Abstract
The objective of this study was an interspecies comparison of free nystatin (NYS) and liposomal NYS (Nyotran) distribution in plasma. NYS and liposomal NYS at concentrations of 5, 10, and 20 μg of NYS/ml were incubated in human, dog, and rat plasma for 5, 60, and 180 min at 37°C. Following these incubations, plasma samples were separated into their high-density lipoprotein (HDL), triglyceride-rich lipoprotein, low-density lipoprotein, and lipoprotein-deficient plasma (LPDP) fractions by density-gradient ultracentrifugation, and each fraction was assayed for NYS by high-pressure liquid chromatography. Total plasma and lipoprotein cholesterol, triglyceride, and protein concentrations in each human, dog, or rat plasma sample were determined by enzymatic assays. When NYS and liposomal NYS were incubated in human, dog, or rat plasma, the majority of the NYS was recovered in the LPDP fraction. For the 5- and 60-min incubation times for all plasmas measured, a significantly greater percentage of NYS was recovered in the lipoprotein fraction (primarily HDL) following the incubation of liposomal NYS than following the incubation of NYS. There was a significant correlation between the lipoprotein lipid and protein profiles in human, dog, and rat plasmas and the distribution of NYS and liposomal NYS in plasma. In particular, differences in the proportion of plasma lipoprotein cholesterol, triglyceride, and apolar lipids (cholesteryl ester and triglycerides) carried by HDL influenced the distribution of NYS and liposomal NYS within plasmas of different species. These findings suggest that the distribution of NYS among plasma lipoproteins of different species is defined by the proportion of lipid carried by HDL, and this is possibly an important consideration when evaluating the pharmacokinetics, toxicities, and activities of these compounds following administration to different animal species.
Plasma lipoproteins are macromolecules of lipid and protein that transport polar and nonpolar lipids through the vascular and extravascular body fluids (6, 23). However, it is well known that plasma lipoprotein profiles vary considerably among different animal species (5). In addition, disease states can significantly influence plasma lipoprotein profiles, possibly resulting in altered therapeutic outcomes. Current research has shown that lipoprotein binding of drug compounds can significantly influence not only the pharmacological and pharmacokinetic properties of the drug but the relative toxicity as well (2, 8, 10, 11, 13–15, 17, 21, 23, 24). An example of one such compound is amphotericin B (AMPB), a polyene antibiotic used in the treatment of systemic fungal infections.
There is growing evidence which suggests that increases in serum cholesterol concentrations increase the renal toxicity of AMPB. Our laboratory has previously observed that when AMPB was administered to hypercholesterolemic insulin-dependent diabetic rats, it was more nephrotoxic than when administered to normolipidemic nondiabetic rats (22). Koldin et al. demonstrated enhanced AMPB-induced nephrotoxicity when AMPB bound to low-density lipoproteins (LDL) was administered to hypercholesterolemic rabbits compared to hypercholesterolemic rabbits administered AMPB alone (7). Lopez-Berestein et al. observed that when AMPB was administered to patients with leukemia (9) and immunocompromised patients who exhibited low serum cholesterol concentrations, the AMPB-induced incidence of renal toxicity was lower (12). Chabot and coworkers observed no measurable renal toxicity when AMPB was administered to cancer patients who exhibited hypocholesterolemia (3). We have further reported that patients with a higher percentage of AMPB bound to serum LDL are more susceptible to AMPB-induced kidney toxicity (19).
Our laboratory has recently observed that when liposomal nystatin (NYS) (Nyotran) was incubated in human plasma with low high-density lipoprotein (HDL) levels for 5 to 120 min at 37°C, the majority of drug was recovered in the HDL fraction (20). These findings are similar to what was observed with AMPB lipid complex (ABLC) and liposomal annamycin (18). A rationale for these similar results may be related to liposome composition. We have observed that the dimyristoyl phosphatidylglycerol (DMPG) component of ABLC and liposomal annamycin predominantly distributes into HDL because of its interaction with the protein components of HDL (apolipoproteins AI and AII) (18). Since liposomal NYS is composed of the same phospholipids as ABLC and liposomal annamycin, the increased distribution of NYS into the HDL fraction, when incorporated into these liposomes, may also be a result of DMPG’s attraction for apolipoproteins AI and AII (18, 20). This rationale is further substantiated by recent findings which demonstrated that as the amount of HDL protein decreased, the amount of liposomal NYS recovered within the HDL fraction proportionally decreased (1).
