Abstract
OBJECTIVE
To examine whether glucagon-like peptide 1 receptor agonists (GLP-1RA) and sodium–glucose cotransporter 2 inhibitors (SGLT2i) are preferentially initiated among patients with cardiovascular disease, heart failure (HF), or nephropathy, where these drug classes have established benefit, compared with dipeptidyl peptidase 4 inhibitors (DPP-4i), for which corresponding benefits have not been demonstrated.
RESEARCH DESIGN AND METHODS
We retrospectively analyzed claims of adults with type 2 diabetes included in OptumLabs Data Warehouse, a deidentified database of commercially insured and Medicare Advantage beneficiaries, who first started GLP-1RA, SGLT2i, or DPP-4i therapy between 2016 and 2019. Using multinomial logistic regression, we examined the relative risk ratios (RRR) of starting GLP-1RA and SGLT2i compared with DPP-4i for those with a history of myocardial infarction (MI), cerebrovascular disease, HF, and nephropathy after adjusting for demographic and other clinical factors.
RESULTS
We identified 75,395 patients who started GLP-1RA, 58,234 who started SGLT2i, and 91,884 who started DPP-4i. Patients with prior MI, cerebrovascular disease, or nephropathy were less likely to start GLP-1RA rather than DPP-4i compared with patients without these conditions (RRR 0.83 [95% CI 0.78–0.88] for MI, RRR 0.77 [0.74–0.81] for cerebrovascular disease, and RRR 0.87 [0.84–0.91] for nephropathy). Patients with HF or nephropathy were less likely to start SGLT2i (RRR 0.83 [0.80–0.87] for HF and RRR 0.57 [0.55–0.60] for nephropathy). Both medication classes were less likely to be started by non-White and older patients.
CONCLUSIONS
Patients with cardiovascular disease, HF, and nephropathy, for whom evidence suggests a greater likelihood of benefiting from GLP-1RA and/or SGLT2i therapy, were less likely to start these drugs. Addressing this treatment/benefit paradox, which was most pronounced in non-White and older patients, may help reduce the morbidity associated with these conditions.
Introduction
More than 34 million adults, or 13% of the U.S. adult population, have diabetes (1), and 80% of those with diagnosed diabetes are taking glucose-lowering medications (2). Optimal patient-centered diabetes care is predicated on treating each patient with medications that are likely to yield the most benefit and risk the least harm, weighing the best available evidence against each patient’s preferences and situation. Cardiovascular disease is the leading cause of death among patients with diabetes (3–5), and kidney disease is one of the most common complications of diabetes and a major risk factor for cardiovascular and all-cause mortality (6). Together, these conditions account for a large proportion of health care expenditures associated with diabetes (7). Thus, while metformin is consistently recommended as the first-line drug in the management of type 2 diabetes, since January 2017 (8), clinical guidelines have advised that the choice of second-line therapy be informed by presence of these key comorbidities, specifically cardiovascular disease, heart failure (HF), and chronic kidney disease or nephropathy, in addition to hypoglycemia risk, considerations of medication adverse effects and affordability, and patient preference (9–12).
Postmarketing randomized controlled trials of newly approved glucose-lowering medications, which had been mandated by the U.S. Food and Drug Administration between 2008 and 2020 to ensure their cardiovascular safety (13), revealed favorable effects of glucagon-like peptide-1 receptor agonists (GLP-1RA) on cardiovascular and kidney outcomes and of sodium–glucose cotransporter 2 inhibitors (SGLT2i) on HF and kidney outcomes. In contrast, dipeptidyl peptidase-4 inhibitors (DPP-4i) were largely cardiovascular/kidney-neutral, while concerns about increased HF risk with saxagliptin therapy were raised. This evidence generated great interest in using GLP-1RA and/or SGLT2i for patients with relevant comorbidities (i.e., cardiovascular disease, HF, or nephropathy) (14,15) and either caution (for saxagliptin) or neutrality regarding the use of DPP4i in these contexts (9). Thus, optimal diabetes management would entail preferential use of GLP-1RA and SGLT2i in the presence of these comorbidities. Yet, whether these drugs are indeed more likely to be used by patients with cardiovascular disease, HF, and/or nephropathy than by patients without these conditions in contemporary clinical practice is unknown.
In an effort to identify opportunities to better align management of diabetes with the patient’s clinical situation and best available evidence, we examine whether commercially insured and Medicare Advantage beneficiaries with type 2 diabetes who have prior history of cardiovascular disease (specifically, myocardial infarction [MI] or cerebrovascular disease), HF, and nephropathy are more likely to start treatment with GLP-1RA and SGLT2i, as opposed to DPP4i, between 2016 and 2019. We also assess for differences in GLP-1RA, SGLT2i, and DPP-4i initiation as a function of nonclinical factors, such as patient age, sex, and racial/ethnic origin, in light of known disparities in diabetes health outcomes among these groups (16–19). These results can inform clinical decision making, the development and implementation of decision support tools, and use of health plan formulary design and patient cost-sharing to support evidence-based management of hyperglycemia to ultimately reduce the morbidity and mortality associated with type 2 diabetes.
Research Design and Methods
Study Design
We retrospectively analyzed deidentified administrative claims data from OptumLabs Data Warehouse (OLDW), which includes medical and pharmacy claims and enrollment records for commercial and Medicare Advantage enrollees (20,21). OLDW contains longitudinal health information on enrollees, representing a diverse mixture of ages, races/ethnicities, and geographic regions across the U.S. This study was exempt from review by the Mayo Clinic Institutional Review Board because it involved research solely on preexisting and deidentified data.
Study Population
We identified adults (aged ≥18 years) with type 2 diabetes who initiated therapy with GLP-1RA, SGLT2i, or DPP-4i class medications (Supplementary Table 1) between 1 January 2016 and 31 December 2019 and were not treated with any of these medications during the preceding 12 months.
