Abstract
A dissertation is a practical exercise that educates students about basics of research methodology, promotes scientific writing and encourages critical thinking. The National Medical Commission (India) regulations make assessment of a dissertation by a minimum of three examiners mandatory. The candidate can appear for the final examination only after acceptance of the dissertation. An important role in a dissertation is that of the guide who has to guide his protégés through the process. This manuscript aims to assist students and guides on the basics of conduct of a dissertation and writing the dissertation. For students who will ultimately become researchers, a dissertation serves as an early exercise. Even for people who may never do research after their degree, a dissertation will help them discern the merits of new treatment options available in literature for the benefit of their patients.
Keywords: Academic dissertations as topic, ethics committee, registries, research design, students, writing
INTRODUCTION
The zenith of clinical residency is the completion of the Master's Dissertation, a document formulating the result of research conducted by the student under the guidance of a guide and presenting and publishing the research work. Writing a proper dissertation is most important to present the research findings in an acceptable format. It is also reviewed by the examiners to determine a part of the criteria for the candidate to pass the Masters’ Degree Examination.
The predominant role in a dissertation is that of the guide who has to mentor his protégés through the process by educating them on research methodology, by: (i) identifying a pertinent and topical research question, (ii) formulating the “type” of study and the study design, (iii) selecting the sample population, (iv) collecting and collating the research data accurately, (v) analysing the data, (vi) concluding the research by distilling the outcome, and last but not the least (vii) make the findings known by publication in an acceptable, peer-reviewed journal.[1] The co-guide could be a co-investigator from another department related to the study topic, and she/he will play an equivalent role in guiding the student.
Research is a creative and systematic work undertaken to increase the stock of knowledge.[2] This work, known as a study may be broadly classified into two groups in a clinical setting:
Trials: Here the researcher intervenes to either prevent a disease or to treat it.
Observational studies: Wherein the investigator makes no active intervention and merely observes the patients or subjects allocated the treatment based on clinical decisions.[3]
The research which is described in a dissertation needs to be presented under the following headings: Introduction, Aim of the Study, Description of devices if any or pharmacology of drugs, Review of Literature, Material and Methods, Observations and Results, Discussion, Conclusions, Limitations of the study, Bibliography, Proforma, Master chart. Some necessary certificates from the guide and the institute are a requirement in certain universities. The students often add an acknowledgement page before the details of their dissertation proper. It is their expression of gratitude to all of those who they feel have been directly or indirectly helpful in conduct of the study, data analysis, and finally construction of the dissertation.
Framing the research question (RQ)
It is the duty of the teacher to suggest suitable research topics to the residents, based on resources available, feasibility and ease of conduct at the centre. Using the FINER criteria, the acronym for feasibility, topical interest, novelty, ethicality and relevance would be an excellent way to create a correct RQ.[4]
The PICOT method which describes the patient, intervention, comparison, outcome and time, would help us narrow down to a specific and well-formulated RQ.[5,6] A good RQ leads to the derivation of a research hypothesis, which is an assumption or prediction of the outcome that will be tested by the research. The research topic could be chosen from among the routine clinical work regarding clinical management, use of drugs e.g., vasopressors to prevent hypotension or equipment such as high flow nasal oxygen to avoid ventilation.
Review of literature
To gather this information may be a difficult task for a fresh trainee however, a good review of the available literature is a tool to identify and narrow down a good RQ and generate a hypothesis. Literature sources could be primary (clinical trials, case reports), secondary (reviews, meta-analyses) or tertiary (e.g., reference books, compilations). Methods of searching literature could be manual (journals) or electronic (online databases), by looking up references or listed citations in existing articles. Electronic database searches are made through the various search engines available online e.g., scholar.google.com, National Library of Medicine (NLM) website, clinical key app and many more. Advanced searches options may help narrow down the search results to those that are relevant for the student. This could be based on synthesising keywords from the RQ, or by searching for phrases, Boolean operators, or utilising filters.
After choosing the topic, an apt and accurate title has to be chosen. This should be guided by the use of Medical Subject Headings (MeSH) terminology from the NLM, which is used for indexing, cataloguing, and searching of biomedical and health-related information.[7] The dissertation requires a detailed title which may include the objective of the study, key words and even the PICOT components. One may add the study design in the title e.g. “a randomised cross over study” or “an observational analytical study” etc.
Aim and the objectives
The Aims and the Objectives of the research study have to be listed clearly, before initiating the study.[8] “Gaps” or deficiencies in existing knowledge should be clearly cited. The Aim by definition is a statement of the expected outcome, while the Objectives (which might be further classed into primary and secondary based on importance) should be specific, measurable, achievable, realistic or relevant, time-bound and challenging; in short, “SMART!” To simplify, the aim is a statement of intent, in terms of what we hope to achieve at the end of the project. Objectives are specific, positive statements of measurable outcomes, and are a list of steps that will be taken to achieve the outcome.[9] Aim of a dissertation, for example, could be to know which of two nerve block techniques is better. To realise this aim, comparing the duration of postoperative analgesia after administration of the block by any measurable criteria, could be an objective, such as the time to use of first rescue analgesic drug. Similarly, total postoperative analgesic drug consumption may form a secondary outcome variable as it is also measurable. These will generate data that may be used for analysis to realise the main aim of the study.