The present studies determined the plasma distribution of free NYS and liposomal NYS following incubation in human, dog, and rat plasmas. Dog and rat plasmas were chosen because many preclinical pharmacokinetic and drug safety studies are done with these species and the data generated are used to define drug dosing in human safety studies. We hypothesize that any observed differences in the plasma distribution of NYS and liposomal NYS between species may be attributed to the different lipoprotein composition of each species.
MATERIALS AND METHODS
Chemicals, lipids, and plasma.
Aronex Pharmaceuticals, Inc. (The Woodlands, Tex.) generously donated NYS powder (Gist Brocades lot NT/3598MR) and liposomal NYS (Aronex lot 503-33-0010). Human plasma was provided by the British Columbia Red Cross from three different nondiseased normolipidemic male volunteers between the ages of 20 and 25. Rat plasma was obtained from Sprague-Dawley male rats (250 to 300 g). Dog plasma was obtained from three individual male beagles (Harlan Bioproducts, Indianapolis, Ind.). Organic solvents (methanol, etc.) were purchased from Fisher Canada. Ultracentrifugation supplies (i.e., centrifuge tubes, density gradient solutions, etc.) were purchased from Beckman Canada. Lipid and protein analysis kits were purchased from Sigma Chemical (St. Louis, Mo.). Affinity lipoprotein separation kits were purchased from Isolab Inc. (St. Louis, Mo.).
Preparation of analytes and solutions.
A 1-mg/ml solution of NYS as previously described (1) was prepared by dissolving 2 mg of NYS powder in 2 ml of methanol. The mixture was covered, protected from light, and stored at 4°C for the duration of the experiment.
Liposomal NYS suspension as previously described (1) was prepared by the addition of 100 mg of accurately weighed liposomal NYS powder (commercially prepared) to 10 ml of 0.9% sodium chloride. The mixture was dispersed by hand shaking for 1 min, incubated at 37°C for 10 min, and then further agitated for another 1 min. The resulting liposomal NYS suspension contained a final NYS concentration of 1 mg/ml and was utilized immediately upon reconstitution.
Harvesting of plasma from blood.
Blood collected from healthy human volunteers (screened by the British Columbia Red Cross), dogs, and rats was placed in drug-free glass test tubes which contained 0.05 M EDTA and was centrifuged in a tabletop centrifuge for 10 min at 2,000 RPM; all plasmas were stored at −20°C until used in the study.
Lipoprotein separation.
The plasma was separated into its HDL, LDL, triglyceride-rich lipoprotein (TRL; these consist of very-low-density lipoprotein [VLDL] and chylomicrons), and lipoprotein-deficient plasma (LPDP) fractions by step gradient ultracentrifugation with sodium bromide as previously described (1).
To assure that the distribution of NYS found in each of these fractions was a result of its association with each lipoprotein or lipoprotein-deficient fraction and not a result of the density of the formulation, the density of free NYS reconstituted in methanol and the liposomal NYS formulation reconstituted in 0.9% sodium chloride (USP) following incubation for 1 h at 37°C in LPDP was determined by ultracentrifugation (1).
Determination of plasma lipoprotein triglyceride, cholesterol, and protein concentrations.
The total plasma triglycerides (TG), cholesterol (TC), and protein (TP), concentrations of the human, rat, and dog plasmas used were determined by enzymatic assays purchased from Sigma Diagnostics (St. Louis, Mo.) as previously described (1). Neither NYS nor liposomal NYS interfered with the lipid and protein assays (data not shown).
NYS quantification.
NYS quantification within each lipoprotein and LPDP fraction was determined by high-pressure liquid chromatography with external calibration curves as previously described (1).
