Abstract
OBJECTIVE
To assess changes in antidiabetes medication class prescriptions over time among patients with diabetic kidney disease (DKD), characteristics of patients prescribed these medications, and prescribers’ specialty.
RESEARCH DESIGN AND METHODS
We conducted a cohort study design using insurance claims data between 2013 and the first quarter of 2020 (2020Q1). Included are adult patients with DKD who initiated a new antidiabetes medication between 2013 and 2020Q1 (N = 160,489 patients). The primary outcome is the yearly and quarterly percent of medication initiation for each antidiabetes medication class over all antidiabetes medication initiations.
RESULTS
For patients with DKD, sodium–glucose cotransporter 2 inhibitor (SGLT2i) and glucagon-like peptide 1 receptor agonist (GLP-1RA) initiations steadily increased between 2013 and 2020Q1. Internists and endocrinologists were the most frequent prescriber specialties. Patients <65 years of age had a larger percentage of all initiations that were SGLT2i or GLP-1RA, 16% and 23%, respectively, in 2019, and patients >75 years of age had a smaller percentage of all initiations that were SGLT2i or GLP-1RA, 11% and 13%, in 2019.
CONCLUSIONS
For patients with DKD, SGLT2i and GLP-1RA prescriptions have increased over time, likely reflecting evolving prescribing patterns in response to the results of recent clinical trials and new clinical guidelines.
Introduction
Diabetes affects more than 30 million Americans—or 10.5% of the U.S. population. Among adult patients with diabetes, the estimated prevalence of moderate to severe kidney disease (stages 3 or 4) is nearly 20% (1). Patients with diabetic kidney disease (DKD) are at increased risk of end-stage kidney disease (ESKD) and cardiovascular disease, increased mortality (2), lower quality of life (3), and higher health care costs and rates of health care use (4).
In randomized controlled clinical trials, medications within the sodium–glucose cotransporter 2 inhibitor (SGLT2i) class and glucagon-like peptide 1 receptor agonist (GLP-1RA) class have demonstrated either or both renal protective and cardiovascular benefits (5–16). The results of the CREDENCE trial demonstrated that the SGLT2i canagliflozin is associated with a 30% reduction in kidney disease progression, as measured by a composite outcome comprised of progression to ESKD, doubling of creatinine level, or death from renal or cardiovascular events (17). Since this land-mark trial, additional research focusing on the SGLT2i dapagliflozin has shown similar cardiovascular benefits for patients with DKD (18). Regarding the GLP-1RA class, the results of the randomized controlled trial AWARD-7 demonstrated that patients taking dulaglutide exhibited a slower decline in estimated glomerular filtration rate (eGFR) (16). Additional studies have shown cardiovascular benefits for dulaglutide, semaglutide, and liraglutide (15,19,20). A list of SGLT2i and GLP-1RA drug approvals and main clinical trials concluded by quarter 1 of 2020 (2020Q1) demonstrating renal protective and cardiovascular benefits can be found in Supplementary Table 1.
While renal protective benefits of SGLT2i and GLP-1RA have been suggested in clinical trials, it remains unknown to what extent these medications are used in real-world settings among patients with DKD. Given the observed renal and cardiovascular benefits, multiple medical societies have recently updated their guidelines to promote SGLT2i and GLP-1RA use in patients with type 2 diabetes mellitus (T2DM) with DKD (21,22). Here, we aim to assess the prescribing patterns of antidiabetes medications among pat-ients with DKD, including trends in initiation of these medications over time, characteristics of patients prescribed these medications, and prescribers’ specialty.
Research Design and Methods
Data Source and Study Population
In this study we used data from Optum’s de-identified Clinformatics Data Mart database, which contains demographic information, health plan enrollment status, and inpatient and outpatient diagnoses and procedures along with pharmacy dispensing records for each enrolled patient. Results for outpatient laboratory tests are available for a subset of beneficiaries (∼40%) through linkage with national laboratory test provider chains.
The study population included patients who initiated one of the following antidiabetes medication classes between 1 January 2013 and 31 March 2020: SGLT2i, GLP-1RA, dipeptidyl peptidase 4 inhibitors (DPP-4i), sulfonylureas (SU), thiazolidinediones (TZD), metformin, and insulins. Patients could contribute to multiple medication classes if the inclusion criteria were met for each new class initiation. The date of medication initiation was designated as the cohort entry date, and all patients were required to have at least a 6-month enrollment prior to cohort entry. Patients were required to be new users (no dispensing of the drug class in the 6 months prior to the index date) and to have a prior diagnosis of DKD. DKD was defined as a diagnosis of T2DM and a diagnosis of chronic kidney disease (CKD). CKD was defined as having either two ICD-9 or ICD-10 codes for CKD (see Supplementary Table 2) or one ICD-9 or ICD-10 code for CKD and a laboratory eGFR value indicative of CKD (i.e., eGFR <60 mL/min/1.73 m2). Only patients with CKD stages 3 and 4 were included in this analysis. Patients were excluded if they were younger than 18 years old or had a diagnosis of type 1 diabetes or gestational diabetes mellitus, acute kidney injury, ESKD, or history of kidney transplant (see Supplementary Table 2).
This study received approval by the Mass General Brigham Institutional Review Board.