Patients with type 1 diabetes were excluded. Diabetes type was ascertained on the basis of International Classification of Diseases (ICD) codes and medications filled during 12 months preceding the index prescription fill date, consistent with previously described methodology (22,23). Specifically, type 1 diabetes was assumed for patients who had 1) more type 1 diabetes than type 2 diabetes diagnosis codes on evaluation and management visit claims and had insulin claims, or 2) an equal number of type 1 and type 2 diabetes diagnosis codes and had bolus insulin claims and no sulfonylurea claims. This approach was selected to minimize misclassification of diabetes type when using claims alone, because patients with type 1 diabetes would be treated with bolus (i.e., rapid-acting) insulin and would not be treated with sulfonylurea medications. Patients meeting diagnosis-based criteria for type 1 diabetes but treated with nonsulfonylurea classes of noninsulin medications were not reclassified as type 2 diabetes because those medications may be used off-label as adjunct therapies in type 1 diabetes. ICD codes indicative of type 1 diabetes included ICD-9th Revision-Clinical Modification 250.x1 and 250.x3, and ICD-10-CM codes E10.xxx and O24.0xx. ICD codes indicative of type 2 diabetes included ICD-9th Revision-Clinical Modification 250.x0 and 250.x2 and ICD-10-CM E11.xxx and O24.1xx. Evaluation and management visits were identified by Current Procedural Terminology codes 99,201–99,499.
To examine the impact of glycemic control on medication choice, we identified the subset of the study population that had an available hemoglobin A1c (HbA1c) result within the 6 months prior to and including the index date. In the event of multiple results, HbA1c closest to the index date was considered. Laboratory test results are available for a subset of the OLDW population based on contractual agreements between OptumLabs and commercial laboratory companies.
Explanatory Comorbidity Variables
Comorbidities for which there are evidence-based indications for preferential use of GLP-1RA (i.e., MI, cerebrovascular disease, and nephropathy) or SGLT2i (i.e., HF and nephropathy), as opposed to DPP-4i, were examined. All were ascertained using primary and secondary ICD diagnosis codes from any claim during the 12 months preceding the index drug fill date (Supplementary Table 2).
Other Covariates
Patient demographic characteristics included age (categorized as 18–44, 45–64, 65–74, and ≥75 years), sex (male or female), race/ethnicity (White, Black, Hispanic, Asian, other/unknown), U.S. region of residency (Midwest, Northeast, South, West), and type of health plan (commercial vs. Medicare Advantage). Clinical variables included prescriber specialty (endocrinology, primary care [comprising family medicine, internal medicine, and pediatrics], cardiology, nephrology, other, or unknown), individual classes of glucose-lowering medications used at the time of index medication initiation (i.e., those filled during 120 days prior to the index date, per Supplementary Table 1), and comorbidities. GLP-1RA, SGLT2i, and DPP-4i were classified as “first-line” if there were no fills for any class of diabetes drugs in the preceding 12 months. For comorbidities, we specifically considered the total count of diabetes complications as a surrogate for diabetes duration and severity, ascertained using the Diabetes Complications Severity Index (retinopathy, nephropathy, neuropathy, cerebrovascular disease, cardiovascular disease, and peripheral vascular disease) (24), as well as the individual presence of retinopathy, neuropathy, peripheral vascular disease, dementia, chronic obstructive pulmonary disease (COPD), cirrhosis, and cancer (except for skin cancer), as well as emergency department (ED) visits or hospitalizations for hypoglycemia and hyperglycemia. Code sets used for all comorbidities are detailed in Supplementary Table 2.
Statistical Analysis
All analyses were conducted at the patient level. Characteristics of GLP-1RA initiators, SGLT2i initiators, and DPP-4i initiators as of the index date were reported as frequencies with percentages for categorical data and as means with standard deviation (SD) or medians with interquartile range (IQR) for continuous variables. Differences across groups were assessed using χ2 tests for categorical and Kruskal-Wallis tests for continuous variables.
Multinomial logistic regression examined factors associated with GLP-1RA and SGLT2i, compared with DPP-4i initiation, with results presented as relative risk ratios (RRR) and 95% confidence intervals (CI). Model covariates included explanatory variables and other covariates detailed above. This model was also used to calculate the adjusted rates of GLP-1RA, SGLT2i, and DPP-4i initiation in each calendar year for the overall study population and for subgroups of patients with MI, cerebrovascular disease, HF, and nephropathy. In a sensitivity analysis, we additionally considered interaction terms between year of medication initiation and the presence of compelling medical comorbidities (i.e., MI, cerebrovascular disease, HF, and nephropathy).
Subgroup analysis was conducted among patients with available HbA1c test results, replicating the above modeling approach but with the inclusion of HbA1c test results as one of the covariates. Analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC) and Stata 15.1 (StataCorp, College Station, TX).
Results
Study Population
We identified 75,395 adults with type 2 diabetes who had initiated a GLP-1RA, 58,234 who initiated a SGLT2i, and 91,884 who initiated a DPP-4i between 2016 and 2019 (Supplementary Fig. 1). Patients starting GLP-1RA and SGLT2i were younger (57.3 [SD, 12.9] years and 59.1 [SD, 12.0] years, respectively) and more often White (62.4% and 60.5%, respectively) compared with DPP-4i initiators, who were 65.0 (SD, 12.9) years old and 55.6% White (Table 1). GLP-1RA initiators were more frequently women (57.6%), whereas SGLT2i were started by women less often (42.6%) compared with DPP-4i initiators (49.1%). Primary care clinicians prescribed these medications at least half of the time, with greater rates of initiation for DPP-4i at 61.1% compared with GLP-1RA at 50.4% and SGLT2i at 58.7%.
Table 1.
