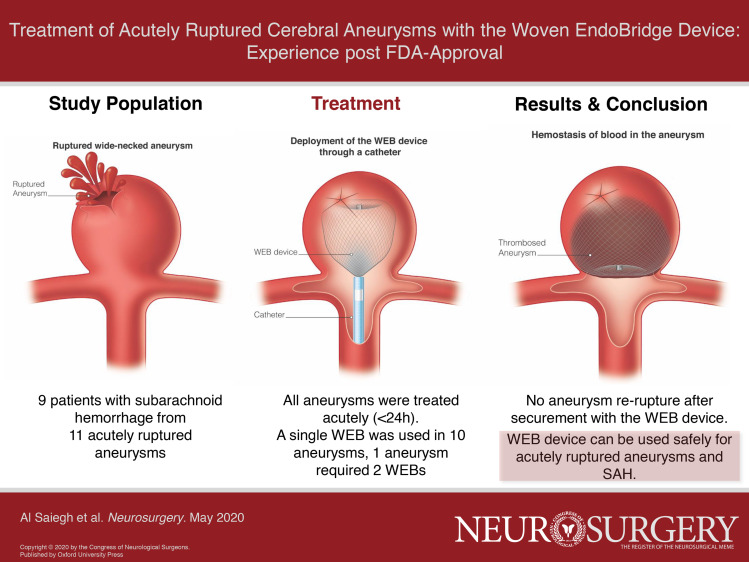
Abstract
BACKGROUND
Coil embolization of ruptured bifurcation aneurysms is challenging and often necessitates adjunctive stenting, which requires antiplatelet therapy in the setting of subarachnoid hemorrhage (SAH). The Woven EndoBridge (WEB; Terumo) device is an alternative self-expanding 3D mesh that does not require antiplatelet agents. However, its use has been mostly reserved for unruptured aneurysms.
OBJECTIVE
To assess the safety and feasibility of ruptured aneurysm treatment with the WEB.
METHODS
Retrospective analysis of 9 SAH patients with 11 aneurysms that were treated with the WEB device at 2 institutions after FDA approval.
RESULTS
Hunt and Hess grades were III and IV in 4 (44%) each and V in 1 (11%). All patients were treated within 24 h of hospitalization, and a single WEB was used in all but one aneurysm. Aneurysms treated were 3 basilar tip, 2 anterior communicating artery, 2 posterior inferior cerebellarartery, 1 middle cerebral artery, 1 carotid-ophthalmic artery, 1 posterior communicating artery, and 1 vertebrobasilar junction. Mean aneurysm height and width were 6.2 ± 2.2 mm (range: 3-10) and 5.6 ± 3.0 mm (range: 3.3-14), respectively. Mean dome-to-neck ratio was 1.7 ± 0.8 (range: 1.0-3.8). There was one intraoperative rupture that occurred because of device dislodgement and was managed with embolization. There were no treatment-related mortalities and no re-rupture after securement of the aneurysms with the WEB.
CONCLUSION
Our preliminary experience indicates that the WEB device can be used safely for ruptured aneurysms of various sizes in the anterior and posterior circulation. Larger series with long-term follow-up are necessary to confirm our findings.
Keywords: Cerebral aneurysm, Embolization, Endovascular, Subarachnoid hemorrhage, WEB device
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- AComm
anterior communicating artery
- MCA
middle cerebral artery
- PComm
posterior communicating artery
- PICA
posterior inferior cerebellar artery
- SL
single laye
- SAH
subarachnoid hemorrhage
- VBJ
vertebrobasilar junction
- WEB
Woven EndoBridge
Coil embolization has become the first-line treatment modality for acutely ruptured cerebral aneurysms, because it has been shown to achieve improved functional outcomes and survival compared to clip ligation.1-4 However, coil embolization can be challenging in certain aneurysms, including wide-neck bifurcation aneurysms with an unfavorable dome-to-neck ratio, as it can result in coil herniation into the parent vessel. Therefore, stent-assisted coiling or flow diverting stents are often used in such cases, but that, in turn, requires a loading dose of antiplatelet agents and prolonged dual antiplatelet therapy in the setting of an acute subarachnoid hemorrhage (SAH).