Inclusion and exclusions
The important aspect to consider after detailing when and how the objectives will be measured is documenting the eligibility criteria for inclusion of participants. The exclusion criteria must be from among the included population/patients only. e.g., If only American Society of Anesthesiologists (ASA) I and II are included, then ASA III and IV cannot be considered as exclusion criteria, since they were never a part of the study. The protocol must also delineate the setting of the study, locations where data would be collected, and specify duration of conduct of the dissertation. A written informed consent after explaining the aim, objectives and methodology of the study is legally mandatory before embarking upon any human study. The study should explicitly clarify whether it is a retrospective or a prospective study, where the study is conducted and the duration of the study.
Sample size: The sample subjects in the study should be representative of the population upon whom the inference has to be drawn. Sampling is the process of selecting a group of representative people from a larger population and subjecting them for the research.[10] The sample size represents a number, beyond which the addition of population is unlikely to change the conclusion of the study. The sample size is calculated taking into consideration the primary outcome criteria, confidence interval (CI), power of the study, and the effect size the researcher wishes to observe in the primary objective of the study. Hence a typical sample size statement can be - “Assuming a duration of analgesia of 150 min and standard deviation (SD) of 15 min in first group, keeping power at 80% and CIs at 95% (alpha error at 0.05), a sample of 26 patients would be required to detect a minimum difference (effect size) of 30% in the duration of analgesia between the two groups. Information regarding the different sampling methods and sample size calculations may be found in the Supplementary file 1 (248KB, pdf) .
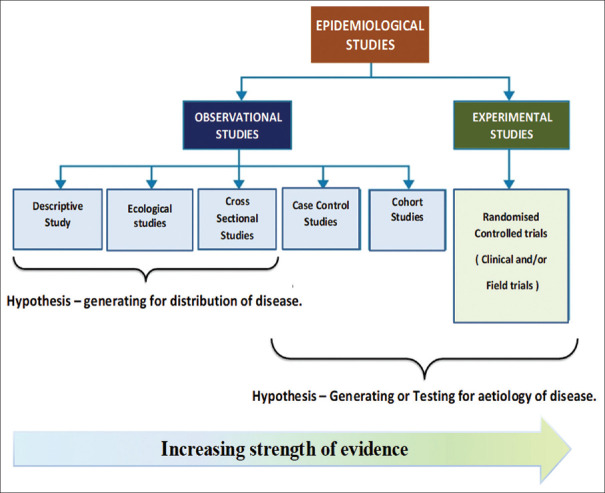
Any one research question may be answered using a number of research designs.[11] Research designs are often described as either observational or experimental. The various research designs may be depicted graphically as shown in Figure 1.
Figure 1.
Graphical description of available research designs
The observational studies lack “the three cornerstones of experimentation” – controls, randomisation, and replication. In an experimental study on the other hand, in order to assess the effect of treatment intervention on a participant, it is important to compare it with subjects similar to each other but who have not been given the studied treatment. This group, also called the control group, may help distinguish the effect of the chosen intervention on outcomes from effects caused by other factors, such as the natural history of disease, placebo effects, or observer or patient expectations.
All the proposed dissertations must be submitted to the scientific committee for any suggestion regarding the correct methodology to be followed, before seeking ethical committee approval.
Ethical considerations
Ethical concerns are an important part of the research project, right from selection of the topic to the dissertation writing. It must be remembered, that the purpose of a dissertation given to a post-graduate student is to guide him/her through the process by educating them on the very basics of research methodology. It is therefore not imperative that the protégés undertake a complicated or risky project. If research involves human or animal subjects, drugs or procedures, research ethics guidelines as well as drug control approvals have to be obtained before tabling the proposal to the Institutional Ethics Committee (IEC). The roles, responsibilities and composition of the Ethics Committee has been specified by the Directorate General of Health Services, Government of India. Documented approval of the Ethics committee is mandatory before any subject can be enroled for any dissertation in India. Even retrospective studies require approval from the IEC. Details of this document is available at: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadEthicsRegistration/Applmhrcrr.pdf.
The candidate and the guide are called to present their proposal before the committee. The ethical implications, risks and management, subjects’ rights and responsibilities, informed consent, monetary aspects, the research and analysis methods are all discussed. The patient safety is a topmost priority and any doubts of the ethical committee members should be explained in medically layman's terms. The dissertation topics should be listed as “Academic clinical trials” and must involve only those drugs which are already approved by the Drugs Controller General of India. More commonly, the Committee suggests rectifications, and then the researchers have to resubmit the modified proposal after incorporating the suggestions, at the next sitting of the committee or seek online approval, as required. At the conclusion of the research project, the ethics committee has to be updated with the findings and conclusions, as well as when it is submitted for publication. Any deviation from the approved timeline, as well as the research parameters has to be brought to the attention of the IEC immediately, and re-approval sought.
Clinical trial registration
Clinical Trial Registry of India (CTRI) is a free online searchable system for prospective registration of all clinical studies conducted in India. It is owned and managed by the National Institute of Medical Statistics, a division of Indian Council of Medical Research, Government of India. Registration of clinical trials will ensure transparency, accountability and accessibility of trials and their results to all potential beneficiaries.
After the dissertation proposal is passed by the scientific committee and IEC, it may be submitted for approval of trial registration to the CTRI. The student has to create a login at the CTRI website, and submit all the required data with the help of the guides. After submission, CTRI may ask for corrections, clarifications or changes. Subject enrolment and the actual trial should begin only after the CTRI approval.