The high-pressure liquid chromatography system consisted of a Shimadzu controller interfaced to an autosampler and tunable absorbance detector. The detector was set at a UV absorbency wavelength of 306 nm and an absorbency sensitivity of 0.05 absorbency units (full scale). All results were recorded on a Shimadzu data module integrator. Samples (100 μl for TRL and LDL and 20 μl for HDL and LPDP) were injected onto a Zorbax SB-C18 column (4.6 by 150 mm; 5-μm particle size) prefitted with a Zorbax Reliance SB-C18 guard column (4.6 by 12.5 mm; 5-μm particle size) (Rockland Technologies, Inc.). Chromatographic separation was carried out at ambient temperature. The mobile phase employed an isocratic flow and consisted of 10 mM sodium phosphate, 1 mM EDTA, 30% methanol, and 30% acetonitrile, pH 6. The flow rate was set at 0.5 ml/min.
Experimental design.
To assess the distribution of NYS and liposomal NYS within rat, dog, and human plasmas, NYS and liposomal NYS (5, 10, and 20 μg of NYS/ml of plasma; concentrations are close to the peak levels in plasma observed in mice [25] and HIV-positive patients [14] following single-dose intravenous administration) were incubated in plasmas from rats, dogs, and humans for 5, 60, and 180 min at 37°C. The plasma samples were removed and assayed for the drug in each of the lipoprotein and LPDP fractions. Control experiments were done in which ethanol and 0.9% sodium chloride without drugs were incubated in plasma. Previous studies have demonstrated that methanol at the incubation volume to deliver 100 μg of NYS per 1 ml of plasma does not alter the composition or concentration of plasma lipoproteins.
Data and statistical analysis.
Correlation coefficients between the amount of NYS recovered within the VLDL, HDL, and LDL plasma fractions and the amount of cholesterol and triglyceride within these fractions and plasma lipoprotein composition were determined by Pearson’s test (see Tables 3 and 4). Differences in the plasma distributions of NYS and liposomal NYS following incubation in plasmas of different species and the lipoprotein lipid and protein concentrations in human, dog, and rat plasmas were determined by two-way analysis of variance without repeated measures (INSTAT; Human Systems Dynamics). Critical differences were assessed by Newman-Keuls and Tukey post hoc tests. A difference was considered significant if the probability of chance explaining the results was reduced to less than 5% (P < 0.05). All data were expressed as means ± standard deviations.
TABLE 3.
Correlation coefficients between the amount of NYS recovered in each lipoprotein fraction and plasma lipoprotein lipid and protein concentration and composition in humans, dogs, and rats following the incubation of free NYS and liposomal NYSa
| Drug | Parameter | Correlationb
|
||
|---|---|---|---|---|
| Nystatin-TRL | Nystatin-LDL | Nystatin-HDL | ||
| NYS | TC | 0.25 NS | −0.41 NS | 0.91 |
| TG | 0.04 NS | −0.03 NS | −0.29 NS | |
| TP | 0.16 NS | −0.19 NS | −0.02 NS | |
| TC/TP | 0.62 NS | −0.70 | 0.80 | |
| TG/TP | −0.49 NS | 0.21 NS | −0.37 NS | |
| TG/TC | −0.60 NS | 0.65 NS | −0.88 | |
| Liposomal NYS | TC | 0.25 NS | −0.13 NS | −0.26 NS |
| TG | −0.07 NS | −0.76 | −0.27 NS | |
| TP | 0.15 NS | 0.13 NS | −0.26 NS | |
| TC/TP | 0.46 NS | −0.46 NS | 0.74 | |
| TG/TP | −0.04 NS | −0.75 | 0.09 NS | |
| TG/TC | −0.82 | −0.73 | −0.49 NS | |
NYS and liposomal NYS at 20 μg of NYS/ml were incubated in either human, dog, or rat plasma for 5 min at 37°C.
Pearson correlation coefficients between free NYS or liposomal NYS associated with lipoproteins and plasma lipoprotein lipid and protein concentration and composition. Results are expressed as r (Pearson correlation coefficient); boldface, P < 0.05; NS, not significant. Correlations were determined from plasma samples from three individual humans, dogs, and rats.