The need for better endovascular treatment options for wide-neck bifurcation aneurysms led to the development of the Woven EndoBridge (WEB, Sequent Medical Inc; Termuo Corp), which was recently FDA approved for internal carotid artery terminus, middle cerebral artery (MCA) bifurcation, anterior communicating artery (AComm), and basilar apex aneurysms with dome diameter 3 to 10 mm, neck size > 4 mm, or dome-to-neck ratio ranging from 1 to 2.0.5 The WEB is a self-expanding microbraided cylindrical or spherical 3D mesh of nitinol wires ranging 19 to 38 μm6,7 that is placed into the aneurysm and leads to stasis at the aneurysm neck that results in thrombosis and endothelialization across the ostium of the aneurysm over time.8
Given its intrasaccular deployment, its use does not require the administration of any antiplatelet agents. Therefore, it makes the WEB device a valuable option for the treatment of acutely ruptured aneurysms. However, its use has been mostly reserved for unruptured aneurysms.9 Herein, we present the feasibility, safety, and outcome of our multicenter experience with acutely ruptured aneurysms that were treated with the WEB device post-FDA approval.
METHODS
Patient Selection, Variables, and Outcomes
We conducted a retrospective analysis and identified 11 aneurysms in 9 consecutive patients who had SAH and underwent treatment with the WEB device (Sequent; Terumo) between March and July 2019 at 2 institutions with high endovascular volumes by dual-trained neurosurgeons with clipping capabilities. The study protocol was approved by the Institutional Review Board. Patients’ electronic medical records were accessed, and relevant data were extracted for analysis. Data analysis and interpretation was performed upon removal of all identifiable personal health information. Because of the retrospective design and analysis of deidentified data, patient consent was not required.
Statistical Analysis
Means and standard deviations are presented for continuous variables, and frequency is presented for categorical variables. The statistical analysis was conducted using IBM SPSS (Version 24.0., IBM Corp, Armonk, New York).
RESULTS
Demographics and Aneurysm Characteristics
Of the 9 patients, 8 were female and 1 was male, with a mean age of 66.4 ± 13.5 yr (range: 47-81). Hunt and Hess grades on presentation were III in 4 (44%), IV in 4 (44%), and V in 1 (11%). All patients were treated in the acute phase within 24 h of hospitalization. Two of the patients had 2 cerebral aneurysms, of which both were treated with the WEB device (Sequent; Terumo), because the causative lesion could not be determined by the subarachnoid blood pattern. The aneurysms treated were 3 basilar tip, 2 AComm (Figure 1), 2 posterior inferior cerebellar artery (PICA), 1 MCA, 1 carotid-ophthalmic artery, 1 posterior communicating artery (PComm), and 1 vertebrobasilar junction (VBJ). Of these, 2 aneurysms (1 basilar tip and 1 PComm) were previously treated with coil embolization. The mean aneurysm height and width were 6.2 ± 2.2 mm (range: 3-10) and 5.6 ± 3.0 mm (range: 3.3-14), respectively (Table). Mean dome-to-neck ratio was 1.7 ± 0.8 (range: 1.0-3.8).
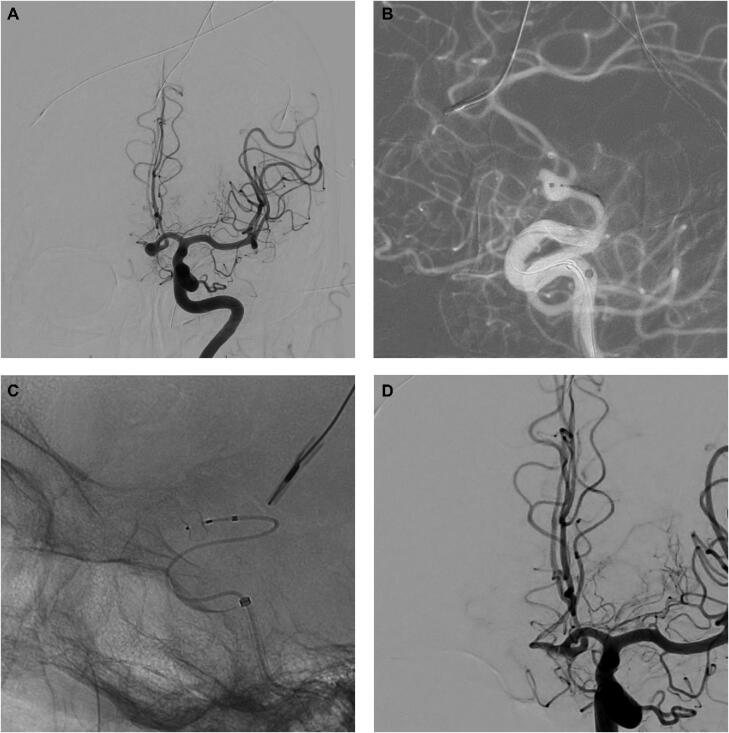
Figure 1.