Patient Characteristics
Information on patient characteristics including demographics, comorbidities, and health care use was assessed for each study participant in the 6 months prior to cohort entry. Patient characteristics are reported for the year 2019, which is the most recent calendar year with a full year of data available; the full population characteristics for all years are included in Supplementary Table 3. States were categorized into regions with use of census-provided definitions (23). All comorbidities were identified by ICD-9, ICD-10, and Current Procedural Terminology, 4th Edition (CPT-4) codes (Supplementary Table 2). Within each 12-month time interval of the study period, we estimated the proportion of patients in each medication class who had one of our measured diagnoses. The eGFR was calculated from available laboratory data in the data set. Our eGFR calculations were based on a modified Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (24) equation that does not include race, as presented below:
- Females:
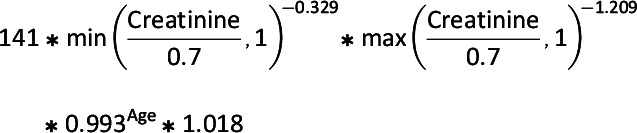
- Males:
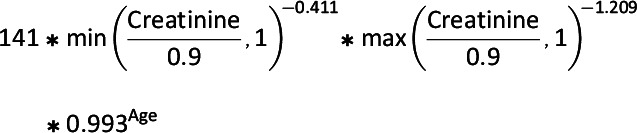
Medication Initiation
Medication initiation for each antidiabetes medication class was reported as a percentage of all antidiabetes medication initiations. Between 2013 and 2018, medication initiation is shown aggregated at the annual level. Starting in 2019, quarterly data are displayed to show the more rapid changes that occurred since the publication of results from recent clinical trials demonstrating renal and cardiovascular protective benefits of SGLT2i and GLP-1RA among patients with DKD. Quarterly data for the entire study period are available in Supplementary Fig. 1.
Subgroup Analysis for SGLT2i and GLP-1RA Initiators
A subgroup analysis was conducted for initiators of SGLT2i and GLP-1RA based on age and eGFR. Age was categorized as <65 years, 65–74 years, or ≥75 years. eGFR was calculated as described above, and categories were as follows: eGFR 15–30 mL/min/1.73 m2 (corresponding to stage 4 DKD), 30–44 mL/min/1.73 m2 (corresponding to stage 3B DKD), and 45–59 mL/min/1.73 m2 (corresponding to stage 3A DKD).
Prescriber Specialty
Prescriber specialty data for each new prescription were categorized according to the taxonomy codes associated with each unique national provider identification (NPI) record, which were extracted from the NPI record associated with the initial prescription for each medication class for a patient. The taxonomy codes were linked to taxonomy descriptions with the crosswalk file provided by the Centers for Medicare & Medicaid Services (25). These were then categorized as internal medicine (including family medicine, general practice, and hospital medicine), nephrology, cardiology, endocrinology, nursing or physician assistant, or other (inclusive of the following: all surgical specialties, obstetrics and gynecology, dermatology, neurology, and not otherwise categorized). If providers had both a code for internal medicine and a more specialized code (nephrology, cardiology, or endocrinology), they were only counted for the more specialized code. There were 85 providers who had two specialized codes (i.e., nephrology and endocrinology), and these providers were counted in both categories. Percentages are reported of all patients who initiated a medication class prescribed by a provider of a certain specialty; i.e., 15% of all patients who initiated a GLP-1RA were prescribed this medication by an endocrinologist.
Results
Patient Characteristics
In 2019, 2,653 patients with DKD initiated SGLT2i and 4,980 initiated GLP-1RA: 8% and 15%, respectively, of all antidiabetes medication initiations during that year (Table 1). The most frequently initiated classes in 2019 were metformin and SU, 21% and 19% of all initiations. On average, patients initiating SGLT2i and GLP-1RA medications were younger than those initiating all other classes. Patients starting insulin had the highest burden of comorbidities, as measured with the average combined comorbidity index (CCI) score (5.4), and these patients had the highest prevalence of all measured baseline comorbidities. Of patients initiating an SGLT2i, 24% had a baseline diagnosis of congestive heart failure (CHF) in 2019 compared with 17% in 2014; additionally, 34% had a baseline diagnosis of ischemic heart disease in 2019 compared with 28% in 2014 (data not shown). Baseline characteristics remained consistent for all other antidiabetes medication classes between 2014 and 2019. Patients who were included based on one ICD-9 or ICD-10 diagnosis code and laboratory eGFR criteria (∼40% of each medication class population) were not significantly different in their baseline characteristics from patients who were included based on two ICD-9 or ICD-10 diagnosis codes (data not shown).
Table 1.
Characteristics of new initiators of antidiabetes medications dispensed in 2019
| SGLT2i | GLP-1RA | DPP-4i | SU | TZD | Metformin | Insulin | |