Study population
| GLP-1RA initiators | SGLT2i initiators | DPP-4i initiators | P value | |
|---|---|---|---|---|
| (n = 75,395) | (n = 58,234) | (n = 91,884) | ||
| Demographics | ||||
| Age, years, mean (SD) | 57.3 (12.9) | 59.1 (12.0) | 65.0 (12.9) | <0.001 |
| Age-group, n (%) | <0.001 | |||
| 18–44 years | 12,805 (17.0) | 6,969 (12.0) | 6,594 (7.2) | |
| 45–64 years | 38,628 (51.2) | 31,016 (53.3) | 33,855 (36.8) | |
| 65–74 years | 17,771 (23.6) | 14,663 (25.2) | 28,450 (31.0) | |
| ≥75 years | 6,191 (8.2) | 5,586 (9.6) | 22,985 (25.0) | |
| Sex, n (%) | <0.001 | |||
| Female | 43,408 (57.6) | 24,779 (42.6) | 45,149 (49.1) | |
| Male | 31,987 (42.4) | 33,455 (57.4) | 46,735 (50.9) | |
| Race/ethnicity, n (%) | <0.001 | |||
| White | 47,017 (62.4) | 35,225 (60.5) | 51,096 (55.6) | |
| Black | 11,742 (15.6) | 8,087 (13.9) | 16,468 (17.9) | |
| Hispanic | 10,148 (13.5) | 9,090 (15.6) | 14,994 (16.3) | |
| Asian | 1,761 (2.3) | 2,463 (4.2) | 4,662 (5.1) | |
| Unknown | 4,727 (6.3) | 3,369 (5.8) | 4,664 (5.1) | |
| U.S. region, n (%) | <0.001 | |||
| Midwest | 18,346 (24.3) | 13,728 (23.6) | 20,679 (22.5) | |
| Northeast | 7,153 (9.5) | 5,760 (9.9) | 12,941 (14.1) | |
| South | 41,283 (54.8) | 32,106 (55.1) | 49,480 (53.9) | |
| West | 8,613 (11.4) | 6,640 (11.4) | 8,784 (9.6) | |
| Insurance type, n (%) | <0.001 | |||
| Commercial | 45,033 (59.7) | 35,543 (61.0) | 35,233 (38.3) | |
| Medicare Advantage | 30,362 (40.3) | 22,691 (39.0) | 56,651 (61.7) | |
| Index year, n (%) | <0.001 | |||
| 2016 | 12,347 (16.4) | 11,515 (19.8) | 22,514 (24.5) | |
| 2017 | 16,887 (22.4) | 14,263 (24.5) | 25,131 (27.4) | |
| 2018 | 20,373 (27.0) | 13,877 (23.8) | 23,041 (25.1) | |
| 2019 | 25,788 (34.2) | 18,579 (31.9) | 21,198 (23.1) | |
| Clinical characteristics | ||||
| Baseline medication fills, n (%) | ||||
| None | 21,132 (28.0) | 12,393 (21.3) | 22,481 (24.5) | <0.001 |
| Metformin | 39,414 (52.3) | 37,050 (63.6) | 52,024 (56.6) | <0.001 |
| Sulfonylureas | 17,475 (23.2) | 17,276 (29.7) | 30,495 (33.2) | <0.001 |
| Thiazolidinediones | 3,615 (4.8) | 3,301 (5.7) | 4,120 (4.5) | <0.001 |
| Insulin (any) | 22,493 (29.8) | 10,522 (18.1) | 13,265 (14.4) | <0.001 |
| Basal insulin | 19,608 (26.0) | 9,107 (15.6) | 11,633 (12.7) | <0.001 |
| Bolus insulin | 11,105 (14.7) | 4,774 (8.2) | 5,253 (5.7) | <0.001 |
| Other medication(s) | 173 (0.2) | 123 (0.2) | 268 (0.3) | 0.004 |
| Treatment type, n (%) | <0.001 | |||
| First-line | 16,164 (21.4) | 7,944 (13.6) | 14,996 (16.3) | |
| Second-line | 59,231 (78.6) | 50,290 (86.4) | 76,888 (83.7) | |
| Diabetes complications count, n, mean (SD) | 1.0 (1.3) | 0.9 (1.1) | 1.2 (1.3) | <0.001 |
| Diabetes complications count, n (%) | <0.001 | |||
| 0 | 35,375 (46.9) | 28,440 (48.8) | 35,792 (39.0) | |
| 1 | 18,860 (25.0) | 15,860 (27.2) | 23,986 (26.1) | |
| 2 | 10,689 (14.2) | 7,958 (13.7) | 15,850 (17.3) | |
| 3 | 6,081 (8.1) | 3,827 (6.6) | 9,413 (10.2) | |
| ≥4 | 4,390 (5.8) | 2,149 (3.7) | 6,843 (7.4) | |
| Comorbidities, n (%) | ||||
| Retinopathy | 9,951 (13.2) | 6,571 (11.3) | 12,645 (13.8) | <0.001 |
| Nephropathy | 14,149 (18.8) | 7,677 (13.2) | 22,773 (24.8) | <0.001 |
| Neuropathy | 19,510 (25.9) | 12,457 (21.4) | 22,786 (24.8) | <0.001 |
| Peripheral vascular disease | 9,462 (12.5) | 6,415 (11.0) | 14,613 (15.9) | <0.001 |
| Dementia | 1,137 (1.5) | 530 (0.9) | 4,109 (4.5) | <0.001 |
| MI | 2,445 (3.2) | 2,277 (3.9) | 4,327 (4.7) | <0.001 |
| Heart failure | 6,453 (8.6) | 4,210 (7.2) | 11,383 (12.4) | <0.001 |
| Cerebrovascular disease | 5,829 (7.7) | 4,360 (7.5) | 11,291 (12.3) | <0.001 |
| Chronic obstructive pulmonary disease | 9,102 (12.1) | 6,052 (10.4) | 13,345 (14.5) | <0.001 |
| Cancer | 4,546 (6.0) | 3,506 (6.0) | 8,087 (8.8) | <0.001 |
| Cirrhosis | 692 (0.9) | 495 (0.9) | 1,108 (1.2) | <0.001 |
| Prior severe hyperglycemia | 449 (0.6) | 230 (0.4) | 587 (0.6) | <0.001 |
| Prior severe hypoglycemia | 545 (0.7) | 245 (0.4) | 1,208 (1.3) | <0.001 |
| Prescriber specialty, n (%) | <0.001 | |||
| Primary care | 38,021 (50.4) | 34,208 (58.7) | 56,152 (61.1) | |
| Endocrinology | 11,267 (14.9) | 6,268 (10.8) | 5,554 (6.0) | |
| Cardiology | 272 (0.4) | 842 (1.4) | 703 (0.8) | |
| Nephrology | 160 (0.2) | 131 (0.2) | 485 (0.5) | |
| Other | 11,339 (15.0) | 7,361 (12.6) | 7,807 (8.5) | |
| Unknown | 14,336 (19.0) | 9,424 (16.2) | 21,183 (23.1) | |
| HbA1c available within prior 6 months | 27,843 (36.9) | 24,189 (41.5) | 37,022 (40.3) | <0.001 |
| HbA1c level, %, median (IQR)* | 8.1 (6.7, 9.7) | 8.3 (7.3, 9.7) | 8.0 (7.1, 9.3) | <0.001 |
| HbA1c category, n (%)* | <0.001 | |||
| ≤5.6% | 1,931 (6.9) | 299 (1.2) | 588 (1.6) | |
| 5.7–6.4% | 3,936 (14.1) | 1,760 (7.3) | 3,424 (9.2) | |
| 6.5–6.9% | 2,249 (8.1) | 2,068 (8.5) | 3,806 (10.3) | |
| 7.0–7.9% | 4,890 (17.6) | 6,075 (25.1) | 10,247 (27.7) | |
| 8.0–8.9% | 4,998 (18.0) | 5,238 (21.7) | 7,956 (21.5) | |
| 9.0–9.9% | 3,705 (13.3) | 3,357 (13.9) | 4,502 (12.2) | |
| ≥10.0% | 6,134 (22.0) | 5,392 (22.3) | 6,499 (17.6) |
Baseline characteristics of adults with type 2 diabetes at the time of their first prescription fill of a GLP-1RA, SGLT2i, or DPP-4i.