Randomisation
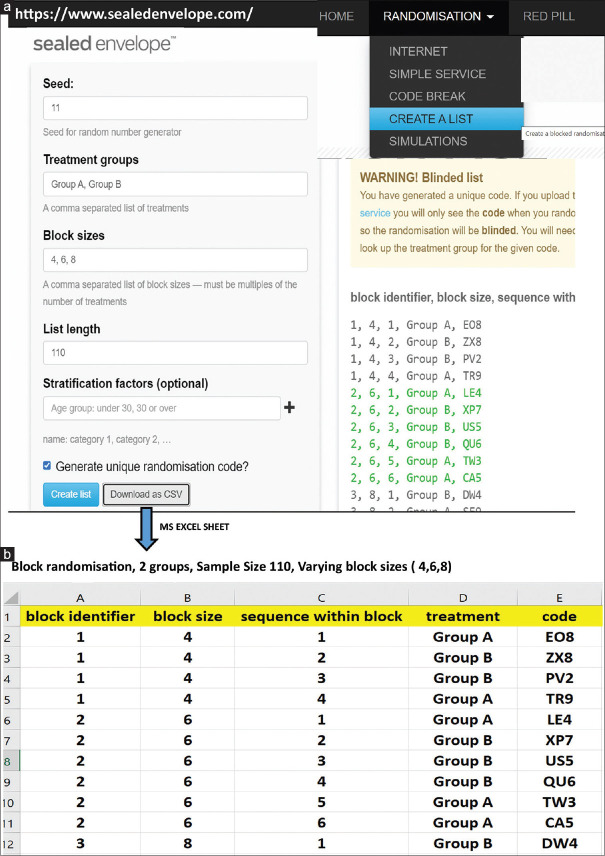
In an experimental study design, the method of randomisation gives every subject an equal chance to get selected in any group by preventing bias. Primarily, three basic types employed in post-graduate medical dissertations are simple randomisation, block randomisation and stratified randomisation. Simple randomisation is based upon a single sequence of random assignments such as flipping a coin, rolling of dice (above 3 or below 3), shuffling of cards (odd or even) to allocate into two groups. Some students use a random number table found in books or use computer-generated random numbers. There are many random number generators, randomisation programs as well as randomisation services available online too. (https://www-users.york.ac.uk/~mb55/guide/randsery.htm).
There are many applications which generate random number sequences and a research student may use such computer-generated random numbers [Figure 2]. Simple randomisation has higher chances of unequal distribution into the two groups, especially when sample sizes are low (<100) and thus block randomisation may be preferred. Details of how to do randomisation along with methods of allocation concealment may be found in Supplementary file 2 (390.5KB, pdf) .
Figure 2.
Figure depicting how to do block randomisation using online resources. (a) generation of a random list (b) transfer of the list to an MS excel file
Allocation concealment
If it is important in a study to generate a random sequence of intervention, it is also important for this sequence to be concealed from all stake-holders to prevent any scope of bias.[12] Allocation concealment refers to the technique used to implement a random sequence for allocation of intervention, and not to generate it.[13] In an Indian post-graduate dissertation, the sequentially numbered, opaque, sealed envelopes (SNOSE) technique is commonly used [Supplementary file 2 (390.5KB, pdf) ].
Blinding
To minimise the chances of differential treatment allocation or assessments of outcomes, it is important to blind as many individuals as possible in the trial. Blinding is not an all-or-none phenomenon. Thus, it is very desirable to explicitly state in the dissertation, which individuals were blinded, how they achieved blinding and whether they tested the success of blinding.
Commonly used terms for blinding are
Single blinding: Masks the participants from knowing which intervention has been given.
Double blinding: Blinds both the participants as well as researchers to the treatment allocation.
Triple blinding: By withholding allocation information from the subjects, researchers, as well as data analysts. The specific roles of researchers involved in randomisation, allocation concealment and blinding should be stated clearly in the dissertation.
Data which can be measured as numbers are called quantitative data [Table 1]. Studies which emphasise objective measurements to generate numerical data and then apply statistical and mathematical analysis constitute quantitative research. Qualitative research on the other hand focuses on understanding people's beliefs, experiences, attitudes, behaviours and thus these generate non-numerical data called qualitative data, also known as categorical data, descriptive data or frequency counts. Importance of differentiating data into qualitative and quantitative lies in the fact that statistical analysis as well as the graphical representation may be very different.
Table 1.
Data collection types
| Quantitative Data Collection | Qualitative Data Collection |
|---|---|
| 1. Experiments | 1. In-depth interviews |
| 2. Surveys | 2. Observation methods |
| 3. Interviews | 3. Document review |
| Telephone interviews | Focus groups |
| Face-to-face interviews | Longitudinal studies |
| Computer Assisted Personal Interview (CAPI) | Case studies |
| 4. Questionnaires | |
| Mail questionnaires | |
| Web-based questionnaires |
In order to obtain data from the outcome variable for the purpose of analysis, we need to design a study which would give us the most valid information. A valid data or measurement tool, is the degree to which the tool measures what it claims to measure. For example, appearance of end tidal carbon dioxide waveform is a more valid measurement to assess correct endotracheal tube placement than auscultation of breath sounds on chest inflation.