A 76-yr-old female who presented with Hunt and Hess grade III SAH. A, AP view of a left ICA injection showing an anterior communicating aneurysm. B, Road-map view and microcatheterization of the aneurysm for deployment of a 5 × 3 WEB device. C, Nonsubtracted view showing the WEB device in place. D, AP view of left ICA injection showing complete occlusion of the aneurysm.
TABLE.
Patient and Aneurysm Characteristics
| Patient | Age sex | Aneurysm location | H&H | Aneurysm width (mm) | Aneurysm height (mm) | Neck width (mm) | Route | WEB SL | Degree of stasisa | Six-mo follow-up angiogram |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58F | VBJ | 5 | 14 | 10 | 9 | Femoral | 11 × 9 and 11 × 8 | Complete occlusion | N/A |
| 2 | 61F | PComm | 3 | 6 | 3 | 2 | Femoral | 4 × 3 | Venous | Aneurysm remnant |
| 3 | 66F | Basilar apex | 4 | 3.5 | 3.9 | 2.5 | Femoral | 4 × 3 | Arterial | N/A |
| 4 | 81F | MCA | 4 | 3.3 | 4.3 | 2.4 | Carotid puncture | 4 × 3 | Venous | N/A |
| 5 | 76F | AComm (Figure 1) | 3 | 5.2 | 4.8 | 5.2 | Radial | 5 × 3 | Complete occlusion | Complete occlusion |
| 6 | 81F | AComm | 4 | 5.5 | 4.7 | 4.5 | Femoral | 5 × 4 | Capillary | Complete occlusion |
| 7 | 79F | Bilobed PICA (Figure 3) | 3 | #1: 3.68 #2: 5.17 | #1: 7.85 #2: 5.1 | #1: 3.7 #2: 4.1 | Radial | #1: 4 × 3 #2: 5 × 4 | Complete occlusionb | Complete occlusion |
| 8 | 49M | #1: carotid ophthalmic #2: basilar tip | 3 | #1: 4.9 #2: 5.4 | #1: 6.25 #2: 7.65 | #1: 5.68 #2: 5.18 | Femoral | #1: 7 × 4 #2: 6 × 5 | #1: capillary #2: venous | N/A |
| 9 | 47F | Basilar tip | 4 | 6.3 | 8.99 | 4.15 | Femoral | 8 × 4 | Venous | N/A |
aEvaluation of degree of stasis as described by O’Kelly et al.9
bIpsilateral vertebral artery deconstruction.
WEB Device Placement and Sizing
WEB device deployment was successful in all aneurysms and—even though available at both institutions—none of the patients had to be converted to a craniotomy and clip ligation of the aneurysm. In 10 of the 11 aneurysms, 1 WEB was sufficient to achieve adequate obliteration. The VBJ aneurysm was 14 mm in size and required placement of 2 WEB devices (Figure 2). In all but one aneurysm, the device was confined to the aneurysm cavity with no parent vessel compromise. However, the second WEB device used for the VBJ aneurysm was protruding into the vertebral artery, and stent placement was required to ensure patency and prevent device herniation. A control angiogram showed no branch occlusion, and the patient was clinically asymptomatic. This was the only patient in whom antiplatelet agents (aspirin and clopidogrel) were necessary because of adjunctive stent placement. An external ventricular drain had already been placed and was successfully weaned off obviating the need for a shunt.