The denominator for HbA1c values are patients with baseline HbA1c data available.
Although traditionally recommended as second-line therapies, GLP-1RA, SGLT2i, and DPP-4i were used as first-line drugs by 21.4%, 13.6%, and 16.3% of patients being started on these respective drug classes. Most of the patients starting these drugs were treated with metformin at the time of initiation: 52.3%, 63.6%, and 56.6% of GLP-1 RA, SGLT2i, and DPP-4i, respectively. Baseline sulfonylurea use was the second-most common, with 23.2%, 29.7%, and 33.2% patients treated with sulfonylurea drugs at the time of GLP-1 RA, SGLT2i, and DPP-4i initiation, respectively.
Choice of Glucose-Lowering Pharmacotherapy
After adjusting for demographic and clinical covariates, we found patients with a history of MI were significantly less likely to start a GLP-1RA (RRR 0.83; 95% CI 0.78–0.88) than a DPP-4i, as were patients with cerebrovascular disease (RRR 0.77; 95% CI 0.74–0.81) (Table 2). Patients with nephropathy were less likely to start both GLP-1RA (RRR 87; 95% CI 0.84–0.91) and SGLT2i (RRR 0.57; 95% CI 0.55–0.60) compared with DPP-4i. Finally, patients with HF were less likely to start a SGLT2i (RRR 0.83; 95% CI 0.80–0.87) than a DPP-4i.
Table 2.
Demographic and clinical factors associated with starting GLP-1RA and SGLT2i therapy compared with DPP-4i therapy
| GLP-1RA vs. DPP-4i | SGLT2i vs. DPP-4i | |||
|---|---|---|---|---|
| RRR (95% CI) | P value | RRR (95% CI) | P value | |
| Age-group | ||||
| 18–44 years | Reference | — | Reference | — |
| 45–64 years | 0.59 (0.57–0.61) | <0.001 | 0.88 (0.85–0.92) | <0.001 |
| 65–74 years | 0.38 (0.36–0.40) | <0.001 | 0.73 (0.69–0.76) | <0.001 |
| ≥75 years | 0.18 (0.17–0.19) | <0.001 | 0.42 (0.39–0.44) | <0.001 |
| Sex | ||||
| Male | Reference | — | Reference | — |
| Female | 1.49 (1.45–1.52) | <0.001 | 0.85 (0.83–0.87) | <0.001 |
| Race/ethnicity | ||||
| White | Reference | — | Reference | — |
| Black | 0.71 (0.69–0.73) | <0.001 | 0.76 (0.74–0.79) | <0.001 |
| Hispanic | 0.64 (0.62–0.66) | <0.001 | 0.81 (0.79–0.84) | <0.001 |
| Asian | 0.39 (0.36–0.41) | <0.001 | 0.72 (0.68–0.76) | <0.001 |
| Unknown | 0.87 (0.83–0.91) | <0.001 | 0.90 (0.86–0.95) | <0.001 |
| U.S. region | ||||
| Midwest | Reference | — | Reference | — |
| Northeast | 0.82 (0.79–0.86) | <0.001 | 0.84 (0.81–0.88) | <0.001 |
| South | 1.02 (1.00–1.05) | 0.11 | 1.09 (1.06–1.12) | <0.001 |
| West | 1.08 (1.03–1.12) | <0.001 | 1.05 (1.01–1.10) | 0.02 |
| Insurance type | ||||
| Commercial | Reference | — | Reference | — |
| Medicare Advantage | 0.71 (0.69–0.74) | <0.001 | 0.61 (0.59–0.64) | <0.001 |
| Index year | ||||
| 2016 | Reference | — | Reference | — |
| 2017 | 1.38 (1.33–1.42) | <0.001 | 1.24 (1.20–1.28) | <0.001 |
| 2018 | 1.97 (1.91–2.04) | <0.001 | 1.40 (1.35–1.44) | <0.001 |
| 2019 | 2.90 (2.81–2.99) | <0.001 | 2.17 (2.10–2.24) | <0.001 |
| Baseline medication fills | ||||
| None | 1.56 (1.50–1.62) | <0.001 | 0.99 (0.95–1.03) | 0.64 |
| Metformin | 1.10 (1.07–1.14) | <0.001 | 1.22 (1.17–1.26) | <0.001 |
| Sulfonylureas | 0.94 (0.91–0.96) | <0.001 | 1.00 (0.97–1.02) | 0.84 |
| Thiazolidinediones | 1.51 (1.44–1.59) | <0.001 | 1.38 (1.31–1.45) | <0.001 |
| Basal insulin | 2.75 (2.66–2.84) | <0.001 | 1.50 (1.45–1.56) | <0.001 |
| Bolus insulin | 2.28 (2.18–2.38) | <0.001 | 1.71 (1.63–1.80) | <0.001 |
| Other medication(s) | 1.18 (0.96–1.46) | 0.12 | 0.93 (0.74–1.16) | 0.51 |
| Diabetes complications count | ||||
| 0 | Reference | — | Reference | — |
| 1 | 1.04 (1.00–1.08) | 0.03 | 1.17 (1.13–1.21) | <0.001 |
| 2 | 1.11 (1.05–1.18) | <0.001 | 1.27 (1.19–1.34) | <0.001 |