The compilation of all data in a ‘Master Chart’ is a necessary step for planning, facilitating and appropriate preparation and processing of the data for analysis. It is a complete set of raw research data arranged in a systematic manner forming a well-structured and formatted, computable data matrix/database of the research to facilitate data analysis. The master chart is prepared as a Microsoft Excel sheet with the appropriate number of columns depicting the variable parameters for each individual subjects/respondents enlisted in the rows.
Statistical analysis
The detailed statistical methodology applied to analyse the data must be stated in the text under the subheading of statistical analysis in the Methods section. The statistician should be involved in the study during the initial planning stage itself. Following four steps have to be addressed while planning, performing and text writing of the statistical analysis part in this section.
Step 1. How many study groups are present? Whether analysis is for an unpaired or paired situation? Whether the recorded data contains repeated measurements? Unpaired or paired situations decide again on the choice of a test. The latter describes before and after situations for collected data (e.g. Heart rate data ‘before’ and ‘after’ spinal anaesthesia for a single group). Further, data should be checked to find out whether they are from repeated measurements (e.g., Mean blood pressure at 0, 1st, 2nd, 5th, 10th minutes and so on) for a group. Different types of data are commonly encountered in a dissertation [Supplementary file 3A (381.5KB, pdf) ].
Step 2. Does the data follow a normal distribution?[14]
Each study group as well as every parameter has to be checked for distribution analysis. This step will confirm whether the data of a particular group is normally distributed (parametric data) or does not follow the normal distribution (non-parametric data); subsequent statistical test selection mainly depends on the results of the distribution analysis. For example, one may choose the Student's’ test instead of the ‘Mann-Whitney U’ for non-parametric data, which may be incorrect. Each study group as well as every parameter has to be checked for distribution analysis [Supplementary File 3B (381.5KB, pdf) ].
Step 3. Calculation of measures of central tendency and measures of variability.
Measures of central tendency mainly include mean, median and mode whereas measures of variability include range, interquartile range (IQR), SD or variance not standard error of mean. Depending on Step 2 findings, one needs to make the appropriate choice. Mean and SD/variance are more often for normally distributed and median with IQR are the best measure for not normal (skewed) distribution. Proportions are used to describe the data whenever the sample size is ≥100. For a small sample size, especially when it is approximately 25-30, describe the data as 5/25 instead of 20%. Software used for statistical analysis automatically calculates the listed step 3 measures and thus makes the job easy.
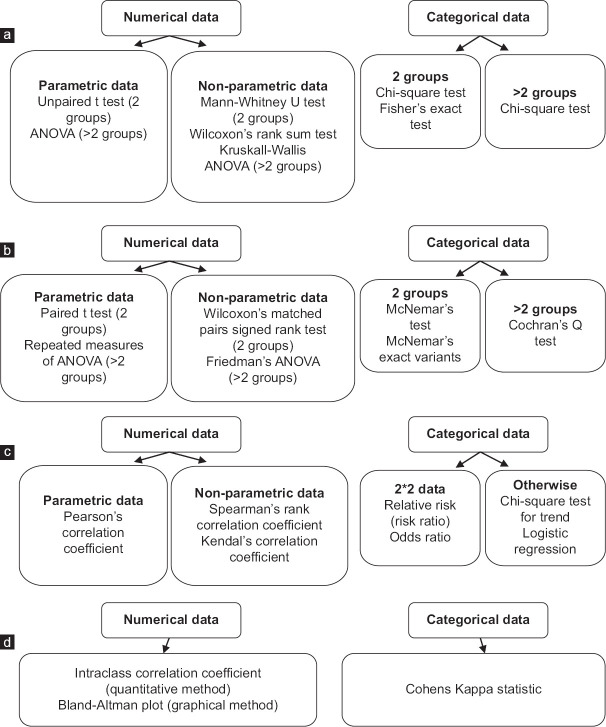
Step 4. Which statistical test do I choose for necessary analysis?
Choosing a particular test [Figure 3] is based on orderly placed questions which are addressed in the dissertation.[15]
Figure 3.
Chosing a statistical test, (a). to find a difference between the groups of unpaired situations, (b). to find a difference between the groups of paired situations, (c). to find any association between the variables, (d). to find any agreement between the assessment techniques. ANOVA: Analysis of Variance. Reproduced with permission from Editor of Indian Journal of Ophthalmology, and the author, Dr Barun Nayak[15]
Is there a difference between the groups of unpaired situations?
Is there a difference between the groups of paired situations?
Is there any association between the variables?
Is there any agreement between the assessment techniques?
Perform necessary analysis using user-friendly software such as GraphPad Prism, Minitab or MedCalc,etc. Once the analysis is complete, appropriate writing in the text form is equally essential. Specific test names used to examine each part of the results have to be described. Simple listing of series of tests should not be done. A typical write-up can be seen in the subsequent sections of the supplementary files [Supplementary files 3C (381.5KB, pdf) –E (381.5KB, pdf) ]. One needs to state the level of significance and software details also.
Role of a statistician in dissertation and data analysis
Involving a statistician before planning a study design, prior to data collection, after data have been collected, and while data are analysed is desirable when conducting a dissertation. On the contrary, it is also true that self-learning of statistical analysis reduces the need for statisticians’ help and will improve the quality of research. A statistician is best compared to a mechanic of a car which we drive; he knows each element of the car, but it is we who have to drive it. Sometimes the statisticians may not be available for a student in an institute. Self-learning software tools, user-friendly statistical software for basic statistical analysis thus gain importance for students as well as guides. The statistician will design processes for data collection, gather numerical data, collect, analyse, and interpret data, identify the trends and relationships in data, perform statistical analysis and its interpretation, and finally assist in final conclusion writing.