|---|---|---|---|---|---|---|---|
| New initiations, n (%) | 2,653 (8) | 4,980 (15) | 4,725 (14) | 6,497 (19) | 2,325 (7) | 7,105 (21) | 5,406 (16) |
| Age in years, mean (SD) | 70.8 (8.8) | 69.9 (8.9) | 73.7 (8.9) | 73.4 (8.7) | 72.9 (8.4) | 72.6 (8.7) | 72.3 (9.4) |
| CCI, mean (SD) | 4.2 (2.2) | 4.2 (2.2) | 4.4 (2.5) | 4.3 (2.4) | 3.7 (2.0) | 4.1 (2.4) | 5.4 (2.9) |
| Female, n (%) | 1,222 (46) | 2,664 (53) | 2,470 (52) | 3,225 (50) | 1,143 (49) | 3,712 (52) | 2,871 (53) |
| Race, n (%)* | |||||||
| White | 1,231 (46) | 2,227 (45) | 1,971 (42) | 2,914 (45) | 986 (42) | 3,103 (44) | 2,503 (46) |
| Black | 352 (13) | 678 (14) | 719 (15) | 819 (13) | 281 (12) | 890 (13) | 738 (14) |
| Asian | 120 (5) | 136 (3) | 189 (4) | 210 (3) | 95 (4) | 237 (3) | 120 (2) |
| Hispanic | 366 (14) | 685 (14) | 747 (16) | 931 (14) | 395 (17) | 959 (13) | 639 (12) |
| Other/unknown | 584 (22) | 1,254 (25) | 1,099 (23) | 1,623 (25) | 568 (24) | 1,916 (27) | 1,406 (26) |
| Region, n (%)* | |||||||
| Northeast | 232 (9) | 390 (8) | 435 (9) | 517 (8) | 129 (6) | 577 (8) | 464 (9) |
| Midwest | 360 (14) | 739 (15) | 659 (14) | 906 (14) | 248 (11) | 1,000 (14) | 993 (18) |
| South | 1,420 (54) | 2,761 (55) | 2,607 (55) | 3,510 (54) | 1,250 (54) | 3,631 (51) | 2,783 (51) |
| West | 641 (24) | 1,090 (22) | 1,024 (22) | 1,564 (24) | 698 (30) | 1,897 (27) | 1,166 (22) |
| Baseline comorbidities, n (%) | |||||||
| Nephropathy | 2,609 (98) | 4,893 (98) | 4,625 (98) | 6,315 (97) | 2,279 (98) | 6,904 (97) | 5,337 (99) |
| Neuropathy | 948 (36) | 1,971 (40) | 1,561 (33) | 2,003 (31) | 775 (33) | 2,145 (30) | 2,472 (46) |
| Retinopathy | 341 (13) | 648 (13) | 535 (11) | 635 (10) | 253 (11) | 686 (10) | 779 (14) |
| Hypertension | 2,470 (93) | 4,634 (93) | 4,401 (93) | 5,965 (92) | 2,101 (90) | 6,426 (90) | 5,116 (95) |
| Heart failure | 629 (24) | 1,177 (24) | 1,130 (24) | 1,511 (23) | 294 (13) | 1,419 (20) | 1,827 (34) |
| Ischemic heart disease | 894 (34) | 1,468 (29) | 1,410 (30) | 1,924 (30) | 502 (22) | 1,994 (28) | 2,042 (38) |
| Stroke/TIA | 244 (9) | 561 (11) | 624 (13) | 761 (12) | 237 (10) | 831 (12) | 874 (16) |
| Medication use | |||||||
| Unique DM Rx, mean (SD) | 2.0 (1.1) | 1.9 (1.1) | 1.3 (1.0) | 1.1 (1.0) | 1.6 (1.1) | 0.9 (1.1) | 1.5 (1.1) |
| SGLT2i, n (%) | — | 521 (10) | 326 (7) | 306 (5) | 185 (8) | 338 (5) | 227 (4) |
| GLP-1RA, n (%) | 560 (21) | — | 305 (6) | 537 (8) | 276 (12) | 624 (9) | 793 (15) |
| DPP-4i, n (%) | 773 (29) | 1,293 (26) | — | 1,332 (21) | 573 (25) | 968 (14) | 982 (18) |
| SU, n (%) | 1,155 (44) | 1,937 (39) | 2,034 (43) | — | 1,239 (53) | 2,235 (31) | 1,665 (31) |
| TZDs, n (%) | 351 (13) | 523 (11) | 414 (9) | 634 (10) | — | 517 (7) | 334 (6) |
| Metformin, n (%) | 1,304 (49) | 1,995 (40) | 2,028 (43) | 3,039 (47) | 1,099 (47) | — | 1,490 (28) |
| Insulin, n (%) | 496 (19) | 1,494 (30) | 585 (12) | 560 (9) | 248 (11) | 791 (11) | — |
| Laboratory data | |||||||
| eGFR, mL/min/1.73 m2, mean (SD) | 48.4 (13.3) | 43.4 (13.4) | 42.8 (13.0) | 43.0 (13.7) | 44.0 (13.5) | 49.8 (12.2) | 41.6 (14.8) |
| Creatinine available, n (%) | 1,222 (46) | 2,194 (44) | 2,201 (47) | 3,017 (46) | 1,124 (48) | 3,086 (43) | 2,101 (39) |
| HbA1c level, %, mean (SD) | 8.0 (1.8) | 8.3 (1.8) | 7.7 (1.6) | 7.6 (1.6) | 8.0 (1.7) | 7.4 (1.7) | 8.2 (2.0) |
| HbA1c available, n (%) | 1,315 (50) | 2,288 (46) | 2,295 (49) | 3,053 (47) | 1,186 (51) | 3,359 (47) | 2,228 (41) |
| Health care use, n (%) | |||||||
| ER visit, ≥1 | 609 (23) | 1,190 (24) | 1,354 (29) | 1,809 (28) | 429 (18) | 1,840 (26) | 2,293 (42) |
| Hospitalization visit, ≥1 | 232 (9) | 417 (8) | 662 (14) | 798 (12) | 150 (6) | 902 (13) | 1,392 (26) |
Patient baseline characteristics, laboratory data, and health care use information are for the 6-month period prior to cohort entry. DM Rx, diabetes medication prescription; ER, emergency room; TIA, transient ischemic attack.
Note that these percentages may not total 100% due to rounding.
Patients initiating an SGLT2i or a GLP-1RA had higher average numbers of unique antidiabetes medication prescriptions in the 6 months prior to initiation: an average of 2.0 and 1.9, respectively. The most commonly prescribed medications during this baseline period were metformin and SU; metformin was previously used by 49% of patients initiating an SGLT2i and 40% of patients initiating a GLP-1RA. SUs were previously used by 44% of patients initiating an SGLT2i and 39% of patients initiating a GLP-1RA. For patients initiating an SGLT2i, 21% had used a GLP-1RA during the baseline window. For patients initiating a GLP-1RA, 10% had used an SGLT2i during the prior 6 months. For patients initiating an SGLT2i, average eGFR was 48.4 mL/min/1.73 m2 in 2019 compared with 51.2 mL/min/1.73 m2 in 2014 (data not shown). Average HbA1c level for these patients was 8.0% in 2019 compared with 8.5% in 2014. For patients initiating a GLP-1RA, average calculated eGFR and HbA1c did not change between 2014 and 2019.