| 3 | 1.21 (1.12–1.32) | <0.001 | 1.38 (1.26–1.51) | <0.001 |
| ≥4 | 1.30 (1.16–1.47) | <0.001 | 1.47 (1.29–1.66) | <0.001 |
| Comorbidities | ||||
| MI | 0.83 (0.78–0.88) | <0.001 | 1.05 (0.99–1.11) | 0.09 |
| Cerebrovascular disease | 0.77 (0.74–0.81) | <0.001 | 0.82 (0.78–0.86) | <0.001 |
| Heart failure | 0.86 (0.82–0.89) | <0.001 | 0.83 (0.80–0.87) | <0.001 |
| Nephropathy | 0.87 (0.84–0.91) | <0.001 | 0.57 (0.55–0.60) | <0.001 |
| Retinopathy | 0.99 (0.95–1.03) | 0.66 | 0.90 (0.86–0.94) | <0.001 |
| Neuropathy | 1.12 (1.08–1.17) | <0.001 | 0.95 (0.91–0.99) | 0.008 |
| Peripheral vascular disease | 0.89 (0.85–0.93) | <0.001 | 0.84 (0.80–0.88) | <0.001 |
| Prior severe hyperglycemia | 0.45 (0.39–0.52) | <0.001 | 0.50 (0.42–0.59) | <0.001 |
| Prior severe hypoglycemia | 0.62 (0.55–0.69) | <0.001 | 0.55 (0.47–0.63) | <0.001 |
| Dementia | 0.58 (0.54–0.62) | <0.001 | 0.41 (0.37–0.45) | <0.001 |
| Chronic obstructive pulmonary disease | 0.97 (0.94–1.01) | 0.12 | 0.92 (0.89–0.95) | <0.001 |
| Cancer | 0.88 (0.84–0.92) | <0.001 | 0.88 (0.84–0.92) | <0.001 |
| Cirrhosis | 0.73 (0.65–0.81) | <0.001 | 0.79 (0.71–0.89) | <0.001 |
| Prescriber specialty | ||||
| Endocrinology | Reference | — | Reference | — |
| Primary Care | 0.43 (0.41–0.44) | <0.001 | 0.60 (0.57–0.62) | <0.001 |
| Cardiology | 0.37 (0.31–0.43) | <0.001 | 1.65 (1.47–1.85) | <0.001 |
| Nephrology | 0.27 (0.22–0.32) | <0.001 | 0.42 (0.34–0.51) | <0.001 |
| Other | 0.58 (0.55–0.61) | <0.001 | 0.65 (0.61–0.68) | <0.001 |
| Unknown | 0.48 (0.46–0.50) | <0.001 | 0.58 (0.55–0.61) | <0.001 |
Multinomial logistic regression examined the RRR of GLP-1RA and SGLT2i compared with DPP-4i initiation, after adjusting for the other variables shown.
Indeed, patients with any of the examined conditions were less likely to start GLP-1RA or SGLT2i as opposed to DPP-4i, with the exception of patients with neuropathy who were more likely to start a GLP-1RA than DPP-4i.
Primary care providers were significantly less likely than endocrinologists to initiate GLP-1RA (RRR 0.43; 95% CI 0.41–0.44) or SGLT2i (RRR 0.60; 95% CI 0.57–0.62) rather than DPP-4i (Table 2). Nephrologists were also less likely to initiate GLP-1RA (RRR 0.27; 95% CI 0.22–0.32) or SGLT2i (RRR 0.42; 95% CI 0.34–0.51) rather than DPP-4i. Cardiologists, however, were more likely to initiate SGLT2i (RRR 1.65; 95% CI 1.47–1.85), although still less likely to initiate GLP-1RA (RRR 0.37; 95% CI 0.31–0.43).
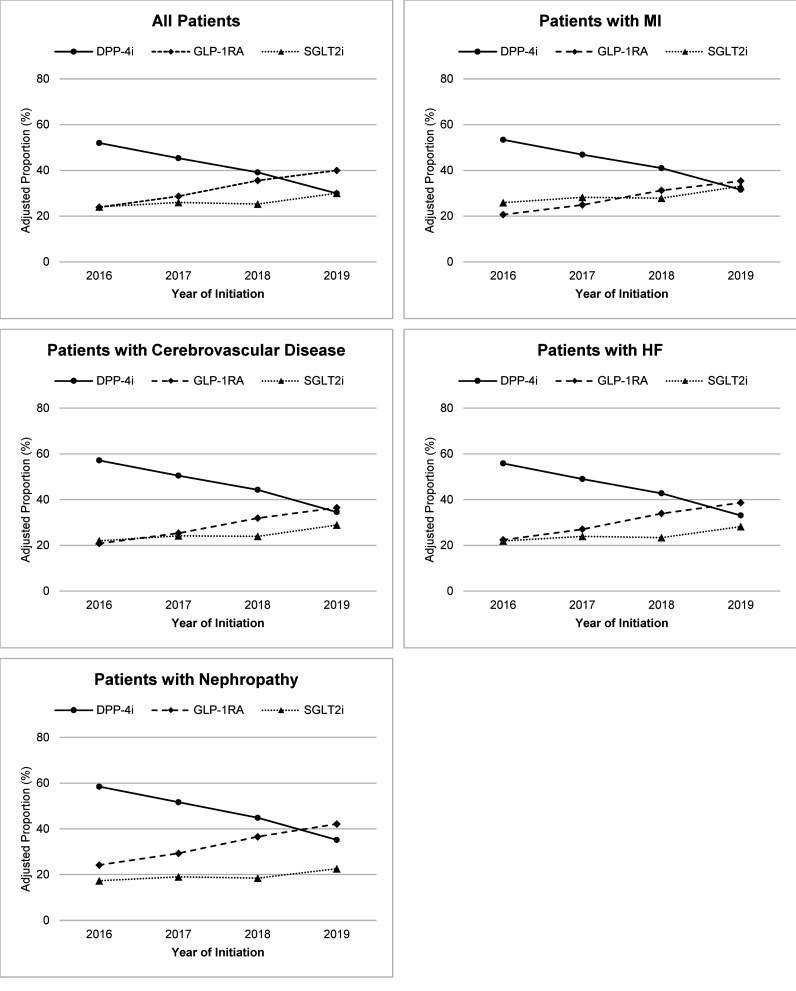
The RRR of starting either a GLP-1RA or a SGLT2i as opposed to a DPP-4i increased over time (Table 2). In 2016, DPP-4i were started more frequently than GLP-1RA or SGLT2i in the overall study population as well in subgroups of patients with MI, cerebrovascular disease, HF, and nephropathy (Fig. 1; Supplementary Table 3). By 2019, the adjusted proportion of patients starting GLP-1RA exceeded the proportion starting SGLT2i or DPP-4i in the overall population and in all of the comorbidity subgroups. However, SGLT2i were started less frequently than DPP-4i by patients with HF and nephropathy even in 2019. We further conducted a sensitivity analysis that considered the interaction between calendar year of medication initiation and the comorbidities calling for preferential use of GLP-1RA and/or SGLT2i as opposed to DPP-4i (Supplementary Table 4). We found no consistently significant interaction between year and GLP-1RA initiation for any of the examined comorbidities. The relative risks of SGLT2i initiation among patients with MI and HF, but not cerebrovascular disease or nephropathy, did become more likely over time.