TABLE 4.
Correlation coefficients between the amount of NYS recovered in the HDL fraction and the proportion of lipoprotein cholesterol, triglyceride, apolar lipids (cholesteryl ester + triglycerides), and protein carried by HDL in human, dog, and rat plasma following incubation of free NYS and liposomal NYSa
| Drug | Correlationb
|
|||
|---|---|---|---|---|
| Cholesterol | Triglyceride | Apolar lipids | Protein | |
| NYS | 0.82 | 0.86 | 0.88 | 0.66 NS |
| Liposomal NYS | 0.73 | 0.89 | 0.78 | 0.23 NS |
NYS and liposomal NYS at 20 μg of NYS/ml were incubated in either human, dog, or rat plasma for 5 min at 37°C.
Pearson correlation coefficients between free NYS or liposomal NYS associated with the proportion of lipoprotein cholesterol, triglyceride, apolar lipids, and protein carried by HDL. The results are expressed as r (Pearson correlation coefficient) values; boldface, P < 0.05; NS, not significant. Correlations were determined from plasma samples from three individual humans, dogs, and rats.
RESULTS
Differences in total and lipoprotein cholesterol, triglyceride, and protein concentrations among human, dog, and rat plasmas were observed (Table 1). Differences in lipoprotein composition between human, dog, and rat plasmas were observed as reported in Table 2.
TABLE 1.
Total and lipoprotein plasma cholesterol (esterified + unesterified), triglyceride, and protein concentrations from three different species
| Component and species | Concn (mg/dl)a in:
|
|||
|---|---|---|---|---|
| TRL | LDL | HDL | Total | |
| Cholesterol (esterified + unesterified) | ||||
| Human | 28.4 ± 2.5 | 73.5 ± 4.8 | 40.1 ± 3.9 | 141.9 ± 4.1 |
| Dog | 6.2 ± 1.6b | 31.5 ± 4.5b | 107.5 ± 18.4b | 145.2 ± 20.0 |
| Rat | 3.9 ± 0.7bc | 29.4 ± 7.9b | 30.4 ± 7.4c | 63.7 ± 2.8bc |
| Triglyceride | ||||
| Human | 39.0 ± 3.0 | 22.9 ± 1.0 | 27.8 ± 2.7 | 89.6 ± 3.8 |
| Dog | 9.9 ± 1.5b | 17.7 ± 1.0b | 21.0 ± 2.5b | 48.6 ± 2.2b |
| Rat | 18.5 ± 1.6bc | 9.6 ± 1.1bc | 16.4 ± 1.4bc | 44.5 ± 2.1c |
| Protein | ||||
| Human | 16.3 ± 2.2 | 51.9 ± 2.0 | 261.6 ± 67.5 | 4,453.1 ± 216.2 |
| Dog | 2.7 ± 1.3b | 30.7 ± 7.4b | 199.6 ± 16.0 | 3,724.4 ± 133.8b |
| Rat | 3.6 ± 0.6b | 30.1 ± 10.8b | 74.9 ± 19.9bc | 3,462.1 ± 19.8bc |
Data are expressed as means ± standard deviations (n = 3 [individual plasma samples from humans, dogs, and rats]).
P < 0.05 versus human profile.
P < 0.05 versus dog profile.
TABLE 2.
Plasma lipoprotein composition in three different species
| Lipoprotein fraction and components | Ratio (wt/wt) ina:
|
||
|---|---|---|---|
| Human | Dog | Rat | |
| TRL | |||
| TC/TP | 1.8 ± 0.1 | 2.2 ± 0.4 | 1.1 ± 0.1bc |
| TG/TP | 2.4 ± 0.2 | 3.5 ± 0.8b | 5.1 ± 0.5bc |
| TG/TC | 1.4 ± 0.1 | 1.6 ± 0.2 | 4.8 ± 0.6bc |
| LDL | |||
| TC/TP | 1.4 ± 0.1 | 1.1 ± 0.1b | 1.0 ± 0.2b |
| TG/TP | 0.4 ± 0.02 | 0.6 ± 0.2 | 0.4 ± 0.2 |
| TG/TC | 0.3 ± 0.03 | 0.6 ± 0.1b | 0.35 ± 0.12c |
| HDL | |||
| TC/TP | 0.17 ± 0.05 | 0.54 ± 0.10b | 0.42 ± 0.08b |
| TG/TP | 0.11 ± 0.04 | 0.11 ± 0.02 | 0.23 ± 0.06bc |
| TG/TC | 0.7 ± 0.1 | 0.20 ± 0.03b | 0.57 ± 0.15c |
Data are expressed as means ± standard deviations (n = 3 for all species and fractions).