Trends in Antidiabetes Medication Initiations by Age and Kidney Function
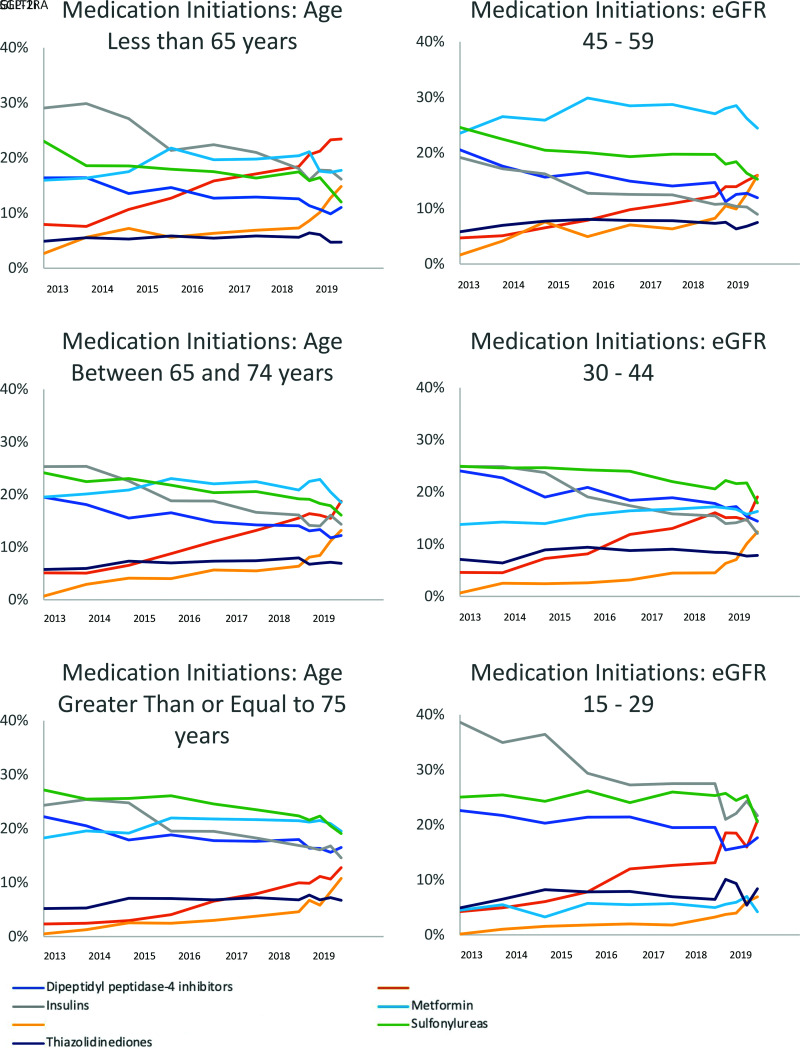
SGLT2i and GLP-1RA initiations steadily increased since 2014 as a percentage of all new antidiabetes medication initiations (Fig. 1). The percentage of new TZD initiations remained stable during this time, while the percentage of all other medication class initiations steadily declined. Stratified analyses were performed for assessment of initiation trends of antidiabetes medication classes by patient age and eGFR (Fig. 2). For every subgroup examined, there were increasing trends of SGLT2i and GLP-1RA initiations between 2013 and the first quarter of 2020, consistent with the increasing trends of SGLT2i and GLP-1RA initiations in our overall study population of patients with DKD. In 2019, for patients age <65 years, 16% of initiations were for an SGLT2i and 23% were for a GLP-1RA compared with 12% and 19% for patients between 65 and 74 years old, respectively, and 11% and 13% for patients 75 years of age and older. Metformin was the most common new medication initiation for patients between 65 and 74 years old, and for patients ≥75 years old, comprising 19% of all medication initiations for both groups. For patients with eGFR between 45 and 59 mL/min/1.73 m2, 15% of prescriptions were for an SGLT2i and 15% were for a GLP-1RA compared with 12% and 18% for patients with eGFR between 30 and 44 mL/min/1.73 m2 and 7% and 21% for patients with eGFR <30 mL/min/1.73 m2.
Figure 1.
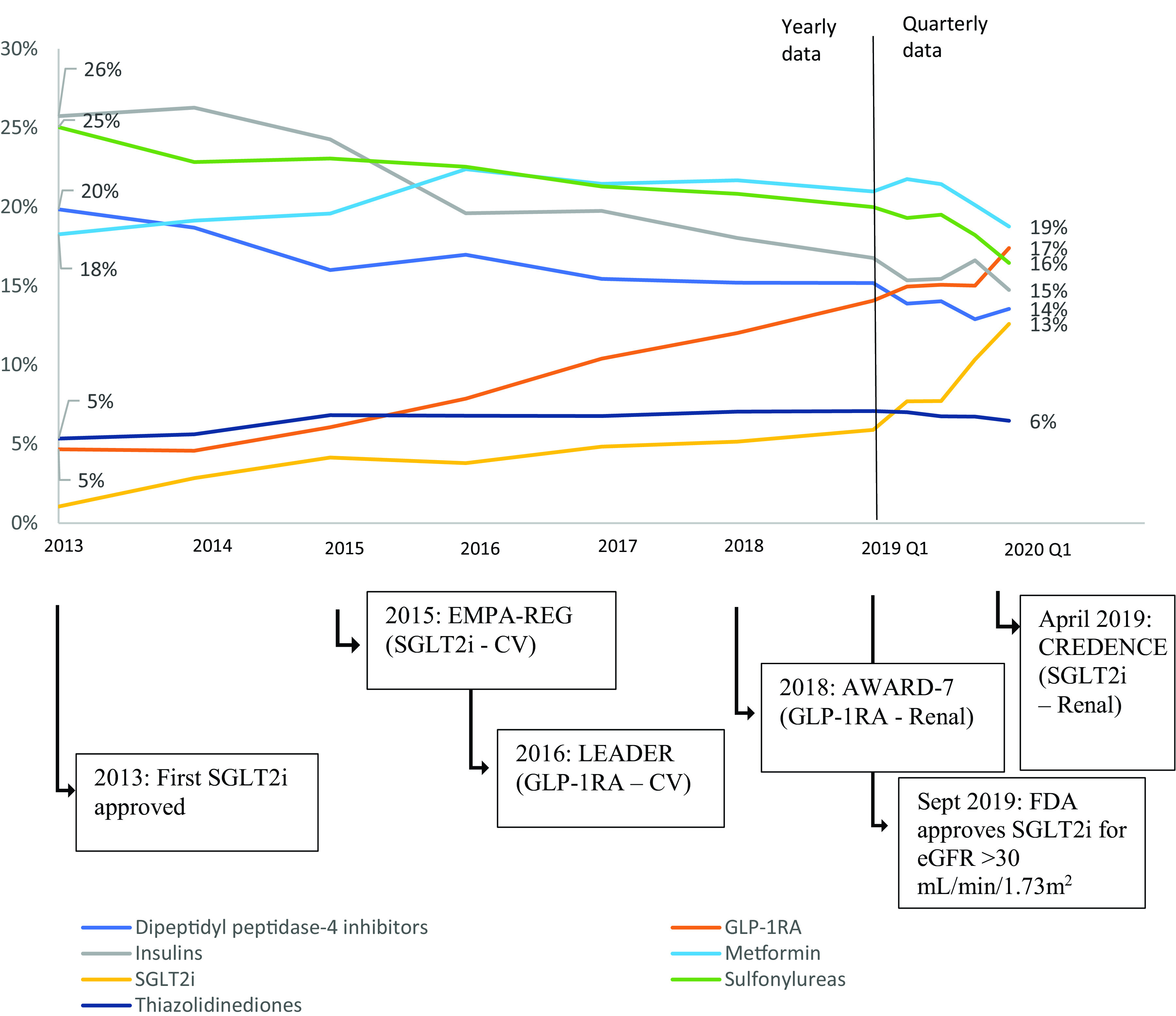
Medication initiation of antidiabetes medications for patients with DKD over time. Medication class initiations are presented as a percentage of all initiations. Between 2013 and 2018, data are shown aggregated at the year level; 2019 and 2020 data are shown at the quarter level. Quarterly data are available in Supplementary Table 1 for 2013–2018. Major dates shown include the first clinical trials for the class of medications with proven cardiovascular (CV) or renal benefits. Additional dates related to FDA SGLT2i approvals are also included. eGFR units are presented as mL/min/1.73 m2. EMPA-REG, EMPA-REG OUTCOME trial; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results.
Figure 2.
Medication initiations as a percentage of all antidiabetes medication initiations by age and calculated eGFR. Between 2013 and 2018, data are shown aggregated at the year level; 2019 and 2020 data are shown at the quarter level. Calculated based on laboratory creatinine values, all eGFR units are mL/min/1.73 m2. Age categories are ≤65 years, 66–74 years, and ≥75 years.
Trends in Antidiabetes Medication Prescribers’ Specialty