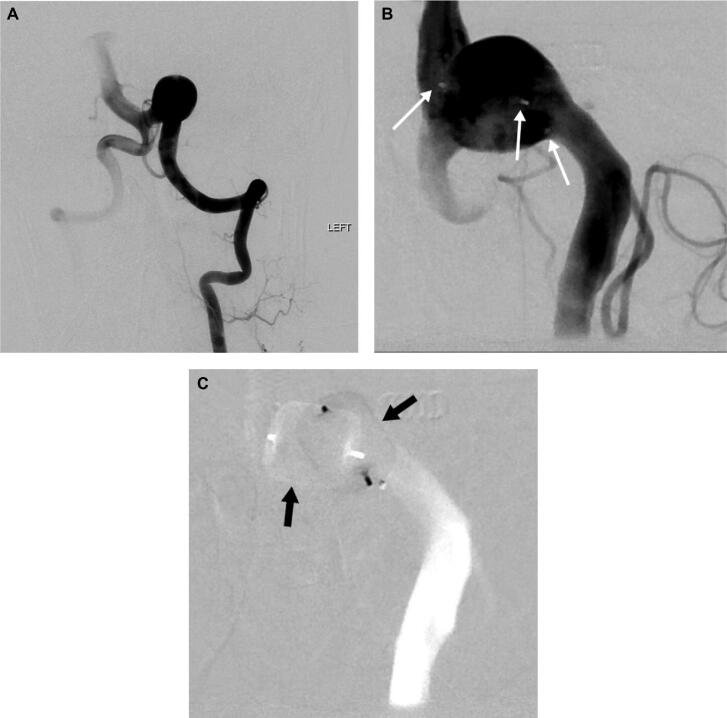
Figure 2.
A, Patient with Hunt and Hess grade V SAH whose left vertebral artery (VA) injection showed a left vertebrobasilar junction (VBJ) aneurysm, which required placement of 2 WEB devices. B, Magnified view of the aneurysm immediately after WEB device deployment (white arrows pointing at WEB device markers). C, Subtracted view showing the outline of the 2 WEB devices in the aneurysm (black arrows).
All aneurysms were treated with a single-layer (SL) WEB device with a size range from 4 × 3 to 11 × 9 (Table). The mean overall procedure time was 85.9 ± 32.6 min (range: 34-156). Six (54.5%) aneurysms were treated via the transfemoral route, whereas access was obtained by radial artery catheterization in 4 (36.6%) and via direct carotid puncture in 1 patient because of extensive vessel tortuosity.
A control angiogram was done in all cases to evaluate intra-aneurysmal flow disruption after deployment of the WEB device. Grading was performed as previously described by O’Kelly et al.10 Stasis in the arterial phase was observed in 1 aneurysm, in the capillary phase in 2 aneurysms, and in the venous phase in 4 aneurysms. Complete occlusion after WEB device deployment was observed in 2 aneurysms (Table). The patient with the PICA aneurysm had complete aneurysm occlusion after deconstruction of the ipsilateral vertebral artery (Table and Figure 3).