P < 0.05 versus human profile.
P < 0.05 versus dog profile.
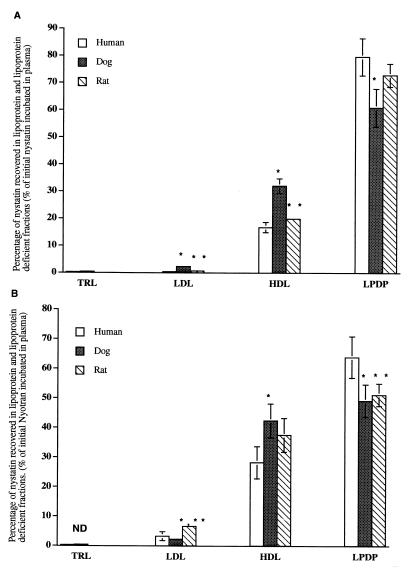
To determine the distribution in plasma lipoprotein of free NYS and NYS incorporated into liposomes, NYS and liposomal NYS (5, 10, and 20 μg of NYS/ml) were incubated for 5, 60, and 180 min at 37°C in normolipidemic human, dog, and rat plasmas. The data for the 20-μg/ml concentration following 5 min of incubation of NYS and liposomal NYS are presented in Fig. 1. The data following incubation for 60 and 180 min are similar (data not shown). In addition, similar results were observed following the incubation of 5 and 10 μg of NYS and liposomal NYS/μl (data not shown). In all three species the majority of NYS is recovered in the LPDP fraction. For the 5-min incubation time for all plasmas measured, a greater percentage of NYS was recovered in the lipoprotein fraction (primarily HDL) following the incubation of liposomal NYS than following the incubation of NYS. The incubation of NYS-free liposomes at 5, 60, and 180 min concurrently with free NYS did not alter NYS distribution in human plasma (data not shown).
FIG. 1.
Distribution of free NYS (A) and liposomal NYS (B) (20 μg/ml) in human, dog, and rat plasmas following incubation for 5 min at 37°C. The data are expressed as percent of total NYS distributed in TRL, LDL, HDL, and LPDP fractions. The data are reported as means ± standard deviations (error bars) (n = 3). ∗, P < 0.05 compared to human plasma; ∗∗, P < 0.05 compared to dog plasma.
NYS.
When correlations between the amount of NYS recovered within the TRL, HDL, and LDL plasma fractions following incubation of free NYS (20 μg/ml) for 5 min at 37°C and the amount of cholesterol (esterified and unesterified), triglyceride, and protein within these fractions were calculated for the plasmas of all three species, the following relationship was observed. As HDL TC increases from rat (lowest) through dog (highest) plasmas (Table 1), the amount of NYS recovered within this fraction proportionally increases (Table 3).
When correlations between the amount of NYS recovered in each lipoprotein fraction and lipoprotein composition were determined, the following relationships were observed. As the LDL TC/TP ratio increased, the amount of NYS recovered in LDL decreased (Table 3). As the HDL TC/TP ratio increased, the amount of NYS recovered in HDL increased, but when the HDL TG/TC ratio increased, the amount of NYS recovered decreased (Table 3).
Liposomal NYS.
