. 2020;26:109‐114. [DOI] [PubMed] [Google Scholar]
- 140. Kanno M, Doi M, Kubota K, Kanoya Y. Risk factors for postoperative delirium and subsyndromal delirium in older patients in the surgical ward: A prospective observational study. PLoS One. 2021;16:e0255607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bramley P, McArthur K, Blayney A, McCullagh I. Risk factors for postoperative delirium: An umbrella review of systematic reviews. Int J Surg. 2021;93:106063. [DOI] [PubMed] [Google Scholar]
- 142. Wei W, Chen X, Yu J, Li XQ. Risk factors for postoperative stroke in adults patients with moyamoya disease: a systematic review with meta‐analysis. BMC Neurol. 2019;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wahlsten LR, Zareini B, Smedegaard L, Gislason GH, Palm H, Brorson S. A medical history of arterial thrombosis is a strong predictor of post‐operative myocardial infarction and stroke in patients with hip fractures—a nationwide cohort study. Age Ageing. 2021;50:1252‐1260. [DOI] [PubMed] [Google Scholar]
- 144. Li L‐Z, Huang Y‐Y, Yang Z‐H, Zhang S‐J, Han Z‐P, Luo Y‐M. Potential microglia‐based interventions for stroke. CNS Neurosci Ther. 2020;26:288‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Okita Y, Ikeno Y, Yokawa K, et al. Direct perfusion of the carotid artery in patients with brain malperfusion secondary to acute aortic dissection. Gen Thorac Cardiovasc Surg. 2019;67:161‐167. [DOI] [PubMed] [Google Scholar]
- 146. Zhao H, Guo F, Xu J, et al. Preoperative imaging risk findings for postoperative new stroke in patients with acute Type A aortic dissection. Frontiers in Cardiovascular Medicine 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhao Q, Yan T, Chopp M, Venkat P, Chen J. Brain‐kidney interaction: Renal dysfunction following ischemic stroke. J Cereb Blood Flow Metab. 2020;40:246‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ghoshal S, Gomez J, Datar SV, Tegeler C, Sarwal A, Freedman BI. The impact of chronic kidney disease on cerebral hemodynamics: A transcranial Doppler study. J Cereb Blood Flow Metab. 2020;40:482‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing post‐operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang KKP, Liu W, Chew STH, Ti LK, Shen L. New‐onset atrial fibrillation after cardiac surgery is a significant risk factor for long‐term stroke: an eight‐year prospective cohort study. J Cardiothorac Vasc Anesth. 2021;35(12):3559‐3564. [DOI] [PubMed] [Google Scholar]
- 151. Junejo RT, Braz ID, Lucas SJ, et al. Neurovascular coupling and cerebral autoregulation in atrial fibrillation. J Cereb Blood Flow Metab. 2020;40:1647‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]