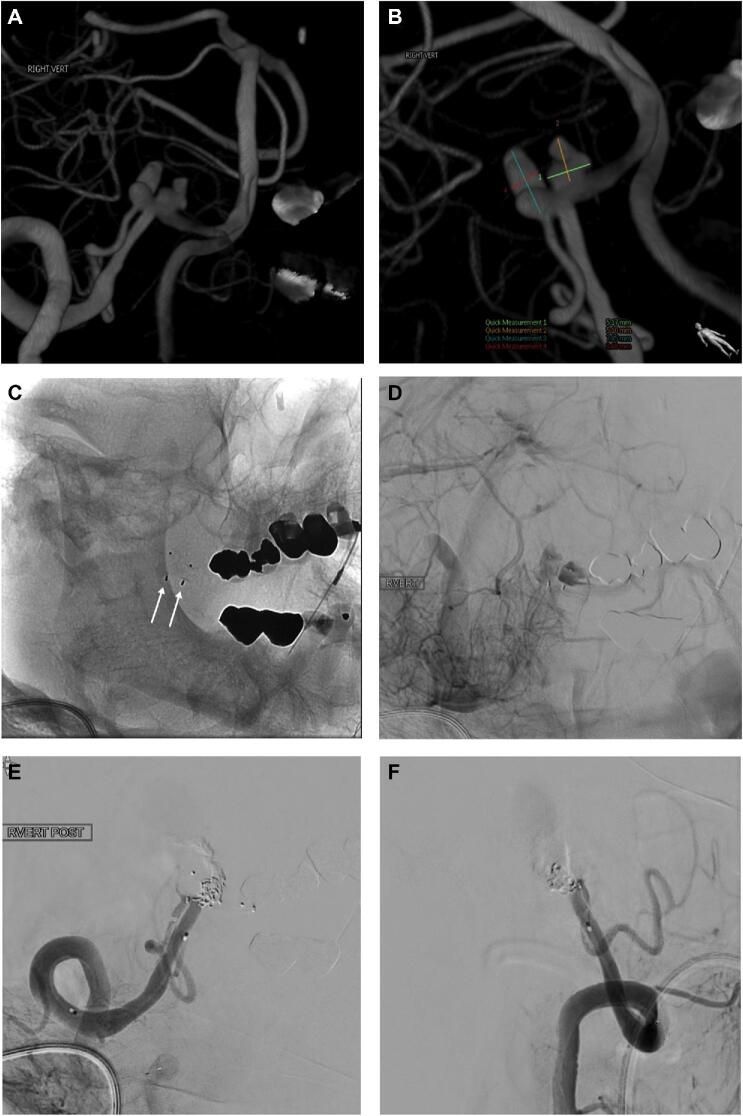
Figure 3.
Patient who had a Hunt and Hess grade III SAH and had intraoperative aneurysm rupture. A and B show a 3D model of a right vertebral artery (VA) injection and 2 dysplastic aneurysms at the origin of the PICA. B, Measurements of both aneurysms are shown in the bottom half of the picture and Table (patient #7). C, Nonsubtracted view showing 2 WEB devices with one in each aneurysm (white arrows). D, Subtracted view in the venous phase after right VA injection showing stasis in both aneurysms. Right VA injection in AP E and lateral F views after deconstruction with Onyx and coils. Note patency of the right PICA on both views.
A 6-mo follow-up, angiogram was available for 4 of the patients. The degree of aneurysm occlusion was evaluated following the Raymond-Roy occlusion classification.11 Complete occlusion was observed in 3 patients. The patient with the PComm aneurysm had an aneurysm remnant, for which she underwent clip ligation (Table).
Adverse Events
One intraoperative rupture occurred in the patient with the PICA aneurysm because of device dislodgement. A combination of Onyx and coils was used to deconstruct the ipsilateral vertebral artery to control the hemorrhage. Postoperatively, the patient had a transient sixth nerve palsy, but no other deficits related to the rupture. There were no treatment-related deaths; one patient died of cardiopulmonary failure later during their hospital course. There was no rebleeding from any of the aneurysms after treatment with the WEB device.
DISCUSSION
The WEB device (Sequent; Terumo) received FDA approval on December 31, 2018, which has led to an important expansion of the armamentarium of the neurovascular surgeon for the treatment of wide-neck bifurcation aneurysms. This is of particular importance in acute SAH, as patients are frequently critically ill and may not tolerate a craniotomy for clip ligation. In addition, it can be used as a stand-alone tool that does not require adjunctive stenting and obviates the need for dual-antiplatelet therapy. Thus, it can potentially lower hemorrhagic complications, especially in high-grade SAH patients who require invasive procedures, such as ventriculostomy, ventriculoperitoneal shunting, tracheostomy, or gastrostomy.
The WEB device is rapidly gaining popularity for the use of wide-necked bifurcation aneurysms, and several studies have established its safety and efficacy in unruptured aneurysms.12-14 The present study is the first one to examine its safety and efficacy in ruptured aneurysms at 2 United States institutions with a high volume of endovascular cases post-FDA approval. Our experience demonstrates that WEB placement was feasible in all patients in a variety of aneurysm sizes in both the anterior and posterior circulation. In addition, deployment was achieved in a wide range of Hunt and Hess grades using the transfemoral or transradial route or via direct carotid puncture.