Results
Results are an important component of the dissertation and should follow clearly from the study objectives. Results (sometimes described as observations that are made by the researcher) should be presented after correct analysis of data, in an appropriate combination of text, charts, tables, graphs or diagrams. Decision has to be taken on each outcome; which outcome has to be presented in what format, at the beginning of writing itself. These should be statistically interpreted, but statistics should not surpass the dissertation results. The observations should always be described accurately and with factual or realistic values in results section, but should not be interpreted in the results section.
While writing, classification and reporting of the Results has to be done under five section paragraphs- population data, data distribution analysis, results of the primary outcome, results of secondary outcomes, any additional observations made such as a rare adverse event or a side effect (intended or unintended) or of any additional analysis that may have been done, such as subgroup analysis.
At each level, one may either encounter qualitative (n/N and %) or quantitative data (mean [SD], median [IQR] and so on.
In the first paragraph of Results while describing the population data, one has to write about included and excluded patients. One needs to cite the Consolidated Standards of Reporting Trials (CONSORT) flow chart to the text, at this stage. Subsequently, highlighting of age, sex, height, body mass index (BMI) and other study characteristics referring to the first table of ‘patients data’ should be considered. It is not desirable to detail all values and their comparison P values in the text again in population data as long as they are presented in a cited table. An example of this pattern can be seen in Supplementary file 3D (381.5KB, pdf) .
In the second paragraph, one needs to explain how the data is distributed. It should be noted that, this is not a comparison between the study groups but represents data distribution for the individual study groups (Group A or Group B, separately)[Supplementary file 3E (381.5KB, pdf) ].
In the subsequent paragraph of Results, focused writing on results of the primary outcomes is very important. It should be attempted to mention most of the data outputs related to the primary outcomes as the study is concluded based on the results of this outcome analysis. The measures of central tendency and dispersion (Mean or median and SD or IQR etc., respectively), alongside the CIs, sample number and P values need to be mentioned. It should be noted that the CIs can be for the mean as well as for the mean difference and should not be interchanged. An example of this pattern can be seen in Supplementary file 3F (381.5KB, pdf) .
A large number of the dissertations are guided for single primary outcome analysis, and also the results of multiple secondary outcomes are needed to be written. The primary outcome should be presented in detail, and secondary outcomes can be presented in tables or graphs only. This will help in avoiding a possible evaluator's fatigue. An example of this pattern can be seen in Supplementary file 3G (381.5KB, pdf) .
In the last paragraph of the Results, mention any additional observations, such as a rare adverse event or side effect or describe the unexpected results. The results of any additional analysis (subgroup analysis) then need to be described too. An example of this pattern can be seen in Supplementary file 3H (381.5KB, pdf) .
The most common error observed in the Results text is duplication of the data and analytical outputs. While using the text for summarising the results, at each level, it should not be forgotten to cite the table or graph but the information presented in a table should not be repeated in the text. Further, results should not be given to a greater degree of accuracy than that of the measurement. For example, mean (SD) age need to be presented as 34.5 (11.3) years instead of 34.5634 (11.349). The latter does not carry any additional information and is unnecessary. The actual P values need to be mentioned. The P value should not be simply stated as ‘P < 0.05’; P value should be written with the actual numbers, such as ‘P = 0.021’. The symbol ‘<’ should be used only when actual P value is <0.001 or <0.0001. One should try avoiding % calculations for a small sample especially when n < 100. The sample size calculation is a part of the methodology and should not be mentioned in the Results section.
Tables
The use of tables will help present actual data values especially when in large numbers. The data and their relationships can be easily understood by an appropriate table and one should avoid overwriting of results in the text format. All values of sample size, central tendency, dispersions, CIs and P value are to be presented in appropriate columns and rows. Preparing a dummy table for all outcomes on a rough paper before proceeding to Microsoft Excel may be contemplated. Appropriate title heading (e.g., Table 1. Study Characteristics), Column Headings (e.g., Parameter studied, P values) should be presented. A footnote should be added whenever necessary. For outputs, where statistically significant P values are recorded, the same should be highlighted using an asterisk (*) symbol and the same *symbol should be cited in the footnote describing its value (e.g., *P < 0.001) which is self-explanatory for statistically significance. One should not use abbreviations such as ‘NS’ or ‘Sig’ for describing (non-) significance. Abbreviations should be described for all presented tables. A typical example of a table can be seen in Figure 4.
Figure 4.