When correlations between the amount of NYS recovered within the TRL, HDL, and LDL plasma fractions following incubation of liposomal NYS (20 μg of NYS/ml) for 5 min at 37°C and the amount of cholesterol (esterified and unesterified), triglyceride, and protein within these fractions were calculated for all three species, the following relationship was observed. As LDL TG increases from rat (lowest) through human (highest) plasmas (Table 1), the amount of NYStatin recovered within this fraction proportionally decreases (Table 3). When correlations between the amount of NYS recovered in each lipoprotein fraction and lipoprotein composition were determined, the following relationships were observed. As the TRL TG/TC ratio increased, the amount of NYS recovered in the TRL fraction proportionally decreased (Table 3). As the LDL TG/TP and TG/TC ratios increased, the amount of NYS recovered in the LDL fraction proportionally decreased (Table 3). As the HDL TC/TP ratio increased, the amount of NYS recovered in the HDL proportionally increased (Table 3). Correlations with free NYS and liposomal NYS concentrations of 5 and 10 μg/ml were done with similar findings (data not shown).
When correlations between the amount of NYS recovered in the TRL, LDL, and HDL fractions following incubation of NYS liposomal NYS (20 μg of NYS/ml) for 5 min at 37°C and the proportion of lipoprotein cholesterol (esterified and unesterified), triglyceride, apolar lipids (cholesteryl ester and triglycerides), and protein carried by TRL, LDL, and HDL in human, dog, and rat plasmas were determined, the following relationships were observed. As the proportion of lipoprotein cholesterol, triglyceride, and apolar lipids carried by HDL increases from rat plasma (lowest) through human plasma (highest), the amount of NYS recovered in the HDL fraction proportionally increases (Table 4). No such correlations were observed in the TRL and LDL fractions (data not shown).
DISCUSSION
The purpose of this study was to determine the distribution in plasma of free NYS and liposomal NYS following incubation in human, rat, and dog plasmas. We observed that when NYS and liposomal NYS were incubated in human, dog, and rat plasmas, the majority of the NYS was recovered in the LPDP fraction. For both the 5- and 60-min incubation times in the rat, dog, or human plasma, a greater percentage of NYS was recovered in the lipoprotein fraction (primarily HDL) following the incubation of liposomal NYS than following the incubation of NYS. Differences between the lipoprotein lipid and protein profiles in human, dog, and rat plasmas correlated with the distribution in plasma of NYS and liposomal NYS. In particular, differences in the proportion of plasma lipoprotein cholesterol, triglyceride, and apolar lipids (cholesteryl ester and triglycerides) carried by HDL influenced the distribution of NYS and liposomal NYS within plasmas of different species.
Previous studies with AMPB have suggested that an alteration in plasma lipid concentrations modifies the drug’s pharmacological behavior. Chavanet and coworkers have demonstrated that an increase in plasma triglyceride concentration leads to a reduction in AMPB toxicity (4). These findings suggested that triglycerides, or their main vehicles in serum, chylomicrons, LDL, and VLDL, were involved in the protective effect against AMPB toxicity. Souza and coworkers have further shown that a triglyceride-rich emulsion that behaves in vivo as chylomicrons was able to reduce the in vivo and in vitro toxicity of AMPB (16). Our laboratory has recently shown enhanced AMPB-induced kidney toxicity within patients who exhibited elevated serum LDL cholesterol concentrations (19).
In the present study, we have observed differences in the distribution in plasma of NYS when free NYS and liposomal NYS are incubated in human plasma compared to when they are incubated in dog and rat plasmas. It appears that these differences can be attributed to differences in the species lipoprotein lipid and protein concentrations (Table 1) and composition (Table 2) profiles. In particular, the higher HDL cholesterol concentration found in dog plasma resulted in more NYS being recovered in this fraction than in human and rat plasmas following the incubation of free NYS (Table 4). However, increases in LDL triglyceride concentrations among the different species resulted in less NYS being recovered in this fraction following the incubation of liposomal NYS (Table 3). These findings suggest that NYS distribution in lipoprotein following the incubation of free NYS is regulated by a different plasma lipoprotein component (HDL cholesterol) than that following the incubation of liposomal NYS, which appears to be regulated by plasma LDL triglyceride.