Figure 1.
Adjusted proportions of patients initiating GLP-1RA, SGLT2i, and DPP-4i therapy between 2016 and 2019.
Demographic Differences in the Choice of Glucose-Lowering Pharmacotherapy
The relative risks of starting a GLP-1RA or a SGLT2i, as opposed to DPP-4i, decreased progressively with age (Table 2). This was most apparent for GLP-1RA initiation, where compared with patients 18–44 years the RRR of GLP-1RA versus DPP-4i initiation were 41%, 62%, and 72% lower among patients 45–64, 65–74, and ≥75 years old. All non-White racial/ethnic groups were less likely to start a GLP-1RA or SGLT2i, as opposed to a DPP-4i, than White patients. Women were more likely to start a GLP-1RA (RRR 1.49; 95% CI 1.45–1.52) than men, but were less likely to start a SGTL2i (RRR 0.85; 95% CI 0.83–0.87). Finally, enrollees in Medicare Advantage plans were less likely to start a GLP-RA (RRR 0.71; 95% CI 0.69–0.74) or a SGLT2i (RRR 0.61; 95% CI 0.59–0.64), as opposed to a DPP-4i, compared with enrollees in a private health plan.
Impact of Glycemic Control on Medication Choice (Subgroup Analysis)
HbA1c test result data were available for ∼40% of the study population (Table 1). Median HbA1c was 8.1% (IQR 6.7–9.7) among patients started on GLP-1RA, 8.3% (IQR 7.3–9.7) among patients starting SGLT2i, and 8.0% (IQR 7.1–9.3) among patients starting DPP-4i. Baseline characteristics of patients in the HbA1c subset (Supplementary Table 5) and associations between each of the baseline variables and the relative risk of GLP-1RA and SGLT2i initiation (Supplementary Table 6) were similar to the overall study population. The RRR of GLP-1RA initiation, as opposed to DPP-4i initiation, were greater among patients with lower HbA1c levels (RRR 3.62 [95% CI, 3.23–4.07] for HbA1c ≤5.6% and RRR 1.81 [95% CI, 1.68–1.96] for HbA1c 5.7–6.4% vs. 6.5–6.9%), lower among patients with moderately elevated HbA1c (RRR 0.76 [95% CI, 0.71–0.81] for HbA1c 7.0–7.9% and RRR 0.92 [95% CI, 0.86–0.99] for HbA1c 8.0–8.9%), and similar for high HbA1c (i.e., ≥9.0%) compared with HbA1c 6.5–6.9%. In contrast, the relative risks of SGLT2i initiation were lower at low HbA1c (RRR 0.75 [95% CI, 0.64–0.88] for HbA1c ≤5.6% and RRR 0.91 [95% CI, 0.84–0.98] for HbA1c 5.7–6.4%), similar at moderate elevated HbA1c, and higher for high HbA1c (RRR 1.14 [95% CI, 1.06–1.23] for HbA1c 9.0–9.9% and RRR 1.13 [95% CI, 1.06–1.21] for HbA1c ≥10%) compared with HbA1c 6.5–6.9%.
Conclusions
High-quality, patient-centered diabetes care is predicated on treating each patient with the drugs that are most likely to benefit and least likely to harm them. Yet, in our study population, patients more likely to benefit from GLP-1RA and/or SGLT2i drug classes were less likely to start them. For example, patients with cardiovascular disease (i.e., history of MI or cerebrovascular disease) and nephropathy were less likely to start a GLP-1RA, while patients with HF and nephropathy were less likely to start a SGLT2i. This treatment/benefit paradox, whereby patients most likely to benefit from a particular drug are not prescribed it, represents an important opportunity to optimize glucose-lowering treatment regimens and improve health outcomes among highest-risk patients with type 2 diabetes.
Several factors may contribute to the underuse of GLP-1RA and SGLT2i relative to DPP-4i by patients with evidence-based indications for their use. Clinician familiarity and comfort with using medications is a strong determinant of their use (25–29). Some clinicians may not be aware of the nonglycemic benefits of GLP-1RA and SGLT2i, and as such preferentially prescribe these newer, costly medications in situations where more intensive management is warranted (i.e., in younger patients and those with less comorbidity). Clinicians may also hesitate to prescribe drugs with which they have less experience to patients with serious health conditions whom they may perceive to be at greater risk for adverse drug reactions or for deterioration in health status as a result of a medication change.