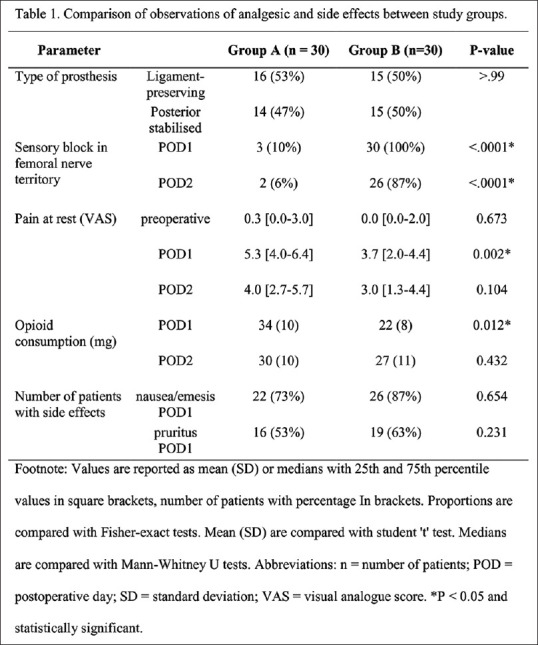
Example of presenting a table
Graphical images
Similar to tables, the graphs and diagrams give a bird's-eye view of the entire data and therefore may easily be understood. bar diagrams (simple, multiple or component), pie charts, line diagrams, pictograms and spot maps suit qualitative data more whereas the histograms, frequency polygons, cumulative frequency, polygon scatter diagram, box and whisker plots and correlation diagrams are used to depict quantitative data. Too much presentation of graphs and images, selection of inappropriate or interchanging of graphs, unnecessary representation of three-dimensional graph for one-dimensional graphs, disproportionate sizes of length and width and incorrect scale and labelling of an axis should be avoided. All graphs should contain legends, abbreviation descriptions and a footnote. Appropriate labelling of the x- and the y-axis is also essential. Priori decided scale for axis data should be considered. The ‘error bar’ represents SDs or IQRs in the graphs and should be used irrespective of whether they are bar charts or line graphs. Not showing error bars in a graphical image is a gross mistake. An error bar can be shown on only one side of the line graph to keep it simple. A typical example of a graphical image can be seen in Figure 5. The number of subjects (sample) is to be mentioned for each time point on the x-axis. An asterisk (*) needs to be put for data comparisons having statistically significant P value in the graph itself and they are self-explanatory with a ‘stand-alone’ graph.
Figure 5.
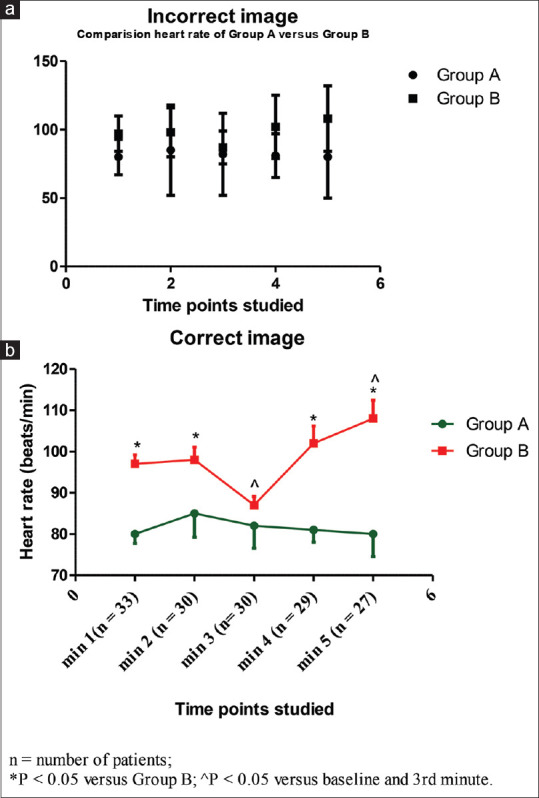
Example of an incorrect (a) and correct (b) image
Discussion
Once the results have been adequately analysed and described, the next step is to draw conclusions from the data and study. The main goal is to defend the work by staging a constructive debate with the literature.[16] Generally, the length of the ‘Discussion’ section should not exceed the sum of other sections (introduction, material and methods, and results).[17] Here the interpretation, importance/implications, relevance, limitations of the results are elaborated and should end in recommendations.
It is advisable to start by mentioning the RQ precisely, summarising the main findings without repeating the entire data or results again. The emphasis should be on how the results correlate with the RQ and the implications of these results, with the relevant review of literature (ROL). Do the results coincide with and add anything to the prevalent knowledge? If not, why not? It should justify the differences with plausible explanation. Ultimately it should be made clear, if the study has been successful in making some contribution to the existing evidence. The new results should not be introduced and any exaggerated deductions which cannot be corroborated by the outcomes should not be made.
The discussion should terminate with limitations of the study,[17] mentioned magnanimously. Indicating limitations of the study reflects objectivity of the authors. It should not enlist any errors, but should acknowledge the constraints and choices in designing, planning methodology or unanticipated challenges that may have cropped up during the actual conduct of the study. However, after listing the limitations, the validity of results pertaining to the RQ may be emphasised again.
Conclusion
This section should convey the precise and concise message as the take home message. The work carried out should be summarised and the answer found to the RQ should be succinctly highlighted. One should not start dwelling on the specific results but mention the overall gain or insights from the observations, especially, whether it fills the gap in the existing knowledge if any. The impact, it may have on the existing knowledge and practices needs to be reiterated.
What to do when we get a negative result?