We further observed that increasing the TC/TP ratio within LDL and the TG/TC ratio within HDL for free NYS resulted in less NYS being recovered in these fractions, while increasing the TC/TP ratio within HDL resulted in more NYS being recovered in HDL (Table 3). However, for liposomal NYS, increasing the TG/TC ratio within TRL and LDL and the TG/TP ratio within LDL resulted in less NYS being recovered in these fractions, while increasing the TC/TP ratio within HDL resulted in more NYS being recovered in this fraction (Table 3). In addition, we have reported that the differences in the proportion of lipid carried by HDL within the different animal species may also dictate NYS binding. Our results suggest that species (i.e., dogs) which have a greater proportion of their plasma lipids carried by HDL will have a greater percentage of NYS recovered in their HDL fraction than those species (i.e., humans and rats) which have a lower proportion of their plasma lipids carried by HDL (Table 4). These findings suggest that not only lipid mass and lipoprotein lipid and protein composition but also the type of lipoprotein in which these changes occur is another possible factor in determining to which lipoprotein NYS binds.
In conclusion, we have determined that the distribution of NYS in plasma is altered when it is incorporated into liposomes composed of dimyristoyl phosphatidylcholine and DMPG. Furthermore, not only the relative levels of individual lipoproteins but also their lipid and protein compositions and the proportions of lipid carried by HDL define the distribution of NYS among plasma lipoproteins of different species, and this may be an important consideration when evaluating the pharmacokinetics, toxicities, and activities, of these compounds following administration to different animal species.
ACKNOWLEDGMENTS
This work was funded by Aronex Pharmaceuticals, Inc., and the Medical Research Council of Canada (grant MT-14484 to K.M.W.).
REFERENCES
- 1.Cassidy S M, Strobel F W, Wasan K M. The plasma lipoprotein distribution of liposomal nystatin is influenced by the protein content of high-density lipoproteins. Antimicrob Agents Chemother. 1998;42:1878–1888. doi: 10.1128/aac.42.8.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cenni B, Meyer J, Brandt R, Betschart B. The antimalarial drug halofantrine is bound mainly to low and high density lipoproteins in human serum. Br J Clin Pharmacol. 1995;39:519–526. doi: 10.1111/j.1365-2125.1995.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabot G G, Pazdur R, Valeriote F A, Baker L H. Pharmacokinetics and toxicity of continuous infusion of amphotericin B in cancer patients. J Pharm Sci. 1989;78:307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- 4.Chavanet J, Joly V, Rigaud D, Bolard J, Carbon C, Yeni P. Influence of diet on experimental toxicity of amphotericin B deoxycholate. Antimicrob Agents Chemother. 1994;38:963–968. doi: 10.1128/aac.38.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha Y C, Barter P J. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp Biochem Physiol. 1982;71:265–269. doi: 10.1016/0305-0491(82)90252-8. [DOI] [PubMed] [Google Scholar]
- 6.Harmony J A K, Aleson A L, McCarthy B M. Chapter 15. In: Scanu A M, Spector A A, editors. Biochemistry and biology of plasma lipoproteins. New York, N.Y: Marcel Dekker, Inc.; 1986. pp. 403–430. [Google Scholar]
- 7.Koldin M H, Kobayashi G S, Brajtburg J, Medoff G. Effects of elevation of serum cholesterol and administration of amphotericin B complexed to lipoproteins on amphotericin B-induced toxicity to rabbits. Antimicrob Agents Chemother. 1985;28:144–145. doi: 10.1128/aac.28.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemaire M, Tillement J P. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J Pharm Pharmacol. 1982;34:715–718. doi: 10.1111/j.2042-7158.1982.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Berestein G. Liposomes as carriers of antifungal drugs. Ann NY Acad Sci. 1988;544:590–597. doi: 10.1111/j.1749-6632.1988.tb40459.x. [DOI] [PubMed] [Google Scholar]