In addition to gaps in GLP-1RA and SGLT2i initiation by patients with clinical indications for their use, we found disparities as a function of race/ethnicity, sex, and age. Compared with White patients, Black, Hispanic, and Asian patients were all less likely to start both GLP-1RA and SGLT2i. Several prior studies demonstrated lower rates of new drug use by Black patients (22,30,31), although until now there were insufficient data in other racial/ethnic groups, and this may contribute to worse diabetes health outcomes in minority populations (32). Older adults were significantly less likely to start both GLP-1RA and SGLT2i compared with younger patients, as were patients with Medicare Advantage as opposed to private health insurance coverage. Indeed, an earlier study comparing patterns of glucose-lowering medication use among older adults with Medicare Advantage and private health plans found Medicare Advantage beneficiaries were less likely to be treated with GLP-1RA or SGLT2i but were more likely to be treated with DPP-4i than similarly aged beneficiaries of private health plans despite similar formulary designs (33). Women were less likely than men to start SGLT2i but more likely to start GLP-1RA. This may reflect women’s concerns about the adverse effect profiles of these drugs, including urinary tract infections with SGLT2i (deterring use) and weight loss with GLP-1RA (favoring use). However, more nuanced and complete understanding of factors driving the observed differences in medication use would require individual engagement and qualitative exploration of clinicians’ and patients’ attitudes and beliefs regarding glucose-lowering medications.
We were surprised to find how frequently all three medications were used as first-line agents, even though they are generally recommended for use as second-line drugs in addition to metformin (9). Overall, 21.4% of GLP-1RA initiators, 13.6% of SGT2i initiators, and 16.3% of DPP-4i initiators had no fills for another glucose-lowering drug in the year prior to starting one of these drugs. Treatment-naïve patients were more likely to start a GLP-1RA than a DPP-4i, potentially reflecting GLP-1RA’s secondary indications for weight loss. We could not verify this in our data, because biometric (i.e., BMI) data are not available in the OLDW claims database. We also did not examine whether first-line initiation of these drugs occurred among patients with clinical indications for them (e.g., SGLT2i among patients with HF, GLP-1RA among patients with cardiovascular disease), but patients with these comorbidities were, overall, less likely to be prescribed the preferred drugs. Moreover, our earlier study assessing SGLT2i adoption found that SGLT2i initiation as a first-line drug was less, not more, likely in patients with underlying HF (22).
Our findings reinforce the need for care delivery models that better support evidence-based diabetes management and use of GLP-1RA and SGLT2i drugs. This is particularly important for primary care clinicians who initiated glucose-lowering medications for the majority of patients. Other specialists may also help support evidence-based prescribing of glucose-lowering therapy. Current guidelines for cardiovascular disease and HF management in patients with type 2 diabetes recommend preferential use of GLP-1RA and SGLT2i in patients with or at high risk for cardiovascular disease and HF, respectively, even with mild hyperglycemia and as first-line therapy (34–37). In our study, cardiologists were more likely to prescribe SGLT2i than any other specialty, but primary care clinicians and nephrologists were less likely to prescribe either GLP-1RA or SGLT2i than endocrinologists.
This is the first study, to our knowledge, to examine contemporary trends in glucose-lowering medication use in the era following cardiovascular outcomes trials, focusing specifically on whether drug choice in patients with type 2 diabetes was optimized to ensure greatest benefit. It does, however, have important limitations. The study population consisted of commercial and Medicare Advantage beneficiaries, and medication use patterns likely differ among patients without insurance, with public or other private health plans, or outside the U.S. Our data captured medications filled through the health benefit, but medications obtained through low-cost generic drug programs (38,39), which do not include GLP-1RA, SGLT2i, or DPP4i, or those obtained as samples, cannot be captured. This may have resulted in an overestimate of patients starting all three classes of medications as first-line therapy, although this would not explain the greater proportion of presumably treatment-naïve patients starting GLP-1RA as opposed to DPP-4i. Finally, we could not assess other factors that may influence the choice of glucose-lowering therapy, including patient interest and acceptance, as these considerations cannot be captured by administrative data.
In conclusion, our findings reveal an important treatment/benefit paradox, whereby patients most likely to benefit from specific classes of glucose-lowering medications are less likely to receive them. Initiation of clinically preferred medications (i.e., GLP-1RA and SGLT2i) was most reduced among older and non-White patients, reinforcing the disparities seen in diabetes management and potentially contributing to poor health outcomes in these populations. Patient and clinician education regarding individualized approaches to diabetes management, care delivery models that support shared decision making (i.e., point-of-care clinical decision support and decision aides), and health plan reimbursement for evidence-based treatment strategies, may therefore help improve access to new diabetes therapeutics, reduce disparities, and improve the health outcomes for patients living with diabetes.
Article Information
Funding. This effort was funded by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK114497 (R.G.M) and Agency for Healthcare Research and Quality’s (AHRQ) Comparative Health System Performance Initiative grants 1U19HS024075 (N.D.S.) and R01HS025164 (P.K.-M., N.D.S.). In the past 36 months, R.G.M. received research support through NIDDK (R03DK114497, P30DK111024) and AARP (Quality Measure Innovation Grant). In the past 36 months, N.D.S. received research support through Mayo Clinic from the U.S. Food and Drug Administration (FDA) to establish the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), the Centers of Medicare and Medicaid Innovation under the Transforming Clinical Practice Initiative (TCPI), AHRQ (R01HS025402, R03HS025517), the National Heart, Lung and Blood Institute (NHLBI) of the NIH (R56HL130496, R01HL131535), the National Science Foundation, and the Patient Centered Outcomes Research Institute (PCORI) to develop a Clinical Data Research Network (LHSNet). In the past 36 months, S.J.R. has received research support from the U.S. FDA to develop methods for postmarket surveillance of medical devices (U01FD004585), FDA to establish the Yale-Mayo Clinic CERSI program (U01FD005938), from AHRQ (R01HS022882), NHLBI (R01HS025164), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International. P.K.-M. serves as the principal investigator to several grants on prescription drug studies from NIH National Institute on Aging (P01AG005842), NHLBI (R56HL130496), and the American Cancer Society (131611-RSGI-17-154-01-CPHPS).
Study contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH.