In order to maximize the benefit of the WEB device in patients with acute SAH, appropriate shape and size selection are crucial elements to secure the aneurysm with the WEB while preserving parent vessel patency without adjunctive stenting and antiplatelet therapy. In our series, the ideal device size was determined by calculating the mean of the neck-to-dome ratio on the anteroposterior and lateral views, then subtracting 1 mm from the height and adding 1 mm to the width. This allows for optimal sidewall apposition of the mesh inside the aneurysm cavity without herniation into the parent vessel. Using this strategy, a single WEB device with no stent was used in all but 1 aneurysm (91%). As described by Klisch et al,15 optimal sizing is also critical, as it produces a seal at the aneurysm-parent vessel interface and reduces blood inflow, which is particularly important in the treatment of ruptured aneurysms. Undersizing of the WEB often leads to insufficient flow disruption seen on the control angiogram. Oversizing the WEB, on the other hand, causes protrusion of the device into the parent artery and carries the risk of thromboembolic events. This is a finding that was also observed by Caroff et al7 in their early series with the WEB for ruptured aneurysms that required the administration of intra-arterial antiplatelet agents. One advantage of the WEB is that—in cases of unsatisfactory flow disruption—the device can be immediately re-sheathed and retrieved, and a different size or another treatment option may be considered. We did not encounter events of symptomatic thromboembolism or branch occlusion in our cohort and had stagnation of contrast in 9 and immediate complete aneurysm occlusion in 2 cases (Figure 1). There was no re-rupture of any of the aneurysms after treatment with the WEB device.
We had one complication in this small series (9%), which occurred because of dislodgement of the device causing vessel injury. Our complication rate is within the range of WEB-related complications previously published in the literature and is not higher when compared to series with unruptured aneurysms.16,17
In summary, the WEB device is a valuable tool in the treatment of acutely ruptured aneurysms and we expect that neurointerventionalists at high-volume centers will have a comparable experience with it.
Limitations
Our study is limited by its retrospective design and the small sample size. Yet, it provides a preliminary experience with the WEB in ruptured aneurysms of the anterior and posterior circulation at 2 institutions after FDA approval and may encourage others to adopt this approach in their practice. Our focus is the procedural safety and feasibility and early protection from a re-rupture, which is why long-term follow-up angiographic and functional outcomes are not available yet as we started using the device in March 2019 after FDA approval.
CONCLUSION
Our preliminary experience shows that the WEB device (Sequent; Terumo) can be used safely to secure aneurysms of various sizes in the anterior and posterior circulation in the acute phase of SAH. When appropriately sized, adjunctive stenting is not necessary, which makes the WEB a valuable alternative to stent-assisted coiling and flow diverters as it obviates the need for antiplatelet therapy. Larger series with long-term follow-up are necessary to confirm the safety of this approach.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
Part of this work was presented at the 2019 Congress of Neurological Surgeons Annual Meeting in San Francisco, California, on October 21, 2019, in the “Section on Cerebrovascular Surgery 1” as an oral presentation.
Contributor Information
Fadi Al Saiegh, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
David Hasan, Department of Neurological Surgery, University of Iowa Hospitals, Iowa City, Iowa.
Nikolaos Mouchtouris, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Mario Zanaty, Department of Neurological Surgery, University of Iowa Hospitals, Iowa City, Iowa.
Ahmad Sweid, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Omaditya Khanna, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Nohra Chalouhi, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Ritam Ghosh, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Stavropoula Tjoumakaris, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
M Reid Gooch, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Robert Rosenwasser, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Pascal Jabbour, Department of Neurological Surgery, Thomas Jefferson University Hospital, Philadelphia, Pennsylvania.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
Neurosurgery Speaks (Audio Abstracts)
Listen to audio translations of this paper's abstract into select languages by choosing from one of the selections below.