In 2019, the most common prescriber specialty for a new initiation of an SGLT2i or a GLP-1RA was internal medicine (73% and 68.5% for SGLT2i and GLP-1RA, respectively); the second most common prescriber specialty was endocrinology (21% and 15% for SGLT2i and GLP-1RA) (Fig. 3). Of new initiations, <5% were prescribed, each, by nephrologists, cardiologists, and nursing or physician assistants for both SGLT2i and GLP-1RA. These percentages remained stable between 2014 and 2019. After the results of Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) were released in April 2019, there was a small increase in the number of SGLT2i initiations prescribed by nephrologists (2% in 2019Q1 to 6% by 2019Q3). No increase was seen in the number of GLP-1RA initiations by nephrologists during the same time period.
Figure 3.
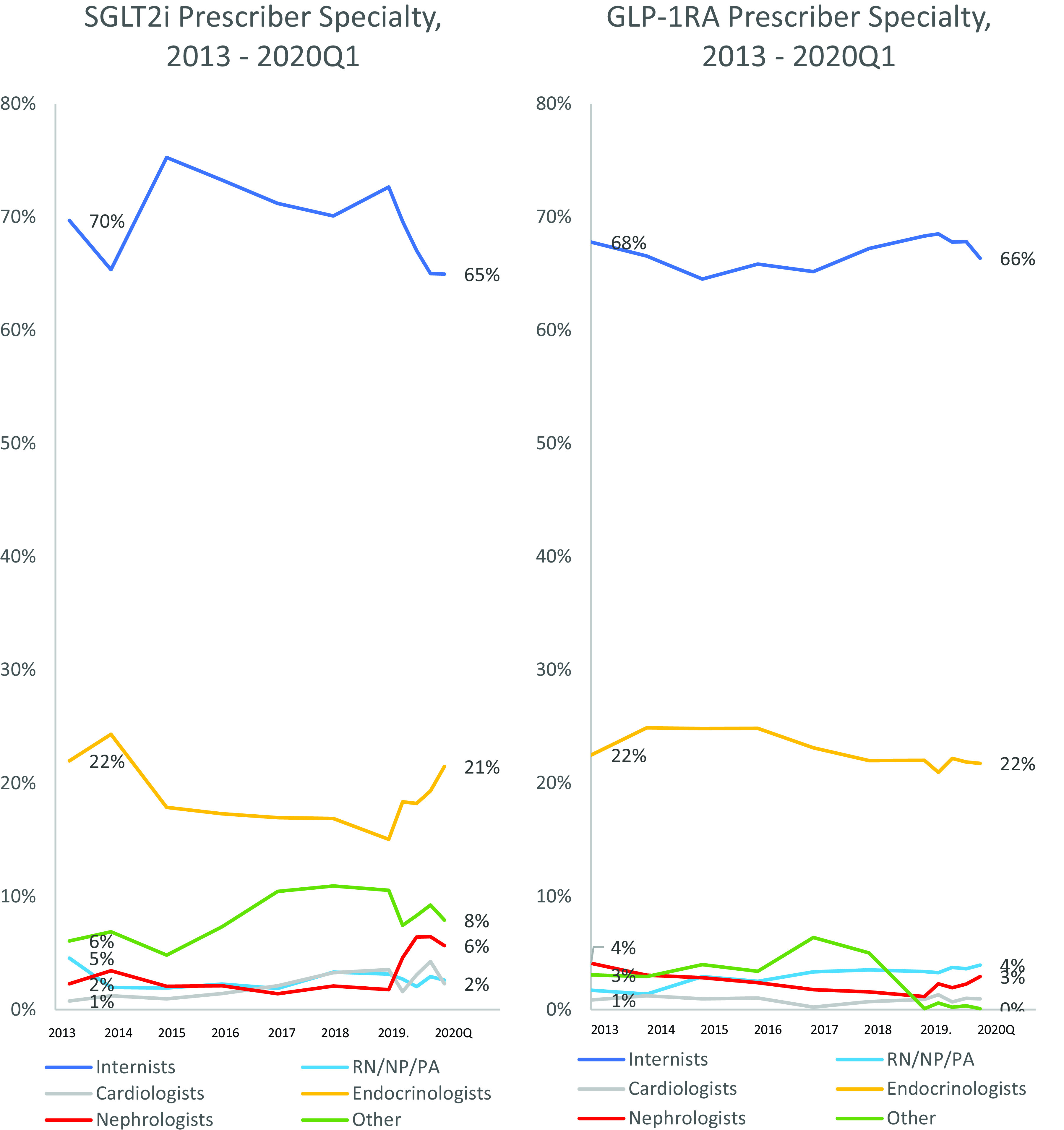
Prescriber specialty for SGLT2i and GLP-1RA initiations over time. Between 2013 and 2018, data are shown aggregated at the year level; 2019 and 2020 data are shown at the quarter level. Internist includes physicians within the following specialties: internal medicine, family medicine, general practice, and hospital medicine. RN/NP/PA, registered nurse/nurse practitioner/physician assistant.
Conclusions
This study describes the trends in the initiation of antidiabetes medications over time among patients with DKD. We found that initiations of SGLT2i and GLP-1RA increased as a percentage of all new antidiabetes medication initiations over time. Among patients initiating an SGLT2i, a higher percentage had a baseline diagnosis of CHF or ischemic heart disease in 2019 compared with patients who initiated an SGLT2i in 2014. Patients initiating an SGLT2i or a GLP-1RA had a higher average number of unique antidiabetes medications in the prior 6 months compared with patients initiating other medication classes. The percentage of SGLT2i initiations in patients with DKD was lower for patients with more severe renal dysfunction, while the percentage of GLP-1RA initiations in patients with DKD was higher for patients with more severe kidney dysfunction. We observed increasing percentages of SGLT2i and GLP-1RA initiations over time across all patient age subgroups. However, these increases were most pronounced for patients age <65 years, and the smallest increase in percentages was seen in patients age ≥75 years. The prescribers of SGLT2i and GLP-1RA medications were commonly internists and endocrinologists, and there was a small increase in the number of nephrologists prescribing SGLT2i medications in 2019.
In our study, patients newly initiating an SGLT2i were more likely to have a prior diagnosis of CHF or ischemic heart disease in 2019 compared with 2014. This suggests a change in prescribing patterns in response to the clinical trials demonstrating a benefit for patients with concurrent cardiovascular disease and diabetes. The BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial and other clinical trials had demonstrated that among patients with a history of T2DM and cardiovascular disease initiating an SGLT2i, there was a reduction in risk of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (6,9).