Sometimes, despite the best research framework, the results obtained are inconclusive or may even challenge a few accepted assumptions.[18] These are frequently, but inappropriately, termed as negative results and the data as negative data. Students must believe that if the study design is robust and valid, if the confounders have been carefully neutralised and the outcome parameters measure what they are intended to, then no result is a negative result. In fact, such results force us to critically re-evaluate our current understanding of concepts and knowledge thereby helping in better decision making. Studies showing lack of prolongation of the apnoea desaturation safety periods at lower oxygen flows strengthened belief in the difficult airway guidelines which recommend nasal insufflations with at least 15 L/min oxygen.[19,20,21]
Publishing the dissertation work
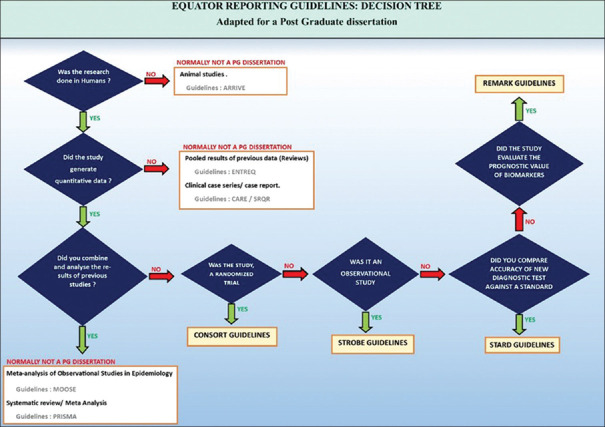
There are many reporting guidelines based upon the design of research. These are a checklist, flow diagram, or structured text to guide authors in reporting a specific type of research, developed using explicit methodology. The CONSORT[22] and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) initiatives,[23] both included in the Enhancing the Quality and Transparency of Health Research (EQUATOR) international network, have elaborated appropriate suggestions to improve the transparency, clarity and completeness of scientific literature [Figure 6].
Figure 6.
Equator publishing tree
All authors are advised to follow the CONSORT/STROBE checklist attached as Supplementary file 4 (338.2KB, pdf) , when writing and reporting their dissertation.
For most dissertations in Anaesthesiology, the CONSORT, STROBE, Standards for Reporting Diagnostic accuracy studies (STARD) or REporting recommendations for tumour MARKer prognostic studies (REMARK) guidelines would suffice.
Abstract and Summary
These two are the essential sections of a dissertation.
Abstract
It should be at the beginning of the manuscript, after the title page and acknowledgments, but before the table of contents. The preparation varies as per the University guidelines, but generally ranges between 150 to 300 words. Although it comes at the very beginning of the thesis, it is the last part one writes. It must not be a ‘copy-paste job’ from the main manuscript, but well thought out miniaturisation, giving the overview of the entire text. As a rule, there should be no citation of references here.
Logically, it would have four components starting with aims, methods, results, and conclusion. One should begin the abstract with the research question/objectives precisely, avoiding excessive background information. Adjectives like, evaluate, investigate, test, compare raise the curiosity quotient of the reader. This is followed by a brief methodology highlighting only the core steps used. There is no need of mentioning the challenges, corrections, or modifications, if any. Finally, important results, which may be restricted to fulfilment (or not), of the primary objective should be mentioned. Abstracts end with the main conclusion stating whether a specific answer to the RQ was found/not found. Then recommendations as a policy statement or utility may be made taking care that it is implementable.
Keywords may be included in the abstract, as per the recommendations of the concerned university. The keywords are primarily useful as markers for future searches. Lastly, the random reader using any search engine may use these, and the identifiability is increased.
Summary
The summary most often, is either the last part of the Discussion or commonly, associated with the conclusions (Summary and Conclusions). Repetition of introduction, whole methodology, and all the results should be avoided. Summary, if individually written, should not be more than 150 to 300 words. It highlights the research question, methods used to investigate it, the outcomes/fallouts of these, and then the conclusion part may start.
References/bibliography
Writing References serves mainly two purposes. It is the tacit acknowledgement of the fact that someone else's written words or their ideas or their intellectual property (IP) are used, in part or in toto, to avoid any blame of plagiarism. It is to emphasise the circumspective and thorough literature search that has been carried out in preparation of the work.
Vancouver style for referencing is commonly used in biomedical dissertation writing. A reference list contains details of the works cited in the text of the document. (e.g. book, journal article, pamphlet, government reports, conference material, internet site). These details must include sufficient details so that others may locate and access those references.[24]
How much older the references can be cited, depends upon the university protocol. Conventionally accepted rule is anywhere between 5-10 years. About 85% of references should be dispersed in this time range. Remaining 15%, which may include older ones if they deal with theories, historical aspects, and any other factual content. Rather than citing an entire book, it is prudent to concentrate on the chapter or subsection of the text. There are subjective variations between universities on this matter. But, by and large, these are quoted as and when deemed necessary and with correct citation.
Bibliography is a separate list from the reference list and should be arranged alphabetically by writing name of the ‘author or title’ (where no author name is given) in the Vancouver style.
There are different aspects of writing the references.[24]
Citing the reference in the form of a number in the text. The work of other authors referred in the manuscript should be given a unique number and quoted. This is done in the order of their appearance in the text in chronological order by using Arabic numerals. The multiple publications of same author shall be written individually. If a reference article has more than six authors, all six names should be written, followed by “et al.” to be used in lieu of other author names. It is desirable to write the names of the journals in abbreviations as per the NLM catalogue. Examples of writing references from the various sources may be found in the Supplementary file 5 (289KB, pdf) .
Both the guide and the student have to work closely while searching the topic initially and also while finalising the submission of the dissertation. But the role of the guide in perusing the document in detail, and guiding the candidate through the required corrections by periodic updates and discussions cannot be over-emphasised.