REFERENCES
- 1. Molyneux A, Kerr R, Stratton Iet al.. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274. [DOI] [PubMed] [Google Scholar]
- 2. McDougall CG, Spetzler RF, Zabramski JMet al.. The barrow ruptured aneurysm trial. J Neurosurg. 2012;116(1):135-144. [DOI] [PubMed] [Google Scholar]
- 3. Li H, Pan R, Wang Het al.. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013;44(1):29-37. [DOI] [PubMed] [Google Scholar]
- 4. Cognard C, Pierot L, Anxionnat R, Ricolfi F, Clarity Study G. Results of embolization used as the first treatment choice in a consecutive nonselected population of ruptured aneurysms: clinical results of the clarity GDC study. Neurosurgery. 2011;69(4):837-841; discussion 842. [DOI] [PubMed] [Google Scholar]
- 5. FDA . Woven EndoBridge (WEB) Aneurysm Embolization System - P170032. https://www.fda.gov/medical-devices/recently-approved-devices/woven-endobridge-web-aneurysm-embolization-system-p170032. Accessed July 31, 2019.
- 6. Ding YH, Lewis DA, Kadirvel R, Dai D, Kallmes DF. The Woven EndoBridge: a new aneurysm occlusion device. AJNR Am J Neuroradiol. 2011;32(3):607-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caroff J, Mihalea C, Dargento Fet al.. Woven EndoBridge (WEB) device for endovascular treatment of ruptured intracranial wide-neck aneurysms: a single-center experience. Neuroradiology. 2014;56(9):755-761. [DOI] [PubMed] [Google Scholar]
- 8. Al Saiegh F, Mouchtouris N, Sweid Aet al.. Placement of the Woven EndoBridge (WEB) device via distal transradial access in the anatomical snuffbox: a technical note. J Clin Neurosci. 2019;69:261-264. [DOI] [PubMed] [Google Scholar]
- 9. Lescher S, du Mesnil de Rochemont R, Berkefeld J. Woven EndoBridge (WEB) device for endovascular treatment of complex unruptured aneurysms-a single center experience. Neuroradiology. 2016;58(4):383-390. [DOI] [PubMed] [Google Scholar]
- 10. O’Kelly C J, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010;16(2):133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kabbasch C, Goertz L, Siebert Eet al.. Factors that determine aneurysm occlusion after embolization with the Woven EndoBridge (WEB). J Neurointervent Surg. 2019;11(5):503-510. [DOI] [PubMed] [Google Scholar]
- 12. van Rooij SB, van Rooij WJ, Peluso JP, Sluzewski M. The Woven EndoBridge (WEB) as primary treatment for unruptured intracranial aneurysms. Interv Neuroradiol. 2018;24(5):475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kabbasch C, Goertz L, Siebert Eet al.. Comparison of WEB embolization and coiling in unruptured intracranial aneurysms: safety and efficacy based on a propensity score analysis. World Neurosurg. 2019;126:e937-e943. [DOI] [PubMed] [Google Scholar]
- 14. Bozzetto Ambrosi P, Gory B, Sivan-Hoffmann Ret al.. Endovascular treatment of bifurcation intracranial aneurysms with the WEB SL/SLS: 6-month clinical and angiographic results. Interv Neuroradiol. 2015;21(4):462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klisch J, Sychra V, Strasilla C, Liebig T, Fiorella D. The Woven EndoBridge cerebral aneurysm embolization device (WEB II): initial clinical experience. Neuroradiology. 2011;53(8):599-607. [DOI] [PubMed] [Google Scholar]
- 16. Tau N, Sadeh-Gonik U, Aulagner G, Turjman F, Gory B, Armoiry X. The Woven EndoBridge (WEB) for endovascular therapy of intracranial aneurysms: update of a systematic review with meta-analysis. Clin Neurol Neurosurg. 2018;166:110-115. [DOI] [PubMed] [Google Scholar]
- 17. Lv X, Zhang Y, Jiang W. Systematic review of Woven EndoBridge for wide-necked bifurcation aneurysms: complications, adequate occlusion rate, morbidity, and mortality. World Neurosurg. 2018;110:20-25. [DOI] [PubMed] [Google Scholar]