Our finding that SGLT2i and GLP-1RA medications are increasing as a percentage of all new antidiabetes medication initiations among patients with DKD is in line with results of prior studies focusing on the overall population of T2DM patients (26–28). We found that GLP-1RA medications were more commonly initiated than SGLT2i and had been previously used in 20% of SGLT2i initiators. In a prior study focused on medication usage in T2DM patients with preserved renal function, a higher percentage was described of SGLT2i use than GLP-1RA use (29). One potential reason for the difference in our study is the decreased eGFR in our patient population. This is further demonstrated by the stratification of our patients with DKD by eGFR; here we saw the largest difference in rates of initiations between these classes (21% of initiations were GLP-1RA, while only 7% of initiations were SGLT2i) among patients in the lowest eGFR group. The 2018 treatment algorithms by the American Diabetes Association and American Heart Association recommended SGLT2i over GLP-1RA as the second-line prescription after metformin for patients with CHF or DKD when eGFR is adequate (22,30). There was initially concern about prescribing SGLT2i in patients with renal impairment given a risk of increased adverse events, and while a few clinical trials had included participants with eGFR 30–45 mL/min/1.73 m2 (2,6,8,12), they were not adequately powered to conclude that these medications could be used safely in patients with severe renal dysfunction. Additionally, studies show that the impact of SGLT2i on lowering HbA1c is reduced for those with eGFR <60 mL/min/1.73 m2 and severely reduced for those with eGFR <45 mL/min/1.73 m2 (2,31). In contrast, the GLP-1RA medication exenatide was approved for use in patients with eGFR >30 mL/min/1.73 m2 in 2014 and the GLP-1RA lixisenatide was approved for those with eGFR >15 mL/min/1.73 m2 in 2017 (2,32,33). The glucose-lowering impact of GLP-1RAs is preserved even in patients with severe renal dysfunction (16). Given this, prior to the release of CREDENCE results and evidence of cardiovascular benefit for SGLT2i, we expected minimal SGLT2i use in our population of patients with DKD.
In our study, patients with DKD with eGFR <30 mL/min/1.73 m2 (2) continued to be initiated on metformin at similar rates before and after the U.S. Food and Drug Administration (FDA) guidance in 2016 to limit use in this population (34). There is minimal research regarding metformin use in this population given the contraindication. Similarly, these patients continued to be initiated on SU despite multiple guidelines recommending reduced SU use in patients with stage 3 CKD and beyond (35). Given the evidence of real-world usage in these populations, further research in these populations to better understand why they are still being prescribed metformin or SU and to understand their associated outcomes would be of clinical interest.