Assessment of dissertations
Rarely, examiners might reject a dissertation for failure to choose a contemporary topic, a poor review of literature, defective methodology, biased analysis or incorrect conclusions. If these cannot be corrected satisfactorily, it will then be back to the drawing board for the researchers, who would have to start from scratch to redesign the study, keeping the deficiencies in mind this time.
Before submission, dissertation has to be run through “plagiarism detector” software, such as Turnitin or Grammarly to ensure that plagiarism does not happen even unwittingly. Informal guidelines state that the percentage plagiarism picked up by these tools should be <10%.
No work of art is devoid of mistakes/errors. Logically, a dissertation, being no exception, may also have errors. Our aim, is to minimise them.
SUMMARY
The dissertation is an integral part in the professional journey of any medical post-graduate student. It is also an important responsibility for a guide to educate his protégé, the basics of research methodology through the process. Searching for a gap in literature and identification of a pertinent research question is the initial step. Careful planning of the study design is a vitally important aspect. After the conduct of study, writing the dissertation is an art for which the student often needs guidance. A good dissertation is a good description of a meticulously conducted study under the different headings described, utilising the various reporting guidelines. By avoiding some common errors as discussed in this manuscript, a good dissertation can result in a very fruitful addition to medical literature.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY FILES
REFERENCES
- 1.Frascati Manual. 2015: Guidelines for Collecting and Reporting Data on Research and Experimental Development. Paris: OECD; 2015. p. 398. [Google Scholar]
- 2.Study designs in biomedical research: An introduction to the different types. Students 4 Best Evidence. 2021. [Last accessed on 2021 Dec 14]. Available from: https://s4be.cochrane.org/blog/2021/04/06/an-introduction-to-different-types-of-study-design/
- 3.Bhaskar SB, Manjuladevi M. Methodology for research II. Indian J Anaesth. 2016;60:646. doi: 10.4103/0019-5049.190620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson JL, Adkins D, Chauvin S. A review of the quality indicators of rigor in qualitative research. Am J Pharm Educ. 2020;84:7120. doi: 10.5688/ajpe7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrugia P, Petrisor BA, Farrokhyar F, Bhandari M. Practical tips for surgical research: Research questions, hypotheses and objectives. Can J Surg. 2010;53:278–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Fandino W. Formulating a good research question: Pearls and pitfalls. Indian J Anaesth. 2019;63:611–6. doi: 10.4103/ija.IJA_198_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding L, Marra CJ, Illes J. Establishing a comprehensive search strategy for Indigenous health literature reviews. Syst Rev. 2021;10:115. doi: 10.1186/s13643-021-01664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg R. Methodology for research I. Indian J Anaesth. 2016;60:640. doi: 10.4103/0019-5049.190619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doody O, Bailey ME. Setting a research question, aim and objective. Nurs Res. 2016;23:19–23. doi: 10.7748/nr.23.4.19.s5. [DOI] [PubMed] [Google Scholar]
- 10.Dhulkhed VK, Tantry TP, Kurdi MS. Minimising statistical errors in the research domain: Time to work harder and dig deeper! Indian J Anaesth. 2021;65:567–71. doi: 10.4103/ija.ija_720_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor M. Types of studies and research design. Indian J Anaesth. 2016;60:626. doi: 10.4103/0019-5049.190616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karanicolas PJ, Farrokhyar F, Bhandari M. Practical tips for surgical research: Blinding: Who, what, when, why, how? Can J Surg. 2010;53:345–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz KF, Grimes DA. Allocation concealment in randomised trials: Defending against deciphering. Lancet. 2002;359:614–8. doi: 10.1016/S0140-6736(02)07750-4. [DOI] [PubMed] [Google Scholar]
- 14.McCluskey A, Lalkhen AG. Statistics I: data and correlations. Continuing Educ Anaesth Crit Care Pain. 2007;7:95–9. [Google Scholar]
- 15.Nayak BK, Hazra A. How to choose the right statistical test? Indian J Ophthalmol. 2011;59:85–6. doi: 10.4103/0301-4738.77005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad HR. How to write a doctoral thesis. Pak J Med Sci. 2016;32:270–3. doi: 10.12669/pjms.322.10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanli O, Erdem S, Tefik T. How to write a discussion section? Turk J Urol. 2014;39:20–4. doi: 10.5152/tud.2013.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negative Results: The Dark Matter of Research |AJE. [Last accessed on 2021 Dec 24]. Available from: https://www.aje.com/arc/negative-results-dark-matter-research/
- 19.Sahay N, Sharma S, Bhadani UK, Sinha C, Kumar A, Ranjan A. Effect of nasal oxygen supplementation during apnoea of intubation on arterial oxygen levels: A prospective randomised controlled trial. Indian J Anaesth. 2017;61:897. doi: 10.4103/ija.IJA_232_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myatra S, Ahmed S, Kundra P, Garg R, Ramkumar V, Patwa A, et al. The all India difficult airway association 2016 guidelines for tracheal intubation in the Intensive Care Unit. Indian J Anaesth. 2016;60:922. doi: 10.4103/0019-5049.195485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta D. Highlight negative results to improve science. Nature. 2019 doi: 10.1038/d41586-019-02960-3. doi: 10.1038/d41586-019-02960-3. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology. 2007;18:800–4. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 24.Guides.lib.monash.edu. 2021. [Last accessed on 2021 Dec 09]. Available from: https://guides.lib.monash.edu/ld.php?content_id=48260115 .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.