Duality of Interest. In the past 36 months, J.S.R. has received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing and from Medtronic, Inc. P.K.-M. reports receiving consulting fees unrelated to this work from Tactile Medical, Sempre Health, and Precision Health Economics. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.G.M. designed the study, interpreted the data, and wrote the manuscript. H.K.V.H. analyzed the data and reviewed and edited the manuscript. P.K.-M., J.S.R., and V.M.M. contributed to the discussion and reviewed and edited the manuscript. N.D.S. supervised study design and data interpretation, contributed to the discussion, and reviewed and edited the manuscript. R.G.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Virtual Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14847717.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, Georgia, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2020 [Google Scholar]
- 2. Centers for Disease Control and Prevention . Diabetes Data & Statistics. Diabetes Atlas. Division of Diabetes Translation, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Accessed 21 July 2020. Available from https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html#
- 3. Baena-Díez JM, Peñafiel J, Subirana I, et al.; FRESCO Investigators . Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care 2016;39:1987–1995 [DOI] [PubMed] [Google Scholar]
- 4. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al.; Emerging Risk Factors Collaboration . Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tancredi M, Rosengren A, Svensson A-M, et al. Excess mortality among persons with Type 2 diabetes. N Engl J Med 2015;373:1720–1732 [DOI] [PubMed] [Google Scholar]
- 6. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S64–S74 [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S98–S110 [DOI] [PubMed] [Google Scholar]
- 10. National Institutes for Health and Care Excellence . NICE Pathways: Managing blood glucose in adults with type 2 diabetes. Published 2019. Updated March 26, 2019. Accessed 23 April 2019. Available from https://pathways.nice.org.uk/pathways/type-2-diabetes-in-adults
- 11. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2019 Executive Summary. Endocr Pract 2019;25:69–100 [DOI] [PubMed] [Google Scholar]
- 12. Conlin PR, Colburn J, Aron D, Pries RM, Tschanz MP, Pogach L. Synopsis of the 2017 U.S. Department of Veterans Affairs/U.S. Department of Defense Clinical Practice Guideline: management of type 2 diabetes mellitus. Ann Intern Med 2017;167:655–663 [DOI] [PubMed] [Google Scholar]
- 13. U.S. Food and Drug Administration . Guidance for industry. Diabetes Mellitus—Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008. FDA-2008-D-0118-0029. Accessed 20 July 2021. Available from https://www.regulations.gov/document/FDA-2008-D-0118-0029
- 14. Ismail-Beigi F, Moghissi E, Kosiborod M, Inzucchi SE. Shifting paradigms in the medical management of type 2 diabetes: reflections on recent cardiovascular outcome trials. J Gen Intern Med 2017;32:1044–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chilton RJ, Dungan KM, Shubrook JH, Umpierrez GE. Cardiovascular risk and the implications for clinical practice of cardiovascular outcome trials in type 2 diabetes. Prim Care Diabetes 2020;14:193–212 [DOI] [PubMed] [Google Scholar]
- 16. Karter AJ, Lipska KJ, O’Connor PJ, et al.; SUPREME-DM Study Group . High rates of severe hypoglycemia among African American patients with diabetes: the Surveillance, and Management of Diabetes Mellitus (SUPREME-DM) network. J Diabetes Complications 2017;31:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang MH, Moonesinghe R, Athar HM, Truman BI. Trends in disparity by sex and race/ethnicity for the leading causes of death in the United States-1999-2010. J Public Health Manag Pract 2016;22(Suppl. 1):S13–S24 [DOI] [PubMed] [Google Scholar]
- 18. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016;37:278–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prospective Studies Collaboration and Asia Pacific Cohort Studies Collaboration . Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol 2018;6:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–1194 [DOI] [PubMed] [Google Scholar]
- 21. OptumLabs . OptumLabs and OptumLabs Data Warehouse (OLDW). Cambridge, MA: May 2019 [Google Scholar]
- 22. McCoy RG, Dykhoff HJ, Sangaralingham L, et al. Adoption of new glucose-lowering medications in the U.S. - the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther 2019;21:702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Paradox of glycemic management: multimorbidity, glycemic control, and high-risk medication use among adults with diabetes. BMJ Open Diabetes Res Care 2020;8:e001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care 2012;18:721–726 [PubMed] [Google Scholar]
- 25. Tamblyn R, McLeod P, Hanley JA, Girard N, Hurley J. Physician and practice characteristics associated with the early utilization of new prescription drugs [erratum appears in Med Care 2003;41:1117]. Med Care 2003;41:895–908 [DOI] [PubMed] [Google Scholar]
- 26. Helin-Salmivaara A, Huupponen R, Virtanen A, Klaukka T. Adoption of celecoxib and rofecoxib: a nationwide database study. J Clin Pharm Ther 2005;30:145–152 [DOI] [PubMed] [Google Scholar]
- 27. Schneeweiss S, Glynn RJ, Avorn J, Solomon DH. A Medicare database review found that physician preferences increasingly outweighed patient characteristics as determinants of first-time prescriptions for COX-2 inhibitors. J Clin Epidemiol 2005;58:98–102 [DOI] [PubMed] [Google Scholar]
- 28. Garjón FJ, Azparren A, Vergara I, Azaola B, Loayssa JR. Adoption of new drugs by physicians: a survival analysis. BMC Health Serv Res 2012;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lo-Ciganic W-H, Gellad WF, Huskamp HA, et al. Who were the early adopters of dabigatran?: An application of group-based trajectory models. Med Care 2016;54:725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Zuckerman IH, Miller NA, Shaya FT, Noel JM, Mullins CD. Utilizing new prescription drugs: disparities among non-Hispanic whites, non-Hispanic blacks, and Hispanic whites. Health Serv Res 2007;42:1499–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jung J, Feldman R. Racial-ethnic disparities in uptake of new hepatitis C drugs in Medicare. J Racial Ethn Health Disparities 2017;4:1147–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2017. Atlanta, GA, Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017 [Google Scholar]
- 33. McCoy RG, Van Houten HK, Deng Y, et al. Comparison of diabetes medications used by adults with commercial insurance vs Medicare Advantage, 2016 to 2019. JAMA Netw Open 2021;4:e2035792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:e177–e232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Das SR, Everett BM, Birtcher KK, et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:1117–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunlay SM, Givertz MM, Aguilar D, et al.; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and the Heart Failure Society of America . Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019;140:e294–e324 [DOI] [PubMed] [Google Scholar]
- 37. Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 [DOI] [PubMed] [Google Scholar]
- 38. Pauly NJ, Talbert JC, Brown J. Low-cost generic program use by Medicare Beneficiaries: implications for Medication exposure misclassification in administrative claims data. J Manag Care Spec Pharm 2016;22:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pauly NJ, Brown JD. Prevalence of low-cost generic program use in a nationally representative cohort of privately insured adults. J Manag Care Spec Pharm 2015;21:1162–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]