In our study, patients with DKD who were <65 years old were more likely to be started on SGLT2i and GLP-1RA medications compared with older patients with DKD. For SGLT2i, there were initial concerns for volume depletion, especially in older patients (36), and the FDA had released warnings of fracture risk and decreased bone mineral density for canagliflozin (37), despite more recent studies showing contrary results (38,39). Another potential reason for this trend suggested by Handelsman et al. (40) is that GLP-1RA may be less desirable in an older population given the predominantly injectable formulations and the gastrointestinal side effects. Additionally, both SGLT2i and GLP-1RA classes are more expensive than older medication classes, and for older populations this cost may be more prohibitive (41).
We also found that the most common prescriber specialties were internists and endocrinologists, with a small increase in prescriptions from nephrologists after the release of the CREDENCE results. This indicates that other specialists are starting to prescribe antidiabetes medications that are predominantly and traditionally prescribed by internists and endocrinologists. Prior investigators, in research on SGLT2i prescriptions, had examined the role of cardiologists in an overall T2DM population and reported that they were responsible for only 5% of prescriptions even after the FDA expanded labeling for use in cardiovascular disease (42). Interestingly, a recent study estimated that for patients with cardiovascular disease, following goal-directed treatment that included prescriptions for SGLT2i had the potential to prevent >25,000 deaths per year (43).
This study has several limitations. First, the study population only included enrollees of commercial and Medicare Advantage insurance plans, and thus our findings may not be generalizable to an uninsured or a non–Medicare Advantage population. Second, we only included patients with at least 6 months of prior enrollment without prior dispensing during that window to capture only new initiations; however, if a patient had been previously prescribed and then discontinued this medication >6 months prior to our captured initiation date, that patient would be classified as a new initiation. Third, we did not have formulary information available that may have influenced the trends that we saw. Fourth, initiation information in the database captures only filled prescriptions; if a medication was prescribed but not filled by a patient, this would not be included as an initiation. Finally, since information on albuminuria or proteinuria was not available in our claims database, our study did not include patients who had a diagnosis of DKD based primarily on these criteria.
Overall, we found increasing initiations of SGLT2i and GLP-1RA antidiabetes medications in a DKD population, especially in younger patients and patients with moderately impaired kidney function. We found that SGLT2i are beginning to be initiated in patients with more severely impaired kidney function, reflecting the change in guidelines and FDA-recommended use. As expected, we also saw that internal medicine and endocrinology were the most common prescriber specialties for these two medications, with a small increase in nephrology prescribers for SGLT2i. Given the evidence that SGLT2i and GLP-1RA benefit patients with DKD, it is important that all prescribers who may be seeing patients with DKD in their clinical practice be aware of the potential benefits (44). Further research regarding the safety of the SGLT2i and GLP-1RA medication classes in older populations with more severe kidney impairment may be helpful to evaluate whether promoting uptake in this population would be beneficial. Future studies investigating characteristics of patients who did not receive a guideline-appropriate medication would be helpful, such as patients who have heart failure and diabetes but are not initiated on an SGLT2i, to identify what barriers to adoption may remain. Additionally, continuing to monitor the uptake of these medications as more prescribers become familiar with these medication classes will be important to gauge whether newer medications are being prescribed when indicated for patients with DKD.
Article Information
Funding. This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School. M.Z. is supported by a National Institutes of Health T32 grant (DK007199), and E.P. was supported by a National Institute on Aging career development grant K08AG055670.
None of these funding sources had any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Duality of Interest. E.P. is principal investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to the topic of the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.T.H., E.P., and J.M.P. contributed to the study concept and design. All authors contributed to acquisition, analysis, or interpretation of data. S.T.H. and J.M.P. drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. S.T.H. and J.M.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14825016.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. Centers for Disease Control and Prevention, Department of Health and Human Services . National Diabetes Statistics Report 2020: Esti-mates of Diabetes and Its Burden in the United States, 2020. Accessed 18 January 2021. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 2. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karner-Hutuleac A. Health related quality of life of diabetic and chronic renal failure patients. Procedia Soc Behav Sci 2012;33:85–89 DOI: 10.1016/j.sbspro.2012.01.088 [Google Scholar]
- 4. Zhou Z, Chaudhari P, Yang H, et al. Healthcare resource use, costs, and disease progression associated with diabetic nephropathy in adults with type 2 diabetes: a retrospective observational study. Diabetes Ther 2017;8:555–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tikkanen I, Narko K, Zeller C, et al.; EMPA-REG BP Investigators . Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015;38:420–428 [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 7. Salsali A, Kim G, Woerle HJ, Broedl UC, Hantel S. Cardiovascular safety of empagliflozin in patients with type 2 diabetes: a meta-analysis of data from randomized placebo-controlled trials. Diabetes Obes Metab 2016;18:1034–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 9. Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Cana-gliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 10. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 11. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39 [DOI] [PubMed] [Google Scholar]
- 12. Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 2018;6:691–704 [DOI] [PubMed] [Google Scholar]
- 13. Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation 2019;140:1693–1702 [DOI] [PubMed] [Google Scholar]
- 14. Mann JFE, Ørsted DD, Brown-Frandsen K, et al.; LEADER Steering Committee and Invest-igators . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377:839–848 [DOI] [PubMed] [Google Scholar]
- 15. Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 16. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6:605–617 [DOI] [PubMed] [Google Scholar]