- 10.Milton K A, Edwards G, Ward S A, Orme M L E, Breckenridge A M. Pharmacokinetics of halofantrine in man: effects of food and dose size. Br J Clin Pharmacol. 1989;28:71–77. doi: 10.1111/j.1365-2125.1989.tb03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northcote R J, Todd I C, Ballantyne D. Beta blockers and lipoproteins: a review of current knowledge. Scott Med J. 1986;31:220–228. doi: 10.1177/003693308603100402. [DOI] [PubMed] [Google Scholar]
- 12.Pontain D R, Sun D, Brim J D, et al. Inhibition of HIV replication by liposomal amphotericin B. Antiviral Res. 1989;13:119–125. doi: 10.1016/0166-3542(89)90023-5. [DOI] [PubMed] [Google Scholar]
- 13.Rajaram O V, Fatterpaker P, Sreenivasan A. Involvement of binding lipoproteins in the absorption and transport of alpha-tocopherol in the rat. Biochem J. 1974;140:509–516. doi: 10.1042/bj1400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rios A, Rosenblum M, Crofoot G, Lenk R P, Hayman A, Lopez-Berestein G. Pharmacokinetics of liposomal nystatin in patients with human immunodeficiency virus infection. J Infect Dis. 1993;168:153–154. doi: 10.1093/infdis/168.1.253. [DOI] [PubMed] [Google Scholar]
- 15.Selvam M P, Blay R A, Geyer S, Buck S M, Pollock L, Mayner R E, Epstein J S. Inhibition of HIV-1 replication in H9 cells by nystatin-A compared with other antiviral agents. AIDS Res Hum Retroviruses. 1993;9:475–481. doi: 10.1089/aid.1993.9.475. [DOI] [PubMed] [Google Scholar]
- 16.Souza L C, Maranhao R C, Schrier S, Campa A. In vitro and in vivo studies of the decrease of amphotericin B toxicity upon association with a triglyceride-rich emulsion. J Antimicrob Agents. 1993;32:123–132. doi: 10.1093/jac/32.1.123. [DOI] [PubMed] [Google Scholar]
- 17.Stevens N M, Eagle C A, Fisher P B, Mechlinski W, Schaffner C P. In vitro antiherpetic activity of water soluble amphotericin methyl ester. Arch Virol. 1975;48:391–394. doi: 10.1007/BF01317438. [DOI] [PubMed] [Google Scholar]
- 18.Wasan K M, Cassidy S M. The role of plasma lipoproteins in modifying the biological activity of hydrophobic drugs. J Pharm Sci. 1998;86:411–424. doi: 10.1021/js970407a. [DOI] [PubMed] [Google Scholar]
- 19.Wasan K M, Conklin J S. Enhanced amphotericin B nephrotoxicity in intensive care patients with elevated levels of low-density lipoprotein cholesterol. Clin Infect Dis. 1997;24:78–80. doi: 10.1093/clinids/24.1.78. [DOI] [PubMed] [Google Scholar]
- 20.Wasan K M, Ramaswamy M, Cassidy S M, Kazemi M, Strobel F W, Thies R L. Physical characteristics and lipoprotein distribution of liposomal nystatin in human plasma. Antimicrob Agents Chemother. 1997;41:1871–1875. doi: 10.1128/aac.41.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasan K M, Lopez-Berestein G. Diversity of lipid-based polyene formulations and their behavior in biological systems. Eur J Clin Microbiol Infect Dis. 1997;16:81–92. doi: 10.1007/BF01575125. [DOI] [PubMed] [Google Scholar]
- 22.Wasan K M, Conklin J S. Evaluation of renal toxicity and antifungal activity of free and liposomal amphotericin B following a single intravenous dose to diabetic rats with systemic candidiasis. Antimicrob Agents Chemother. 1996;40:1806–1810. doi: 10.1128/aac.40.8.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasan K M. Modifications in plasma lipoprotein concentration and lipid composition regulate the biological activity of hydrophobic drugs. J Pharmacol Toxicol Methods. 1996;36:1–11. doi: 10.1016/1056-8719(96)00054-8. [DOI] [PubMed] [Google Scholar]
- 24.Wasan K M, Brazeau G A, Keyhani A, Hayman A C, Lopez-Berestein G. Role of liposome composition and temperature in distribution of amphotericin B in serum lipoproteins. Antimicrob Agents Chemother. 1993;37:246–250. doi: 10.1128/aac.37.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasan, K. M., et al. Unpublished results.