- 17. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 18. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 19. Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 20. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Boer IH, Caramori ML, Chan JCN, et al.; Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes manage-ment in chronic kidney disease. Kidney Int 2020;98:S1–S115 [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . 9. Pharma-cologic approaches to glycemic treatment: Standards of Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S98–S110 [DOI] [PubMed] [Google Scholar]
- 23. U.S. Census Bureau . Census Regions and Divisions of the United States. Accessed 24 September 2020. Available from https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- 24. Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Medicare and Medicaid Services . Crosswalk Medicare Provider/Supplier to Healthcare Provider Taxonomy, 2020. Accessed 16 August 2020. Available from https://data.cms.gov/Medicare-Enrollment/CROSSWALK-MEDICARE-PROVIDER-SUPPLIER-to-HEALTHCARE/j75i-rw8y
- 26. Fadini GP, Frison V, Rigato M, et al. Trend 2010-2018 in the clinical use of GLP-1 receptor agonists for the treatment of type 2 diabetes in routine clinical practice: an observational study from Northeast Italy. Acta Diabetol 2020;57:367–375 [DOI] [PubMed] [Google Scholar]
- 27. Ahuja V, Chou C-H. Novel therapeutics for diabetes: uptake, usage trends, and comparative effectiveness. Curr Diab Rep 2016;16:47–47 [DOI] [PubMed] [Google Scholar]
- 28. Dave CV, Schneeweiss S, Wexler DJ, Brill G, Patorno E. Trends in clinical characteristics and prescribing preferences for SGLT2 inhibitors and GLP-1 receptor agonists, 2013–2018. Diabetes Care 2020;43:921–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dennis JM, Henley WE, McGovern AP, et al.; MASTERMIND consortium . Time trends in prescribing of type 2 diabetes drugs, glycaemic response and risk factors: a retrospective analysis of primary care data, 2010-2017. Diabetes Obes Metab 2019;21:1576–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelly MS, Lewis J, Huntsberry AM, Dea L, Portillo I. Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Postgrad Med 2019;131:31–42 [DOI] [PubMed] [Google Scholar]
- 32. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neumiller JJ, Alicic RZ, Tuttle KR. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Nephrol 2017;28:2263–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. U.S. Food and Drug Administration DSC . FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Accessed 7 May 2021. Available from https://www.fda.gov/files/drugs/published/Drug-Safety-Communication-FDA-revises-warnings-regarding-use-of-the-diabetes-medicine-metformin-in-certain-patients-with-reduced-kidney-function-%28PDF%29.pdf
- 35. Ioannidis I. Diabetes treatment in patients with renal disease: is the landscape clear enough? World J Diabetes 2014;5:651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf 2019;18:295–311 [DOI] [PubMed] [Google Scholar]
- 37. U.S. Food and Drug Administration . FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density, 2015. Accessed 13 October 2020. Available from https://www.fda.gov/media/93815/download
- 38. Fralick M, Kim SC, Schneeweiss S, Kim D, Redelmeier DA, Patorno E. Fracture risk after initiation of use of canagliflozin: a cohort study. Ann Intern Med 2019;170:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abrahami D, Douros A, Yin H, Yu OHY, Azoulay L. Sodium–glucose cotransporter 2 inhibitors and the risk of fractures among patients with type 2 diabetes. Diabetes Care 2019;42:e150–e152 [DOI] [PubMed] [Google Scholar]
- 40. Handelsman Y, Muskiet MHA, Meneilly GS. Combining GLP-1 receptor agonists and basal insulin in older adults with type 2 diabetes: focus on lixisenatide and insulin glargine. Adv Ther 2019;36:3321–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mackey K, Parchman ML, Leykum LK, Lanham HJ, Noël PH, Zeber JE. Impact of the chronic care model on medication adherence when patients perceive cost as a barrier. Prim Care Diabetes 2012;6:137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaduganathan M, Sathiyakumar V, Singh A, et al. Prescriber patterns of SGLT2i after expansions of U.S. Food and Drug Administration labeling. J Am Coll Cardiol 2018;72:3370–3372 [DOI] [PubMed] [Google Scholar]
- 43. Bassi NS, Ziaeian B, Yancy CW, Fonarow GC. Association of optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy with outcome for patients with heart failure. JAMA Cardiol 2020;5:948–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tuttle KR, Cherney DZ; Diabetic Kidney Disease Task Force of the American Society of Nephrology . Sodium glucose cotransporter 2 inhibition heralds a call-to-action for diabetic kidney disease. Clin J Am Soc Nephrol 2020;15:285–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Center for Drug Evaluation and Research . Approval Letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021773_Byetta_approv.PDF
- 46. Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Center for Drug Evaluation and Research . Approval Letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022341s000approv.pdf
- 48. Center for Drug Evaluation and Research . Approval Letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125431Orig1s000Approv.pdf
- 49. Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular out-comes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 50. Center for Drug Evaluation and Research . Highlights of Prescribing Information. Dulaglutide NDA approval letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125469s036lbl.pdf
- 51. Center for Drug Evaluation and Research . Highlights of Prescribing Information. Lixisenatide NDA approval letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_ docs/label/2016/208471Orig1-s000lbl.pdf
- 52. Pfeffer MA, Claggett B, Diaz R, et al.; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 53. Center for Drug Evaluation and Research . Highlights of Prescribing Information. Semaglutide NDA approval letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf
- 54. Husain M, Birkenfeld AL, Donsmark M, et al.; PIONEER 6 Investigators . Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851 [DOI] [PubMed] [Google Scholar]
- 55. Center for Drug Evaluation and Research . Highlights of Prescribing Information. Canagliflozin NDA approval letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/20402s036lbl.pdf
- 56. Center for Drug Evaluation and Research . Dapagliflozin NDA approval letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202293s020lbl.pdf
- 57. McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Invest-igators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 58. Center for Drug Evaluation and Research . Highlights of Prescribing Information. Empagliflozin NDA approval letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204629s023lbl.pdf
- 59. Center for Drug Evaluation and Research . Highlights of Prescribing Information. Ertugloflozin NDA approval letter. Accessed 7 May 2021. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209803s002lbl.pdf